Abstract
Previous studies have shown that ambient ultrafine particles with diameters less than 100 nm (UFPs) can pass from the lungs to the circulation because of their very small diameter, and induce lung oxidative stress with a resultant dysfunction of lung endothelial cells. However, no studies have addressed the potential combined effects of UFPs and cigarette smoke on vascular endothelial cells. We hypothesized that co-exposure to UFPs and cigarette smoke extract (CSE) may cause combined effects on activation of endothelial cells and dysfunction of endothelium by oxidative stress through activation of NADPH oxidase. We determined the effects of UFPs with or without CSE on mouse pulmonary microvascular endothelial cells (MPMVEC) obtained from C57BL/6J (wild-type) and gp91phox knock-out mice (gp91phox is one of the key components of NADPH oxidase, one of ROS generators). Our results showed that exposure of MPMVEC from wild-type mice to UFPs or CSE, at a non-toxic dose, induced reactive oxygen species (ROS) generation, increased phosphorylation of p38 and Erk1/2, and up-regulated early growth response -1 (Egr-1) and IL-6 genes. These effects were significantly enhanced when cells were co-exposed to both UFPs and CSE. However, exposure of MPMVEC from gp91phox knock-out mice did not induce the above effects. Furthermore, UFPs- and/or CSE-induced Egr-1 mRNA upregulation was attenuated significantly when cells were pre-treated with p38 specific inhibitor, SB 203580, or MEK1/2 inhibitor, PD98059, and Egr-1 siRNA treatment abolished UFPs- and/or CSE- induced overexpression of IL-6. Our results suggest that UFPs and/or CSE caused activation of NADPH oxidase, resulting in ROS generation that led to activation of MAPKs through induced phosphorylation of p38 and ERK1/2 MAPKs and upregulation of Egr-1. Those effects may further result in endothelial dysfunction through production of cytokines such as IL-6. Our results suggest that co-exposure to UFPs and CSE causes enhanced injury to endothelial cells.
Keywords: Ultrafine particles, cigarette smoke extract, reactive oxygen species, NADPH oxidase, gp91phox, MAPKs, Egr-1, IL-6
Introduction
Particulate air pollution is generated from both anthropogenic sources and natural sources. Epidemiological evidence has shown a clear and consistent association between concentrations of ambient particulate matter (PM) and increases in pulmonary and cardiovascular morbidity and mortality (Dockery et al., 1993; Pope et al., 2002, 2004). However, the underlying mechanisms are still unclear. Ultrafine particles (UFPs) with diameters less than 100 nm are important component of PM since they have small diameter and large specific surface area. Several studies have shown that they have the ability to translocate from the lungs into the circulation, where they may mediate their effects on endothelium in lungs and other organs (Araujo et al., 2008; Calderon-Garciduenas et al., 2001; Frampton et al., 2001, 2006; Shimada et al., 2006; Upadhay et al., 2008). Our previous studies demonstrated that exposure of endothelial cells to UFPs caused reactive oxygen species (ROS) generation, thus resulting in endothelial dysfunction by oxidative stress through activation of NADPH oxidase (Mo et al., 2009).
Cigarette smoke is widely recognized as one of the major causes of coronary artery disease, cancer (particularly lung cancer), and chronic obstructive pulmonary disease (COPD) in adults. Cigarette smoke extract (CSE) has been shown to impact the function of endothelium and cause altered gene expression in endothelial cells via their ability to generate ROS and nitrogen species (Barnoya and Glantz, 2005; Bercher et al., 2007; Brunekreef and Holgates, 2002; Donaldson et al., 2001; Edirisinghe et al. 2008). CSE could activate NADPH oxidase and increase endothelial superoxide anion generation, thereby resulting in endothelial dysfunction (Jaimes et al., 2004). Previous studies also showed that exposure to CSE caused the loss of endothelial integrity resulting in expression of leukocyte adhesion proteins, reduced anticoagulant activity, and the release of growth factors, inflammatory mediators and cytokines (Hoshino et al., 2005; Low et al., 2007; Milara et al., 2010; Orose et al., 2007; Shih et al., 2010).
Excessive ROS generation, known as a state of oxidative stress, has been linked to cardiovascular diseases. Several mechanisms have been proposed to explain how excessive production of ROS leads to vascular pathology: (1) ROS are able to promote the oxidation of low-density lipoprotein (LDL) (Lusis, 2000); (2) O2- rapidly inactivates endothelium-derived nitric oxide (NO), a molecule with intrinsic anti-atherogenic properties, leading to endothelial dysfunction, a hallmark of early atherosclerosis (Cai and Harrison, 2000; Griendling et al., 2000; Lum and Roebuck, 2001; Lusis, 2000); and (3) the reaction between O2-.and NO generates peroxynitrite (ONOO-), which has been found to be cytotoxic to endothelial cells (Cai and Harrison, 2000; Griendling et al. 2000; Lum and Roebuck, 2001; Lusis, 2000). There are four main potential sources of superoxide and H2O2 in the vascular wall: vascular NADPH oxidase; xanthine oxidase; NO synthase; and mitochondria. Several reports have shown that NADPH oxidase is the main ROS generator in vascular endothelial cells (Bokoch and Knaus, 2003; Cai and Harrison, 2000; Griendling et al., 2000; Lum and Roebuck, 2001; Mo et al., 2009; Orosz et al., 2007).
Although there are links between the cardiovascular diseases and cigarette smoke or PM, data on the combined effects for cardiovascular diseases from co-exposure to UFPs and cigarette smoke is lacking. And the mechanisms underlying endothelial dysfunction caused by cigarette smoke and/or UFPs are still obscure. In the present study, we hypothesized that co-exposure to UFPs and cigarette smoke extract would cause enhanced effects on pulmonary endothelium by oxidative stress through activation of endothelial NADPH oxidase, thus resulting in activation of MAPKs and upregulation of early growth response 1 gene (Egr-1) and proinflammatory cytokines such as interleukin-6 (IL-6). Two kinds of endothelial cells were used in this study. One was mouse pulmonary microvascular endothelial cells (MPMVEC) isolated from C57BL/6J mice (wild-type), while the other was MPMVEC isolated from gp91phox knock out (KO) mice. Gp91phox is one of the membrane components of NADPH oxidase. First, we investigated whether co-exposure of endothelial cells to UFPs and CSE have combined effects on causing endothelial cell ROS generation, MAPKs activation, and Egr-1 and IL-6 upregulation. Then, we determined the possible pathways involved in UFPs- and/or CSE-induced endothelial cell dysfunction.
Materials and Methods
Chemicals and reagents
Antibodies against total p38, phospho-p38, total ERK1/2 and phospho-ERK1/2 were obtained from Cell Signaling Technology (Beverly, MA), monoclonal mouse anti-β-actin antibody from Sigma (Saint Louis, MO), polyclonal rabbit anti-Egr1 antibody and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG from Santa Cruz Biotechnology (Santa Cruz, CA), and HRP-conjugated goat anti-rabbit IgG from CHEMICON (Temecula, CA). 2', 7'-dichlorodihydrofluorescein diacetate (H2-DCFDA) was purchased from Molecular Probes (Eugene, OR), SB 203580 from TOCRIS (Ellisville, MO) and PD98059 from Cell Signaling Technology (Beverly, MA). Reagents for cell culture were purchased from Mediatech, Inc. (Herndon, VA) including Dulbecco's Modification of Eagle's Medium (DMEM), fetal bovine serum (FBS), 0.05% trypsin EDTA, nonessential amino acids, and penicillin-streptomycin solution. All other chemicals were purchased from Fisher Scientific (Fair Lawn, NJ) except when otherwise stated.
Animals
C57BL/6J mice (wild-type) were used as control of gp91phox KO mice (B6.129S6-Cybbtm1Din/J) since the gp91phox KO mice were in a C57BL/6 background. Both mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal use was reviewed and approved by the University of Louisville Institutional Animal Care and Use Committee.
Source and characteristics of particles
UFPs were collected and prepared as described in our previous study (Mo et al., 2009) by using a nano-MOUDI cascade impactor (MSP Corporation, Shoreview, MN) in downtown Louisville, Kentucky, USA. The characteristics of UFPs were determined as in our previous study (Mo et al., 2009).
Preparation of CSE solution
The CSE was prepared according to a previous report (Edirisinghe et al., 2008). Research grade cigarettes (3R4F) were obtained from Kentucky Tobacco Research & Development Center, University of Kentucky, Lexington, Kentucky. Each cigarette contains 9.4 mg tar, 0.73 mg nicotine, and 11.0 mg total particulate matter (TPM). Mainstream smoke of 20 cigarettes was withdrawn steadily via a pump and bubbled in 20 ml DMEM without phenol red and fetal bovine serum. The concentration of 100% CSE has a 0.6 absorbance at 320 nm. The CSE was then aliquoted and stored at –80°C immediately. The CSE was diluted to the desired percentage with DMEM prior to cell exposure.
Endothelial cell culture
Mouse pulmonary microvascular endothelial cells (MPMVEC) were isolated from lungs of wild-type C57BL/6J or gp91phox KO mice as described previously (Mo et al., 2009; Yu et al., 2010; Zhang et al., 2005, 2008). The endothelial phenotype was confirmed by demonstrating cellular uptake of DiI-labeled acetylated low-density lipoprotein (DiI-Ac-LDL) and reactivity to anti-PECAM-1 (Zhang et al., 2005, 2008).MPMVEC were grown in a 5% CO2 atmosphere at 37°C in DMEM with 4.5 g/l glucose, L-glutamine and sodium pyruvate, supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, and 1% nonessential amino acids.
Cytotoxicity assays
Two different methods were used to determine the cytotoxicity of UFPs and/or CSE in MPMVEC according to manufacturer's instructions. One is CellTiter 96 AQueous non-radioactive cell proliferation assay (MTS assay) (Promega, Madison, WI) while another is In Vitro Toxicity Assay Kit, Sulforhodamine B (SRB) Based (SRB assay) (Sigma, St. Louis, MO). MTS assay is a colorimetric method for determining the number of metabolically active cells in which dehydrogenase enzymes can convert a tetrazolium compound (MTS) into an aqueous, soluble, and colored formazan. SRB assay is a means of measuring total biomass by staining cellular proteins with the Sulforhodamine B. Briefly, 3 × 103 cells were seeded into each well of 96-well plates and were allowed to attach to the growth surface by culturing overnight. The cytotoxicity was measured after 24 h at different concentrations of UFPs (0, 10, 20, 50, 100 and 200 μg/ml), CSE (0, 1, 2.5, 5, 7.5, 10, 15 and 20 %) or UFPs with CSE treatment. The experiment was repeated three times with six replicates in each experiment. The absorbance was recorded by a Multi-Detection Microplate Reader (Synergy™ HT, BioTek, Winooski, VT). The cell viability was expressed as the percentage of the control which was without any treatments.
Endothelial ROS generation
2', 7'-dichlorodihydrofluorescein diacetate (H2-DCFDA) was used to determine intracellular oxidants generated by UFPs and/or CSE exposure as previously described (Wan et al., 2008, 2011; Yu et al., 2010; Mo et al., 2009; Zhang et al., 2005, 2008). H2-DCFDA is nonfluorescent and cell-permeant. It can rapidly diffuse through the cell membrane and is hydrolyzed by intracellular esterases to an oxidative sensitive form, dichlorodihydrofluorescein (H2-DCF). This serves as a substrate for intracellular oxidants to generate highly fluorescent DCF with a fluorescent intensity proportional to intracellular ROS (Mo et al., 2009). Briefly, 1 × 104 cells were seeded into each well of 96-well plates and cultured overnight to allow the cells to attach to the growth surface. Then cells were incubated for 2 hours with 5 μM of H2-DCFDA at 37°C in the dark following treatment with different concentration of UFPs and/or CSE for four hours. The fluorescence of DCF was measured using a Multi-Detection Microplate Reader (Synergy™ HT, BioTek, Winooski, VT) at 485 nm excitation (λex) and 528 nm emission (λem). Cell fluorescence without the addition of H2-DCFDA was used as background fluorescence level and subtracted out from the sample fluorescence level. Cells with H2-DCFDA pretreatment but without UFPs and/or CSE exposure were used as control. Results were expressed as the percentage of the arbitrary fluorescence units (AFU) in control cells.
Protein isolation and Western blot
Cytosolic proteins were extracted by using RIPA lysis buffer (Santa Cruz, CA) supplemented with PMSF, protease inhibitor cocktail and sodium orthovanadate (Santa Cruz). Cells were lysed in RIPA on ice for 40 min. The supernatant which contains cytosolic proteins was collected after centrifuging at 10,000 × g and 4°C for 15 min. The protein concentration in the supernatant was measured by Bradford method with a DU730 Spectrophotometer (Beckman Coulter, Fullerton, CA). Total proteins were extracted according to our previous study (Mo et al., 2009; Yu et al., 2010; Zhang et al., 2008). Western blot was performed as previously described with minor modifications (Mo et al., 2003, 2009; Yu et al., 2010; Zhang et al., 2008). Briefly, 5-40 μg protein was loaded in each lane of 12% (w/v) polyacrylamide gel in the presence of 0.1% SDS and electrotransferred into Immun-Blot polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The blot was blocked by incubation in 5% milk in 1× TBST (10 mM Tris/150 mM NaCl/0.05% Tween-20, pH 7.5) for 2 h at room temperature, then incubated with primary antibodies at 4°C overnight, followed with HRP-conjugated secondary antibodies (1:2000) for 1 h. Immunoreactive bands were detected by using ECL™ Western Blotting Detection Reagents (GE Healthcare, Amersham™, Buckinghamshire, UK) following exposure to x-ray film (Kodak, Rochester, NY) and quantified by using NIH image J software.
Total RNA isolation, reverse transcription (RT) and real-time PCR
TRI Reagent (SIGMA, St. Louis, MO) was used to isolate total RNA according to the manufacturer's instruction. RNA concentration was measured by absorbance at 260 nm with a DU 730 Spectrophotometer (Beckman Coulter, Fullerton, CA). 2 μg total RNA was reverse-transcribed into cDNA using 1μl M-MLV reverse transcriptase (Promega, Madison, WI) in a total volume of 25 μl which contains 2 μl of 0.5 μg/μl oligo(dT)18 primer, 1.25 μl of 10 mM dNTP, 0.75 μl RNasin Ribonuclease inhibitor, and 5 μl of 5 × M-MLV reaction buffer. Real-time PCR was performed by using a Bio-Rad iQ5 iCycler as previous described (Mo et al., 2003, 2009). Briefly, 1 μl cDNA from each sample was mixed with 1 μl of 5 μM of each primer, 10 μl of 2 × SYBR Green Supermix (Bio-Rad) in a total volume of 20 μl. The experimental protocol consisted of four programs: (1) denaturation of the cDNA/RNA hybrid at 95°C for 3 min; (2) amplification of cDNA for 50 cycles, each cycle using sequentially 95°C for 10 s, 58°C (β-actin and Egr-1) or 59.3°C (IL-6) for 30 s and 72°C for 30 s; (3) analysis of the melting curve to confirm the single product amplification during the PCR assay; and (4) cooling the rotor and thermal chamber at 25°C. The specific primers for mouse IL-6, Egr-1 and β-actin (as the internal control) were: IL-6 sense 5′-TTG GGA CTG ATG CTG GTG ACA-3’; IL-6 antisense 5′-TTG GAA ATT GGG GTA GGA AGG A-3′; Egr-1 sense 5’-GGG GAG CCG AGC GAA CAA CC-3’; Egr-1 antisense 5’-GAG GCA GAG GAA GAC GAT GAA GCA G-3’; β-actin sense 5′-GGC ATT GTT ACC AAC TGG GAC-3′; and β-actin antisense 5′-ACC AGA GGC ATA CAG GGA CAG-3′ (Mo et al., 2009). The relative expression level of each gene was calculated as fold dilution by using a standard curve for each gene. Standard curves were obtained by real-time PCR using 3 μl, 1 μl, and 1 μl of 10-fold dilution, 100-fold dilution and 1000-fold dilution respectively of cDNA obtained from MPMVEC. The expression levels of IL-6 and Egr-1 were then normalized with the relative expression level of mouse β-actin in the same sample. Each sample was performed in triplicate.
IL-6 measurement by ELISA. 2 × 105 cells were seeded into each well of 6-well plates. After overnight culture, cells were treated with UFPs, CSE, or UFPs with CSE in 1 ml medium for 24 h. IL-6 levels in the cell culture supernatants were determined by Endogen Mouse IL-6 ELISA Kit (Pierce Biotechnology, Inc., Rockford, IL) according to the manufacturer's instructions. In order to eliminate possible cell number differences under each experimental condition, cell lysates were also collected by using RIPA lysis buffer (Santa Cruz, CA) supplemented with PMSF, protease inhibitor cocktail and sodium orthovanadate (Santa Cruz). Total protein was measured using a BioRad protein assay. IL-6 secretion into media was represented as pictograms released into media per mg total cell protein.
Transfection of MPMVEC with Egr-1 siRNA
Egr-1 siRNA transfection was performed as our previous study (Mo et al., 2009). Egr-1 siRNA (Santa Cruz, CA) is a pool of three target-specific 20-25 nt dsRNAs. Control siRNA–A (Santa Cruz) was used to see if there is “off-targeting” effect. Briefly, cells were transfected with Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) and 100 nM Egr-1 siRNA or Control siRNA-A in antibiotic-free and FBS-free DMEM for 5-7 h. Then equal volumes of fresh DMEM containing 20% FBS and 2 times antibiotics concentration were added. After culture for 18~24 h, the medium was aspirated and normal DMEM was added. Then two experiments were performed. (1) To evaluate the efficiency of the Egr-1 siRNA knock-down, transfected cells were cultured for an additional 12 h. Then cells were collected for Western blot as described above. (2) To determine the effects of Egr-1 siRNA on IL-6 expression, transfected MPMVEC were exposed to 50 μg/ml of UFPs and/or 2.5% CSE for 12 h. Cells were collected for real-time PCR as described above.
Statistical analysis
Data were expressed as mean ± SD and analyzed by ANOVA. Significance was considered present when a p-value was less than 0.05. Statistical analysis was carried out using SigmaStat software (Jandel Scientific, San Raphael, CA).
Results
Cytotoxicity of UFPs, CSE and UFPs with CSE in MPMVEC
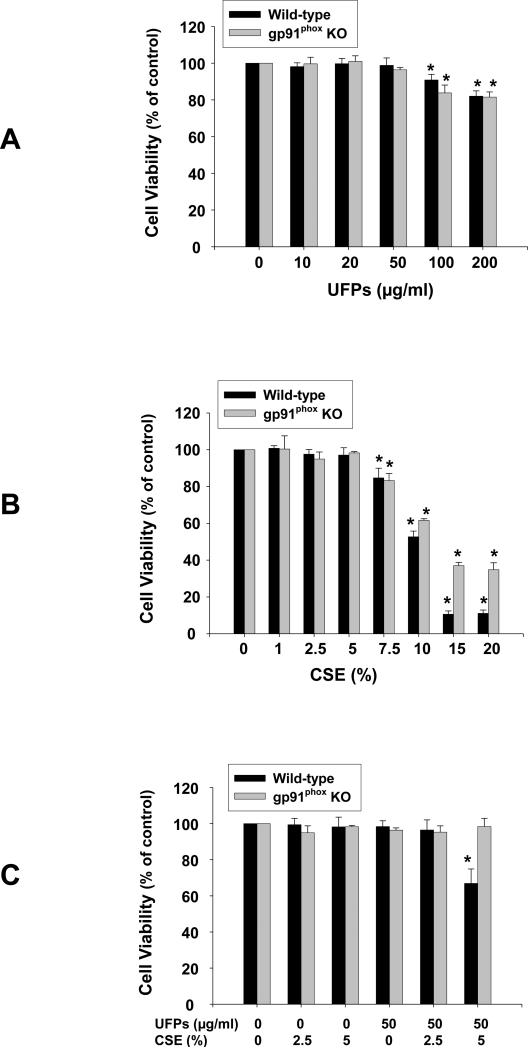
In MPMVEC from both wild-type and gp91phox KO mice, exposure to 50 μg/ml or less of UFPs and 5 % or less of CSE for 24 h did not cause significant cytotoxicity as determined by MTS assay (Fig. 1 A & B). Significant cytotoxicity was observed when cells were exposed to 100 and 200 μg/ml of UFPs and 7.5% or more of CSE for 24 h (Fig. 1 A & B). In MPMVEC obtained from wild-type mice, significant cytotoxicity was observed when cells were co-exposed to both 50 μg/ml of UFPs and 5% of CSE although anyone of them alone did not induce significant cytotoxicity (Fig. 1C). Of note, this combined effect was not observed in MPMVEC obtained from gp91phox KO mice (Fig. 1C). The above results were further confirmed by SRB assay (Data not shown). In the following experiments, non-toxic doses were chosen to investigate the effects of UFPs and/or CSE on endothelial cells.
Figure 1. Cytotoxicity of UFPs (A), CSE (B) and UFPs with CSE (C) on MPMVEC from wild-type or gp91phox KO mice.
3 × 103 cells were seeded into each well of 96-well plates. After overnight culture, cells were treated with UFPs, CSE or UFPs with CSE, respectively. Cytotoxicity was determined with MTS assay kit (Promega) after 24 h treatment. Cells without UFPs or CSE treatment were used as controls. Data are shown as mean ± SD of three experiments with six replicates in each experiment. * Significant difference as compared with the control, p < 0.05.
Co-exposure to UFPs and CSE caused increased ROS generation
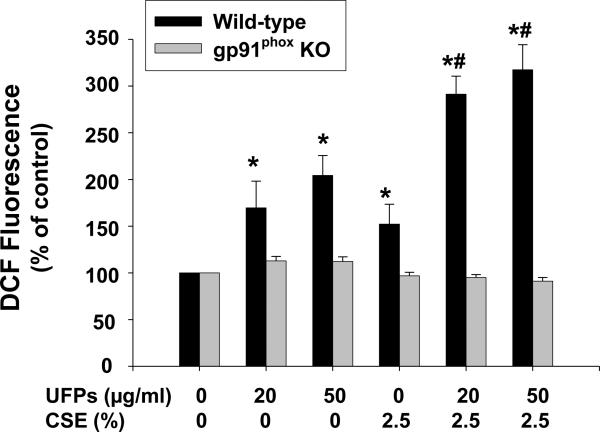
ROS generation after exposure of MPMVEC to UFPs and/or CSE was determined by measuring DCF fluorescence intensity in the cell culture system as described previously (Mo et al., 2009; Wan et al., 2008, 2011; Yu et al., 2010). In MPMVEC from wild-type mice, exposure to 20 and 50 μg/ml of UFPs and 2.5% of CSE for four hours generated significant ROS (Fig. 2). ROS was significantly enhanced when cells were co-exposed to both UFPs and CSE (Fig. 2). In MPMVEC obtained from gp91phox KO mice, no significantly increased ROS generation were observed when cells were either exposed to UFPs, CSE or UFPs with CSE (Fig. 2). Since gp91phox is one of key components of NADPH oxidase, our results demonstrated that NADPH oxidase appears to be responsible for ROS generation in MPMVEC exposed to UFPs and/or CSE.
Figure 2. Effects of UFPs, CSE and UFPs with CSE on ROS generation in MPMVEC from wild-type or gp91phox KO mice.
1 × 104 cells were seeded into each well of 96-well plates. After overnight culture, cells were pretreated with 5 μM H2-DCFDA for 2 h prior to exposure to UFPs, CSE or UFPs with CSE for 4 h. Data are shown as mean ± SD of three experiments with six replicates in each experiment. DCF fluorescence in cells with 5 μM H2-DCFDA pre-treatment but without UFPs and CSE treatment was used as control. * Significant difference as compared with the control, p < 0.05; # Significant difference as compared with the UFPs alone or CSE alone treated group, p < 0.05.
Co-exposure to UFPs and CSE resulted in increased phosphorylation of MAPKs
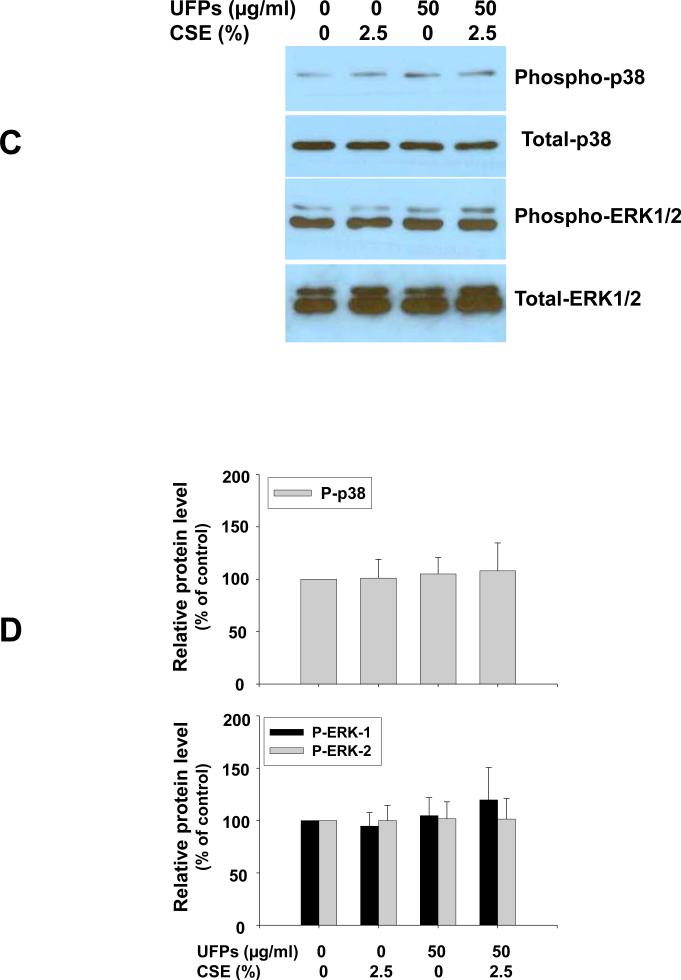
To investigate whether UFPs and/or CSE-induced ROS generation could activate endothelial cells, the effects of UFPs and/or CSE on p38 and ERK1/2 mitogen-activated protein kinase (MAPK) activation in MPMVEC were studied by Western blot. Our results showed that in MPMVEC from wild-type mice, the phosphorylation of p38 and ERK1/2 MAPKs increased significantly after 20 and 50 μg/ml of UFPs and 2.5% of CSE exposure for one hour (Fig. 3 A & B). This effect was enhanced when cells were co-exposed to both UFPs and CSE (Fig. 3 A & B). However, no such effects were observed when MPMVEC from gp91phox mice were exposed to UFPs and/or CSE (Fig. 3 C & D). These results suggest that ROS generation in MPMVEC exposed to UFPs and/or CSE is involved in the activation of MAPKs.
Figure 3. Increased phosphorylation of p38 and ERK1/2 in MPMVEC from wild-type mice exposed to UFPs, CSE and UFPs with CSE (A & B). In MPMVEC from gp91phox KO mice, UFPs and CSE failed to induce such effects (C & D).
Cells were treated with UFPs, CSE or UFPs with CSE for one hour. Cells without UFPs and CSE treatment were used as control. 30 μg protein (phospho-p38 and p38) and 10 μg protein (phospho-ERK1/2 and ERK1/2) were loaded in each lane. For ERK, two different isoforms were identified, ERK1 (p44) and ERK2 (p42). P-p38, phospho-p38; P-ERK1/2, phospho-ERK1/2. A and C show the results of a single Western blot experiment. B and D represent normalized band densitometry readings averaged from 3 independent experiments ± SD of Western results. * Significant difference as compared with the control, p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05.
Co-exposure to UFPs and CSE led to increased Egr-1 expression
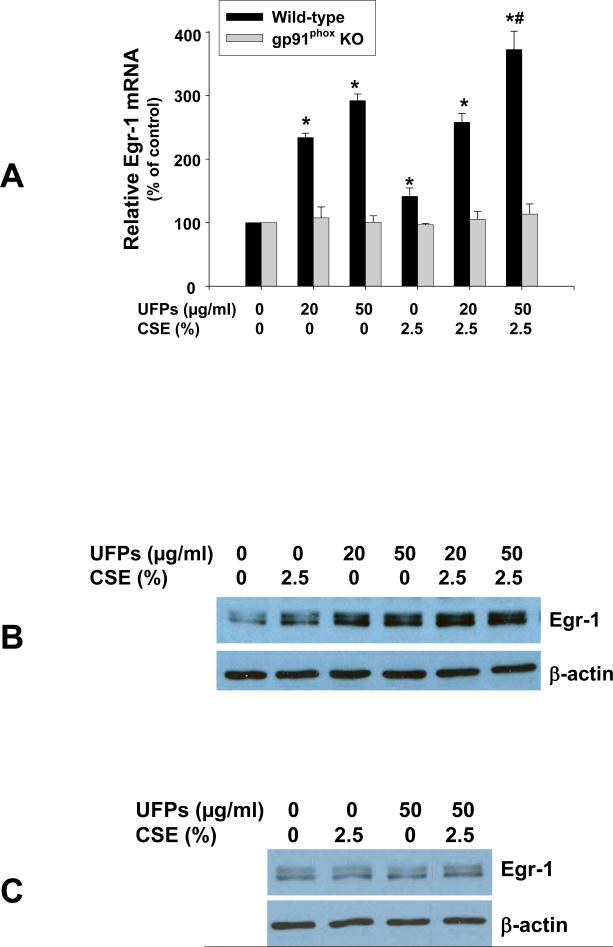
Exposure of MPMVEC from wild-type mice to 20 and 50 μg/ml of UFPs and 2.5% of CSE caused increased Egr-1 expression at both mRNA (Fig. 4A) and protein (Fig. 4B) levels. Co-exposure to UFPs and CSE enhanced these effects (Fig. 4 A & B). To investigate whether activation of NADPH oxidase was involved in the above effects, MPMCEV from gp91phox KO mice were exposed to UFPs and/or CSE. Our results showed that neither UFPs nor CSE induced Egr-1 over-expression in MPMVEC from gp91phox KO mice (Fig. 4 A & C).
Figure 4. Egr-1 induction in MPMVEC from wild-type mice exposed to UFPs, CSE and UFPs with CSE (A & B), but not in MPMVEC from gp91phox KO mice (C).
2 × 105 cells were seeded into each well of 6-well plates. After overnight culture, cells were treated with UFPs, CSE, or UFPs with CSE. Cells were collected after one hour treatment for real-time PCR (A) or after 3 hours treatment for Western blot (B & C). (A) Data are shown as mean ± SD of three experiments with three replicates in each experiment. * Significant difference as compared with the control, p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05.
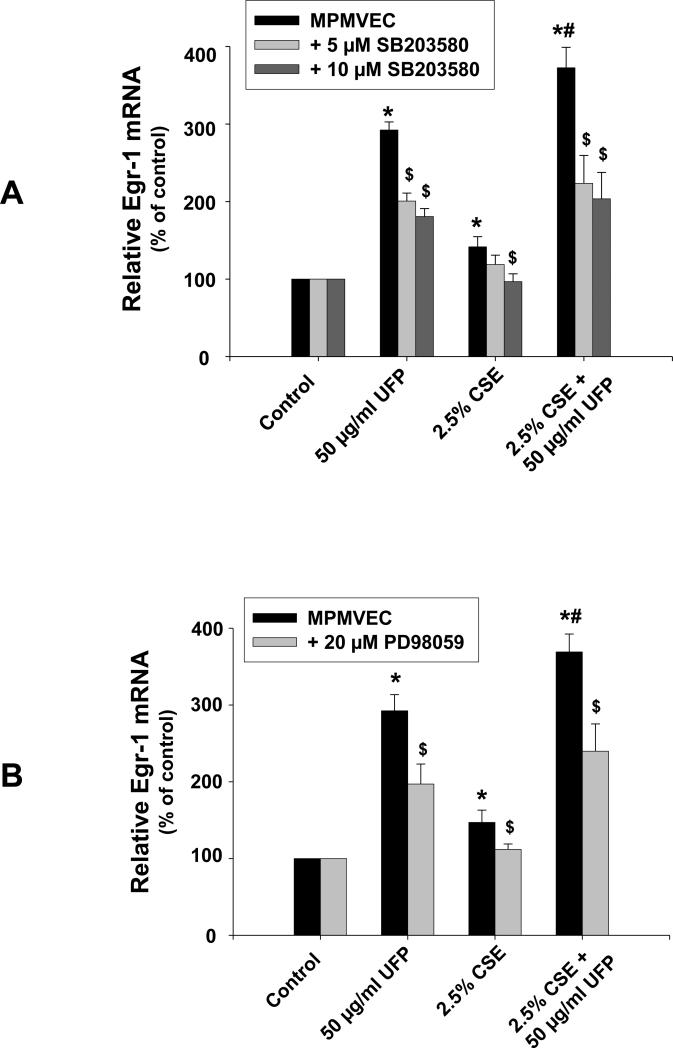
To further examine the potential pathways involved in UFPs- and/or CSE-induced Egr-1 up-regulation, a specific p38 inhibitor, SB203580 (5 μM or 10 μM), or an MEK1/2 inhibitor, PD98059 (20 μM), were used to pre-treat MPMVEC from wild-type mice 3 h prior to exposure to UFPs and/or CSE for 1 h. Our results indicated that pre-treatment of MPMVEC from wild-type mice with the p38 specific inhibitor (Fig. 5A) or MEK1/2 inhibitor (Fig. 5B) significantly attenuated UFPs- and/or CSE- induced Egr-1 up-regulation. These results suggest that activation of MAPK pathways was involved in the increased Egr-1 expression after exposure to UFPs and/or CSE.
Figure 5. UFPs- and/or CSE- induced Egr-1 upregulation in MPMVEC from wild-type mice was abolished by pre-treatment of cells with SB203580 (A) and PD98059 (B).
2 × 105 cells were seeded into each well of 6-well plates. After overnight culture, cells were pre-treated with 5 μM or 10 μM of SB203580 (A), or 20 μM of PD98059 (B) for 3 h, then treated with UFPs and/or CSE for one hour. Total RNA was extracted and used for real-time PCR. Data are shown as mean ± SD of three experiments with three replicates in each experiment. * Significant difference as compared with the control (without any treatment), p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05; $ Significant difference as compared with those with UFPs and/or CSE treatment but without SB203580 or PD98059 pre-treatment, p < 0.05.
Co-exposure to UFPs and CSE upregulated IL-6 expression
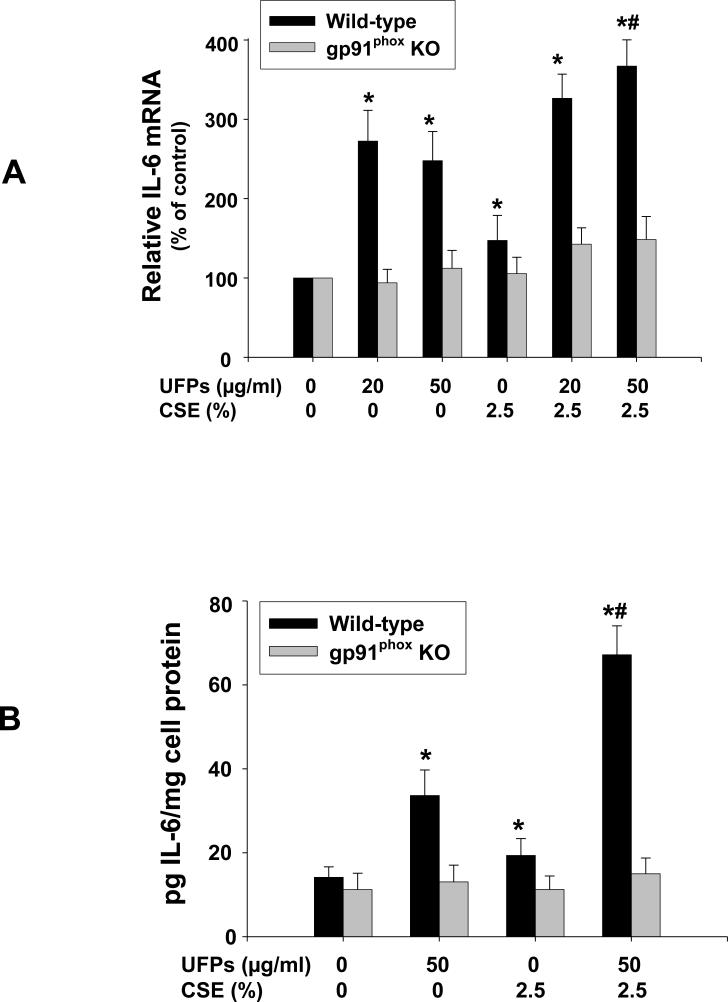
To determine whether UFPs and/or CSE will induce endothelial cell inflammation and dysfunction, we investigated the expression level of proinflammatory cytokine, IL-6, in MPMVEC exposed to UFPs and/or CSE. The results showed that exposure of MPMVEC from wild-type mice to 20 and 50 μg/ml of UFPs and 2.5% of CSE for 12 h caused increased IL-6 mRNA expression by real-time PCR which was further increased when cells were co-exposed to 50 μg/ml of UFPs and 2.5% of CSE (Fig. 6A). The effects of UFPs and/or CSE on IL-6 were further confirmed at the protein level by ELISA (Fig. 6B). However, IL-6 expression level did not increase when MPMVEC from gp91phox KO mice were exposed to UFPs and/or CSE (Fig. 6 A & B). These results demonstrated the important role of ROS in UFPs- and/or CSE- induced IL-6 upregulation.
Figure 6. Increased expression of IL-6 in MPMVEC from wild-type mice exposed to UFPs, CSE and UFPs with CSE.
2 × 105 cells were seeded into each well of 6-well plates. After overnight culture, cells were treated with UFPs, CSE, or UFPs with CSE for 12 h (A) or 24 h (B). Cells without any treatment were used as controls. (A) IL-6 mRNA was analyzed by real-time PCR. Data are shown as mean ± SD of three experiments with three replicates in each experiment. (B) IL-6 in the cell culture medium was determined by ELISA. Data are shown as mean ± SD of three experiments with duplicates in each experiment. * Significant difference as compared with the control, p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05.
To investigate whether MAPKs activation was involved in UFPs- and/or CSE- induced IL-6 upregulation, a p38 specific inhibitor, SB203580 (10 μM), was used to pre-treat MPMVEC from wild-type mice for 3 h prior to exposure to UFPs and/or CSE for an additional 12 h. The results showed that SB203589 pre-treatment significantly attenuated UFPs- and/or CSE- induced IL-6 up-regulation (Fig. 7), which demonstrated the involvement of p38 pathway in the IL-6 up-regulation.
Figure 7.
Increased expression of IL-6 in MPMVEC from wild-type mice exposed to UFPs and/or CSE was attenuated by pre-treatment of cells with p38 inhibitor. 2 × 105 cells were seeded into each well of 6-well plates. After overnight culture, cells were pre-treated with 10 μM SB203580 for 3 h, then treated with UFPs and/or CSE for 12 h. Cells without any treatment were used as controls. Real-time PCR was performed with iQ5 Cycler (Bio-Rad). Data are shown as mean ± SD of three experiments with three replicates in each experiment. * Significant difference as compared with the control, p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05; $ Significant difference as compared with those with UFPs and/or CSE treatment but without SB203580 pre-treatment, p < 0.05.
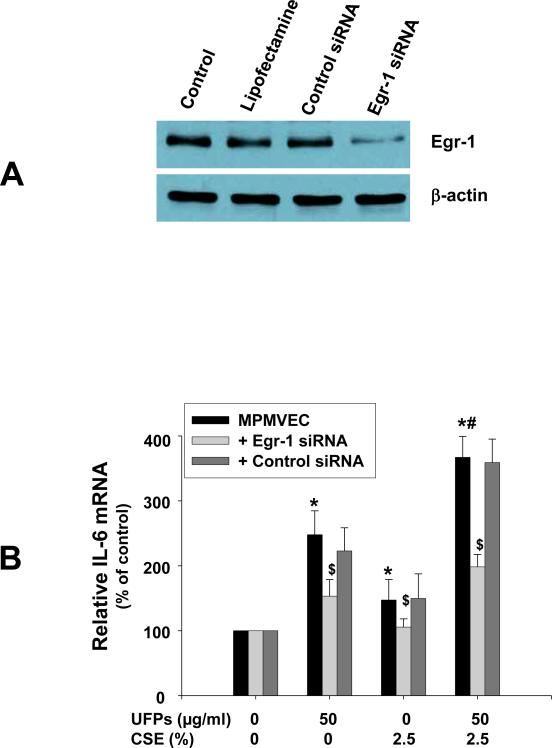
In order to determine if Egr-1 was involved in the increased expression of IL-6 after UFPs and/or CSE exposure, Egr-1 siRNA was transfected into MPMVEC from wild-type mice. Our results showed that Egr-1 siRNA treatment significantly inhibited Egr-1 protein expression (Fig. 8A) and abolished UFPs- and/or CSE- induced IL-6 upregulation (Fig. 8B).
Figure 8. Egr-1 siRNA treatment inhibited Egr-1 expression (A) and abolished increased expression of IL-6 in MPMVEC from wild-type mice exposed to UFPs, CSE and UFPs with CSE (B).
(A) MPMVEC were collected for Western blot after transfected with 100 nM of Egr-1 or control siRNA. 40 μg (Egr-1) or 5 μg (β-actin) protein was loaded in each lane. Control, cells without transfection; Lipofectamine, cells treated with Lipofectamine 2000 Reagent (Invitrogen) but without any siRNA treatment; Control siRNA, cells transfected with control siRNA-A (Santa Cruz). (B) Cells were transfected with Egr-1 siRNA for 24 h following treatment with UFPs, CSE, or UFPs with CSE for 12h. Real-time PCR was performed with iQ5 Cycler (Bio-Rad). Data are shown as mean ± SD of three experiments with three replicates in each experiment. * Significant difference as compared with the control (without any treatment), p < 0.05; # Significant difference as compared with UFPs alone or CSE alone treated group, p < 0.05; $ Significant difference as compared with non-siRNA transfected UFPs- and/or CSE- treated group, p < 0.05.
Discussion
While cigarette smoke is widely recognized as a major cause of pulmonary and cardiovascular diseases, there are other causes as well, some acting in concert with smoking to increase risk (Barnoya et al., 2005; Witschi et al., 1997). For example, epidemiological studies have demonstrated a strong link between exposure to high concentrations of PM10 (mean aerodynamic diameter less than 10 μm) or PM2.5 (diameter less than 2.5 μM) and the increased incidence of pulmonary and cardiovascular diseases (Dockery et al., 1993; Pope et al., 2002, 2004). In fact, smoking and PM10/PM2.5 both have been found to be important risk factors in the development of early atherosclerosis (Dockery et al., 1993; Knoflach et al., 2003; Pope et al., 2002, 2004). Therefore, a better understanding of the effects of smoking and PM on endothelial cells is an important step that may lead to a better understanding of the potential mechanisms involved in these effects and may suggest novel therapies or preventative strategies for smoking- and PM- induced cardiovascular diseases.
Previous studies showed that ultrafine particles can pass from the lungs to the circulatory system (Hanoir et al., 2003; Nemmar et al., 2001, 2002a, 2002b, 2003). Our studies demonstrated that exposure to UFPs, an important component of PM, can cause the dysfunction of endothelial cells through activation of NADPH oxidase (Mo et al., 2009). Although exposure to UFPs or cigarette smoking alone has been shown to cause activation of endothelial cells, little is known about the combined effects of UFPs and cigarette smoking. In the present study, we not only measured the effects of UFPs or CSE alone on endothelial cells, but also determined their combined effects after cells were co-exposed to both UFPs and CSE including their cytotoxicity and ROS generation ability. We also explored whether co-exposure to UFPs and CSE increased endothelial dysfunction by UFPs- and CSE- induced oxidative stress, activation of protein kinases (MAPKs), and up-regulation of early growth response 1 and proinflammatory cytokines such as IL-6.
One of our findings is that either UFPs or CSE, at a non-toxic dose, induced significant ROS generation in endothelial cells obtained from wild-type mice, and co-exposure to both UFPs and CSE caused significantly higher ROS generation than that when cells were exposed to UFPs or CSE alone. CSE and PM have been shown to impact the function of endothelium and cause altered gene expression in endothelial cells via their ability to generate ROS and nitrogen species (NOS) (Brunekreef, 2002; Donaldon et al., 2002; Hoshino et al., 2005; Mo et al., 2009; Orosz et al., 2007; Sumanasekera et al. 2007). Several established risk factors for cardiovascular diseases have been linked to excessive ROS generation, known as a state of oxidative stress. Oxidative stress occurs when the flux of ROS or free radical generation exceeds available antioxidant defenses. Importantly, ROS, including O2.- and hydrogen peroxide, have been implicated in proatherogenic vascular phenotypic alterations, including induction of proinflammatory gene expression (Ohsuzu, 2004; Ross, 1999). Oxidative stress has been shown to cause cell injuries with exposure to various particles, including PM. Therefore, our findings that co-exposure of endothelial cells to UFPs and CSE caused significantly increased ROS generation may be one of reasons why there was a combined cytotoxic effect of UFPs and CSE on endothelial cells.
There are several potential ROS generation sources in pulmonary endothelial cells (Cai and Harrison, 2000; Griendling et al., 2000; Lum and Roebuck, 2001). Our previous studies showed that NADPH oxidase was involved in ROS generation in endothelial cells exposed to UFPs (Mo et al., 2009). The NADPH oxidase is composed of two integral membrane proteins (p22phox and gp91phox) and four cytosolic proteins (Rac, p40phox, p47phox and p67phox). In order to verify whether activation of NADPH oxidase was involved in the ROS generation when cells were co-exposed to UFPs and CSE, we employed endothelial cells isolated from gp91phox knock-out mice. In this kind of cells, the ROS generation ability of NADPH oxidase was totally destroyed. Our results showed that “knocking out” NADPH oxidase (gp91phox) in endothelial cells abolished ROS production with co-exposure to UFPs and CSE. This suggests that NADPH oxidase is involved in ROS generation in endothelial cells co-exposed to both UFPs and CSE.
The mitogen-activated protein kinase (MAPK) pathway is involved in a variety of biological events and is also responsible for cytokine production in response to various particles. MAPKs can be activated through phosphorylation. UFPs or CSE are known to induce oxidative stress and subsequently activate MAPKs (Karoly et al., 2007; Kim et al., Li et al., 2003; Li et al., 2006, Low et al., 2007; Orosz et al., 2007). The present study showed that co-exposure of endothelial cells to UFPs and CSE caused a significant increase in phosphorylation of Erk1/2 and p38 as compared to that when cells were exposed to UFPs or CSE alone. Further, we demonstrated that activation of NADPH oxidase to generate ROS was also involved in the activation of MAPKs because exposure of endothelial cells from gp91phox knock-out mice to UFPs and/or CSE did not cause increased phosphorylation of Erk1/2 and p38.
Our results demonstrated that UFPs or CSE significantly up-regulated Egr-1 expression at both the transcriptional and translational levels in endothelial cells. Co-exposure to UFPs and CSE resulted in a significant increase in the expression of Egr-1 as compared to that with exposure to UFPs or CSE alone. Egr-1 is a zinc finger transcription factor, which has 80 to 82 kDa nuclear phosphoprotein consisted of 533 amino acids. The DNA-binding domain of Egr-1 consists of three zinc finger motifs located between amino acid 332 and 416 towards the carboxyl-terminal region of the protein (Khachigian et al., 1996). Egr-1 expression is highly regulated by many factors including oxidative stress, cellular signaling molecules such as MAPKs, shear stress, mechanical injury, and Ang II, which can further stimulate transcription of several proinflammatory genes, including TNF-α, IL-2, MCP-1 and ICAM-1 (Houston et al., 1999; Jeong et al., 2010; Khachigian et al., 1996; Yan et al., 1999). Our present studies clearly demonstrated that oxidative stress and activation of MAPKs were involved in up-regulation of Egr-1 expression since either UFPs or CSE failed to upregulate Egr-1 expression in endothelial cells from gp91phox knock-out mice, and the Egr-1 expression was abolished when endothelial cells were pre-treated with SB203580, a specific p38 inhibitor, or PD98059, a MEK 1/2 inhibitor. Egr-1 also can stimulate expression of growth factors such as PDGF, TGF-β, bFGF, tissue factor, and proinflammatory cytokines (Houston et al., 1999; Jeong et al., 2010; Khachigian et al., 1996; Reynolds et al., 2006; Yan et al., 1999). Knocking down of Egr-1 by siRNA significantly attenuated UFPs- and/or CSE- induced IL-6 upregulation. IL-6 is a key proinflammatory cytokine, and IL-6-induced endothelial cell dysfunction has been implicated in cardiovascular diseases (Ohsuzu, 2004; Ross, 1999). Thus, co-exposure to UFPs and CSE may activate Egr-1, and further cause endothelial cell inflammation through production of cytokines such as IL-6.
Taken together, our studies showed that ROS, generated by exposure of endothelial cells to UFPs and/or CSE, activated MAPKs which may further induce Egr-1 upregulation and proinflammatory cytokine generation, thus resulting in endothelial inflammation and dysfunction. Co-exposure to both UFPs and CSE greatly enhanced these effects.
Highlights.
We determined ROS, MAPKs activation, and Egr-1 and IL-6 upregulation in endothelial cells with exposure to UFPs and/or CSE. >ROS generation in cells exposed to UFPs and/or CSE activated MAPKs induced Egr-1 upregulation and IL-6 generation. >Co-exposure to UFPs with CSE greatly enhanced these effects, resulting in endothelial inflammation and dysfunction.
Acknowledgements
This work was partly supported by American Lung Association (RG-872-N), American Heart Association (086576D), KSEF-1686-RED-11, Health Effects Institute (4751-RFA-05-2/06-12), CTSPGP 20018 from University of Louisville and T32-ES011564. Part of the results was presented at the SOT annual meeting, March 6-10, 2011, Washington, D.C. Some of the research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly founded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-8281101) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers.
Abbreviations
- UFPs
ultrafine particles
- CSE
cigarette smoke extract
- Egr-1
early growth response-1
- MPMVEC
mouse pulmonary microvascular endothelial cells
- ROS
reactive oxygen species
- H2-DCFDA
2', 7'-dichlorodihydrofluorescein diacetate
- DCF
dichlorofluorescein
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in ultrafine range promote early atherosclerosis and systemic oxidative stress. Cir. Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2648–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- Bercher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, Schwarze PE. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal.Toxicol. 2007;19:645–655. doi: 10.1080/08958370701353528. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:233–1244. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Gambling TM, Acuna H, García R, Osnaya N, Monroy S, Villarreal-Calderon A, Carson J, Koren HS, Devlin RB. Canines as sentinel species for assessing chronic exposures to air pollutants: Part 2. Cardiac pathology. Toxicol. Sci. 2001;61:356–367. doi: 10.1093/toxsci/61.2.356. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd., Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr., Speizer FE. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ. Health Perspect. 2001;109(Suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edirisinghe I, Yang SR, Yao H, Rajendrasozhan S, Caito S, Adenuga D, Wong C, Rahman A, Phipps RP, Jin ZG, Rahman I. VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction. FASEB J. 2008;22:2297–310. doi: 10.1096/fj.07-099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW. Systemic and cardiovascular effects of airway injury and inflammation: ultrafine particle exposure in humans. Environ. Health Perspect. 2001;109(suppl 4):529–532. doi: 10.1289/ehp.01109s4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Stewart JC, Oberdörster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang LS, Utell MJ. Inhalation of ultrafine particles alters blood leukocytes expression of adhesion molecules in humans. Environ. Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hamoir J, Nemmar A, Halloy D, Wirth D, Vincke G, Vanderplasschen A, Nemery B, Gustin P. Effect of polystyrene particles on lung microvascular permeability in isolated perfused rabbit lungs: role of size and surface properties. Toxicol. and Appl. Pharmacol. 2003;190:278–285. doi: 10.1016/s0041-008x(03)00192-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Matsumoto K, Gon Y, Maruoka S, Takeshita I, Hayashi S, Koura T, Kujime K, Horie T. p38 mitogen-activated protein kinase regulates IL-8 expression in human pulmonary vascular endothelial cells. Eur. Respir. J. 1999;13:1357–1364. [PubMed] [Google Scholar]
- Hoshino S, Yoshida M, Inoue K, Yano Y, Yanagita M, Mawatari H, Yamane H, Kijima T, Kumagai T, Osaki T, Tachiba I, Kawase I. Cigarette smoke extract induces endothelial cell injury via JNK pathway. Biochem. Biophys. Res. Commun. 2005;329:58–63. doi: 10.1016/j.bbrc.2005.01.095. [DOI] [PubMed] [Google Scholar]
- Houston P, Dickson MC, Ludbrook V, White B, Schwachtgen JL, McVey JH, Mackman N, Reese JM, Gorman DG, Campbell C, Braddock M. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arterioscler. Thromb. Vasc. Biol. 1999;19:281–9. doi: 10.1161/01.atv.19.2.281. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24(6):1031–6. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- Jeong SH, Park JH, Kim JN, Park YH, Shin SY, Lee YH, Kye YC, Son SW. Up-regulation of TNF-alpha secretion by cigarette smoke is mediated by Egr-1 in HaCaT human keratinocytes. Exp. Dermatol. 2010;19:e206–12. doi: 10.1111/j.1600-0625.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- Karoly ED, Li Z, Dailey LA, Hyseni X, Huang YC. Up-regulation of tissue factor in human pulmonary artery endothelial cells after ultrafine particle expousre. Environ. Health Perspect. 2007;115:535–540. doi: 10.1289/ehp.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271:1427–31. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- Kim YM, Reed W, Lenz AG, Jaspers I, Sibajoris R, Nick HS, Samet JM. Ultrafine carbon particles induce interleukin-8 gene transcription and p38 MAPK activation in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L432–L441. doi: 10.1152/ajplung.00285.2004. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters). Circulation. 2003;108:1064–9. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induced oxidative stress and mitochondrial damage. Envrion. Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hyseni X, Carter JD, Soukup JM, Dailey LA, Huang YC. Pollutant particles enhanced H2O2 production from NAD(P)H oxidase and mitochondria in human pulmonary artery endothelial cells. Am. J. Physiol. Cell. Physiol. 2006;291:C357–C365. doi: 10.1152/ajpcell.00365.2005. [DOI] [PubMed] [Google Scholar]
- Low B, Liang M, Fu J. p38 mitogen-activated protein kinase mediates sidestream cigarette smoke-induced endothelial permeability. J. Pharmacol. Sci. 2007;104:225–31. doi: 10.1254/jphs.fp0070385. [DOI] [PubMed] [Google Scholar]
- Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milara J, Juan G, Ortiz JL, Guijarro R, Losada M, Serrano A, Morcillo EJ, Cortijo J. Cigarette smoke-induced pulmonary endothelial dysfunction is partially suppressed by sildenafil. Eur. J. Pharm. Sci. 2010;39:363–72. doi: 10.1016/j.ejps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Mo Y, Feinstein SI, Manevich Y, Zhang Q, Lu L, Ho YS, Fisher AB. 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless gene. FEBS Letter. 2003;555(2):192–198. doi: 10.1016/s0014-5793(03)01199-2. [DOI] [PubMed] [Google Scholar]
- Mo Y, Zhu X, Hu X, Tollerud DJ, Zhang Q. Cytokine and NO release from peripheral blood neutrophils after exposure to metal nanoparticles: in vitro and ex vivo studies. Nanotoxicology. 2008;2:79–87. [Google Scholar]
- Mo Y, Wan R, Chien S, Tollerud DJ, Zhang Q. Activation of endothelial cells after exposure to ambient ultrafine particles: the role of NADPH oxidase. Toxicol. Appl. Pharmacol. 2009;236:183–193. doi: 10.1016/j.taap.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PHM, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamsters. Am. J. Respir. Crit. Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoep PH, Dinsdale S, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 2002a;166:998–1004. doi: 10.1164/rccm.200110-026OC. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002b;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Vermylen J, Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol. Appl. Pharmacol. 2003;186:38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. Atheroscler. Thromb. 2004;11:313–321. doi: 10.5551/jat.11.313. [DOI] [PubMed] [Google Scholar]
- Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am. J. Physiol. Heart Circ. Physiol. 2007;29:H130–9. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd., Burnett RT, Tur MJ, Calle EE, krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd., Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Reynolds PR, Cosio MG, Hoidal JR. Cigarette smoke-induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am. J. Respir. Cell. Mol. Boil. 2006;35:314–319. doi: 10.1165/rcmb.2005-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis is-an inflammatory disease. Am Heart J. 1999;138:S419–420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- Shih RH, Lee IT, Hsieh HL, Kou YR, Yang CM. Cigarette smoke extract induces HO-1 expression in mouse cerebral vascular endothelial cells: involvement of c-Src/NADPH oxidase/PDGFR/JAK2/STAT3 pathway. J. Cell. Physiol. 2010;225:741–50. doi: 10.1002/jcp.22270. [DOI] [PubMed] [Google Scholar]
- Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol. Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Ivanova MM, Johnston BJ, Dougherty SM, Sumanasekera GU, Myers SR, Ali Y, Kizu R, Klinge CM. Rapid effects of diesel exhaust particulate extracts on intracellular signaling in human endothelial cells. Toxicol Lett. 2007;174:61–73. doi: 10.1016/j.toxlet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Stoeger T, Harder V, Thomas RF, Schladweiler MC, Semmler-Behnke M, Takenaka S, Karg E, Reitmeir P, Bader M, Stampfl A, Kodavanti UP, Schulz H. Exposure to ultrafine carbon particles at levels below detectable pulmonary inflammation affects cardiovascular performance in spontaneously hypertensive rats. Part. Fibre Toxicol. 2008;5:19. doi: 10.1186/1743-8977-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mo Y, Zhang X, Chien S, Tollerud DJ, Zhang Q. Matrix metalloproteinase-2 and -9 are induced differently by metal nanoparticles in human monocytes: The role of oxidative stress and protein tyrosine kinase activation. Toxicol. Appl. Pharmacol. 2008;233:276–285. doi: 10.1016/j.taap.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mo Y, Chien S, Li Y, Li Y, Tollerud DJ, Zhang. Q. The role of hypoxia inducible factor- 1α in MMP-2 and MMP-9 production by human monocytes exposed to nickel nanoparticles. Nanotoxicology. 2011 doi: 10.3109/17435390.2010.537791. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H, Joad JP, Pinkerton KE. The toxicology of environmental tobacco smoke. Ann. Rev. Pharmacol. Toxicol. 1997;37:29–52. doi: 10.1146/annurev.pharmtox.37.1.29. [DOI] [PubMed] [Google Scholar]
- Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM. Hypoxia-associated induction of early growth response-1 gene expression. J. Biol. Chem. 1999;274:15030–40. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- Yu M, Mo Y, Wan R, Chien S, Zhang X, Zhang Q. Regulation of plasminogen activator inhibitor-1 expression in endothelial cells with exposure to metal nanoparticles. Toxicol. Lett. 2010;195:82–89. doi: 10.1016/j.toxlet.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L954–L961. doi: 10.1152/ajplung.00210.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chatterjee S, Wei Z, Liu WD, Fisher AB. Rac and PI3 kinase mediate endothelial cells-reactive oxygen species generation during normoxic lung ischemia. Antioxid. Redox. Signal. 2008;10:679–689. doi: 10.1089/ars.2007.1521. [DOI] [PubMed] [Google Scholar]