Abstract
Myeloid-derived suppressor cells (MDSCs) accumulate in the spleen and tumors and contribute to tumor growth, angiogenesis and progression. In this study, we examined the effects of curcumin on the activation and differentiation of MDSCs, their interaction with human cancer cells and related tumor growth. Treatment with curcumin in the diet or by i.p. injection significantly inhibited tumorigenecity and tumor growth, decreased the percentages of MDSCs in the spleen, blood and tumor tissues, reduced IL-6 levels in the serum and tumor tissues in a human gastric cancer xenograft model and a mouse colon cancer allograft model. Curcumin treatment significantly inhibited cell proliferation and colony formation of cancer cells and decreased the secretion of murine interleukin (IL)-6 by MDSCs in a co-culture system. Curcumin treatment inhibited the expansion of MDSCs, the activation of Stat3 and NF-κB in MDSCs, and the secretion of IL-6 by MDSCs when MDSCs were cultured in the presence of IL-1β, or with cancer cell- or myofibroblast-conditioned medium. Furthermore, curcumin treatment polarized MDSCs toward a M1-like phenotype with an increased expression of CCR7 and decreased expression of dectin 1 in vivo and in vitro. Our results demonstrate that curcumin inhibits the accumulation of MDSCs and their interaction with cancer cells and induces the differentiation of MDSCs. The induction of MDSC differentiation and inhibition of the interaction of MDSCs with cancer cells are potential strategies for cancer prevention and therapy.
Keywords: Curcumin, MDSCs, gastric cancer, colon cancer, tumor growth
Introduction
Myeloid-derived suppressor cells (MDSCs) have been identified as a heterogeneous population of immature myeloid cells with the ability to regulate innate immune responses and suppress T-cell activation in humans and animal models (1-4). MDSCs, accumulated in the blood, lymph tissues and tumor sites have been shown to promote tumor growth, angiogenesis, and metastasis (5-8). Our previous results show that the mobilization and activation of MDSCs are critical early events for IL-1β-induced gastric carcinogenesis (6). IL-1β directly activates MDSCs through the NF-κB pathway, resulting in enhanced IL-6 production in MDSCs. Inhibition of MDSC activation by NF-κB inhibitor reduced the production of IL-6, inhibited MDSC mobilization and suppressed the development of gastric cancer in IL-1β transgenic mice (7). These data suggest that inhibition of MDSC activation may be an effective approach for gastric cancer prevention.
The expansion and activation of MDSCs are influenced by a number of factors (9, 10). Most of these factors trigger signaling pathways in MDSCs that converge on Janus kinase (JAK) protein family members and signal transducer and activator of transcription 3 (Stat3) (11, 12). MDSCs from tumor-bearing mice have markedly increased levels of phosphorylated Stat3 (p-Stat3) compared to immature myeloid cells from naïve mice (12). Moreover, ablation of Stat3 expression markedly reduces the expansion of MDSCs and increases T-cell responses in tumor-bearing mice (12, 13). These data elucidate the important role of Stat3 in MDSC function. Some approaches have been explored to inhibit MDSC activation, promote MDSC differentiation, and decrease MDSC expansion and accumulation (1, 10, 14-18). For example, selective depletion of Gr-1+ MDSCs by in vivo administration of monoclonal antibodies against Gr-1+ has resulted in restoring T-cell anti-tumor activity (19). The potential risk of this approach is that tumor-bearing hosts treated with such depleting antibodies may undergo opportunistic infections due to granulocyte deficiency. Thus, searching for safe and effective agents to inhibit MDSCs for cancer prevention is of importance.
Curcumin, derived from the perennial herb Curcuma longa Linn, exhibits cancer preventive and therapeutic properties (20-23). It has a long history of human use in food and as a medication. Curcumin has been shown to be an anti-tumor agent by modulating multiple targets (20), including suppressing the activity of NF-κB (24-27) and JAK2/Stat3 signaling in tumor cells and immune cells (26, 28). Curcumin is also a potent immunomodulator (24). However, the effect of curcumin on MDSCs remains to be studied.
Our previous study has shown that IL-1β induces MDSC activation to produce IL-6 through the NF-κB pathway (6). The IL-6 secreted by MDSCs may directly activate a Stat3 pathway in cancer cells and promote tumor growth (18). However, the interactions between MDSCs and cancer cells have not been adequately delineated. In this study, we investigated such interactions and their inhibition by curcumin. Our results demonstrate that curcumin inhibits the accumulation and activation of MDSCs, interferes with the interaction between gastric cancer cells and MDSCs, induces differentiation of MDSCs, and suppresses tumor growth.
Materials and Methods
Cell culture and reagents
Human gastric cancer cell line MKN-45 purchased from RIKEN (29, 30) was verified by morphology, growth curve analysis, and tested for mycoplasma in March 2010. Mouse colon cancer cell line CT26 was obtained from American Type Culture Collection (ATCC) in June 2010. We did not do further characterization. Bone marrow-derived gastric myofibroblasts (BMD-MF) from IL-1β transgenic mouse were generated in our lab (31, 32). EGFP+ MKN-45 cells were generated through infection of EGFP-retrovirus in our lab. Mycoplasma negative in all four cell lines was confirmed by a PCR method (stratagene) in April 2011. All cell lines were maintained in a RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco BRL). Curcumin (a gift from Sabinsa Corporation) was dissolved in dimethyl sulfoxide (DMSO) and stored at -20 °C.
Tumor graft models and treatments
Four-week old athymic nude mice and BALB/c mice from Jackson Laboratory (Bar Harbor, Maine) were maintained on the AIN93M diet in a sterilized animal room in our animal facility. Gastric cancer cells MKN-45 (1 × 106) were injected subcutaneously (s.c.) into the flank of athymic nude mice. Mouse CT26-colonic cancer cells (1 × 106) syngenic to BALB/c mice were injected s.c. into the flank of BALB/c mice. Starting on the same day of injection, the athymic nude or BALB/c mice were put on the 2% curcumin diet (20 g curcumin/kg AIN93M diet) for 4 weeks. In another study, the mice received daily i.p. injection of curcumin at 50 mg/kg body weight for 3 weeks after tumors reached a size of 100 mm3. Tumor volume was measured by a caliper once every 3 days. Spleens, blood and tumor tissues were harvested at the end of the experiments. Animal protocols were approved by the Animal Care and Facilities Committee (ACFC) of Rutgers, the State University of New Jersey (Protocol No. 09-050).
Detection of MDSCs in the spleens, blood and tumor tissues
The isolation of MDSCs from spleens, blood and tumor tissues was performed as described previously (7). In brief, splenocytes were obtained by passing through a 40-μm cellular strainer (BD Biosciences). Total nucleated cells in peripheral blood were isolated after erythrocyte lysis. To obtain tumor-infiltrating cells, tumors were cut into small pieces, and digested with 5 mg/mL collagenase type IV (Sigma-Aldrich) at 37°C for 15 minutes. Cells were passed through a 40-μm cellular strainer. Cell suspensions were stained with fluorescence-labeled antibodies, then subjected to flow cytometry (FACS) analysis using FACS 500 or MoFlo cell sorter (Beckman Coulter) at the Cytometry and Imaging Core Facility. All antibodies for FACS analysis were purchased from e-Biosciences. Data were analyzed using the Summit software (Beckman Coulter).
Studies with MDSCs and cancer cells in vitro
Splenic MDSCs from mice were sorted by MoFlo cell sorter (Beckman Coulter) using PE-CD11b and PE-cy7-Gr-1 antibodies; or by MACS Separators using magnetic labeled MDSC isolation kit (Miltenyi, Biotec). EGFP+ MKN-45 cells (1 × 105/well) was co-cultured with splenic MDSCs (1 × 106/well) in a RPMI 1640 complete medium in the absence or presence of different concentrations of curcumin. Forty-eight hours after co-culture, cell colonies were counted under a light microscope or fluorescence microscope. Cell supernatant was collected for ELISA analysis. Then, cells were stained with fluorescence-labeled antibodies for FACS analysis. In some experiments, MDSCs were cultured in the presence or absence of IL-1β for 36 hours, or with normal medium (NM) or cancer cell-conditional medium (CC-M) or myofibroblast-medium (MF-M) in the presence or absence of curcumin for 48 hours. Culture medium was collected for ELISA. Cells were harvested for FACS analysis, or for extracting proteins and mRNA.
Measurement of cytokine levels by ELISA
The levels of murine IL-6 in the supernatant, serum and xenograft tumor tissues were determined by using an Enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences). Absorbance was measured at 450 nm by a Multiscan MC reader, and the samples were analyzed by DELTA SOFT II software (BioMetallics).
Real-time PCR
Ribonucleic acid (RNA) was extracted with Trizol (Invitrogen). Complementary deoxyribonucleic acid (cDNA) was synthesized (6). Real-time Polymerase Chain Reaction (PCR) was performed with 1 μl cDNA, TaqMan Universal PCR Master Mix (Applied Biosystems), and target gene assay mix containing sequence-specific primers and 6-carboxyfluorescein dye–labeled TaqMan minor groove binder probe (Applied Biosystems). Data quantitation was performed using the relative standard curve method. Expression levels of the genes were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Apoptosis assays
To detect in vivo apoptosis of MDSCs, splenocytes were isolated from tumor-bearing mice. Splenocytes were stained with APC-CD11b, PE-cy7-Gr-1, PE-Annexin V and 7-AAD and then cells were analyzed by FACS. The apoptotic cells were accounted among the CD11b+Gr-1+ population.
Western Blot analysis
MDSCs were lysed with lysis buffer. Protein samples were subjected to SDS-polyacrylamide gels (Bio-Rad) electrophoresis. The gels were transferred onto nitrocellulose membranes (Bio-Rad). The membranes were probed with specific primary antibodies against Stat3, p65, p-Stat3, p-p65, iNOS, survivin, COX-2 and β-actin (Cell Signaling Technology), incubated with secondary antibodies conjugated to IR fluorophore, Alexa Fluor 680 (Molecular Probes), or IRdye 800 (Rockland Immunochemicals), and scanned using the Odyssey Infrared Imaging System (Li-Cor Biosciences).
Statistical analysis
Unless indicated otherwise, all experiments were conducted at least twice, with at least five mice per group. Results expressed as mean ± SD and were analyzed for differences between two groups using a two-tailed t test for assuming equal variances, with a p value of < 0.05 deemed significant. One-way ANOVA test was used for comparing the results of three or more groups.
Results
Curcumin inhibits tumor growth in a gastric cancer xenograft model
We first investigated the effect of curcumin on gastric cancer growth in a MKN-45 cell xenograft model. Treatment with curcumin in the diet delayed tumor growth in the 4 week experimental period (Fig. 1A) and reduced tumor weight by 46.3% at the end of the experiment (p < 0.05) (Fig. 1B). The treatment with curcumin by i.p. injection significantly inhibited the established tumor growth (Fig. 1C) and reduced tumor weight by 49.2% as compared to the control group at the end of the experiment (Fig. 1D). No differences in food intake, body weight and pathological alterations in major organs were observed between the curcumin-treated groups and control groups.
Fig. 1. Curcumin inhibits gastric cancer xenograft tumor growth.
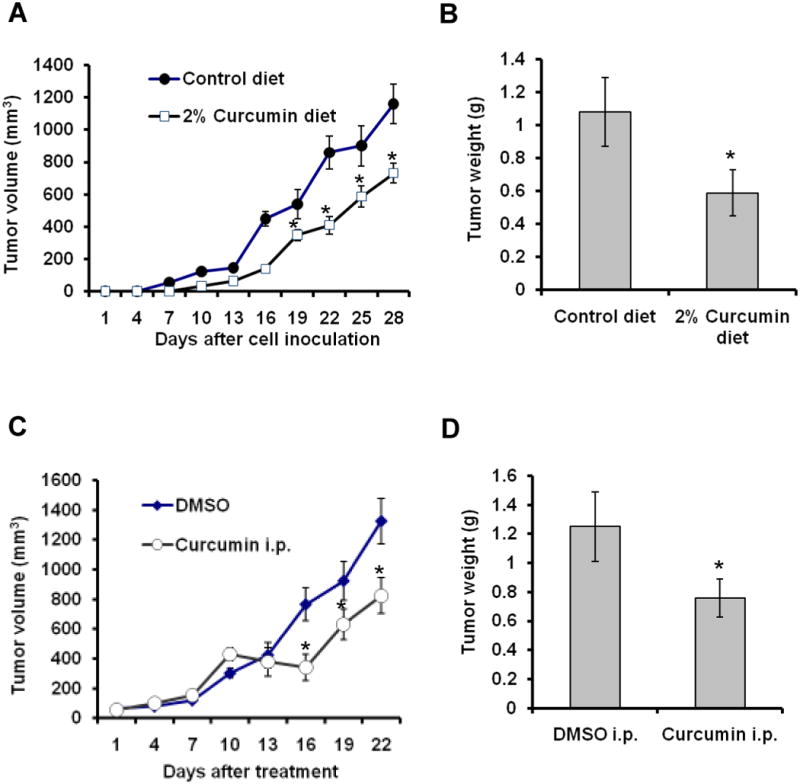
(A-B) Treatment with curcumin in the diet delayed tumorigenesis in athymic nude mice (A) and reduced tumor weight at the termination of the experiment (B). (C-D) Treatment with curcumin by i.p. injection inhibited the established tumor growth (C) and reduced tumor weight (D). Data are the means ± SD of tumor weight (n = 5). *p < 0.05, compared to control group.
Curcumin inhibits the mobilization and accumulation of MDSCs in a xenograft model
To investigate the mechanism by which curcumin inhibits tumor growth, we examined the effect of curcumin on MDSCs. We found that MDSCs were more abundant in the spleens of tumor-bearing mice than in tumor-free mice, consistent with a previous report (2). The percentages of MDSCs in the spleen (Fig. 2A and 2B) and blood (Fig. 2C) were significantly decreased in the tumor-bearing mice that were treated with curcumin in the diet (14 days) or by i.p. injection (5 days) compared to the control groups. The tumor volumes in each group were not significantly different at this time point.
Fig. 2. Curcumin inhibits mobilization and recruitment of MDSCs in a xenograft tumor model.
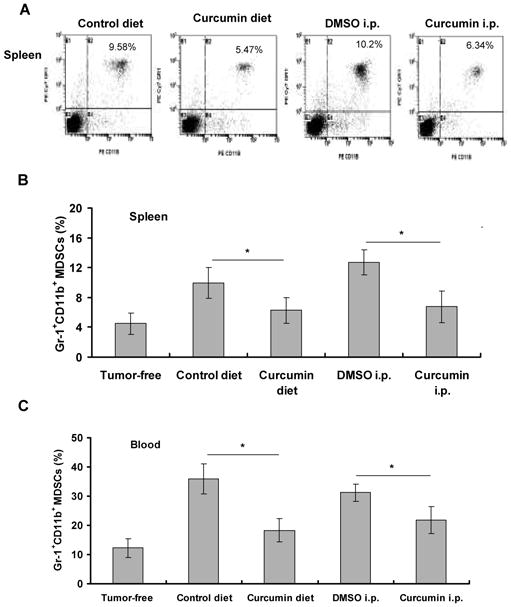
The athymic nude mice injected s.c. with MKN-45 cells received 2% curcumin in the diet for 14 days. The blood and spleens of mice were harvested. In another experiment, tumor-bearing mice received daily i.p. injection of curcumin for 5 days. Spleens (A, B) and blood (C) were harvested. Single cells were stained with PE-CD11b and PE-cy7-Gr-1 antibodies and analyzed by FACS. Representative FACS plots were shown in (A). Data in (B) and (C) are means ± SD of 5 mice per group. *p < 0.05, compared to control treatment.
Curcumin inhibits allograft tumor growth and the accumulation of MDSCs
To determine whether curcumin inhibits tumor growth in an allograft model, BALB/c mice injected with CT26 cells were treated with 2% curcumin in the diet for 4 weeks or by daily i.p. injection of curcumin for 3 weeks. Both treatments significantly decreased tumor volume (Fig. 3A), tumor weight (Fig. 3B) and the percentages of MDSCs in the spleens and tumor tissues (Fig. 3C, supplementary Fig. S1A-S1B) as compared to the control groups.
Fig. 3. Curcumin inhibits allograft tumor growth and MDSC accumulation.
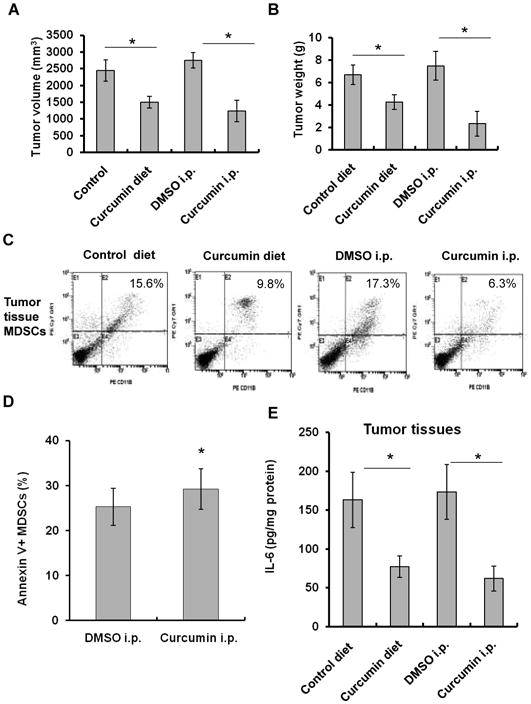
(A-B) Treatment with curcumin in the diet or by i.p. injection inhibited allograft tumor growth (A) and reduced tumor weight, measured at the termination of the experiment (B) in BALB/c mice injected s.c. with CT-26 cells. (C) Curcumin treatment reduced MDSCs accumulation in tumor tissues. BALB/c mice injected s.c. with CT-26 cells received 2% curcumin in the diet for 10 days, or daily i.p. injection for 5 days. Single-cell suspensions of tumor tissues were stained with PE-CD11b, PE-cy7-Gr-1 antibodies and 7-AAD, and analyzed by FACS. Representative FACS plots were shown. (D) Splenic MDSCs isolated from curcumin-injected tumor-bearing mice were stained with PE-CD11b and PE-cy7-Gr-1 antibodies, FITC-Annexin V and 7-AAD, and then analyzed for apoptosis by FACS. (E) The levels of IL-6 in the tumor tissues from tumor-bearing mice treated with curcumin in the diet or by i.p. injection were measured by ELISA. Data are the means ± SD of 5 mice (*p < 0.05, compared to control group).
Next, we investigated whether the decrease in MDSC percentages in spleens was due to a direct pro-apoptotic effect of curcumin. We found that the apoptotic rate of splenic MDSCs from the tumor-bearing mice was significantly lower compared to the naïve mice, indicating that MDSCs in the tumor-bearing mice may have a prolonged lifespan in vivo (data not shown). However, curcumin treatment of the tumor-bearing mice for 5 days significantly increased the early apoptosis of the freshly isolated splenic MDSCs (Fig. 3D). Consistent with the results, treatment with curcumin in the diet also increased apoptosis of MDSCs in the spleen (35.3% vs 21.2% of the control). The data suggest that curcumin induces apoptosis of MDSCs in vivo.
To demonstrate that curcumin treatment has an effect on the activation of MDSCs in vivo, The splenic MDSCs from the curcumin-treated tumor-bearing mice were re-stimulated by CC-M. The MDSCs from the curcumin-treated mice produced lower amounts of IL-6 in response to CC-M than the MDSCs from the DMSO-injected tumor-bearing mice (Supplementary Fig. S1C), indicating that in vivo curcumin treatment has a subsequent effect on MDSC activation ex vivo. Treatment of curcumin in the diet or by i.p. injection significantly reduced the levels of murine IL-6 in the tumor tissues (Fig. 3E) and serum (Supplementary Fig. S1D).
Curcumin induces the polarization of MDSCs toward a M1-like phenotype
We determined the effect of curcumin on the subsets of MDSCs - granulocytic MDSCs (CD11b+Ly6G+Ly6Clow) and monocytic MDSCs (CD11b+Ly6G-Ly6Chigh) (1). We found that curcumin treatment decreased the percentage of granulocytic MDSCs, but not monocytic-MDSCs in the tumor tissues (Fig. 4A, supplementary Fig. S2A). Similar results were obtained from the spleen tissues (Supplementary Fig. S2B). The results indicate that curcumin mainly reduces the accumulation of granulocytic MDSCs in tumor-bearing mice.
Fig. 4. Curcumin induces the polarization of MDSCs toward M1-like phenotype.
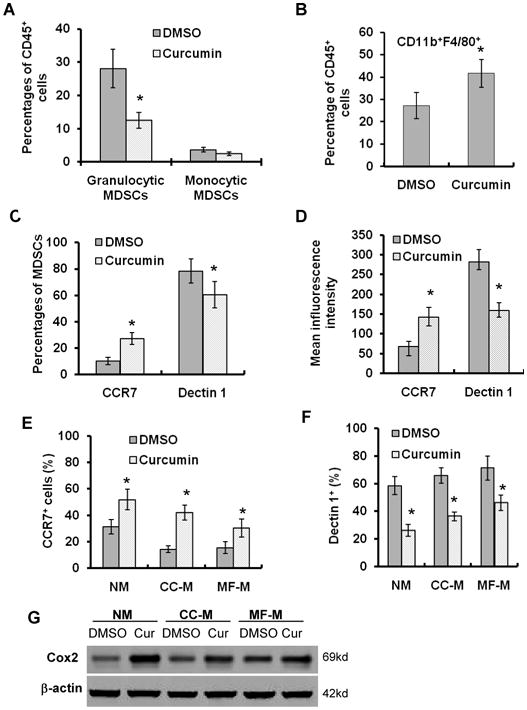
(A-B) Tumor-bearing athymic nude mice received daily i.p. injection of curcumin for 5 days. The single-cell suspensions from tumor tissues were stained with PerCP-CD45, PE-CD11b, PE-Cy7-Ly6G, FITC-F4/80, APC-Ly6C and DAPI, and subjected to FACS analysis using MoFlo cell sorter. The percentages of granulocytic MDSCs and monocytic MDSCs (A) and CD11b+F4/80+ (B) among total CD45+ cells were analyzed. Data are the means ± SD of 5 mice (*p < 0.05, compared to control). (C-D) The single-cell suspensions from tumor tissues were stained with PerCP-CD11b, PE-Cy7-Gr-1, APC-CCR7 and PE-dectin 1 antibodies and subjected to FACS analysis. The percentages of CCR7+ cells and dectin 1+ cells among CD11b+Gr-1+ cells are shown in (C). The mean fluorescence intensities of CCR7 and dectin 1 expression are shown in (D). n=5, *p < 0.05, compared to control. (E-F) Splenic MDSCs isolated from tumor-bearing mice were cultured in indicated media with curcumin (12.5 μM) for 48 hours. MDSCs were stained with APC-CCR7 or PE-dectin 1 antibodies and subjected to FACS analysis. The percentages of CCR7+ cells (E) and dectin 1+ cells (F) were shown. Data are means ± SD of 3 independent experiments. *p < 0.05, compared to control treatment. (G) Western blot determined COX-2 expression in splenic MDSCs cultured in indicated media in the absence or presence of curcumin (12.5 uM) for 48 hours.
Recent studies suggest that MDSCs may differentiate into tumor-associated macrophages (TAMs) expressing alternative macrophage activation (M2) markers (33). We found that curcumin treatment increased the percentage of CD11b+F4/80+ monocytes/macrophages in the tumor tissues compared to DMSO treatment (Fig. 4B, supplementary Fig. S2C). Then, we asked whether curcumin could induce the differentiation of MDSCs toward M1-like monocytes/macrophages. Curcumin treatment increased the percentage of CCR7+ (M1 marker) cells and decreased the percentage of dectin 1+ (M2-marker) cells (Fig. 4C, supplementary Fig. S3A) among MDSCs population of tumor tissues (34, 35), and increased the mean inflorescence intensity (MIF) of CCR7 and decreased the MIF of dectin 1 in MDSCs from curcumin-treated mice (Fig. 4D). The data suggest that curcumin promotes the polarization of MDSCs toward M1-like phenotype in vivo. Furthermore, treatment with curcumin in vitro increased the percentage of CCR7+ cells (Fig. 4E) and reduced the percentage of dectin 1+ cells in sorted splenic MDSCs (from tumor-bearing mice) (Fig. 4F). Real-time PCR showed that curcumin treatment increased mRNA expressions of iNOS (Supplementary Fig. S3B) and COX-2 (Supplementary Fig. S3C) (M1 marker) in MDSCs and decreased expression of YM-1 (M2 marker) (Supplementary Fig. S3D) (34, 35). Western blot showed that curcumin upregulated expression of COX-2 protein in MDSCs (Fig. 4G). The data suggest that curcumin induces the polarization of MDSCs toward M1-like phenotype in vivo and in vitro.
Curcumin inhibits the interaction of MDSCs with cancer cells
Next, we investigated the interaction of cancer cells with MDSCs. The EGFP+ MKN-45 cells were co-cultured with splenic MDSCs isolated from the tumor-bearing mice. There was a 50% increase in the number of MKN-45 cells in the co-culture system compared to cancer cells that were cultured alone (Fig. 5A). The co-cultured cancer cells formed a number of large colonies, whereas MDSCs or gastric cancer cells cultured alone did not form any colony (Fig. 5A-5B). Fluorescence microscopy further confirmed that only EGFP+ MKN-45 cells formed colonies (Fig. 5A, lower panel). The results indicate that cancer cells acquire a growth advantage via interactions with MDSCs.
Fig. 5. Curcumin inhibits the interaction of gastric cancer cells with MDSCs.
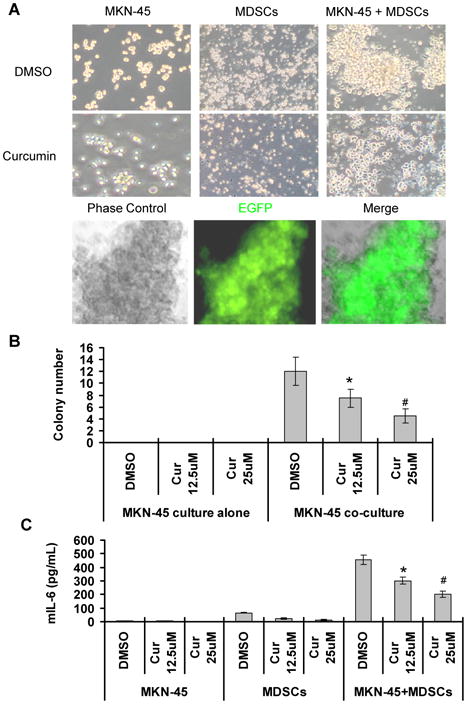
The EGFP+ MKN-45 cells and splenic MDSCs were cultured alone or co-cultured in the absence or presence of curcumin. (A) Representative images (magnification 200 ×) were taken 48 hours after curcumin treatment (25 μM) (upper panel). The merged images indicate that the colonies originated from EGFP+ MKN-45 cells (lower panel). Quantification of colonies of cancer cells were shown in (B). The production of IL-6 by MDSCs and its inhibition by curcumin are shown in (C). The data are means ± SD of 3 independent experiments (*p < 0.05, #p < 0.01, compared to DMSO treatment).
Then, we examined the effect of curcumin on the interactions of MDSCs with cancer cells. The curcumin treatment inhibited proliferation of both MDSCs and cancer cells alone or co-cultured (Fig. 5A), but the inhibitory effect of curcumin on the co-cultured cells was less extensive compared to those cultured alone, suggesting that the co-culture of MDSCs with cancer cells produces a survival advantage for both types of cells. However, the treatment with curcumin markedly reduced colony growth of gastric cancer cells in a co-culture system (Fig. 5A-5B). In this co-culture system, curcumin (12.5 μM) decreased survival of MKN-45 cells by 60% ± 5.4%, but the apoptotic rate of MKN-45 cells was only induced by 25% ± 3.2%, suggesting that the decreased cell growth by curcumin is largely due to abolishing the growth advantage of cancer cells acquired from the interaction with MDSCs.
To investigate the effect of gastric cancer on MDSC activation, we determined the number of MDSCs and the level of murine IL-6 in the cell supernatant. When co-cultured with gastric cancer cells, the number of MDSCs increased 1.8-fold, and the level of murine IL-6 secreted by MDSCs increased 9-fold in the co-culture cell supernatant (Fig. 5C), suggesting that human gastric cancer cells activate mouse MDSCs. Moreover, the treatment with curcumin significantly reduced murine IL-6 secretion from MDSCs in this co-culture system (Fig. 5C). The data indicate that curcumin inhibits cancer cell-induced expansion of MDSCs and secretion of IL-6.
Curcumin suppresses the activation of Stat3 and NF-κB in MDSCs
To further investigate the mechanisms by which curcumin inhibits the interaction of MDSCs with cancer cells, we determined the effect of curcumin on Stat3 and NF-κB in MDSCs. Curcumin significantly inhibited IL-1β-stimulated production of IL-6 by MDSCs (Fig. 6A). To determine whether curcumin inhibited MDSC activation through the NF-κB pathway, we isolated EGFP+ splenic MDSCs from IL-1β; NF-κBEGFP transgenic mice (7), in which EGFP expression was controlled by a cis-element of the NF-κB promoter. The expression of EGFP indicates the activation of cis-element of the NF-κB promoter. Treatment with curcumin reduced the number of EGFP+ cells (Fig. 6B), suggesting that curcumin inhibits the activation of the NF-κB promoter. The exosomes released from tumor cells have been shown to induce expansion of MDSCs and IL-6 production in MDSCs in a Toll-like receptor 2/Stat3-dependent manner (36). Myofibroblasts are a major type of stroma cells in tumor microenvironment (31). We found that CC-M and MF-M induced expansion of MDSCs from naïve mice and that curcumin inhibited CC-M and MF-M-induced expansion of MDSCs (Fig. 6C). Furthermore, CC-M and MF-M increased the expressions of p-Stat3 and p-p65 (Fig. 6D) and production of IL-6 in MDSCs (Fig. 6E). Curcumin inhibited the expressions of Stat3, p-Stat3, p65, p-65 and survivin (Fig. 6D) and reduced IL-6 levels in MDSCs (Fig. 6E).
Fig. 6. Curcumin inhibits the activation of Stat3 and NF-κB in MDSCs.
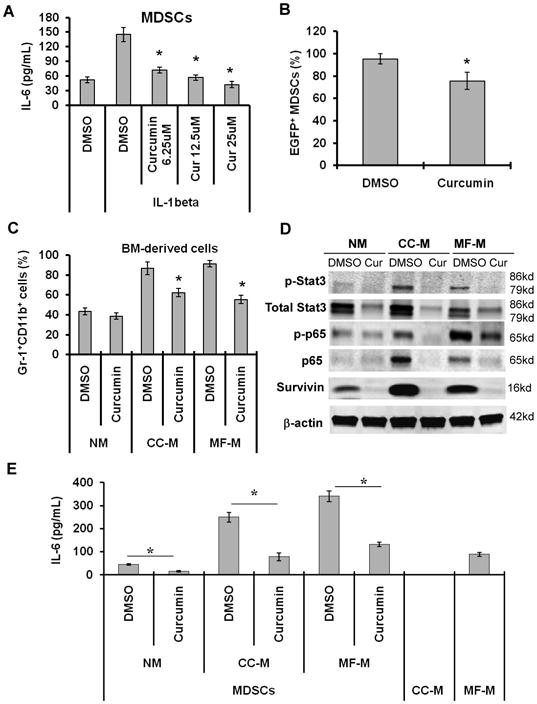
(A) The splenic MDSCs from tumor-free mice were treated with IL-1β in the absence or present of curcumin. The levels of IL-6 in the supernatant were measured by ELISA. (B) Curcumin inhibited NF-κB in MDSCs. The EGFP+ MDSCs isolated from spleens of IL-1β; NF-κBEGFP transgenic mice were treated with curcumin for 48 hours. EGFP+ MDSCs were analyzed by FACS. (C) Curcumin inhibited expansion of MDSCs. Bone marrow-derived cells from tumor-free mice were cultured for 3 days in NM, or CC-M or MF-M in the absence or presence of curcumin. The percentages of Gr-1+CD11b+ cells were determined by FACS. (D, E) The splenic MDSCs isolated from tumor-bearing mice were cultured in indicated media in the absence or presence of curcumin (12.5 μM) for 48 hours. Protein levels were determined by Western blot (D). IL-6 level in the cell supernatant was measured by ELISA (E). The data are means ± SD of 3 independent experiments (*p < 0.01).
Discussion
MDSCs play important roles in cancer development and progression (1, 8). In this study, we demonstrated that treatment with curcumin in the diet or by i.p. injection inhibited tumor growth and the mobilization and activation of MDSCs, and polarized MDSCs toward M1-like phenotype in both BALB/c mouse allograft and athymic nude mouse xenograft models. Curcumin suppressed Stat3 and NK-κB activation and reduced IL-6 production in MDSCs. Our results reveal new mechanisms for the anti-tumor actions of curcumin.
MDSC accumulation in the tumor microenvironment promotes tumor growth (7). Curcumin in the diet or by i.p. injection was able to inhibit MDSC accumulation in the spleen and in the tumor bed, mainly reducing the accumulation of PMN-MDSCs. IL-6 has been reported to induce the expansion and mobilization of MDSCs (10). Curcumin treatment significantly reduced the level of murine IL-6 in the serum and tumor tissues of mice, and induced MDSCs apoptosis in vivo. Thus, the reduction of MDSC accumulation by curcumin may be due to inhibition of MDSC expansion and induction of apoptosis of MDSCs.
An important finding in this study is that curcumin inhibits the activation of Stat3 and NF-κB in MDSCs. The activation of Stat3 and NF-κB regulates the expression of anti-apoptotic, pro-proliferative, immune response genes (37-40). Cytokines, such as IL-6, which are induced by NF-κB activation in stromal cells, often activate Stat3 in both malignant cells and stromal cells (37). Inhibition of NF-κB and Stat3 activation is becoming effective therapeutic strategies for cancer (39, 41, 42). Stat3 has been shown to regulate MDSC expansion (11). We found that both Stat3 and NF-κB were activated in MDSCs, resulting in increased levels of IL-6, and the activation was inhibited by curcumin. Consistent with the in vitro results, treatment of mice with curcumin decreased the level of IL-6 in the tumor tissues and serum. Our results are consistent with recent reports showing that curcumin decreased the expressions of Stat3 and NF-κB in cancer cells (43) and the peripheral blood monocytes of cutaneous T-cell lymphoma patients (28). Thus, inhibition of the activation of Stat3 and NF-κB in MDSCs may be an important anti-tumor mechanism of curcumin.
Another important finding is that curcumin can inhibit the interaction of MDSCs with gastric cancer cells. Previous studies of MDSCs have focused on their interaction with T-cells (1). While some studies have shown that tumor cell-secreted exosomes induce expansion of MDSCs, the direct effect of MDSCs on tumor cells has not been sufficiently studied (36, 44, 45). We found that the MDSCs stimulated gastric cancer cell proliferation and colony formation; in return, the gastric cancer cells activated MDSCs to secrete IL-6. Notably, curcumin treatment significantly inhibited cancer cell proliferation and colony formation and decreased the secretion of murine IL-6 by MDSCs in this co-culture system. Based on our and other results, we propose that cancer cell-secreted factors (such as exosome and IL-1β) activate Stat3 and NF-κB signaling pathways in MDSCs, resulting in enhanced production of IL-6. IL-6 secreted by MDSCs then activates Stat3 pathway in cancer cells, resulting in promoting cancer cell proliferation. Curcumin inhibits the Stat3 and NF-κB pathways in MDSCs and reduced IL-6 production, resulting in the inhibition of IL-6/Stat3 pathway in cancer cells and tumor growth. Thus, curcumin disrupts the interaction of cancer cells with MDSCs through inhibition of IL-6/Stat3 and NF-κB pathways.
A new finding in this study is that curcumin polarizes MDSCs toward a M1-like phenotype both in vivo and in vitro. M1 macrophages promote the cytotoxicity and inhibits tumor growth, whereas M2 macrophages induce immune suppression and tumor progression (46). M1 macrophages express some characteristic molecules such as iNOS, COX-2, CCR7, and pro-inflammatory cytokines (TNF-α and IL-1β), and M2 macrophages express arginase-1, YM-1, mannose receptor, dectin 1 and cytokine IL-4 and IL-10 (34, 35, 46). Curcumin increased expressions of iNOS and COX-2 and decreased expression of YM-1 in MDSCs. Thus, the polarization of MDSCs toward a M1-like phenotype may be a mechanism of antitumor actions of curcumin. One potential mechanism underlying polarization of MDSCs to M1-like phenotype is that curcumin inhibited Stat3 pathway. Stat3 activation has been shown to be associated with M2 macrophage polarization (46, 47). Stat3 may be activated by M2-macrophage-polarizating cytokines such as IL-10 and IL-4 (46, 47). Our results are consistent with a previous report that docetaxel and cucurbitacin I, a Stat3 pathway inhibitor, inhibited p-Stat3 activation, resulting in induction of differentiation of MDSCs toward M1-like phenotype (48). However, further studies are needed to define the detailed mechanisms of curcumin-polarizing MDSCs to M1 macrophages.
In summary, our results have demonstrated that curcumin inhibits the activation of MDSCs, induces the differentiation of MDSCs, interferes with the interaction between MDSCs and cancer cells, and suppresses tumor growth. These results reveal a new mechanism for curcumin to inhibit tumorigenesis, and suggest that targeting MDSCs may be a promising strategy for cancer prevention and therapy.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Yu Hai Sun and FACS analysis of Hyejeong Choi in this work. We thank Z.H. Yang and H. Wang for suggestions in the preparation of this manuscript, D.A. Brenner for providing NF-κBEGFP transgenic mice, C.J. Guo for providing CCR7 and dectin 1 antibodies and Real-time PCR primers, and Sabinsa Corporation for providing curcumin (Curcumin C3 Complex®). This work was supported by grants from the U.S. NIH R21CA149865 (S.P. TU) and RO1 CA120915, RO1 CA122474 and RO1 CA133021 (C.S. Yang) as well as by Shared Facilities funded by NCI Cancer Center Support Grant (CA72720) and National Institute of Environmental Health Center Grant (ES05022).
Footnotes
Disclose Conflict of Interest: No potential conflicts of interest
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 3.Haile LA, von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–81. 81e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 5.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 6.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolcetti L, Marigo I, Mantelli B, Peranzoni E, Zanovello P, Bronte V. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 2008;267:216–25. doi: 10.1016/j.canlet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 10.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nefedova Y, Huang M, Kusmartsev S, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 12.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 13.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 17.Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 70:3526–36. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang XD, Ai W, Asfaha S, et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borie DC, Larson MJ, Flores MG, et al. Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates. Transplantation. 2005;80:1756–64. doi: 10.1097/01.tp.0000184634.25042.ea. [DOI] [PubMed] [Google Scholar]
- 20.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 21.Shureiqi I, Baron JA. Curcumin chemoprevention: the long road to clinical translation. Cancer Prev Res (Phila) 4:296–8. doi: 10.1158/1940-6207.CAPR-11-0060. [DOI] [PubMed] [Google Scholar]
- 22.Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark CA, McEachern MD, Shah SH, et al. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev Res (Phila) 3:1586–95. doi: 10.1158/1940-6207.CAPR-09-0244. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Liu X. Effect of curcumin on immune function of mice. J Huazhong Univ Sci Technolog Med Sci. 2005;25:137–40. doi: 10.1007/BF02873559. [DOI] [PubMed] [Google Scholar]
- 25.Ranjan D, Chen C, Johnston TD, Jeon H, Nagabhushan M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling. J Surg Res. 2004;121:171–7. doi: 10.1016/j.jss.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: potential role for STAT-3 and NF-kappaB signaling. J Invest Dermatol. 130:2110–9. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 27.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissenberger J, Priester M, Bernreuther C, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 16:5781–95. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 29.Tu SP, Cui JT, Liston P, et al. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128:361–75. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 30.Tu SP, Jiang XH, Lin MC, et al. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63:7724–32. [PubMed] [Google Scholar]
- 31.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 34.Liao X, Sharma N, Kapadia F, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefevre L, Gales A, Olagnier D, et al. PPARgamma ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS ONE. 2010;5:e12828. doi: 10.1371/journal.pone.0012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775–87. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov. 2004;3:555–64. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- 42.Gupta SC, Prasad S, Reuter S, et al. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285:35406–17. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 28:166–71. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 44.Xiang X, Liu Y, Zhuang X, et al. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol. 177:1606–10. doi: 10.2353/ajpath.2010.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Shea JJ, Park H, Pesu M, Borie D, Changelian P. New strategies for immunosuppression: interfering with cytokines by targeting the Jak/Stat pathway. Curr Opin Rheumatol. 2005;17:305–11. doi: 10.1097/01.bor.0000160781.07174.db. [DOI] [PubMed] [Google Scholar]
- 48.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


