Abstract
Immune privilege is used by the eye, brain, reproductive organs and gut to preserve structural and functional integrity in the face of inflammation. The eye is arguably the most vulnerable, and therefore also the most “privileged” of tissues, but paradoxically, remains subject to destructive autoimmunity. It has been proposed, although never proven in vivo, that the eye can induce T regulatory cells (Tregs) locally. Using FoxP3-GFP reporter mice expressing a retina-specific T cell receptor, we now show that uncommitted T cells rapidly convert in the living eye to FoxP3+ Tregs in a process involving retinal antigen recognition, de novo FoxP3 induction and proliferation. This takes place within the ocular tissue and is supported by retinoic acid, which is normally present in the eye due to its function in the chemistry of vision. Non-converted T cells showed evidence of priming, but appeared restricted from expressing effector function in the eye. Preexisting ocular inflammation impeded conversion of uncommitted T cells into Tregs. Importantly, retina-specific T cells primed in vivo before introduction into the eye were resistant to Treg conversion in the ocular environment, and instead caused severe uveitis. Thus, uncommitted T cells can be disarmed, but immune privilege is unable to protect from uveitogenic T cells that have acquired effector function prior to entering the eye. These findings shed new light on the phenomenon of immune privilege and on its role, as well as its limitations, in actively controlling immune responses in the tissue.
INTRODUCTION
Immune privilege was once thought to be the property of a few select sites that include the eye, testis, the pregnant uterus and (of all things) the hamster cheek pouch, and was believed to be based mainly on sequestration behind blood-tissue barriers. This view has changed over the years. Immune privilege is now considered to be a general phenomenon through which many tissues are able to actively direct and control immune responses in order to preserve their physical and functional integrity in the face of inflammatory processes (1, 2). Not only the eye and testis, but also the brain, the liver, and mucosal sites including the gut, lung and female reproductive tract are examples of organs that have recently been intensely studied in this regard (3–8)
The eye, perhaps more than any other tissue, must control local expression of immunity. Vision is a very strong evolutionary selective pressure, and to sustain it, multiple mechanisms have evolved to regulate immune responses affecting the eye. The healthy eye is sequestered behind an efficient blood-retina barrier (BRB), has a virtual absence of lymphatic drainage and a profoundly immunosuppressive ocular microenvironment (3, 9). Under some circumstances the eye can also elicit systemic regulatory circuits known as anterior chamber associated immune deviation (ACAID) (10). These safeguards are necessary because the very sequestration of the eye from the immune system impedes peripheral tolerance to retinal antigens (11), allowing persistence in the circulation of non-tolerant retina-specific T cells, which can gain entry into the eye passively (as a result of trauma and bleeding into the eye) or actively (following a priming event in the periphery).
Experimental autoimmune uveitis (EAU) elicited in mice by retina-specific T cells is a model for human autoimmune uveitis, which is often accompanied by responses of patient lymphocytes to retinal antigens. Uveitis has an incidence and prevalence similar to multiple sclerosis and is thought to be responsible for 10–15% of blindness in the US (12). Adoptive transfer experiments in laboratory rodents revealed that infiltration of as few as 10 activated retina-specific uveitogenic T cells into a healthy eye is sufficient to start the inflammatory process leading to EAU (11). It has been an open question why immune privilege, which protects the eye efficiently from day-to-day minor inflammatory insults and traumas, and is thought to underlie the extraordinary success of retinal (allo)grafts, which enjoy close to 90% acceptance at the 1 year mark without any tissue matching (13), is unable to prevent onset of uveitis.
Local induction of regulatory T cells (Tregs) by the eye as a manifestation of immune privilege has been a topic of much interest and even more debate. There is a considerable body of data showing that ocular fluids and ocular resident cells can inhibit activation of T cells in culture and can even induce them to become Tregs (14–18, 19). However, while systemic induction in the spleen of Tregs as part of the eye-driven ACAID phenomenon is based on in vivo findings (10), the notion that Tregs can be induced locally within the eye has been based entirely on in vitro data that were never critically examined in vivo, because the tools for this have simply not been available.
In the present study we use newly developed retina-specific T cell receptor transgenic mice and stringent experimental paradigms to demonstrate for the first time that the living eye efficiently converts naïve retina-specific T cells to FoxP3+ Tregs. Recognition of retinal antigen is required for this process. This indicates that T cell priming (a) can occur locally within the tissue and (b) can effectively “disarm” uncommitted T cells with the potential to cause pathology. Despite presence of TGF-β in ocular fluids, conversion of T cells to Tregs in vivo requires retinoic acid (RA), which is normally present in the eye due to its function in the visual cycle. Thus, RA in the living eye plays a dual role: in vision and in immune privilege. Notably, antigen-experienced T cells appear to be resistant to the immunoregulatory effects of the ocular microenvironment, and in addition, Treg conversion of uncommitted cells is hindered in inflamed eyes. These findings may explain why uveitis can be induced by retina-specific T cells activated in the periphery, in the face of ocular immune privilege.
MATERIALS AND METHODS
Mice
The following 5 mouse strains on B10.RIII background were used in this study: (1) FoxP3-GFP reporter mice on an otherwise conventional background, used as donors of polyclonal T cells; (2) IRBP TCR Tg mice on a conventional background, used as cell donors; (3) IRBP TCR Tg mice on a RAG2−/− background, used as cell donors; (4) CD90.1 congenics, otherwise on a conventional background, used as recipients of cells from the first 3 strains. (5) IRBP−/− mice, used as cell recipients. C57BL/6 wild type (WT) mice were used only to analyze tissue expression of retinaldehyde dehydrogenase (RALDH) enzymes
B10.RIII FoxP3-GFP reporter mice were produced by backcrossing FoxP3-GFP reporter mice on 129xC57BL/6 mixed background (Dr. A. Rudensky, Sloan-Kettering Cancer Ctr, New York, NY) (20) onto the B10.RIII background. IRBP TCR transgenic (Tg) FoxP3-GFP reporter mice on conventional B10.RIII or Rag2−/− B10.RIII background were bred in house, as were IRBP−/− B10.RIII (21) and CD90.1-congenic B10.RIII mice. C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed under SPF conditions, fed standard laboratory chow ad libitum and used at 6 to 10 weeks of age. Treatment of animals was in compliance with Institutional Guidelines and all animal study protocols were approved by the NEI IACUC, ASP #NEI-581.
CD4+ T cell isolation and analysis
T cells were enriched from pooled splenocytes and lymph nodes by using T cell columns (R&D Systems). From these, CD4+GFP− T cells were sorted on FACS Aria (Becton Dickinson, San Jose, CA) to >99% purity. Antibodies used for cell sorting and flow cytometry analysis were all from eBioscience (San Diego, CA).
Immunofluorescent staining and confocal microscopy
Eye sections (10μm) were fixed in 4% paraformaldehyde, permeabilized, and blocked with normal goat serum. The sections were stained with a polyclonal antibody against retinaldehyde dehydrogenases (detecting all isoforms RALDH1A1, RALDH1A2, RALDH1A3, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h, followed by incubation for 30 min in Alexa Fluor 568-conjugated secondary antibody (Invitrogen-Molecular Probes) and 4′,6′-diamidino-2-phenylindole (DAPI, 1 μg/ml, Invitrogen-Molecular Probes).
In vivo Treg conversion
CD4+GFP− T cells were purified from lymph nodes and spleens of IRBP TCR Tg mice expressing a FoxP3-GFP reporter gene. Donor mice were on a Rag2−/− background where specified, otherwise, mice on a conventional background were used when not required by the experiment for reasons of availability (~30% TCR Tg T cells) (R. Horai, unpublished). About 0.5 million cells were injected through the pars plana into the posterior chamber of each eye of CD90.1 congenic recipients. Eyes that sustained unintentional damage to the lens or other structures as a result of the injection were excluded from analysis. To block RA signaling, CD4+GFP− T cells were pretreated with LE540 (50μM or 100nM) for 1 h before injection. On the indicated days after cell injection, recipient eyes were harvested and dispersed into single cell suspension as described (22).
Immunization of mice for uveitis
To prepare WT recipients with uveitis or to prepare primed cells from IRBP TCR Tg (Rag2−/−) donors, mice were immunized with 10 μg of IRBP161-180 peptide emulsified in complete Freund's adjuvant (CFA, Difco, Detroit, MI) that had been supplemented with Mycobacterium tuberculosis strain H37RA to 2.5 mg/ml, as described (23). The WT mice received an intraocular cell transfer two weeks after immunization, after confirmation of disease by fundus examination (23).
Treg suppression assay
CD4+FoxP3− T cells isolated from IRBP TCR Tg FoxP3-GFP reporter mice were injected into eyes of CD90.1 congenic recipients. After 8 days FoxP3+ or FoxP3− CD4+CD90.2+ donor T cells were sorted out (ca. 2,000~4,000 cells/well) and were co-cultured with sorted naïve CD4+CD44lowCD62Lhigh responder cells from IRBP TCR Tg mice (Rag2−/−, 50,000/well). Cells were stimulated with 1μg/ml IRBP161-180 peptide in the presence of irradiated T cell-depleted spleen cells (100,000/well) for 72hr. Proliferation was determined by 3H thymidine incorporation and scintillation counting.
Donor cell proliferation within the eye
CD4+FoxP3− T cells isolated from IRBP TCR Tg FoxP3-GFP reporter mice were labeled with eFluor® 670 (5μM, eBioscience) and were injected into the eyes of naïve congenic recipients, or naïve recipients, fed with Vitamin A (VitA)-deficient diet, or VitA control diet. On day 4, recipient eyes were harvested, dispersed into single cell suspension, and stained for CD4, CD90.2. Propidium iodide-excluding (live) cells were analyzed for FoxP3 expression and eFluor® 670 dilution in CD4+CD90.2+ by flow cytometry.
Cytokine production by donor cells
CD4+GFP–− T cells from IRBP TCR Tg FoxP3-GFP reporter mice (Rag2−/−) background were injected into eyes of CD90.1 congenic recipients. Six days after injection, cells from recipient eyes were isolated and were stimulated with or without PMA (10 ng/ml) and Ionomycin (500 ng/ml) in the presence of Brefeldin A (GolgiPlug, BD Pharmingen, San Diego, CA). After 4h, cells were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% BSA and 0.05% Triton X-100 and stained with PerCP-Cy5.5-conjugated anti-CD4, PE-conjugated CD90.2, APC-conjugated anti-IFN-γ or APC-conjugated anti-IL-17. Up to 500,000 events were acquired using BD CellQuest software and analyzed using FlowJo software (TreeStar Inc, Ashland, OR). As a positive control, FoxP3-GFP reporter mice were actively immunized for EAU as described above. On day 16 after immunization (7 d after onset), inflamed eyes were prepared for single cells and stimulated with PMA and Ionomycin, and analyzed for cytokine production.
Real-time PCR for cytokine mRNA expression by non-converted cells
CD4+GFP–− T cells from IRBP TCR Tg FoxP3-GFP reporter mice were injected into eyes of CD90.1 congenic recipients. Eight days after injection, cells from recipient eyes were isolated, donor CD4+CD90.2+GFP− cells were purified by sorting and were subjected to real-time PCR. As positive control, FoxP3-GFP reporter mice were actively immunized for EAU as described above. On day 17 after immunization (8 days after onset), inflamed eyes were isolated and sorted for CD4+GFP− T cells, and used for real-time PCR. As standardization control, CD4+GFP− T cells from pooled spleens and lymph nodes were sorted from naive FoxP3-GFP reporter mice as naïve control. Total RNA from the sorted cells was extracted with RNeasy Micro kit (QIAGEN, Valencia, CA) and cDNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed with TaqMan 7500 sequence detection system (Applied Biosystems, Foster City, CA) using endogenous control for GAPDH and primer/probe sets from Applied Biosystems. Data were normalized to GAPDH and expressed relative to naïve control (set as 1).
Induction of VitA deficiency
VitA deficient mice were produced as described (24). Briefly, pregnant dams were placed on a diet lacking vitamin A (cat# TD.09838, Harlan, Madison, WI) as soon as pregnancy could be detected (ca. day 14 of gestation). Control mice were fed the same diet supplemented with 20,000 IU Vitamin A/kg (cat# TD.09839, Harlan, Madison, WI). Pups were maintained on the same diet after weaning. Mice were used after 9 weeks of age.
Statistics and experimental reproducibility
Values are presented as mean ± SD where indicated. Statistical differences were calculated with an unpaired Student's t-test, two-tailed (GraphPad Prism version 5.0b). Statistical significance was set at p ≤ 0.05. Experiments were repeated at least 2 and usually more times. Groups numbered 3–7 mice, depending on the experiment (usually 4–5). Results were highly reproducible.
RESULTS
Retina-specific conventional T cells convert to FoxP3+ Tregs cells in the living eye
Naïve as well as antigen-experienced T cells can gain entry into the eye as a result of trauma and bleeding into the eye, or during inflammation after the BRB is disrupted. Thus, their ability to convert to Tregs in vivo is highly relevant to maintenance of ocular homeostasis. To examine this issue we used T cells from FoxP3-GFP reporter mice which also express a transgenic (Tg) T cell receptor (TCR) specific for a retinal protein, the interphotoreceptor retinoid binding protein (IRBP) (R. Horai, unpublished). IRBP is an abundant protein in the retina, whose role is to bind and transport retinoids within the interphotoreceptor space and which serves as an autoimmune target in uveitis (23).
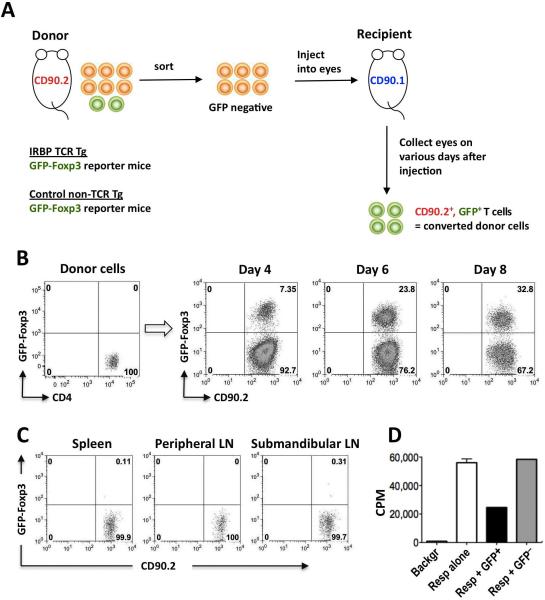
The experimental approach is depicted in Fig 1A. CD4+GFP− (non-Treg) cells were purified from IRBP TCR Tg FoxP3-GFP RAG2−/− CD90.2+ donors, or from control FoxP3-GFP non-Tg donors, and approximately 0.5 million cells were injected into each eye of CD90.1 congenic recipients. GFP-FoxP3 expression in donor-derived (CD90.2+) cells became detectable on day 4 after injection, and by day 8 up to one-third of donor cells expressed FoxP3 (Fig 1B). Very few donor T cells could be detected during this period of time in the eye-draining submandibular lymph node, spleen or pooled peripheral lymph nodes distant from the eye (Fig 1C), supporting the notion that conversion to FoxP3 positivity occurred locally within the eye, without involving recirculation of the cells through the periphery.
Figure 1. Naïve IRBP-specific T cells convert to FoxP3+ Tregs in the eye.
(A) Experimental paradigm to examine in vivo Treg induction. (B) Progressive conversion of IRBP-specific T cells to FoxP3+ cells in eyes of naïve recipient mice. CD4+GFP–− T cells from IRBP TCR Tg FoxP3-GFP reporter mice on Rag2−/− background were injected into eyes of CD90.1 congenic recipients. On the indicated days after injection, FoxP3 expression in CD4+CD90.2+ cells is shown. Representative experiment of three is shown. (C) Paucity of donor FoxP3+ cells in peripheral lymphoid tissues. Sorted GFP-negative CD4+ T cells from IRBP TCR Tg FoxP3-GFP reporter mice (Rag2−/−) were injected into eyes of CD90.1 congenic recipients. Five and 8 days after injection, cells harvested from the indicated lymphoid organs were stained with CD4-APC and CD90.2-PE. Shown is FoxP3 expression in (7-AAD-excluding) CD4+CD90.2+ donor cells on day 5 after cell injection, when there were approximately 15% converted cells in the eye. Data on day 8 were similar, with 30% converted cells in the eye. Representative experiment of two is shown. (D) In vivo converted cells are functionally suppressive. CD4+FoxP3− T cells isolated from IRBP TCR Tg FoxP3-GFP reporter mice were injected into eyes of CD90.1 congenic recipients. After 8 days CD90.2+FoxP3+ or CD90.2+FoxP3− donor T cells were sorted out and examined in an Ag specific in vitro proliferation inhibition assay against fresh IRBP TCR Tg (Rag2−/−) responder cells. Shown is one of 2 repeat experiments, depicting the proliferation of 50,000 responder cells in presence or absence of the converted or non-converted donor cell populations. Only one well each with donor cells could be set up per experiment.
Although in mice T cells expression of FoxP3 is practically synonymous with regulatory function, we wished to confirm that the converted T cells are indeed suppressive. To examine this, we injected IRBP TCR Tg FoxP3-GFP-negative T cells into eyes of CD90.1 congenic recipient mice, as described in Fig 1A. After 8 days the GFP+ and the GFP-negative donor-derived cells were sorted from these eyes and were used in an Ag-specific suppression assay with fresh IRBP TCR Tg target T cells. In 2 repeat experiments, 2,000 and 4,000 sorted GFP+ T cells (all that could be obtained from 18 and 29 eyes, respectively) suppressed 50,000 responder T cells in an antigen-specific assay, whereas the GFP-negative donor cells that had not been converted, did not suppress (Fig 1D). Notably, 50% and 57% suppression, respectively, were achieved at a 1:25 and 1:12.5 Treg to responder ratio, indicating that the in vivo converted cells were highly functional suppressors.
Conversion requires local antigen recognition and is accompanied by proliferation
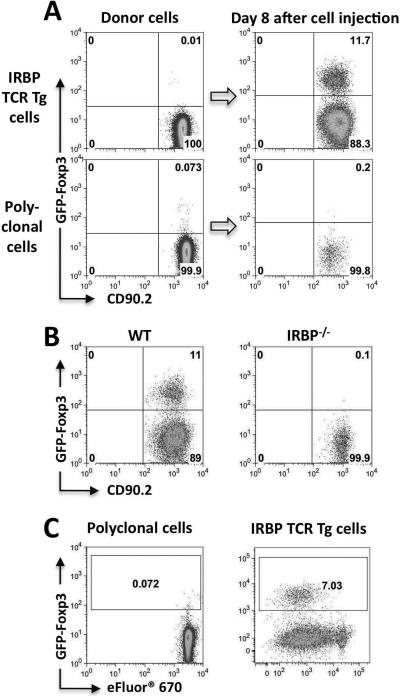
We wished to examine whether in situ Ag recognition was required for conversion, CD4+GFP− cells from IRBP TCR Tg FoxP3-GFP or WT FoxP3-GFP reporter mice were injected into eyes of CD90.1 congenic recipients and conversion was examined 7–8 days later. As before, IRBP TCR Tg T cells efficiently converted to Tregs in the eye, whereas polyclonal CD4+ T cells completely failed to convert (Fig 2A). Polyclonal cells did not convert even if they were incubated with anti-CD3/CD28 before injection into the eye (not shown), indicating that a sustained Ag stimulation is required. Similarly, there was no conversion in eyes of IRBP−/− recipient mice, which lack the target Ag (21, 25) (Fig 2B).
Figure 2. Conversion requires local antigen recognition and is accompanied by proliferation.
(A) Eyes of CD90.1 congenic recipients were injected with CD4+GFP− T cells from IRBP TCR Tg (Rag2−/−) or non-TCR Tg FoxP3-GFP reporter donors, and were analyzed on day 8 after injection. Representative experiment of three is shown. (B) CD4+GFP−T cells sorted from IRBP TCR Tg, Foxp3-GFP reporter mice were injected into eyes of naïve CD90.1 congenic recipients who were either WT (n=4 eyes) or IRBP−/− (n=8 eyes). On day 7 after cell injection, eyes were analyzed for Foxp3 expression on donor cells. Representative experiment of three is shown. (C) Conversion in vivo involves proliferation. Donor cells were labeled with eFluor® 670 and were injected into the eyes of naïve recipients. On day 4, FoxP3 expression and eFluor® 670 dilution in CD4+CD90.2+ was analyzed by flow cytometry. Data are representative of at least three independent experiments.
We next asked whether the Ag-dependent Foxp3 conversion within the eye involved proliferation. Our recent study in which we examined conversion of naïve T cells to Tregs in vitro by aqueous humor (AH) demonstrated that although proliferation took place, it was not necessary for conversion (19). To address this question in vivo, GFP-negative donor cells labeled with the proliferation dye eFluor® 670 were injected into eyes of healthy recipients. On day 4 after injection GFP expression vs proliferation dye dilution were examined. In IRBP TCR Tg cells, FoxP3-GFP expression was detected only in cells that had undergone several rounds of division (Fig 2C). Polyclonal cells again did not convert and did not proliferate.
T cells that had not been converted are primed, but do not express effector function in the eye
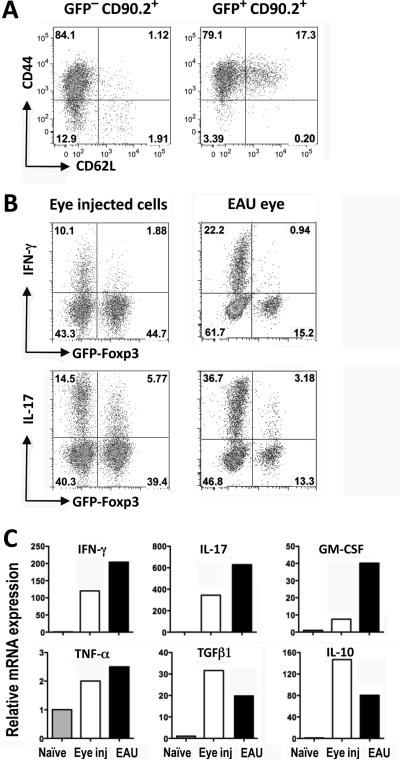
In all the experiments shown thus far, it stands out that only a part of the donor cells became converted to Tregs. We therefore asked what is the functional status of the non-converted cells. To address this, converted and non-converted CD90.2+ IRBP TCR Tg donor cells from eyes of CD90.1 recipients were examined a week after injection for expression of activation markers and for ability to produce the uveitis-relevant cytokines IFN-γ and IL-17 ex vivo (Fig 3). Most non-converted (as well as converted) cells expressed CD44 and were CD62L-negative, both parameters indicating that they had been primed (Fig. 3A). Nevertheless, expression of IFN-γ and IL-17 by these cells was consistently lower than in FoxP3−CD4+ T cells isolated from eyes of mice with actively induced EAU approximately a week after onset (Fig 3B). At the transcriptional level, non-converted donor cells expressed across the board less message for pro-inflammatory cytokines (IFN-γ, IL-17, GM-CSF and TNF-α) and more message for anti-inflammatory cytokines (IL-10 and TGF-β1) (Fig 3C). These results suggest that IRBP specific cells that had not been converted to FoxP3-positivity are restricted from expressing effector function in the eye. This conclusion is borne out by fundoscopic examination of the injected eyes, which showed retinal architecture with only minor changes even 3 weeks after IRBP TCR Tg cell injection, a time when mice actively immunized for uveitis are already past the peak of their disease.
Figure 3. Non–converted cells are primed but are restricted from expressing effector function in the eye.
CD4+GFP–− T cells from naïve IRBP TCR Tg FoxP3-GFP reporter mice (Rag2−/−) were injected into eyes of CD90.1 congenic recipients. (A) Donor cells injected into the eye express activation markers. Seven days after injection, cells from the recipient eyes were analyzed for CD44 vs. CD62L expression on donor cells by flow cytometry. Starting population contained <1% CD44highCD62Llow cells. Shown is a representative one of three independent experiments. (B) Eye-injected donor T cells express low effector function compared to T cells from eyes with actively induced EAU. Seven days after injection, cells isolated from the recipient eyes were pulsed ex vivo with PMA/Ionomycin for 4 hours in the presence of Brefeldin A. Cells stained intracellularly were analyzed for IFN-γ and IL-17 and expression in FoxP3− and in FoxP3+ populations. Cells from eyes of GFP-FoxP3 reporter mice (non-TCR Tg) in which EAU was actively induced by IRBP161–180 immunization were similarly analyzed 7 days after disease onset. Shown are representative plots of four (eye injected) and three (EAU) repetitions. (C) Non-converted GFP− IRBP TCR Tg donor T cells were purified by sorting 8 days after injection into eyes and were analyzed by real-time PCR for expression of pro- and anti-inflammatory cytokines (white columns) compared to CD4+ GFP− cells sorted from eyes of WT mice 8 days after onset of EAU induced by active immunization (black columns). Cytokine mRNA expression is normalized to freshly isolated WT naïve CD4+FoxP3− cells (grey columns).
Retinoic acid is required for conversion of T cells to FoxP3+ Tregs in vivo
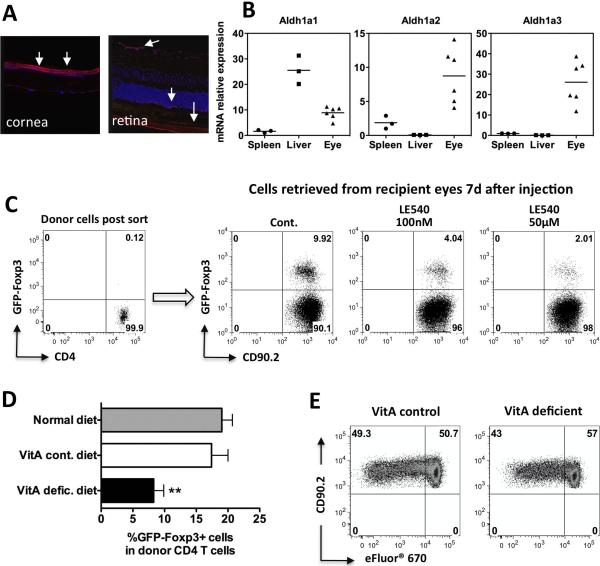
Numerous studies in vitro and in vivo have substantiated the role of TGF-β in conversion of T cells to Tregs (26). The eye contains high levels of TGF-β, mostly as TGF-β2 and mostly in latent form (9, 27). The eye also contains high levels of RA, as RA and its derivatives function in the visual cycle (28). Conversion of the VitA metabolite, retinal, into RA is accomplished by the enzyme retinaldehyde dehydrogenase (RALDH) which is present in three isoforms: ALDH1a1, ALDH1a2, ALDH1a3 (29). By immunohistochemical staining RALDH activity was present in the retina and cornea, and all 3 isoforms were detected in ocular tissues by PCR (Fig 4A,B).
Figure 4. Retinoic acid is required for optimal Treg conversion in vivo.
(A) Immunofluorescence staining for RALDHs in the healthy eye. Eyes from C57BL/6 mice were stained with a polyclonal antibody against all RALDH isoforms (red color, arrows), or with DAPI nuclear stain (blue) (×25). (B) Expression of retinaldehyde dehydrogenases (Aldh1a1, Aldh1a2, Aldh1a3) in the eye compared to spleen and liver of C57BL/6 mice by qRT-PCR. Target gene expression was normalized to its own GAPDH expression, then standardized to expression in a spleen (set as 1). Each symbol represents an individual mouse. A representative experiment of three is shown. (C) Donor CD4+GFP− cells from IRBP TCR Tg FoxP3-GFP reporter mice were pretreated with LE540 (50μM or 100 nM) for 1 h before injection. FoxP3 expression in CD4+CD90.2+ cells was examined 7 days later. Shown is a representative experiment of 5. (D) Reduced Treg conversion in mice fed with VitA deficient diet. CD4+GFP− T cells from IRBP TCR Tg mice were injected into the eyes of naïve congenic recipients, fed with regular diet, VitA control diet, or VitA deficient diet. On day 7 after cell injection, eyes were analyzed for FoxP3 expression on donor cells. IRBP TCR Tg donors were on a conventional background. Shown is one representative experiment of three with 3–7 mice per group. ** P ≤ 0.01. (E) Donor cell proliferation in eyes of VitA deficient vs. control mice. Donor CD4+GFP− cells from IRBP TCR Tg FoxP3-GFP reporter mice were labeled with eFluor® 670 and were injected into the eyes of naïve recipients, fed with VitA control diet, or VitA deficient diet. On day 4, FoxP3 expression and eFluor® 670 dilution in CD4+CD90.2+ were analyzed by flow cytometry. Shown is a representative experiment of two.
In a recent study, we showed that both TGF-β and RA in AH contribute to Treg conversion by ocular fluids in vitro (19). To explore whether RA was needed for the antigen-specific FoxP3 conversion in the more complex ocular environment in vivo, CD4+GFP− donor cells from IRBP TCR Tg mice were pretreated with LE540 for 1 h and were immediately injected into the eyes of naïve recipients. Blockade of RA signaling resulted in a reduction in FoxP3 conversion (Fig 4C).
To further examine the contribution of RA to Foxp3 conversion in the eye, we utilized VitA deficient mice generated by feeding a VitA deficient diet. Conversion of retina-specific T cells to FoxP3+ Tregs in eyes of VitA deficient mice was significantly less efficient than in animals fed the VitA control diet or the conventional diet (Fig 4D). Proliferation of the injected cells in eyes of VitA deficient and control mice was similar (Fig 4E), indicating that reduced conversion was not secondary to a general T cell activation defect resulting from VitA deficiency (30). In the aggregate, these findings indicate that RA is critical for optimal conversion of T cells to Tregs within the living eye.
Treg conversion is impaired in inflamed eyes
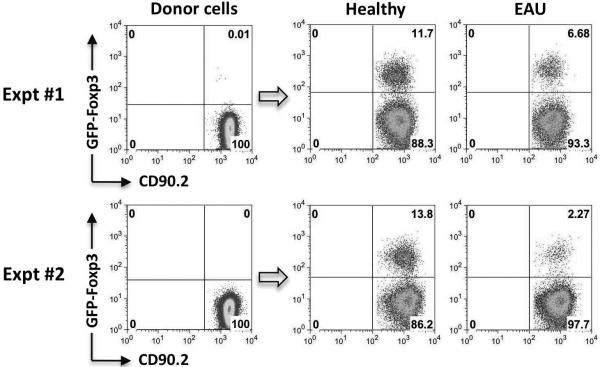
To examine whether conversion of T cells to Tregs can occur in eyes that are already inflamed, uveitis was induced in recipient mice by immunization with IRBP161-180 peptide. Fresh IRBP TCR Tg cells were injected into their eyes at the peak of disease, 14 days after immunization. Unlike in healthy eyes, FoxP3 induction was markedly impaired in eyes with uveitis (Fig 5). This contrasts with a report that the ability to induce ACAID, a systemic response mediated by Treg cells generated in the spleen in response to antigen injected into the eye, persists if antigen is injected into inflamed eyes (31). Our data indicate that in contrast to any systemic effects, local immune privilege, as manifested by conversion of conventional T cells to Tregs, becomes compromised during acute inflammation.
Figure 5. Conversion to Treg is impaired in inflamed eyes.
Eyes of CD90.1 congenic recipients, either untreated or immunized with IRBP161–180 two weeks earlier and exhibiting active uveitis, were injected with CD4+GFP− T cells from IRBP TCR Tg (Rag2−/−) FoxP3-GFP reporter donors, and were analyzed on day 8 after injection. Data are shown from two independent experiments with 3–4 animals per group.
Previously primed T cells are resistant to conversion in the ocular microenvironment
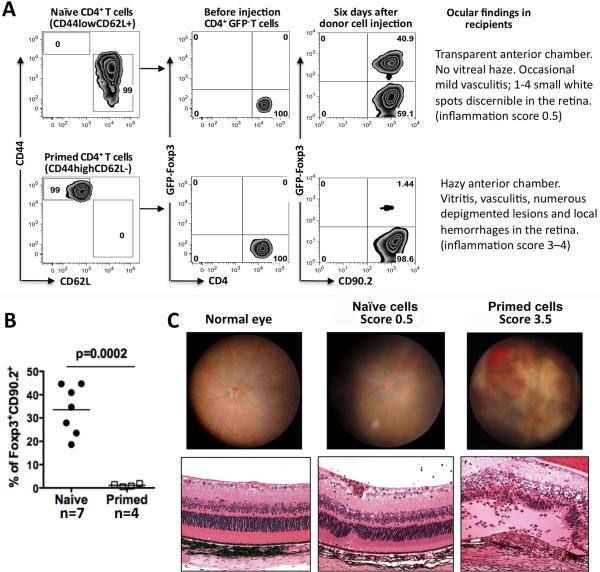
The data described above are compatible with the interpretation that a retina specific T cell that enters the eye and is exposed to its cognate Ag there, may be induced to adopt a Treg fate and/or is inhibited from expressing effector function. If so, the question immediately arises, how can uveitis develop? Because uveitogenic T cells are primed in the periphery before they encounter the ocular microenvironment, we decided to examine the effect of the ocular microenvironment on antigen-experienced, as opposed to antigen-naïve T cells. Naïve (CD44lowCD62Lhigh) cells were sorted from lymph nodes and spleens of unmanipulated IRBP TCR Tg RAG2−/− FoxP3-GFP reporter mice. Primed (CD44highCD62Llow) T cells were similarly sorted from spleens of donors that had been immunized with IRBP161-180 10–14 days earlier. Both populations of cells were then injected into eyes of CD90.1 congenic recipients and 6 days later eyes were examined by fundoscopic examination and collected for cell analysis. Whereas the naïve population efficiently converted to FoxP3 positivity, as expected, the in vivo primed IRBP-specific T cells failed to convert to FoxP3 positivity (Fig 6A, 6B) and instead induced severe inflammation in the recipient's eyes. In contrast, eyes injected with naïve cells retained a largely normal appearance of the retina even 3 weeks after cell injection. Figure 6C shows illustrative examples of the clinical and histopathological appearance of eyes at the observed scores of inflammation. These results provide an in vivo correlate to our recent finding that effector/memory T cells resist conversion into Tregs by ocular fluids (19) and demonstrate that this has functional consequences that are directly relevant to disease.
Figure 6. Previously primed T cells are resistant to Treg conversion in the ocular microenvironment and instead induce uveitis.
(A) CD62LhighCD44low (naïve) or CD62LlowCD44high (primed) CD4+FoxP3− T cells were sorted from naïve or from IRBP161–180-immunized IRBP TCR Tg Rag2−/− FoxP3-GFP reporter mice (8 and 12 animals, respectively). The sorted cells were injected into eyes of CD90.1 congenic recipients (2–5 mice/group). FoxP3 induction was assessed 6 days later. Data are representative of 3 experiments (naïve) and 2 experiments (primed). (B) Compiled data from two repeat experiments showing percent conversion. Each point is one mouse (pool of 2 eyes). Statistical analysis was by student's t-test. (C) Typical fundus and histological appearance of EAU scores illustrating changes typically observed in the injected eyes. Note that these are illustrative examples taken from a different experiment, as the eyes of the mice in panels A and B were used for flow cytometry analysis.
Taken together, the data suggest that T cells specific to retinal Ag with the potential to cause uveitis, can be “disarmed” upon entry into the ocular microenvironment by conversion to a FoxP3+ Treg phenotype and/or inhibition of their function. However, in contrast to uncommitted cells, previously primed cells are resistant to the immunosuppressive and Treg-inducing effects of the ocular microenvironment. They retain their effector function and can participate actively in induction of uveitis.
DISCUSSION
The present study provides the first in vivo evidence that the local ocular microenvironment of the eye can “disarm” uncommitted T cells with the potential to cause pathology by converting them to FoxP3+ Tregs and/or by dampening their expression of effector function. Importantly, because conversion of naïve T cells to FoxP3+ Treg phenotype requires antigen recognition and priming (32, 33), our data support the interpretation that naïve T cells which gain access to the tissue can be primed in situ. This finding contrasts with the prevailing notion that naïve T cells are preferentially primed in lymph nodes. While some would argue that naïve T cells are normally excluded from healthy tissues, minor or even major internal bleeding as a consequence of a fall or contusion can and does occur. However, in most tissues in situ priming cannot be easily studied, because cell migration cannot be excluded as a factor. As a relatively closed compartment of small dimensions, the eye is uniquely suited to addressing this question. Our data suggest that if and when naïve cells do gain access to the tissue, under some conditions they may indeed be primed there.
We considered the possibility that the converted T cells were in fact primed in the eye-draining lymph node. Although we cannot completely exclude that a few injected T cells may leave the eye, become converted in the lymph nodes and come back, exceedingly few donor-derived FoxP3+ cells are detectable in the eye-draining submandibular lymph node, or other peripheral lymphoid organs (Fig 1C) and they do not express FoxP3. Furthermore, T cells that leave the eye would have to recirculate through the body to return to the eye. Experimental data indicate that an activated retina-specific T cell in the peripheral circulation has a 0.000015 chance of finding its way to the uninflamed eye (11, 34). Finally, our recent in vitro data show that Treg converted by AH of the eye are initially unstable and lose FoxP3 expression if removed from the AH-containing environment (19). Thus, for all these reasons, exit from the eye and re-entry as a prerequisite for conversion seems very unlikely. It would be of considerable interest to identify the antigen-presenting cells in the eye that participate in the priming and conversion process, but this is a massive undertaking in itself, which is beyond the scope of the current study. That said, Lehmann et al. recently identified a population of rare CD11c+ DC in the retina that were rapidly activated in response to retinal injury and could be involved in this process (35).
The biological relevance of Treg conversion within the eye hinges on the antigen exposure history of the infiltrating T cell. It is accepted that naïve T cells do not cross blood vessels to enter into tissues. However, naïve T cells can, and do, gain entry into the eye passively. Bleeding into the eye can be induced by trauma or by vascular abnormalities accompanying conditions such as macular degeneration, diabetic retinopathy, retinopathy of prematurity, etc. (36). Naïve T cells also gain entry into the eye under conditions of inflammation. During spontaneous EAU in IRBP TCR Tg mice, up to 10% of IRBP161-180-specific T cells that infiltrate inflamed eyes are naïve by CD44 and CD62L expression (R. Horai, unpublished). This is in line with reports of other investigators, who showed that naïve T cells are actively recruited into various chronically inflamed tissues through CCL21 expressed by endothelial cells (37). Our data show that conversion of T cells to Tregs is severely impaired in the inflammatory environment, therefore, it is possible that rather than being “disarmed”, naïve T cells entering the eye during inflammation may become effector cells and at least initially actively contribute to disease. If that is the case, Tregs recruited into the eye from the periphery may be critical to tip the balance and (together with local conversion, however limited) help bring about resolution of the disease.
It is notable that, despite evidence of having been primed, the non-converted donor T cells within the eye appear to be restricted from expressing effector function and are not precipitating acute uveitis. Non-converted donor T cells might also be kept in check by the converted Foxp3+ Treg cells, which appear to be extremely potent suppressors. Furthermore, increased TGF-β1 and IL-10 message in the non-converted population after 8 days in the eye implies that they themselves may contain Tregs that do not express FoxP3. However, because both time and proliferation are required for Tregs to be primed and differentiated, it is likely that additional inhibitory influences in the eye might also contribute directly to dampening their effector potential. Ocular fluids contain neuropeptides such as α-melanocyte stimulating hormone (α-MSH), vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP) and somatostatin (SOM) (38). The ability of ocular resident cells (including retinal glial Müller cells, choroidal cells and various pigment epithelial cells) to inhibit T cell activation and function in culture has been known for some time (14, 17, 39). Both Müller cells and RPE cells in the back of the eye are reported to suppress effector function of primed T cells in culture (14, 40). However, only co-culture with RPE cells was reported to convert T cells to Tregs, partly through contact and partly through soluble mediators (16) (although some have contested this (41)).
Another notable finding is the role of RA in the conversion within the eye in vivo. RA produced by CD103+ DC was shown, together with TGF-β, to contribute to Treg conversion in the gut (42, 43). In the eye, RA and its derivatives are highly abundant in ocular fluids due to their participation in the visual cycle. Photoreceptors continuously take up 11-cis retinal and after using it in the process of phototransduction, release all-trans RA, which is taken up by RPE and regenerated into 11-cis-retinal (28). Thus, lymphocytes that find their way into the eye are literally “bathed” in RA. In an in vitro study, we recently reported that TGF-β and RA present in AH synergize in converting naïve T cells to Tregs, with TGF-β enhancing expression of RARα in the T cells that are being converted (19). It is likely that the inherent function of RPE to turn over RA contributes to their reported ability to induce Tregs in culture. Nevertheless, it was not a given that RA would be needed in the eye in vivo, for at least 2 reasons. (a) In the in vitro study, the AH had been acid-treated so as to activate TGF-β, whereas most of the TGF-β in the eye is by necessity in latent form, as due to its pro-fibrotic effects TGF-β activation must be tightly controlled (44); (b) It is conceivable that the presence of other potential Treg inducing factors in the eye, i.e, inhibitory neuropeptides and ocular resident cells (14–18, 19) might replace the need for RA. While we are not able to tease out the relative contribution of ocular resident cells vs. ocular fluids and their complex components to the conversion process in vivo, our data demonstrate that RA is a required component for optimal Treg conversion in the living eye. We hypothesize that, because the amount of bioactive TGF-β in the eye is limiting, the role of RA takes on particular importance. Thus, RA functions not only in visual signal transduction, but also in maintaining immune homeostasis within the eye.
An issue that has perplexed the field for many years is why does immune privilege, that efficiently protects the eye from consequences of day-to-day minor insults and traumas, and is believed to underlie the extraordinary success of corneal (allo)grafts (3, 13), apparently fall short of preventing an autoimmune attack on the eye. Our current data may help to explain this conundrum. First, we demonstrate that in contrast to uncommitted cells, Ag-experienced T cells are resistant to conversion in the ocular microenvironment and instead induce uveitis. While accidental or surgical disruption of a blood vessel within the eye would be expected to mostly bring naïve (easily converted) T cells into the eye, uveitis is elicited by effector T cells that had been activated outside the eye, which then acquire the ability to penetrate the BRB and start the inflammatory process (34). Second, de novo conversion of uncommitted T cells to Tregs is hindered in eyes that are already inflamed, further compounding the situation. Impaired conversion to Tregs may at least in part be attributed to profound changes in the composition of ocular fluids during uveitis. During uveitis there is a reduction in inhibitory mediators such as TGF-β and likely other small molecule components including RA, due to loss of the blood-retinal barrier (45). At the same time, presence of inflammatory mediators, including IFN-γ, IL-1 and IL-6 would tend to tip the balance to Th1 and Th17 rather than to Treg differentiation (45, 46).
Our findings shed new light on the phenomenon of immune privilege and on its role, as well as its limitations, in actively controlling inflammatory responses within the tissue and maintaining immune homeostasis.
ACKNOWLEDGEMENTS
The authors thank Dr. Alexander Y. Rudensky (Sloan-Kettering, NY) for the FoxP3-GFP reporter mice and Dr. Gregory Liu of the Medical College of GA for IRBP−/− mice. We thank Drs. Yasmine Belkaid, Jason Hall and Cheng-Ming Sun (NIAID, NIH) for useful discussions and help with VitA deficient mice. We are grateful to Dr. Chi-Chao Chan (NEI, NIH) for histological evaluation and photomicrographs and to our colleagues Drs. Barry Rouse (U Tennessee), Joost Oppenheim, Xin Chen (NCI) and Dr. Hidehiro Yamane (NIAID) for critical review of the manuscript. We thank the staff of the NEI Flow Cytometry Core Facility and the NEI Imaging Core Facility for cell sorting and for confocal microscopy, respectively.
This work was supported by NIH/NEI Intramural funding, Project No. EY000184.
Abbreviations
- AH
aqueous humor
- BRB
Blood-retinal barrier
- RA
retinoic acid
- Treg
regulatory T cells
- TCR
T cell receptor
- FoxP3
forkhead box P3 transcription factor
- RAR
retinoic acid receptor
- CFA
complete Freund's adjuvant
- RALDHs
retinaldehyde dehydrogenases
- IRBP
interphotoreceptor retinoid-binding protein
- EAU
experimental autoimmune uveitis
- 7-AAD
7-aminoactinomycin D
REFERENCES
- 1.Forrester JV. Privilege revisited: an evaluation of the eye's defence mechanisms. Eye (Lond) 2009;23:756. doi: 10.1038/eye.2008.259. [DOI] [PubMed] [Google Scholar]
- 2.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Mason KL, Huffnagle GB, Noverr MC, Kao JY. Overview of gut immunology. Adv Exp Med Biol. 2008;635:1. doi: 10.1007/978-0-387-09550-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5:164. doi: 10.1530/ror.0.0050164. [DOI] [PubMed] [Google Scholar]
- 7.Macaubas C, DeKruyff RH, Umetsu DT. Respiratory tolerance in the protection against asthma. Curr Drug Targets Inflamm Allergy. 2003;2:175. doi: 10.2174/1568010033484304. [DOI] [PubMed] [Google Scholar]
- 8.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201. [PubMed] [Google Scholar]
- 10.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 11.Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 12.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Influence of donor and histocompatibility factors on corneal graft outcome. Transplantation. 1994;58:1210. [PubMed] [Google Scholar]
- 14.Caspi RR, Roberge FG, Nussenblatt RB. Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science. 1987;237:1029. doi: 10.1126/science.2956685. [DOI] [PubMed] [Google Scholar]
- 15.Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. 1997;16:900. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 16.Sugita S, Horie S, Nakamura O, Futagami Y, Takase H, Keino H, Aburatani H, Katunuma N, Ishidoh K, Yamamoto Y, Mochizuki M. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol. 2008;181:7525. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. 2003;11:91. doi: 10.1076/ocii.11.2.91.15914. [DOI] [PubMed] [Google Scholar]
- 18.Ke Y, Sun D, Jiang G, Kaplan HJ, Shao H. PD-L1hi retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells. J Leukoc Biol. 2010;88:1241. doi: 10.1189/jlb.0610332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou R, Horai R, Mattapallil MJ, Caspi RR. A new look at immune privilege of the eye: dual role for the vision-related molecule, retinoic acid. J Immunol. 2011 doi: 10.4049/jimmunol.1101634. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 21.Avichezer D, Grajewski RS, Chan CC, Mattapallil MJ, Silver PB, Raber JA, Liou GI, Wiggert B, Lewis GM, Donoso LA, Caspi RR. An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms. J Exp Med. 2003;198:1665. doi: 10.1084/jem.20030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol Chapter. 2003;15(Unit 15):6. doi: 10.1002/0471142735.im1506s53. [DOI] [PubMed] [Google Scholar]
- 24.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Liou GI, Fei Y, Peachey NS, Matragoon S, Wei S, Blaner WS, Wang Y, Liu C, Gottesman ME, Ripps H. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J Neurosci. 1998;18:4511. doi: 10.1523/JNEUROSCI.18-12-04511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Konkel JE. TGF-beta and `adaptive' Foxp3(+) regulatory T cells. J Mol Cell Biol. 2010;2:30. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi Y, Kawahara J, Iwasaki T, Usui M. Retinoic acid transport to lens epithelium in human aqueous humor. Jpn J Ophthalmol. 1994;38:400. [PubMed] [Google Scholar]
- 29.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 30.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 34:435. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo JS, Streilein JW. Immune privilege persists in eyes with extreme inflammation induced by intravitreal LPS. Eur J Immunol. 2001;31:3806. doi: 10.1002/1521-4141(200112)31:12<3806::aid-immu3806>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4−/−mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci U S A. 2007;104:13756. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754. [PubMed] [Google Scholar]
- 35.Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS. Dendritic cells are early responders to retinal injury. Neurobiol Dis. 2010;40:177. doi: 10.1016/j.nbd.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besharse J, Dana R, Dartt DA. Encyclopedia of the Eye. Vol 2. Academic Press; Oxford: 2010. [Google Scholar]
- 37.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 38.Taylor A. A review of the influence of aqueous humor on immunity. Ocul Immunol Inflamm. 2003;11:231. doi: 10.1076/ocii.11.4.231.18269. [DOI] [PubMed] [Google Scholar]
- 39.Hooper P, Bora NS, Kaplan HJ, Ferguson TA. Inhibition of lymphocyte proliferation by resident ocular cells. Curr Eye Res. 1991;10:363. doi: 10.3109/02713689108996342. [DOI] [PubMed] [Google Scholar]
- 40.Kaestel CG, Jorgensen A, Nielsen M, Eriksen KW, Odum N, Nissen M. Holst, Ropke C. Human retinal pigment epithelial cells inhibit proliferation and IL2R expression of activated T cells. Exp Eye Res. 2002;74:627. doi: 10.1006/exer.2002.1183. [DOI] [PubMed] [Google Scholar]
- 41.Gregerson DS, Heuss ND, Lew KL, McPherson SW, Ferrington DA. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest Ophthalmol Vis Sci. 2007;48:4654. doi: 10.1167/iovs.07-0286. [DOI] [PubMed] [Google Scholar]
- 42.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 43.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saika S. TGFbeta pathobiology in the eye. Lab Invest. 2006;86:106. doi: 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- 45.Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, Rauz S, Wallace GR, Salmon M, Murray PI. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- 46.Ongkosuwito JV, Feron EJ, van Doornik CE, Van der Lelij A, Hoyng CB, La Heij EC, Kijlstra A. Analysis of immunoregulatory cytokines in ocular fluid samples from patients with uveitis. Invest Ophthalmol Vis Sci. 1998;39:2659. [PubMed] [Google Scholar]