Abstract
The purpose of this study was to assess insoluble salts containing gadolinium (Gd3+) for effects on human dermal fibroblasts. Responses to insoluble Gd3+ salts were compared to responses seen with Gd3+ solubilized with organic chelators, as in the Gd3+-based contrast agents (GBCAs) used for magnetic resonance imaging. Insoluble particles of either Gd3+-phosphate or Gd3+-carbonate rapidly attached to the fibroblast cell surface and stimulated proliferation. Growth was observed at Gd3+ concentrations between 12.5 and 125 μM, with toxicity at higher concentrations. Such a narrow window did not characterize GBCA stimulation. Proliferation induced by insoluble Gd3+ salts was inhibited in the presence of antagonists of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways (similar to chelated Gd3+) but was not blocked by an antibody to the platelet-derived growth factor receptor (different from chelated-Gd3+). Finally, high concentrations of the insoluble Gd3+ salts failed to prevent fibroblast lysis under low-Ca2+ conditions while similar concentrations of chelated-Gd3+ were effective. In conclusion, while insoluble Gd3+ salts are capable of stimulating fibroblast proliferation, one should be cautious in assuming that GBCA dechelation must occur in vivo to produce the profibrotic changes seen in association with GBCA exposure in the subset of renal failure patients that develop nephrogenic systemic fibrosis.
Keywords: Gadolinium phosphate, gadolinium carbonate, gadolinium-based contrast agent, dermal fibroblast, intracellular signaling, proliferation
INTRODUCTION
The lanthanoids comprise a group of cationic metals with atomic numbers between 57 and 71. These closely related elements have an ionic radius similar to that of Ca2+, but with a higher overall charge density [1,2]. In biological fluids, lanthanoid metals form insoluble salts with available anions, but solubility can be maintained by forming a complex between the lanthanoid metal and a metal ion chelator. The gadolinium (Gd3+) based contrast agents (GBCAs) used in magnetic resonance imaging are examples of this [3–5]. Although GBCAs are rapidly cleared from the circulation by the kidneys, the contrast agents may remain in the circulation for several hours in individuals with end-stage renal disease (ESRD) [3]. In a small number of ESRD patients, fibrotic skin changes have occurred following GBCA exposure; the clinical syndrome is referred to as nephrogenic systemic sclerosis (NSF) [6–19]. Although the disease has been likened to scleroderma based on the presence of excess collagen deposition, many pathologists describes the lesions as more fibroproliferative than fibrotic [6,7,18,19].
As part of an effort to understand disease patho-physiology, we and others have examined GBCAs for effects on dermal fibroblast function. These studies have demonstrated that GBCA exposure leads to increased fibroblast proliferation [20–23]. Our own studies have shown that growth induction is not correlated with increased procollagen synthesis per se, but is associated with altered collagen deposition due to modulation of matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) levels [21,24,25]. While this provides an understanding of disease patho-physiology, several important questions remain to be addressed. One of the unanswered questions pertains to the nature of the Gd3+ species that is directly responsible for fibroblast activation. The prevailing assumption is that during prolonged GBCA circulation in patients with renal failure, the metal ion separates from the chelator [3–5] and that “free” metal produces the fibrogenic changes. However, Gd3+ does not remain free for very long. Rather, the cationic metal rapidly binds to available anions (phosphate, carbonate, hydroxide etc.) in biological fluids and forms insoluble salts. Previous studies have demonstrated deposits of Gd3+ in lesional tissue of patients with NSF, and sophisticated analytical techniques have demonstrated that tissue Gd3+ is closely associated with phosphate [26–29]. Recently, George et al. [30] used synchrotron X-ray analysis to demonstrate phosphate – bound Gd3+ in NSF skin. Insoluble salts can be phagocytosed by tissue macrophages [31–33], and it has been suggested that activated inflammatory cells release cytokines and other pro-inflammatory mediators to initiate fibrotic changes [34–36]. It is generally assumed, however, that such salts are inert with respect to fibroblasts. The present study tested that assumption directly. Here we have prepared insoluble salts consisting of Gd3+-phosphate and Gd3+-carbonate and have examined the insoluble salts for effects on human dermal fibroblast function. Results obtained with these salts were compared to effects seen when the same cells were exposed to chelated forms of Gd3+ or when Gd3+-chloride was added directly to the culture medium [21,24,25,37,38]. Findings from this study and their potential significance to the fibrogenic changes in NSF are described herein
MATERIALS AND METHODS
Reagents
Gd3+-chloride was obtained from Sigma Chemical Company (St. Louis, MO). A 0.5 M solution in distilled water was prepared from this. Omniscan (GE Healthcare), Magnevist (Berlex Imaging) and Multihance (Bracco diagnostics) were obtained through Experimental Drug Services at the University of Michigan Hospitals. These are sterile aqueous solutions of chelated-Gd3+ used as MRI contrast agents. All three agents are 0.5M with respect to Gd3+. Gadodiamide (0.5 M Gd3+) was obtained as a gift from Dr. Ben Newton (GE Healthcare). U0126 and LY294002 were obtained from Cell Signaling Technologies (Beverly, MA) and a blocking antibody to the PDGF receptor was obtained from R&D Systems (Minneapolis, MN). Antibodies against phospho-ERK, total-ERK, phospho-AKT and total-AKT were obtained from Cell Signaling Technologies.
Preparation of Gd3+-phosphate and Gd3+-carbonate salts
Insoluble salts containing Gd3+ were prepared by mixing 200 μL of a 0.5 M solution of Gd3+-chloride with excess sodium dibasic phosphate. The precipitate that formed was centrifuged and washed 10 times in distilled water and finally re-suspended in 800 μL of distilled water (final concentration of Gd3+ = 125 mM). Gd3+-carbonate was prepared in a similar fashion using sodium dibasic carbonate. Ca2+-phosphate was also prepared in a similar fashion and used as a control.
Isolation of human dermal fibroblasts and human epidermal keratinocytes
Human dermal fibroblasts and human epidermal keratinocytes were isolated from neonatal foreskin as described previously [39]. De-identified tissue was obtained from circumcisions performed at the University of Michigan Hospital under an exemption from IRB oversight. Fibroblasts were grown using Dulbecco’s Modified Minimal Essential Medium supplemented with nonessential amino acids and 10% fetal bovine serum as culture medium (DMEM-FBS). Keratinocytes were grown in Keratinocyte Growth Medium (KGM) (Lonza; Walkersville, MD). KGM consists of MCDB-153 medium as base and is supplemented with a mixture of growth factors including EGF, insulin, hydrocortisone, and bovine pituitary extract. Maintenance of both cell types was at 37°C in an atmosphere of 95% air and 5% CO2. Cells were used at passage 2–3.
Proliferation
Fibroblasts were harvested from 75-cm2 culture flasks by brief (1 minute) exposure to a solution of 0.05% trypsin with 0.2 mg/mL ethylenediaminetetraacetic acid (EDTA). Cells were added to wells of a 24-well dish at 3×104 cells per well in DMEM-FBS. After the cells attached, the wells were washed two times with 1 mL of KGM supplemented with Ca2+ to a final concentration of 1.5 mM. Duplicate wells were counted to provide accurate “zero-time” values. The remaining wells were treated as follows: One mL of culture medium (Ca2+-supplemented KGM) was added to each well. Gd3+-phosphate or Gd3+-carbonate was diluted to the desired amount in serum-free, Ca2+-supplemented KGM and added to the well in a 10 μL volume. Ca2+-phosphate served as control. Incubation was for 3 days at 37°C in an atmosphere of 95% air and 5% CO2. At the end of the incubation period, culture fluids were harvested for assessment of matrix metalloproteinase-1 (MMP-1) as described below. Cells were harvested with trypsin-EDTA and counted.
Keratinocyte proliferation was assessed in the same manner except the culture medium consisted of KGM (0.15 mM Ca2+).
Fibroblast survival assay
Fibroblasts were harvested as above and added to wells of a 24-well dish at 3×104 cells per well in DMEM-FBS. The cells were allowed to attach and then washed two times with 1 mL of Keratinocyte Basal Medium (KBM) supplemented with Ca2+ to a final concentration of 0.1 mM. KBM consists of the same basal medium as KGM but has no growth factors. Duplicate wells were counted to provide accurate “zero-time” values. The remaining wells were used in experiments. One mL of culture medium (KBM with 0.1 mM Ca2+) was added to each well. Gd3+-phosphate or Gd3+-carbonate salts were diluted to the desired amount in culture medium and added to the well in a 10 μL volume. Gd3+-chloride and chelated-Gd3+ (Omniscan, gadodiamide, Magnevist or Multihance) were examined in parallel. Incubation was for 3 days at 37°C in an atmosphere of 95% air and 5% CO2. At the end of the incubation period, cells were harvested with trypsin-EDTA and counted.
MMP-1
Western blotting with a rabbit polyclonal anti-MMP-1 antibody (Millipore/Chemicon, Temicula, CA) was used to assess MMP-1 levels [21,24]. Briefly, samples were separated in SDS-PAGE under denaturing and reducing conditions and transferred to nitrocellulose membranes. After blocking with a 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) at 4°C overnight, membranes were incubated for one hour at room temperature with the antibody diluted 1:1000 in 0.5% nonfat milk/0.1% TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Phototope-HRP Western blot detection kit (Cell Signaling Technologies, Inc.). Images were scanned, digitized and quantified. Prior to loading the gels, protein levels in each sample were determined using the BCA protein determination kit (Pierce Biotechnology; Rockford, IL) and equal amounts of protein were loaded onto each lane. Following electrophoresis and protein transfer to the nitrocellulose filters, Ponceau S reversible staining solution (Pierce Biotechnology) was used to visualize the transferred proteins in order to confirm that comparable amounts of total protein were transferred.
MMP-2
The same culture fluids were assessed for MMP-2 (gelatinase A) by gelatin zymography [21]. Briefly, SDS-PAGE gels were prepared with the incorporation of gelatin (1 mg/mL) at the time of casting. After electrophoresis under non-reducing conditions to separate proteins and overnight incubation to allow for substrate digestion, zones of hydrolysis were identified as “holes” in Coomassie Blue-stained gels and quantified. Values were obtained following scanning and digitization. MMP-2 tends to be expressed at a basal level under most conditions in fibroblasts. Therefore, MMP-2 can be used as a control for secreted proteins in much the same way that actin or β-tubulin is utilized as a control for intracellular proteins.
Intracellular signaling intermediates
Cells were added to wells of a 6-well dish at 3×105 cells per well, and incubated as above. After 5, 10 and 15 minutes, the cell layer was washed one time in PBS and then treated with a “lysis buffer” (Pierce Biotechnology) according to the manufacturer’s instructions. After protein determination, equal amounts of protein were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Lysates were probed for phospho- and total-ERK and phospho- and total-AKT by Western blotting essentially as described above with MMP-1.
Light and electron microscopy
Fibroblasts were exposed to Gd3+-phosphate and incubated for one day ad described above. Following this, the cells were washed exhaustively to remove unbound salt and then prepared for light microscopy or electron microscopy as described below.
Light microscopy
Cells were fixed for one hour in 10% buffered formalin and then stained with hematoxylin and eosin. The cells were examined and imaged using a Zeiss Axio Imager, M1 microscope with the Axio Vision Imaging System.
SEM
Samples were fixed in 2.5 percent glutaraldehyde in 0.1 M Sorensen’s buffer, pH 7.4, overnight at 4°C. After several rinses in buffer, samples were post-fixed in one percent osmium tetroxide in Sorensen’s buffer for one hour. After additional rinses in buffer, the samples were dehydrated through increasing concentrations of ethanol. The samples were then immersed in four, 15-minute changes of hexamethyldisilazane (HMDS). After the fourth change, the samples were incubated with just enough HMDS to cover the tissue and the HMDS was allowed to evaporate overnight. The samples were then mounted on SEM stubs, allowed to off-gas in a vacuum desiccator for at least two hours and sputter coated with gold. Samples were examined with an Amray 1910 FE Scanning Electron Microscope and digitally imaged using Semicaps 2000 software.
TEM
Samples were fixed in 2.5 percent glutaraldehyde in 0.1 M Sorensen’s buffer, pH 7.4, overnight at 4°C. After several rinses in buffer, samples were post-fixed in one percent osmium tetroxide. The samples were then rinsed in double distilled water. Following this, samples were dehydrated in ascending concentrations of ethanol, rinsed two times in 100% ethanol, and embedded in epoxy resin. The samples were ultra-thin sectioned (70 nm thickness) and stained with uranyl acetate and lead citrate. The sections were examined using a Philips CM100 electron microscope at 60 kV. Images were recorded digitally using a Hamamatsu ORCA-HR digital camera system operated with AMT software (Advanced Microscopy Techniques Corp., Danvers, MA).
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post-test for selected pairs (GraphPad Prism version 4.00 for Windows, GraphPad Software). Data were considered significant at p< 0.05. Asterisks have been added to the appropriate data points to denote values that are significantly different from the respective control.
RESULTS
Effects of Gd3+-phosphate and Gd3+-carbonate on fibroblast proliferation
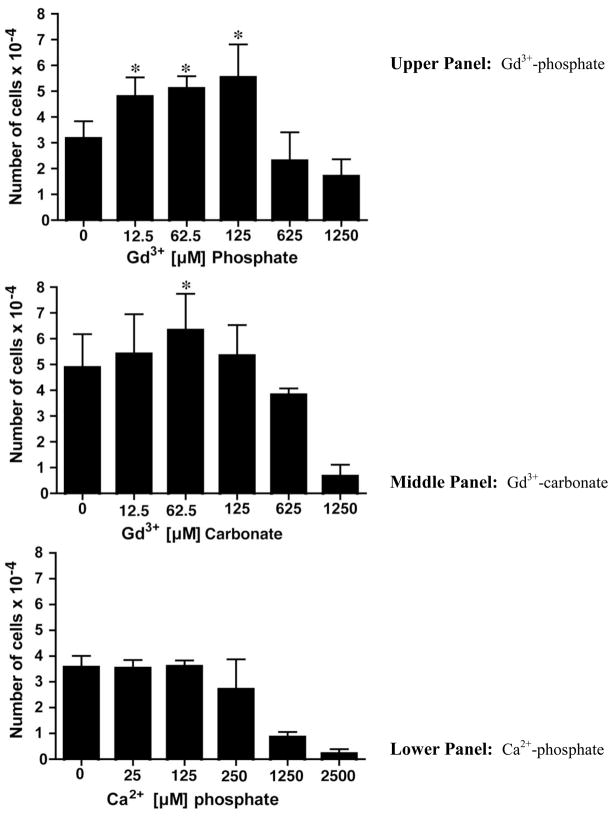
Gd3+- phosphate and Gd3+-carbonate were suspended in fibroblast culture medium (KGM supplemented with Ca2+ to a final concentration of 1.5 mM) and examined for ability to stimulate fibroblast proliferation. The results of this study are shown in Figure 1, where it can be seen that fibroblast proliferation was induced by both types of Gd3+ salts. At stimulatory concentrations with either salt, the total amount of Gd3+ in the culture medium was 12.5 – 125 μM. At concentrations below 12.5 μM, proliferation fell to basal levels and at concentrations greater than 125 μM, both types of salt were cytotoxic. Ca2+-phosphate was utilized as a control for these experiments. When added to fibroblasts at high concentration (equivalent to greater than 125 μM Ca2+), growth-suppression and cytotoxicity were seen. Lower concentrations were not toxic, but there was no growth stimulation at any concentration (Figure 1, lower panel). Gd3+-chloride and chelated-Gd3+ (Omniscan) were used as additional controls. Consistent with our previous results [18,22], fibroblast proliferation was stimulated with Gd3+-chloride over the range of 5–10 μM with a fall off in growth at concentrations above this (Table 1). It should be noted that when Gd3+-chloride was added to culture medium, a precipitate formed with (presumably) various anionic constituents of the culture medium including phosphate, carbonate, citrate, sulfate and hydroxide. Although we have not analyzed the relative amounts of each anion, previous studies by Li et al. [40] found that phosphate was the predominant anion. Also consistent with previous findings [21,24], exposure to Omniscan led to an increase in fibroblast growth over a wide concentration range (0.5 μM to 2.5 mM) (Table 1).
Figure 1. Proliferation of human dermal fibroblasts in response to insoluble Gd3+ salts.
Upper panel: Gd3+-phosphate; Middle panel: Gd3+-carbonate; Lower panel: Ca2+-phosphate. Values shown are means and standard deviations based on n=4 separate experiments with duplicate or triplicate samples per data point. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to control at p<0.05 level.
Table 1.
Proliferation of human dermal fibroblasts in response to Gd3+ - chloride and Omniscan.
| Intervention | Number of cells X10−4 | |
|---|---|---|
| Gd3+ - chloride | Omniscan | |
| Control | 3.9 ± 0.3 | 4.2 ± 0.1 |
| 0.5 μM Gd3+ | 4.0 ± 0.2 | 4.4 ± 0.2 |
| 1 μM Gd3+ | 4.0 ± 0.2 | 4.7 ± 0.1* |
| 5 μM Gd3+ | 5.5 ± 0.1* | 5.2 ± 0.3* |
| 10 μM Gd3+ | 6.3 ± 0.2* | 7.5 ± 0.3* |
| 50 μM Gd3+ | 3.9 ± 0.4 | 6.8 ± 0.3* |
| 100 μM Gd3+ | 2.3 ± 0.3 | 6.9 ± 0.3* |
| 500 μM Gd3+ | <0.1 | 5.9 ± 0.4* |
| 1000 μM Gd3+ | < 0.1 | 5.1 ± 0.2* |
Values shown are means and standard errors based on n=3 separate experiments. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons.
indicates statistically significant increase relative to control at p<0.05 level.
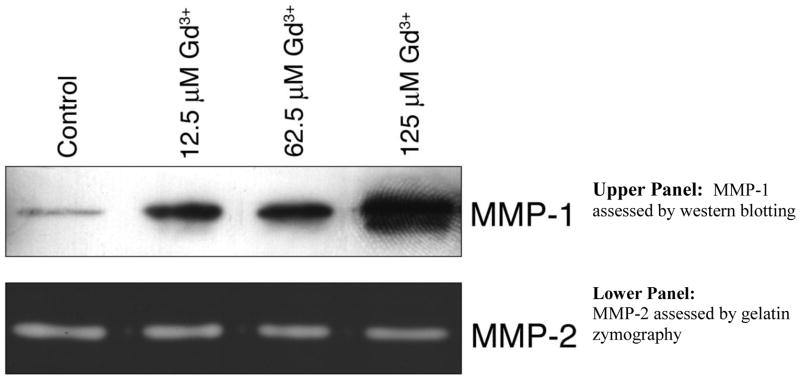
At the time of harvest - i.e., after 72-hours of incubation - the culture fluids were collected from control cells and cells treated with Gd3+-phosphate or Gd3+-carbonate. The culture fluids were examined for MMP-1 by Western blotting. The enzyme was up-regulated by growth-stimulating concentrations of the inorganic Gd3+ salts (Figure 2). As a control for MMP-1, we also assessed the same culture fluids for levels of MMP-2. Previous studies have demonstrated that MMP-2 is not modulated by factors that up-regulate MMP-1 [41]. Other than a fall-off in MMP-2 at cytotoxic concentrations, there was no effect on this enzyme (Figure 2).
Figure 2. MMP changes in human dermal fibroblasts exposed to Gd3+-phosphate.
Upper panel. MMP-1 assessed by western blotting. Lower panel. MMP-2 assessed by gelatin zymography. Gels are from a single experiment representative of three separate experiments with similar results.
Epidermal keratinocytes were assessed for proliferation in response to the same mineral salts. Neither type of Gd3+-containing salt induced proliferation in these cells. Neither did Ca2+-phosphate (not shown). Consistent with our previous results [21], the addition of Gd3+-chloride or Omnican also had no stimulatory effect on keratinocyte proliferation (not shown).
Effects of Gd3+-phosphate and Gd3+-carbonate on fibroblast survival under low-Ca2+ conditions
It is known from past studies [42] that human dermal fibroblast viability is lost at Ca2+ concentrations below approximately 0.15 mM. We took advantage of this to compare inorganic Gd3+ salts with chelated-Gd3+ for ability to replace Ca2+ in this assay. Cells were incubated in KBM with 0.1 mM Ca2+ and treated with the desired reagent over a range of concentrations. Table 2 shows the results from these studies. Fibroblast viability was not maintained in the presence of the Gd3+ - salts. Nor was viability maintained with Gd3+-chloride was added directly to the culture medium. In contrast, several formulations of chelated-Gd3+ were protective over the range of concentration from 0.5 to 5 mM. Of interest, Omniscan, which consists of gadodiamide along with an excess of the chelator, was effective at a lower concentration than was observed with gadodiamide alone. Our (tentative) interpretation of these results is that when Gd3+ in gadodiamide becomes separated from its chelator, it rapidly forms an inorganic salt and is effectively removed from solution. In contrast, when this occurs in Omniscan, the released Gd3+ binds to the free chelator and remains in solution. This is consistent with the notion that chelated Gd3+ is critical to fibroblast protection.
Table 2.
Human dermal fibroblast survival under low-Ca2+ conditions: Effects of Gd3+ salts and chelated-Gd3+.
| Intervention | Number of cells ×10−4 | ||||
|---|---|---|---|---|---|
| 50 μM | 100 μM | 500 μM | 1 mM | 5 mM | |
| Gd3+ - chloride | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Gd3+ - phosphate | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Gd3+ - carbonate | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Omniscan | <0.5 | 0.7 ± 0.2 | 3.9 ± 0.2* | 4.0 ± 0.1* | 4.3 ± 0.1* |
| Magnevist | <0.5 | <0.5 | <0.5 | 0.3 ± 0.2 | 1.9 ± 0.1* |
| Multihance | <0.5 | <0.5 | <0.5 | <0.5 | 0.8 ± 0.1 |
| Gadodiamide | <0.5 | <0.5 | 1.1 ± 0.1 | 2.8 ± 0.3* | 3.3 ± 0.2* |
Values shown are means and standard deviations based on n=3 separate experiments for each reagent. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons.
indicates statistically significant increase relative to control at p<0.05 level.
Interaction of Gd3+-phosphate with fibroblasts: Light and electron microscopy
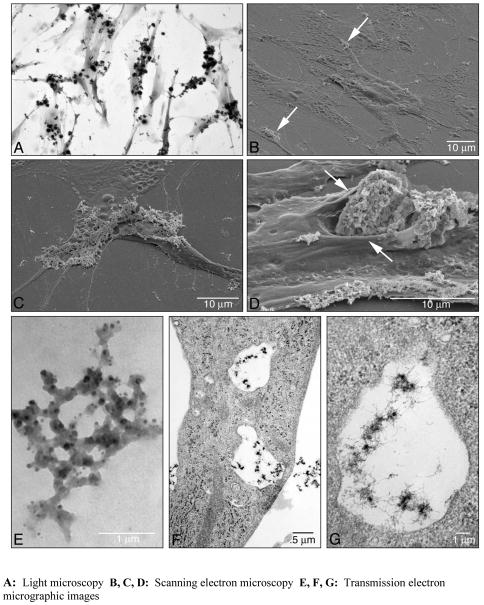
Dermal fibroblasts were exposed to Gd3+-phosphate equivalent to 125 μM Gd3+ (final concentration) and incubated. One day later, the cells were washed extensively to remove unbound salt and then examined by light microscopy and electron microscopy (Figure 3). Panel A shows a light microscopic image of fibroblasts with Gd3+-phosphate attached. Virtually every cell in the field has salt bound to it. Panel B shows a scanning electron micrographic image of fibroblasts with a small amount of the Gd3+ salt attached to the surface (arrows). In panel C, a scanning electron micrographic image of a cell that is more extensively covered with the Gd3+-phosphate is shown. Panel D shows a fibroblast to which a large three-dimensional particle is bound. The cell membrane is enfolded around the particle as if the cell is trying to engulf it. As can be seen in both the light microscopic and scanning electron microscopic images, the Gd3+ salt binds primarily to cells or cell processes. Rarely did we observe salt attached to the bare plastic surface. It should be noted that in spite of the large amount of Gd3+-phosphate attached to some cells, the cells still appeared to be cytologically and ultrastructurally intact. However, when the amount of Gd3+ salt added to the culture was increased further, cell destruction rapidly occurred (not shown). Panels E–G are transmission electron micrographs. Panel E demonstrates the appearance of a typical Gd3+-phosphate particle in a cell-free environment. The fenestrated nature of the particle is apparent. Panels F and G are low and high magnifications demonstrating Gd3+ salt within cytoplasmic vacuoles. Membrane lining of the vacuoles can be seen, but whether the salt particles have been completely engulfed by the cell cannot be conclusively demonstrated from this analysis.
Figure 3. Appearance of human dermal fibroblasts with bound Gd3+-phosphate.
A: Light microscopy. Virtually every cell has salt bound to its surface. B, C and D: Scanning electron microscopy. The cells shown in B have the appearance of control fibroblasts (flattened shape) with only a small amount of salt on the surface (arrows). The cell shown in Panel C is similar to the cells in Panel B except that more of the surface is salt-covered. Panel D shows a cell to which a large particle is bound. The cell membrane appears to be enfolding around the particle (arrows). Panels E–G are transmission electron micrographic images. Panel E shows a cell-free Gd3+ particle (unstained). The fenestrated nature of the particle is apparent. Panels F and G are low and high magnifications of a fibroblast with Gd3+ salt within vacuoles.
Fibroblast response to Gd3+-phosphate: Intracellular signaling events
Two sets of experiments were carried out to assess intracellular signaling pathways activated by exposure of fibroblasts to Gd3+-phosphate. First, ERK phosphorylation as an indicator of MAP kinase activity and AKT phosphorylation as an indicator of PI3 kinase activity were assessed. In parallel, MAP kinase and PI3 kinase antagonists were examined for ability to interfere with Gd3+-phosphate induction of proliferation. ERK phosphorylation is presented in the upper panel of Figure 4. An increase in phosphorylation was observed as early as five minutes after exposure, and was increased at 15 and 30 minutes. There was, as expected, no significant change in total ERK expression over the same time period. The lower panel of Figure 4 shows phospho-AKT expression in the same cells. A similar increase in AKT phosphorylation was seen. As expected, there was no change in total AKT protein at any time-point.
Figure 4. Intracellular signaling in fibroblasts exposed to Gd3+-phosphate.
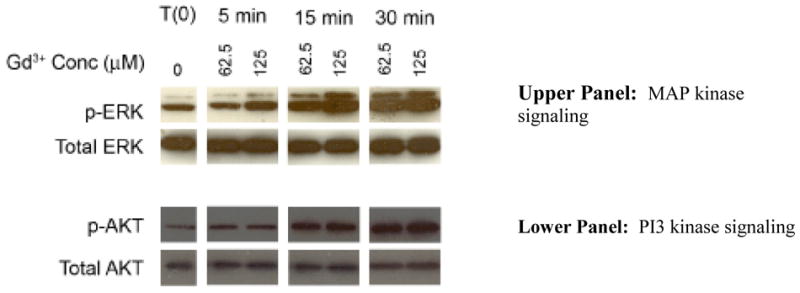
Upper panel: MAP kinase signaling: Cells were treated with Gd3+-phosphate (125 μM Gd3+) for the indicated times. At the end of the incubation period, cell lysates were prepared and assayed for phospho-ERK and total-ERK protein by western blotting. Phospho-ERK and total-ERK from one of three replicate experiments is shown. Lower panel: PI3 kinase signaling. Cells were treated with Gd3+-phosphate (125 μM Gd3+) for the indicated times. At the end of the incubation period, cell lysates were prepared and assayed for phospho-AKT and total AKT protein by western blotting. Phospho-AKT and total-AKT from one of three replicate experiments is shown.
In parallel studies, human dermal fibroblasts were exposed to Gd3+-phosphate in the presence of U0126 (10 μM) or LY294002 (25 μM). Effects on proliferation were assessed. Table 3 demonstrates that in the presence of U0126 (inhibitor of ERK activation) or in the presence of LY294002 (PI3 kinase inhibitor), proliferation was suppressed. In additional studies, fibroblasts were exposed to Gd3+-phosphate in the presence of an antibody that blocks PDGF-induced fibroblast proliferation. Targeting the PDGF receptor was shown in our previous study to suppress proliferation in response to Omniscan [37]. As seen in Table 3, the antibody had no measurable effect on proliferation induced by the insoluble Gd3+ -phosphate salt.
Table 3.
Effects of MAP kinase and PI3 kinase inhibitors and a PDGF receptor-blocking antibody on proliferation of human dermal fibroblasts in response to Gd3+-phosphate.
| Treatment groups | Number of cells ×10−4 |
|---|---|
| Control | 5.0 ± 1.1 |
| Gd3+-phosphate | 6.2 ± 1.5* |
| + U0126 (10 μM) | 4.6 ± 1.4** |
| Control | 5.8 ± 0.6 |
| Gd3+-phosphate | 7.0 ± 0.9* |
| +LY294002 (25 μM) | 5.3 ± 1.8** |
| Control | 5.1 ± 0.5 |
| Gd3+-phosphate | 6.6 ± 0.7* |
| + anti-PDGFr antibody (10 μg/ml) | 6.7 ± 0.7 |
Values shown are means and standard deviations based on four separate experiments with duplicate or triplicate samples per data point. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons.
indicates statistically significant increase compared to negative control at p<0.05 level.
indicates statistically significant decrease compared to Gd3+ -phosphate alone.
DISCUSSION
When Gd3+-chloride is added to a biological fluid, the cationic metal forms insoluble precipitates with available anions including phosphate, carbonate, acetate, ascorbate and hydroxide. Li et al. [40] used energy-dispersive X-ray analysis to demonstrate that phosphate was the predominant anion when Gd3+-chloride was added to cell culture medium. While insoluble salts of many different metals can be phagocytosed by tissue macrophages [31–33,43,44], the insoluble salts are generally assumed to be inert with fibroblasts. The present findings demonstrate that insoluble Gd3+ salts are not inert. Both Gd3+ -phosphate and Gd3+- carbonate rapdily attached to the surface of human dermal fibroblasts. Perturbation of the cell surface was clearly visible by scanning electron microscopy, and (at least partially) engulfed particles could be seen within cells by transmission electron microscopy. Binding was associated with signaling through growth-promoting pathways. Concentrations that stimulated MAP kinase and PI3 kinase signaling also induced a proliferative response. Higher concentrations were cytotoxic.
Are these findings relevant to understanding the patho-physiology of NSF? It has been suggested that prolonged GBCA circulation in patients with end-stage renal disease results in separation of Gd3+ from the chelator [3–5]. The released Gd3+ is presumed to rapidly bind available anions, and be phagocytosed by circulating inflammatory cells or by tissue macrophages. According to this model, the subsequent release of pro-inflammatory and pro-fibrotic cytokines [34–36] mediates the fibrogenic response. Alternatively, it has been shown that Gd3+ - containing compounds (as well as Gd3+ -chloride) can directly induce responses in fibroblasts that are potential pro-fibrotic [20–25,37,38]. While the present findings are consistent with a direct activation of fibrogenic cells by insoluble Gd3+ salts, the data would suggest caution in interpreting the findings in this manner. While Gd3+ - containing salts were capable of inducing fibroblast proliferation, there were significant differences in how fibroblasts responded to Gd3+ in one form or another that should be considered. For example, while chelated-Gd3+ (with a number of different chelators [20–24]) and insoluble Gd3+ salts were both capable of stimulating fibroblast proliferation, there were striking dose-dependent differences. With Omniscan (chelated-Gd3+), fibroblast proliferation could be seen at concentrations as low as 0.5 μM [21] (and present report) while with Gd3+-containing inorganic salts and Gd3+ -chloride, induction was not seen below approximately 10 μM. Seemingly, therefore, if Gd3+ separation from the chelator was critical for inducing a response, there would be insufficient “free” Gd3+ (at 0.5 μM Omniscan) to initiate the events that lead to proliferation.
Difference in biological response at high concentrations were also seen. With chelated-Gd3+, concentrations as high as 500–1000 μM were stimulatory, while with the Gd3+ salts, toxicity was observed at concentrations slightly above those that stimulated growth. Of interest, even higher concentrations of chelated-Gd3+ (0.5 – 5 mM) were effective in preventing fibroblast lysis in a low-Ca2+ environment, while Gd3+-chloride and preformed Gd3+ salts were unable to duplicate this effect. Finally, while our previous studies have shown that fibroblast proliferation induced by Omniscan was inhibited in the presence of an antibody to the PDGF receptor [29], the same antibody did not prevent growth in the presence of the insoluble Gd3+ salts. Taken together, these findings suggest that fibroblast proliferation can be stimulated by chelated-Gd3+ as well as by insoluble salts of the metal. Whether these in vitro findings are relevant to the patho-physiology of NSF is not known at this time. In so far as they are, however, one would not conclude a priori that dechelation must occur in vivo for activity. Quite the contrary, our data support the hypothesis recently put forth by Newton and Jiminez [45] that chelated-Gd3+ compounds such as Omniscan and Magnevist can directly stimulate responses in cells that contribute to fibrosis.
Exposure to high concentrations of Gd3+ through the intravenous route is unique to individuals undergoing contrast-enhanced MRI, but exposure to Gd3+ (and other lanthanoids) can occurs via other routes as well. Lanthanoids are used in several industrial applications (beyond medical imaging) and exposure to metal dusts occurs via inhalation. Pneumocosis is associated with such exposure, as it is with inhalation of other metals [31–33,43,44]. Environmental exposure via inhalation has also been documented to occur naturally in areas where lanthanoid (cerium) dust is rich in the soil. As with industrial exposure, environmental contact with lanthanoid dusts is associated fibrotic tissue injury. Of interest, environmental exposure to cerium is linked to fibrotic cardiomyopathy rather than to lung fibrosis [46–48]. The underlying mechanism of this is not known, but it has been demonstrated that cardiac fibroblasts respond to challenge with a greater proliferative response than do lung fibroblasts [49,50]. Finally, while skin contamination with a variety of metal dusts is common, there is essentially no penetration of the inorganic dusts through healthy skin. However, a florid fibroproliferative response has been seen when abraded skin is exposed [51]. Thus, the direct stimulation of fibroblast proliferation (and other potentially fibrogenic responses) by insoluble salts containing Gd3+ and/or other lanthanoid metals may have relevance in a variety of settings.
A final question concerns the cellular mechanism leading from Gd3+ salt exposure to fibroblast proliferation. Because of its similarity to Ca2+ in ionic radius, but with an overall higher charge density [1–3], Gd3+ interacts with Ca2+-binding sites on a variety of molecules. In many cases, the affinity is higher [52]. In epithelial cells, the extracellular Ca2+-sensing receptor is an important target [53–56]. Features of Ca2+-mediated epithelial differentiation can be induced by low micromolar amounts of Gd3+ or other lanthanoid [57,58]. In other types of cells, including fibroblasts, Gd3+ interacts with Ca2+ channels including voltage-gated, receptor-gated and mechanical stress-gated channels [59–61]. Other regulatory molecules influenced by lanthanoids include calcineurin, Ca2+-dependent and Ca2+/Mg2+-dependent ATPases, protein kinase C and choline esterases [62]. Binding to any of the intracellular moieties could be expected to affect cell function, but it is difficult to envision how large insoluble particles could gain access to cytoplasmic proteins. One possibility is that, as suggested by Li et al. [40], the Gd3+-containing salts constitute a reservoir of Gd3+ and that conditions at the cell surface allow for solubilization of enough metal ion to elicit the responses noted. This might be particularly interesting in light of the electron microscopic findings presented here, which show salt bound to the cell surface and at least partially sequestered within intracellular vacuoles. An alternative possibility is a direct effect on mechanical stress-activated ion channels brought about by binding of the highly-cationic material to the cell surface [61]. These possibilities are, of course, not mutually exclusive. Additional studies will be required to distinguish between these (and other) possibilities.
In summary, understanding the patho-physiologic changes that underlie NSF and other fibrotic diseases associated with lanthanoid exposure is contingent upon defining which, and to what degree, different forms of the metals are capable of eliciting responses of interest. Our results with regard to Gd3+ per se suggest that while different forms can elicit responses in fibroblasts, chelated-Gd3+ has unique properties not shared with insoluble Gd+3 salts that could contribute to the events that contribute to NSF.
Acknowledgments
This study was supported in part by grant CA140760 from the National Institutes of Health, Bethesda, MD, and by grant 11-0577 from the Agency for International Cancer Research
References
- 1.Evans CH, editor. Biochemistry of the Elements. New York: Plenum Press; 1990. Biochemistry of the lanthanides. [Google Scholar]
- 2.Gschneidner KA Jr, Eyring L, editors. Handbook on the Physics and Chemistry of Rare Earths. Elsevier Science & Technology Books; Amsterdam, The Netherlands: 2000. [Google Scholar]
- 3.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998;5(7):491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 4.Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging. 2007;25(5):884–899. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]
- 5.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66(2):175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 7.Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114(7):563–572. doi: 10.1016/s0002-9343(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 8.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 9.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yerram P, Saab G, Karuparthi PR, et al. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2(2):258–263. doi: 10.2215/CJN.03250906. [DOI] [PubMed] [Google Scholar]
- 12.Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (omniscan) Invest Radiol. 2007;42(2):139–145. doi: 10.1097/01.rli.0000253505.88945.d5. [DOI] [PubMed] [Google Scholar]
- 13.Collidge TA, Thomson PC, Mark PB, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology. 2007;245(1):168–175. doi: 10.1148/radiol.2451070353. [DOI] [PubMed] [Google Scholar]
- 14.Rydahl C, Thomsen HS, Marckmann P. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol. 2008;43(2):141–144. doi: 10.1097/RLI.0b013e31815a3407. [DOI] [PubMed] [Google Scholar]
- 15.Shabana WM, Cohan RH, Ellis JH, et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol. 2008;190(3):736–741. doi: 10.2214/AJR.07.3115. [DOI] [PubMed] [Google Scholar]
- 16.Wertman R, Altun E, Martin DR, et al. Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four American universities. Radiology. 2008;248(3):799–806. doi: 10.1148/radiol.2483072093. [DOI] [PubMed] [Google Scholar]
- 17.Broome DR. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol. 2008;66(2):230–234. doi: 10.1016/j.ejrad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.McNeill AM, Barr RJ. Scleromyxedema-like fibromucinosis in a patient undergoing hemodialysis. Int J Dermatol. 2002;41(6):364–367. [PubMed] [Google Scholar]
- 19.Neudecker BA, Stern R, Mark LA, et al. Scleromyxedema-like lesions of patients in renal failure contain hyaluronan: a possible pathophysiological mechanism. J Cutan Pathol. 2005;32(9):612–615. doi: 10.1111/j.0303-6987.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 20.Edward M, Quinn JA, Mukherjee S, et al. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008;214(5):584–593. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- 21.Varani J, DaSilva M, Warner RL, et al. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. 2009;44(2):74–81. doi: 10.1097/RLI.0b013e31818f76b5. [DOI] [PubMed] [Google Scholar]
- 22.Edward M, Quinn JA, Burden AD, et al. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology. 2010;256(3):735–743. doi: 10.1148/radiol.10091131. [DOI] [PubMed] [Google Scholar]
- 23.Wiesinger B, Kehlbach R, Bebin J, et al. Effects of MRI contrast agents on human embryonic lung fibroblasts. Invest Radiol. 2010;45(9):513–519. doi: 10.1097/RLI.0b013e3181eb2fe7. [DOI] [PubMed] [Google Scholar]
- 24.Bhagavathula N, DaSilva M, Aslam MN, et al. Regulation of collagen turnover in human skin fibroblasts exposed to a gadolinium-based contrast agent. Invest Radiol. 2009;44(8):433–439. doi: 10.1097/RLI.0b013e3181a4d7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perone P, Weber S, DaSilva M, et al. Collagenase activity is suppressed by elevated tissue inhibitor of metalloproteinase-1 (TIMP-1) in human skin exposed to a gadolinium-based MRI contrast agent. Invest Radiol. 2010;45:42–48. doi: 10.1097/RLI.0b013e3181bf95eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21–26. doi: 10.1016/j.jaad.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 27.High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:710–712. doi: 10.1016/j.jaad.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Boyd AS, Sanyal S, Abraham JL. Tissue gadolinium deposition and fibrosis mimicking nephrogenic systemic fibrosis – subclinical NSF? J Am Acad Dermatol. 2010;62:337–342. doi: 10.1016/j.jaad.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Abraham JL, Thakral C, Skov L, et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol. 2008;158:273–280. doi: 10.1111/j.1365-2133.2007.08335.x. [DOI] [PubMed] [Google Scholar]
- 30.George SJ, Webb SM, Abraham JL, Cramer SP. Synchrotron X-ray analyses demonstrate phosphate-bound gadolinium in skin in nephrogenic systemic fibrosis. Br J Dermatol. 2010;163:1077–1081. doi: 10.1111/j.1365-2133.2010.09918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(Suppl 1):85–95. doi: 10.1289/ehp.96104s185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heckert EG, Seal S, Self WT. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ Sci Technol. 2008;42(13):5014–5019. doi: 10.1021/es8001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Wang S, Liu J, et al. The oxidative damage in lung of mice caused by lanthanoide. Biol Trace Elem Res. 2010;134(1):68–78. doi: 10.1007/s12011-009-8448-0. [DOI] [PubMed] [Google Scholar]
- 34.Steger-Hartmann T, Raschke M, Riefke B, et al. The involvement of pro-inflammatory cytokines in nephrogenic systemic fibrosis - a mechanistic hypothesis based on preclinical results from a rat model treated with gadodiamide. Exp Toxicol Pathol. 2009;61(6):537–552. doi: 10.1016/j.etp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Wermuth PJ, Del Galdo F, Jimenez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508–1518. doi: 10.1002/art.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Galdo F, Wermuth PJ, Addya S, et al. NFkappaB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69(11):2024–2033. doi: 10.1136/ard.2010.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhagavathula N, Dame MK, Dasilva M, et al. Fibroblast Response to Gadolinium: Role for Platelet-Derived Growth Factor Receptor. Invest Radiol. 2010;45(12):769–777. doi: 10.1097/RLI.0b013e3181e943d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DaSilva M, O’Brien Deming M, Fligiel SE, et al. Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects. Invest Radiol. 2010;45(11):733–739. doi: 10.1097/RLI.0b013e3181e9436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varani J, Perone P, Griffiths CE, et al. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest. 1994;94(5):1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JX, Liu JC, Wang K, et al. Gadolinium-containing bioparticles as an active entity to promote cell cycle progression in mouse embryo fibroblast NIH3T3 cells. J Biol Inorg Chem. 2010;15(4):547–557. doi: 10.1007/s00775-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 41.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 42.Varani J, Shayevitz J, Perry D, et al. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- 43.Kitamura H, Ichinose S, Hosoya T, et al. Inhalation of inorganic particles as a risk factor for idiopathic pulmonary fibrosis--elemental microanalysis of pulmonary lymph nodes obtained at autopsy cases. Pathol Res Pract. 2007;203(8):575–585. doi: 10.1016/j.prp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Rice RH, Vidrio EA, Kumfer BM, et al. Generation of oxidant response to copper and iron nanoparticles and salts: Stimulation by ascorbate. Chem Biol Interact. 2009;181(3):359–365. doi: 10.1016/j.cbi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton BB, Jimenez SA. Mechanism of NSF: New evidence challenging the prevailing theory. J Magn Reson Imaging. 2009;30(6):1277–1283. doi: 10.1002/jmri.21980. [DOI] [PubMed] [Google Scholar]
- 46.Kumar BP, Shivakumar K, Kartha CC, et al. Magnesium deficiency and cerium promote fibrogenesis in rat heart. Bull Environ Contam Toxicol. 1996;57(4):517–524. doi: 10.1007/s001289900220. [DOI] [PubMed] [Google Scholar]
- 47.Valiathan SM, Kartha CC. Endomyocardial fibrosis--the possible connexion with myocardial levels of magnesium and cerium. Int J Cardiol. 1990;28(1):1–5. doi: 10.1016/0167-5273(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 48.Valiathan MS, Kartha CC, Panday VK, et al. A geochemical basis for endomyocardial fibrosis. Cardiovasc Res. 1986;20(9):679–682. doi: 10.1093/cvr/20.9.679. [DOI] [PubMed] [Google Scholar]
- 49.Preeta R, Nair RR. Stimulation of cardiac fibroblast proliferation by cerium: a superoxide anion-mediated response. J Mol Cell Cardiol. 1999;31(8):1573–1580. doi: 10.1006/jmcc.1999.0994. [DOI] [PubMed] [Google Scholar]
- 50.Nair RR, Preeta R, Smitha G, et al. Variation in mitogenic response of cardiac and pulmonary fibroblasts to cerium. Biol Trace Elem Res. 2003;94(3):237–246. doi: 10.1385/BTER:94:3:237. [DOI] [PubMed] [Google Scholar]
- 51.Haley TJ, Komesu N, Efros M, et al. Pharmacology and Toxicology of Lutetium Chloride. J Pharm Sci. 1964;53:1186–1188. doi: 10.1002/jps.2600531011. [DOI] [PubMed] [Google Scholar]
- 52.Pidcock E, Moore GR. Structural characteristics of protein binding sites for calcium and lanthanide ions. J Biol Inorg Chem. 2001;6(5–6):479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, Zhou Y, Castiblanco A, et al. Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009;48(2):388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273(23):14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- 55.McLarnon SJ, Riccardi D. Physiological and pharmacological agonists of the extracellular Ca2+-sensing receptor. Eur J Pharmacol. 2002;447(2–3):271–278. doi: 10.1016/s0014-2999(02)01849-6. [DOI] [PubMed] [Google Scholar]
- 56.Riccardi D, Maldonado-Perez D. The calcium-sensing receptor as a nutrient sensor. Biochem Soc Trans. 2005;33(Pt 1):316–320. doi: 10.1042/BST0330316. [DOI] [PubMed] [Google Scholar]
- 57.Pillai S, Bikle DD. Lanthanum influx into cultured human keratinocytes: Effect on calcium flux and terminal differentiation. Journal of Cellular Physiology. 1992;151(3):623–629. doi: 10.1002/jcp.1041510323. [DOI] [PubMed] [Google Scholar]
- 58.Chakrabarty S, Radjendirane V, Appelman H, et al. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63(1):67–71. [PubMed] [Google Scholar]
- 59.Estacion M, Mordan LJ. Competence induction by PDGF requires sustained calcium influx by a mechanism distinct from storage-dependent calcium influx. Cell Calcium. 1993;14(6):439–454. doi: 10.1016/0143-4160(93)90003-o. [DOI] [PubMed] [Google Scholar]
- 60.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95(4):679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol. 1998;275(2 Pt 1):C619–621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 62.Palasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47(4):1107–1114. [PubMed] [Google Scholar]