Abstract
Interleukin(IL)-27 is a member of the IL-6 and IL-12 family composed of the IL-27p28 and Epstein Barr-induced virus 1 subunits. While IL-27 was originally identified as a pro-inflammatory factor, subsequent studies have revealed the pleotropic nature of this cytokine. This review discusses recent work that explores the effect of IL-27 on CD4+ T cell subsets, including T regulatory type 1 cells, T follicular helper cells, and Foxp3+ T regulatory cells. Additionally, we highlight studies that identify a role for the IL-27p28 subunit as a cytokine receptor antagonist. Much of the recent work on IL-27 has been relevant to human disease states characterized by inappropriate or excessive inflammation, and this review will discuss potential opportunities to use IL-27 as a therapeutic.
Biology of interleukin (IL)-27
IL-27 is a heterodimeric cytokine of the IL-6 and IL-12 family composed of the IL-27p28 and Epstein-Barr virus-induced gene 3 (EBI3) subunits. IL-27p28 and EBI3 are produced primarily by antigen-presenting cells after stimulation by microbial products or inflammatory mediators [1]. The IL-27 receptor (IL-27R) is composed of WSX-1 (also known as T cell cytokine receptor or TCCR), a type I cytokine receptor, and glycoprotein 130 (gp130), a receptor subunit utilized by several other IL-6 and IL-12 family members [1, 2]. While gp130 expression is ubiquitous, WSX-1 expression is largely restricted to leukocytes, including T cells, NK cells [3], human monocytes, and human mast cells [2]. IL-27 appears to bind specifically to WSX-1, and EBI3 is required for signal transduction [2, 4].
IL-27 was initially described as a pro-inflammatory cytokine that promoted T helper 1 (Th1) responses [1]. Early studies indicated that IL-27-IL-27R binding led to phosphorylation of signal transducer and activator of transcription (STAT)1 in naïve CD4+ T cells, causing them to proliferate and become polarized Th1 cells [5]. As an intact Th1 response is necessary to control infection with intracellular protozoa, mice deficient in IL-27 signaling infected with protozoan parasites were expected to have a reduced Th1 response. Surprisingly, Il27ra (encoding WSX-1)−/− mice challenged with parasitic protozoa had intact Th1 responses, yet succumbed to CD4+ T cell-mediated immune-pathology, suggesting that IL-27 was required to limit inflammation in vivo [6, 7]. Subsequent studies in multiple models of infectious and autoimmune disease have confirmed an anti-inflammatory role for IL-27 in Th1, Th2, and Th17 responses [5], and recent work has shown that IL-27 can induce T cells to produce the anti-inflammatory cytokine IL-10 [8–10] (for reviews of early work on IL-27, we refer the reader to [5, 11–14]). Thus, while IL-27 was originally characterized as a pro-inflammatory cytokine, subsequent reports have focused on its anti-inflammatory effects. Of note, more recent studies that examined the influence of IL-27 on T regulatory cell (Treg) populations have revealed new pro-inflammatory properties of IL-27.
While the studies described above highlight that IL-27, like other IL-6 and IL-12 family members, can be pro- or anti-inflammatory, dissecting the basis for these apparently contradictory activities has not been straightforward. The consequence of IL-27 signaling appears to depend on the immunological context, the temporal regulation of IL-27 production, and tissue- and cell-specific expression of components of the IL-27R; however, many questions remain regarding the influence of IL-27 on innate and adaptive immune cell populations during different types of immune responses and in maintaining homeostasis [5, 14]. Very recent work has described the effects of IL-27 signaling on specific T cell subsets, including Foxp3− T regulatory type 1 (Tr-1) cells, T follicular helper cells (Tfh), and Foxp3-expressing Treg. New studies have also identified a role for the IL-27 subunit IL-27p28 as a receptor antagonist, which may explain the differential effects of IL-27 subunit expression in various systems. This review will describe the latest findings that have increased our understanding of the biology of IL-27 and discuss how these data may direct future studies and influence the development of IL-27-based biologic therapeutics for human disease.
IL-27 regulates the development and function of Tr-1 cells
Since the discovery of IL-27, much of the work characterizing this factor has focused on its ability to promote Th1 responses and suppress Th17 differentiation [5, 14]. More recent work has focused on a role for IL-27 in inducing IL-10 production [9, 10]. IL-10 is a potent anti-inflammatory cytokine that restrains inflammation in a variety of contexts. While much is known regarding the function of IL-10, the factors that control IL-10 production by T cells are less clear [15]. Recently, Foxp3− regulatory CD4+ T cells, referred to as Tr-1 cells, have been identified as an important source of IL-10 that limit inflammation [16] (Box 1). In 2007, four separate groups published that IL-27 could induce Tr-1-like CD4+ T cells to produce IL-10 in vitro and in vivo in mice [8–10] and humans [17]. Recent reports have shown that IL-27 promotes the generation of Tr-1 cells that produce IL-10 by inducing expression of the AP-1 family transcription factor c-Maf. c-Maf directly transactivates the Il10 promoter [18–20] to upregulate IL-10 and also binds to the promoter of the common γ chain cytokine Il21 [21] to elicit IL-21 production that maintains IL-10 producers [22]. Moreover, IL-27 signaling upregulates expression of the aryl hydrocarbon receptor, which partners with c-Maf to optimize interactions with the Il10 and Il21 promoters, further supporting Tr-1 development [23]. IL-27-mediated IL-10 production also depends on STAT1 and STAT3 signaling [19, 24] and the inducible costimulator (ICOS) [22]. These data highlight the variety of transcription factors involved in these events (Figure 1). To date, large-scale array studies of the gene expression profile of IL-27-induced Tr-1 cells have not been performed, and it is likely that additional factors will be identified that play a role in controlling IL-10 expression by CD4+ T cells in response to IL-27. Additionally, the source and regulation of the IL-27 that promotes IL-10 in vivo remain unclear, though there is evidence that DC [25–27] and microglia [28] are relevant sources of IL-27 that influence the development of tolerogenic IL-10-producing T cells. Finally, it is unclear whether the same mechanisms that underlie these effects of IL-27 also operate in other cell types, such as CD8+ T cells [29] and macrophages [30]. Together, these future studies will provide an appreciation of immunological contexts in which IL-27 promotes IL-10 and acts as an anti-inflammatory factor.
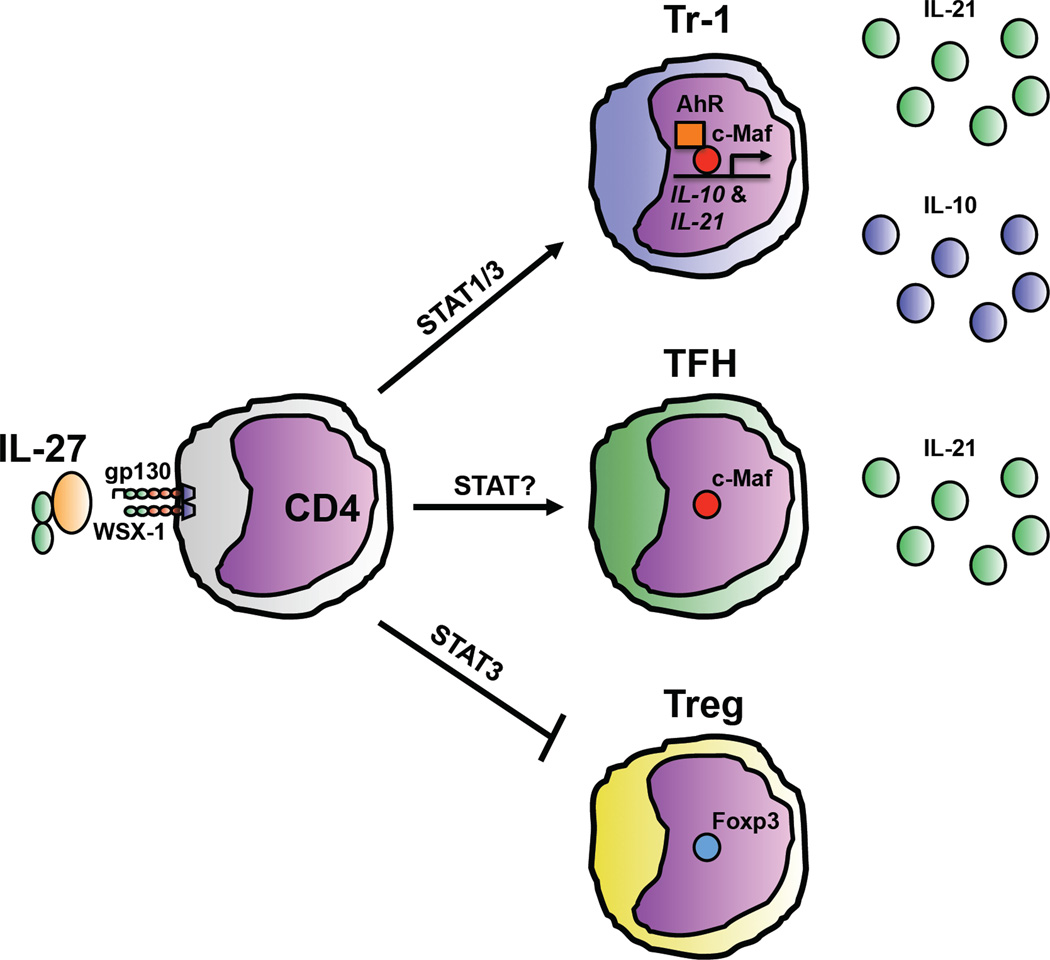
Figure 1. IL-27 promotes the differentiation of Tr-1 cells and Tfh and inhibits Treg generation.
IL-27 signaling on naïve CD4+ T cells can promote the differentiation of IL-10-producing Tr-1 cells by inducing expression of the transcription factor c-Maf in a STAT3-dependent manner. c-Maf transactivates the Il10 promoter to induce IL-10 production and promotes IL-21 production that supports the maintenance of Tr-1 cells. IL-27-induced AhR cooperates with c-Maf to optimize IL-10 and IL-21 expression. IL-27 signaling also can induce the generation of Tfh by increasing c-Maf expression and IL-21 production that promotes the survival and function of TFH as they interact with B cells. Finally, IL-27 signaling, in a partially STAT3-dependent manner, can also inhibit the generation of Foxp3-expressing Treg, although whether IL-27 acts directly or indirectly on differentiating Treg remains unclear.
IL-27 influences humoral responses
New studies have provided evidence that IL-27, like its cousin IL-6, may also influence the activities of Tfh, a T helper subset critical in assisting B cells to class switch and produce high-affinity antibodies. While CD4+ T cells have been known to be necessary for high quality antibody responses for many years, recent work has better characterized the factors required for Tfh development and function [31] (Box 1). Though Tr-1 cells and Tfh are considered to be distinct Th cell fates, there are common factors, including c-Maf and IL-21, which affect the differentiation and function of these two subsets. As already discussed, IL-27 signaling on CD4+ T cells increases expression of c-Maf, which induces IL-10 production [22]. c-Maf signaling is also important in supporting Tfh responses [32, 33] by upregulating IL-21 [22, 32, 34], a critical player that mediates Tfh differentiation and function [35, 36]. Although the effect of IL-27 on Tfh has not been directly tested, the implication is that IL-27 may regulate some of the factors that are important for Tfh differentiation. In concert with this idea, a new report has shown that CD4+ T cells cultured with IL-27 produced IL-21 that supported Tfh differentiation. This study also demonstrated that, following a double immunization protocol, mice deficient in WSX-1 had impaired IL-21 levels, decreased numbers of Tfh, and reduced production of high affinity class-switched antibody and that IL-27 served as an important survival factor for Tfh in vitro and in vivo [37]. Together, these studies suggest that IL-27 signaling may elicit Tfh responses by inducing c-Maf and IL-21 that promote Tfh activity (Figure 1). However, IL-27 alone does not cause CD4+ T cells to differentiate into functional Tfh [37], and IL-27 signaling is not required for the generation of antibody responses in models of infection, allergy, and autoimmunity [38–40]. To further complicate this issue, IL-27 also has direct effects on B cells [41–44]. Ultimately, additional studies regarding the influence of IL-27 signaling on Tfh and B cell populations will be required to better understand how the stimulatory or inhibitory effects of IL-27 may shape the development of antibody responses.
Regulation of Tr-1 cells, Tfh, and Treg differentiation and function.
Tr-1 cells, Tfh, and Treg are Th subsets involved in immune regulation. While Tr-1 cells and Treg suppress responses, Tfh promote antibody production. Though these cells have divergent functions, the regulation of these subsets is controlled by a number of common factors, including ICOS, IL-6, IL-2, IL-21, and STAT3.
Tr-1 cells
Tr-1 cells differentiate in the presence of IL-10 and IL-27 to become potent IL-10 producers [16]. ICOS plays an important role in the stimulation of IL-10 producers [94], and Icos−/− cells do not become Tr-1 cells in the presence of IL-27 [22]. IL-6 also promotes the differentiation of IL-10-producing Tr-1 cells[10], and IL-21 can mediate Tr- 1 survival and proliferation [22] and induces IL-10 production [95]. The differentiation of IL-10 producing Tr-1 cells involves STAT3 signaling [19, 24].
Tfh
The transcription factor Bcl-6 is required for Tfh development [31]. Tfh that express ICOS interact with GC B cells to promote GC formation [35]. IL-6 regulates Tfh development and function [35], though it is not always required [96, 97]. IL-21 promotes Tfh differentiation, proliferation, and survival [35], and IL-21 produced by Tfh is a vital factor for GC B cell survival and proliferation [36]. Both IL-21 and IL-6 signal through STAT3, and this molecule is required for Tfh differentiation [35].
Treg
The transcription factor Foxp3 governs the development and function of the Treg pool [98]. Though Treg development is not absolutely dependent upon ICOS, mice that lack ICOS have decreased Treg frequencies [99]. IL-6 signaling through STAT3 causes Treg to downregulate Foxp3 and gain the ability to produce IL-17 [100]. IL-2 is the most critical cytokine in modulating Treg homeostasis. Mice that lack IL-2 and IL-2Rβ do not possess a full Treg compartment and succumb to lethal autoimmunity [54].
IL-27 regulates Treg
Treg are suppressive CD4+ T cells that express the transcription factor Foxp3, maintain self-tolerance during homeostasis, and limit immune-pathology following infection. The Treg pool consists of natural Treg that are generated in the thymus and inducible Treg that upregulate suppressive function in the periphery [45, 46]. As Treg are a vital line of defense against inappropriate inflammation, there is significant interest in identifying the factors that influence Treg homeostasis and function [47] (Box 1). Multiple cytokines modulate Treg, including IL-6, TGF-β, and IL-2 [48], and early studies showed that IL-27 can also influence Treg populations. Thus, when inducible Treg, generated by culturing naïve CD4+ T cells with TGF-β and IL-2, were exposed to IL-27 during differentiation, the percentage of cells that expressed Foxp3 was reduced [49–51]. In addition, splenic Treg express high WSX-1 levels [3], and IL-27 can inhibit the production of IL-2 [52, 53], a vital cytokine for Treg populations [54]. Together, these reports suggest that IL-27 may be an important regulator of Treg generation and maintenance, and recent studies have used new tools to investigate the influence of IL-27 on Treg populations in vivo. A report from 2009 showed that IL-27 inhibited IL-2-mediated Foxp3+ Treg responses and promoted anti-tumor cytotoxic T lymphocyte (CTL) responses in mice, although no definitive link was established between IL-27-mediated loss of Treg and enhanced CTL activity [55]. A more direct analysis was provided by a study that monitored the effect of IL-27 on Treg in vivo using the CD4+CD45RBhi cell transfer model of colitis. Transfer of Il27ra−/− cells led to reduced colitis symptoms and an increased percentage of transferred cells that upregulated Foxp3+ in the gut and secondary lymphoid organs compared to transfer of wild-type (WT) cells, indicating that IL-27 plays a pathogenic, pro-inflammatory role in this model. Additionally, an oral tolerance model was used to show that higher Treg frequencies in transferred Il27ra−/− cells were due to increased Treg conversion, indicating that IL-27 is a potent antagonist of the differentiation of inducible Treg [56]. In addition to this study, a transgenic mouse model has been utilized in which the IL-27p28 and EBI3 subunits are over-expressed (IL-27 tg mice) to investigate the role of IL-27 in regulating the size of the Treg population in vivo. IL-27 tg mice almost completely lacked Treg in lymphoid organs and succumbed to systemic inflammation. IL-27 signaling, unlike IL-6 signaling [57], did not cause Treg to downregulate Foxp3; however, when IL-27 was present during the generation of the Treg pool, Treg reconstitution was inhibited. Associated with the lack of Treg, IL-27 tg mice had a marked defect in their capacity to produce IL-2, suggesting that IL-27 may influence differentiating Treg indirectly through IL-2 modulation [58].
The studies discussed here suggest a paradigm in which IL-27 is an antagonist of Treg populations (Figure 1), but questions remain regarding the mechanisms that control this pro-inflammatory effect of IL-27. For example, it is unknown whether IL-27 influences CD4+ T cells converting or differentiating to the Treg fate directly or indirectly. The report using Il27ra−/− T cells in the setting of colitis proposed that IL-2 is not involved [56]; however, the study of IL-27 tg mice provides evidence for an indirect mechanism, in which the loss of Treg in an IL-27-rich environment is associated with reduced IL-2 [58]. Additionally, how IL-27 influences inducible versus natural Treg populations remains unknown, though Il27ra−/− mice have normal Treg frequencies [3]. Also, the signaling pathways that control the ability of IL-27 to limit Treg conversion are largely unknown, although STAT3 has been implicated [49–51]. Finally, whether IL-27 can also influence Treg suppressive activities is unclear. In classical Treg suppression assays, IL-27 does not inhibit suppression [56, 59, 60]. However, Treg can suppress other cells by producing anti-inflammatory cytokines, including IL-10 [48], and because IL-27 induces IL-10 production from both Foxp3− and Foxp3+ CD4+ T cells [10], IL-27 might regulate Treg suppressive function via modulation of IL-10-producing capacity. Although IL-27 is efficient at stimulating Foxp3− T cells to produce IL-10 [10], in a model of Staphylococcal enterotoxin and lipopolysaccharide injection, Il27ra−/− Foxp3+ Treg produced more IL-17 and less IL-10 than WT Treg, suggesting that IL-27 signaling can affect the cytokines that Treg produce [61]. Further studies will be required to conclusively determine how IL-27 influences differentiating Treg populations and whether IL-27 regulates Treg activities. Finally, future work will be needed to determine how the pro-inflammatory ability of IL-27 to limit Treg populations is balanced by its anti-inflammatory capacity to induce IL-10.
Biological functions of the IL-27p28 subunit
The IL-6 and IL-12 family, of which IL-27 is the newest member, consists of a number of type I cytokines that are structurally homologous and utilize gp130 or a related receptor subunit in their receptor complexes [62, 63]. The structural homology of the cytokine and receptor subunits of this family allows for a complex combinatorial biology in which one subunit might be able to pair with a number of homologous subunits to form functionally distinct cytokine heterodimers or multimeric receptor complexes. This combinatorial biology suggests that the IL-27p28 and EBI3 subunits may have additional functions aside from participating in the IL-27 complex. In support of this idea, the subunits that make up other heterodimeric family members associate via strong disulfide bonds, whereas the nature of the association between IL-27p28 and EBI3 is less clear. Additionally, the observation that IL-27p28 and EBI3 can be differentially expressed in different cell types implies that IL-27p28 and EBI3 might associate with other factors to form novel cytokines or have biological functions of their own [1]. Consistent with this hypothesis, EBI3 can interact with the IL-12p35 subunit to form IL-35 [64–67] (Box 2). New evidence suggests that the IL-27p28 subunit also binds to a soluble cytokine receptor, cytokine-like factor 1 (CLF), to form a novel heterodimer that interacts with a receptor composed of the IL-6Rα, WSX-1, and gp130. IL-27p28/CLF signaling activated STAT1 and STAT3 phosphorylation and induced cytokine production by T and NK cells [68]. Whether there are other IL-27p28 binding partners in addition to CLF remains to be determined.
Biology of IL-35.
The IL-12p35 subunit was initially thought to be specific for IL-12p70, but this subunit also interacts with EBI3, forming IL-35 [64, 65, 67]. Initial reports indicated that an EBI3/p35-Fc fusion protein could promote the differentiation and proliferation of Foxp3+ Treg and could inhibit the development of Th17 responses in vitro and in vivo [64]. In addition, Foxp3+ Treg expressed high levels of IL-35 that was necessary and sufficient for suppression [65, 101]. IL-35 produced by Treg also promoted the differentiation of Foxp3− regulatory T cell populations [66]. Despite these reports characterizing IL-35, the nature and extent of the influence of IL-35 in vivo remain unclear. Studies have implicated IL-35 as a potent anti-inflammatory factor in the tumor microenvironment [66], in collagen-induced arthritis [102], and during the development of allergic asthma [103]. These data, in addition to studies that identified IL-35-expressing CD4+ T cells in chronic hepatitis patients [104] and showed that human Treg suppress in an IL-35-dependent manner [105], suggest that this factor may be an important immunomodulatory cytokine in vivo in mice and humans. However, human Treg do not constitutively express IL-35 [106], and EBI3 deficiency does not affect Treg function in a murine model of delayed-type hypersensitivity [107]. Additionally, treatment with a recombinant IL-35 fusion protein exacerbated the development of arthritis in mice, suggesting that IL-35 might have inflammatory roles as well [108]. These conflicting reports, coupled with a lack of reagents to measure levels of specific monomeric subunits versus heterodimeric complexes, present a continuing challenge. Additional studies and the development of IL-35-specific antibodies and well-characterized IL-35 reagents will be needed to elucidate the role of IL-35 in human disease.
Recent studies also suggest that IL-27p28 has a biological function on its own in the absence of EBI3. While IL-27p28 alone does not appear to initiate STAT signaling [1], exposure to IL-27p28 antagonized IL-27-mediated STAT1 signaling and IFN-γ production from CD4+ T cells in vitro. Forced expression of IL-27p28 abrogated the anti-tumor effects of IL-27 and suppressed IL-27-mediated graft rejection, suggesting that IL-27p28 can antagonize pro-inflammatory IL-27 signaling in vivo [69]. In addition, a mutation in IL-27p28 generates an IL-27 heterodimer mutant that antagonized WT IL-27 signaling, indicating that crucial amino acid residues in IL-27p28 can interact with the IL-27R to inhibit IL-27 signaling [70]. Finally, a recent study showed that IL-27p28 acts as a low-affinity antagonist for IL-6, IL-11, and IL-27 signaling. These factors all signal through gp130, and structural modeling studies predicted that IL-27p28 alone would be capable of interacting with gp130. Additionally, IL-27p28 was able to block the biological affect of IL-6 signals through gp130 independently of EBI3. The physiological relevance of these observations was illustrated by the finding that over-expression of IL-27p28 antagonized gp130-dependent B cell responses [71]. These studies indicate that IL-27p28 can inhibit the biological activities of several IL-6 family members, though further study will be required to determine the source and regulation of monomeric secreted IL-27p28, to dissect the precise nature of the interactions between IL-27p28, gp130, and the cytokines that utilize this receptor, and to determine whether antagonism of gp130 signaling results in pro- or anti-inflammatory effects.
Therapeutic implications
The studies reviewed above continue to expand our understanding of how IL-27 and its subunits influence the development, outcome, and resolution of inflammation in different contexts. One of the challenges that the field now faces is to determine whether manipulation of IL-27 could be used therapeutically to modulate inflammation that occurs during various human disease states. Thus, the observation that IL-27 plays a critical role in modulating Tr-1 cells, Tfh, and Treg suggests that IL-27 may be utilized therapeutically to influence these populations. Currently, the utility of targeting Tr-1 cells or Tfh to treat inflammatory diseases remains largely unexplored. However, these cell populations are critical in controlling inflammation and in generating antibody responses, respectively, and the ability to selectively regulate their activities using IL-27 may be useful in ameliorating inappropriate immune responses or promoting humoral immunity. In addition, while numerous groups have suggested that Treg could be manipulated to control inflammation during autoimmune disease [72], the studies described here that support a role for IL-27 in limiting Treg populations suggest that IL-27 may be used as a therapeutic agent in situations where Treg activities are deleterious. Thus, Treg expansion, accumulation, or activity could be curtailed to promote the development of desirable immune responses in a number of scenarios, including during vaccination or immune-therapy for cancer [73–75].
In the context of cancer therapy, IL-27 might be useful as a Treg inhibitor to enhance anti-tumor immunity in the suppressive tumor microenvironment. The utility of this approach has been shown in a murine model of neuroblastoma, in which IL-27 therapy inhibited IL-2-induced Treg expansion in the tumor, promoting anti-tumor immune responses [55]. In addition, IL-27 has also been shown to directly support the generation of potent anti-tumor CTL [76, 77]. Recent reports have shown that IL-27 acts as a pro-inflammatory factor in this context to elicit IFN-γ production from CD8+ T cells in vivo in mice [78, 79] and induce IFN-γ production and CTL activity in human CD8+ T cells [80]. Also, IL-27 has direct anti-proliferative effects on some tumors, including melanoma, lung carcinoma, and multiple myeloma [81–83]. Finally, in addition to its potential use as an anti-tumor agent, IL-27 could be used as a potential therapy to reduce inflammation in the context of autoimmune and inflammatory disease. IL-27 treatment ameliorated symptoms in a murine colitis model [84], reduced the inflammation associated with experimental autoimmune arthritis [85, 86], and inhibited the development of inflammation following induction of experimental autoimmune encephalitis [87]. Similar approaches have also demonstrated the ability of IL-27 to ameliorate delayed-type hypersensitivity [88] and airway hyperresponsiveness in a model of OVA-induced asthma [89].
The recent studies that highlight the ability of the IL-27p28 subunit to act as a gp130 cytokine-signaling antagonist are perhaps the most exciting in terms of drug development. Gp130 signaling is involved in a wide variety of immunological functions and is a vital regulator of inflammation in the context of various diseases, including autoimmune disease, inflammatory bowel disease, asthma, and cancer [90]. Thus, IL- 27p28 might be a promising therapeutic agent that acts to limit inflammation and resulting disease by inhibiting gp130 signaling. In support of a role for IL-27p28 in controlling inflammation in human disease, increased susceptibility to asthma or colitis is associated with IL-27p28 polymorphisms [91–93]; however, these studies do not determine whether heterodimeric IL-27 or IL-27p28 alone plays a role in these disease states. While considerable work remains to be done to understand the nature of the association between IL-27p28 polymorphisms and disease development and progression, these studies suggest that IL-27p28 could be used as a specific modulator of inflammation in human patients. Of note, while studies indicate that murine IL-27p28 can be secreted in the absence of EBI3, transfection studies in vitro have suggested that human IL-27p28 is not secreted on its own, indicating that there are species-specific differences in the regulation of the expression and secretion of the IL-27 subunits. Thus, further studies in murine models and with primary human cells and the development of new reagents to evaluate the IL-27 subunits will result in a better assessment of the mechanisms utilized by IL-27 and IL-27p28 to either limit or promote inflammation.
Concluding remarks
Originally described as a pro-inflammatory factor, IL-27 has since been identified as a pleiotropic cytokine with both pro- and anti-inflammatory properties. It can promote inflammation by inducing Th1 polarization of CD4+ T cells and IFN-γ production from CD8+ T cells and by inhibiting Foxp3 upregulation in differentiating Treg populations. On the anti-inflammatory side, a lack of IL-27 signaling results in the development of immune-mediated pathology during responses to certain pathogens, and IL-27 can induce IL-10 production from multiple cell types, including Tr-1 cells.
A few seminal issues currently remain unexplored in the biology of IL-27. First, it is unclear how the ability of IL-27 to promote Th1 polarization and CTL generation may be regulated in the context of the anti-inflammatory activities of IL-27. Whether these two activities are mutually exclusive is currently unknown. Moreover, how the ability of IL-27 to limit Treg populations fits into this interplay is uncertain. In addition, the exact nature of the effects of IL-27 on Tfh, B cell responses and antibody production are incompletely defined. Finally, the molecular and biochemical basis for the ability of IL-27p28 to inhibit signaling through gp130, and the downstream consequences of such a blockade in vivo, remain to be assessed. Future studies that address these questions should positively impact on our understanding of the contribution of IL-27 to the regulation of inflammation in vivo in the context of multiple inflammatory disease settings. Ultimately, these studies will hopefully lead to the development of IL-27 for therapeutic use to treat various human diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 3.Villarino AV, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J. Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 4.Scheller J, et al. No inhibition of IL-27 signaling by soluble gp130. Biochem. Biophys. Res. Commun. 2005;326:724–728. doi: 10.1016/j.bbrc.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 5.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarino A, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 7.Hamano S, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 10.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 11.Brombacher F, et al. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinchieri G, et al. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 13.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 14.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 15.Moore KW, et al. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 17.Murugaiyan G, et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao S, et al. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J. Immunol. 2005;174:3484–3492. doi: 10.4049/jimmunol.174.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, et al. c-Maf regulates IL-10 expression during Th17 polarization. J. Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraiva M, et al. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochman Y, et al. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, et al. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol. Lett. 2011;136:21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 26.Shiokawa A, et al. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol. Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Murugaiyan G, et al. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YS, et al. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, et al. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat. Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer SS, et al. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J. Immunol. 2010;185:6599–6607. doi: 10.4049/jimmunol.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 32.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ise W, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiramatsu Y, et al. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J. Leuk. Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- 35.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batten M, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artis D, et al. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J. Immunol. 2004;172:4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki Y, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J. Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J. Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimoto T, et al. Induction of IgG2a class switching in B cells by IL-27. J. Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 42.Boumendjel A, et al. IL-27 induces the production of IgG1 by human B cells. Eur. Cytokine Netw. 2006;17:281–289. [PubMed] [Google Scholar]
- 43.Larousserie F, et al. Differential effects of IL-27 on human B cell subsets. J. Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 44.Canale S, et al. Interleukin-27 inhibits pediatric B-acute lymphoblastic leukemia cell spreading in a preclinical model. Leukemia. 2011 doi: 10.1038/leu.2011.158. [DOI] [PubMed] [Google Scholar]
- 45.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Wohlfert E, Belkaid Y. Plasticity of T reg at infected sites. Mucosal Immunol. 2010;3:213–215. doi: 10.1038/mi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Neufert C, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eu.r J. Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 50.Stumhofer JS, et al. Negative regulation of Th17 responses. Semin. Immunol. 2007;19:394–399. doi: 10.1016/j.smim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber M, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Inr. Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 52.Owaki T, et al. IL-27 suppresses CD28-medicated IL-2 production through suppressor of cytokine signaling 3. J. Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 53.Villarino AV, et al. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 54.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J. Leuk. Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 55.Salcedo R, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J. Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox JH, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J. Exp. Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tait Wojno ED, et al. A Role for IL-27 in Limiting T Regulatory Cell Populations. J. Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 60.Goodman WA, et al. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J. Immunol. 2011;186:3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAleer JP, et al. The WSX-1 pathway restrains intestinal T-cell immunity. Int. Immunol. 2011;23:129–137. doi: 10.1093/intimm/dxq464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boulay JL, et al. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19:159–163. doi: 10.1016/s1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 63.Heinrich PC, et al. Interlukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niedbala W, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 65.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 66.Collison LW, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devergne O, et al. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crabe S, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J. Immunol. 2009;183:7692–7702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 69.Shimozato O, et al. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. 2009;128:e816–e825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rousseau F, et al. IL-27 structural analysis demonstrates similarities with ciliary neurotrophic factor (CNTF) and leads to the identification of antagonistic variants. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19420–19425. doi: 10.1073/pnas.1005793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stumhofer JS, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brusko TM, et al. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol. Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 73.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 74.Jaron B, et al. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PloS One. 2008;3:e2833. doi: 10.1371/journal.pone.0002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruter J, et al. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Front. Biosci. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 76.Hisada M, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 77.Salcedo R, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J. Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 78.Cao Y, et al. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J. Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 79.Mayer KD, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J. Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 80.Schneider R, et al. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur. J. Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 81.Yoshimoto T, et al. Antiproliferative activity of IL-27 on melanoma. J. Immunol. 2008;180:6527–6535. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 82.Ho MY, et al. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J. Immunol. 2009;183:6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 83.Cocco C, et al. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin. Cancer Res. 2010;16:4188–4197. doi: 10.1158/1078-0432.CCR-10-0173. [DOI] [PubMed] [Google Scholar]
- 84.Sasaoka T, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiol. Gastroenterol. Liver Physiol. 2011;300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 85.Rajaiah R, et al. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J. Biol. Chem. 2011;286:2817–2825. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niedbala W, et al. Interleukin 27 attenuates collagen-induced arthritis. Ann. Rheum. Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 87.Fitzgerald DC, et al. Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells and the Effector Phase of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 88.Miyazaki Y, et al. Amelioration of delayed-type hypersensitivity responses by IL-27 administration. Biochem. Biophys. Res. Commun. 2008;373:397–402. doi: 10.1016/j.bbrc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 89.Yoshimoto T, et al. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 90.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J. Leuk. Biol. 2010;88:1145–1156. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chae SC, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J. Hum. Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 92.Imielinski M, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li CS, et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J. Gastroenterol. Hepatol. 2009;24:1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- 94.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 95.Spolski R, et al. IL-21 Mediates Suppressive Effects via Its Induction of IL-10. J. Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J. Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eto D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herman AE, et al. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang XO, et al. Regulation of inflammatory responses by IL-17F. The J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collison LW, et al. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J. Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kochetkova I, et al. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J. Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang CH, et al. Airway Inflammation and IgE Production Induced by Dust Mite Allergen-Specific Memory/Effector Th2 Cell Line Can Be Effectively Attenuated by IL-35. J. Immunol. 2011;187:462–471. doi: 10.4049/jimmunol.1100259. [DOI] [PubMed] [Google Scholar]
- 104.Liu F, et al. Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin. Immunol. 2011;139:1–5. doi: 10.1016/j.clim.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 105.Chaturvedi V, et al. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J. Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Bardel E, et al. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J. Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 107.Tong H, et al. Exacerbation of delayed-type hypersensitivity responses in EBV-induced gene-3 (EBI-3)-deficient mice. Immunol. Lett. 2010;128:108–115. doi: 10.1016/j.imlet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Kuo J, et al. Interleukin-35 enhances lyme arthritis in borrelia-vaccinated and -infected mice. Clin. Vaccine Immunol. 2011;18:1125–1132. doi: 10.1128/CVI.00052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]