Abstract
Compelling pre-clinical and pilot clinical data support the role of green tea polyphenols in prostate cancer prevention. We conducted a randomized, double-blind, placebo controlled trial of Polyphenon E (enriched green tea polyphenol extract) in men with prostate cancer scheduled to undergo radical prostatectomy. The study aimed to determine the bioavailability of green tea polyphenols in prostate tissue and to measure its effects on systemic and tissue biomarkers of prostate cancer carcinogenesis. Participants received either Polyphenon E (containing 800 mg epigallocatechin gallate) or placebo daily for 3–6 weeks before surgery. Following the intervention, green tea polyphenol levels in the prostatectomy tissue were low to undetectable. Polyphenon E intervention resulted in favorable but not statistically significant changes in serum prostate specific antigen, serum insulin-like growth factor axis, and oxidative DNA damage in blood leukocytes. Tissue biomarkers of cell proliferation, apoptosis, and angiogenesis in the prostatectomy tissue did not differ between the treatment arms. The proportion of subjects who had a decrease in Gleason score between biopsy and surgical specimens was greater in those on Polyphenon E but was not statistically significant. The study's findings of low bioavailability and/or bioaccumulation of green tea polyphenols in prostate tissue and statistically insignificant changes in systemic and tissue biomarkers from 3–6 weeks of administration suggests that prostate cancer preventive activity of green tea polyphenols, if occurring, may be through indirect means and/or that the activity may need to be evaluated with longer intervention durations, repeated dosing, or in patients at earlier stages of the disease.
Keywords: Polyphenon E, green tea polyphenols, prostate cancer, chemoprevention
Introduction
Prostate cancer is the most common cancer affecting men with an annual age-adjusted incidence of 165.8 cases per 100,000 men in 2007 and a lifetime risk of 1 in 6.25 men [SEER CSR 1975–2007]. A need for prostate cancer prevention is predicted based on the aging society with estimates of a 4-fold increase in the number of people over the age of 65 years by the year of 2050. As many men choose to monitor their prostate health, chemoprevention could be used for both prevention of prostate cancer development as well as prevention of prostate cancer progression.
Chemoprevention of prostate cancer has shown promise using hormonal agents including the 5-alpha-reductase inhibitors finasteride and dutasteride, which block the conversion of testosterone to dihydrotestosterone. The Prostate Cancer Prevention Trial (PCPT) randomized over 8,000 men to either finasteride 5 mg or placebo and showed a 25% relative reduction in the incidence of prostate cancer in the finasteride arm (18.4% versus 24.4% placebo group) [1]. The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study randomized over 6,000 men with a prior negative prostate biopsy to receive dutasteride or placebo and found a 23% reduction in the dutasteride arm in the risk of prostate cancer after 4 years [2]. However, concerns about the possible selection of more aggressive cancers and side effects from these agents have limited their clinical adoption and point to the need for alternative chemoprevention agents [3].
Epidemiologic data showing wide variations in prostate cancer incidence worldwide have suggested that agents derived from dietary sources may represent a possible mechanism underlying these observations. Supplementation with these candidate dietary agents may represent a possible alternative strategy for prostate cancer chemoprevention. Some agents under consideration have included lycopenes in tomatoes, leguminous plant derived isoflavones, flaxseed, and green tea derived polyphenols.
Green tea polyphenols have shown promise in inhibiting prostate cancer growth in several pre-clinical studies. Mukhtar and colleagues have shown that administration of green tea polyphenols (0.1% in drinking fluid) to transgenic adenocarcinoma of the mouse prostate (TRAMP) mice for 24 weeks markedly inhibits prostate cancer development and distant site metastases [4, 5]. The green tea effect is associated with a decrease in cell proliferation and an increase in apoptosis in the prostate tissue, a favorable change in the insulin-like growth factor (IGF)-axis, and a significant suppression of angiogenic and metastatic markers. A more recent study examined the effect of green tea polyphenols (0.1% in drinking fluid) in the TRAMP mouse model and found that the chemoprevention potential decreased with advancing stage of the disease [6]. When green tea polyphenols were administered at the early stage, IGF-1 and its downstream targets were more effectively inhibited [6]. Similar effects on early stage, but not late stage prostate cancer were observed with epigallocatechin gallate (EGCG), the most abundant green tea polyphenol, in the TRAMP model [7]. It is not clear whether tea catechins inhibit prostate carcinogenesis by a direct action that requires the presence of tea catechins at significant levels or by an indirect means, such as affecting circulating cytokines and hormones related to prostate cancer carcinogenesis.
A clinical study by Bettuzi and colleagues has suggested that green tea polyphenols may also be effective in reversal or delay of prostate carcinogenesis, with men with biopsy proven high-grade prostatic intraepithelial neoplasia receiving green tea polyphenol tablets for 12 months developing fewer cases of prostate cancer than those given placebo [8, 9].
Based on the compelling pre-clinical evidence and promising pilot clinical data, we hypothesized that green tea catechin oral supplementation will delay or regress prostate cancer development and progression. To test this hypothesis, we conducted a randomized, double-blind, and placebo controlled trial of green tea polyphenols (formulated as Polyphenon E) in a group of men undergoing prostatectomy for their prostate cancer. The primary aim of the study was to determine the bioavailability of green tea polyphenols in prostate tissue with secondary endpoints being measurement of modulation of systemic and tissue biomarkers related to prostate carcinogenesis process.
Materials and Methods
Study Design
The study was a randomized, double-blind, placebo controlled intervention trial. Patients with a diagnosis of prostate cancer scheduled to undergo radical prostatectomy were randomly assigned to receive either Polyphenon E or placebo for 3–6 weeks before surgery. The primary objective is to determine the bioavailability of green tea polyphenols in prostate tissue after Polyphenon E intervention. The secondary objectives are to determine the effect of Polyphenon E intervention on cell proliferation, apoptosis, and angiogenesis in prostatectomy tissues, serum prostate specific antigen (PSA), serum insulin-like growth factor (IGF) axis, oxidative DNA damage in blood leukocytes, and plasma catechin concentrations. The study was approved by the University of Arizona Institutional Review Board. Written informed consent was obtained from all participants.
Study Drugs
Polyphenon E drug substance contains 85–95% total catechins, with 56–72% as EGCG, and <1.0% caffeine. The drug substance was provided to National Cancer Institute, Division of Cancer Prevention (NCI, DCP) by Mitsui Norin. This study used Polyphenon E oral capsules, standardized to contain 200 mg EGCG per capsule, and matched placebo capsules, supplied by NCI, DCP. The study capsules were stored at room temperature and protected from environmental extremes.
Study Population
Patients with biopsy-confirmed prostate carcinoma electing prostatectomy as their primary treatment and at least 3 weeks from scheduled surgery were enrolled onto the study. To be eligible, patients must have had biopsy-proved prostate cancer, had not received other therapy for their prostate cancer, had a current PSA less than 50 ng/ml, be over the age of 18, had no history of chemotherapy and/or radiation for any malignancy in the previous 5 years, had good performance status, and had normal renal (creatinine ≤ institutional upper limits of normal) and hepatic function(total bilirubin, AST (SGOT)/ALT (SGPT) ≤ institutional upper limits of normal). Patients were excluded if they drank tea regularly within one month of enrollment (more than 6 servings of hot tea or 12 servings of iced tea or equivalent combination per week), were receiving other investigational agents, had a history of allergic reactions attributed to compounds of similar chemical to Polyphenon E, or had uncontrolled intercurrent illness.
Study Procedures
During the initial visit, participants underwent eligibility evaluation. Each participant underwent an interview and brief physical exam to obtain medical history, performance status, height, weight, blood pressure, pulse, and temperature measurements. Blood samples were collected for complete blood count with differentials, comprehensive metabolic panel, and systemic research endpoints. Blood samples for clinical labs were collected and processed according to the diagnostic lab's standards. Blood samples for serum biomarkers were collected into SST vacutainer tubes, allowed to clot for at least 30 min, and centrifuged. Serum was collected and stored as multiple aliquots at −80°C until analysis. Blood samples for plasma catechin concentration measurement and leukocyte oxidative DNA damage biomarker assay were collected into vacutainer tubes containing sodium heparin. After centrifugation, plasma was collected and mixed with Vc-EDTA solution (0.4 M NaH2PO4 buffer containing 20% vitamin C and 0.1% EDTA, pH 3.6) in the volume ratio of 1:0.02 and stored immediately at −80°C for catechin concentration analysis. The remaining buffy coat was collected and stored immediately at −80°C for leukocyte oxidative DNA damage biomarker analysis.
Upon determination of eligibility, participants were randomized (1:1) to receive Polyphenon E or placebo. Participants were instructed to take 4 study capsules each morning with food for 3–6 weeks until the day before surgery. Participants were also required to keep an intake calendar and adverse event diary throughout the study participation. Participants returned to the clinic within 3 days prior to surgery to undergo assessment of adverse events and compliance, and blood collection for complete blood count with differentials, comprehensive metabolic panel, and systemic research endpoints. Blood samples were processed and stored as described above. The NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used to for adverse event description and grading. Agent intervention compliance was evaluated by capsule count and intake calendar. Participants were considered compliant if they had taken at least 80% of their assigned study doses.
At surgery, a prostate tissue sample was collected, immediately snap-frozen, and stored at −80°C for measurements of catechin concentrations. Paraffin-embedded tissue blocks/slides were requested from institution's clinical pathology lab for tissue biomarker analysis.
Green Tea Polyphenol Concentration Analysis
Green tea polyphenol standards were purchased from Sigma-Aldrich Corp. and Nacalai USA Inc. The identity of the green tea polyphenol standards was confirmed by mass spectrometry. Prostate tissue was weighed and processed according to a procedure validated for tissue green tea polyphenol concentration analysis [10] with minor modifications. Briefly, prostate tissue (~300 mg) was homogenized in a mixture consisting of 50 μL of water, 750 μL of methanol:ethyl acetate (2:1), 250 μL of 0.3 M sodium dithionite /0.1% EDTA, and 25 μL of internal standard solution (625 ng/ml ethyl gallate in water). The homogenate was centrifuged, supernatants collected and concentrated by vaccum centrifugation to remove the organic solvents. The remaining aqueous phase was buffered with 250 μL of 0.4 M phosphate buffer (pH = 6.8) and incubated with 250 units of β-glucuronidase and 10 units of sulfatase at 37°C for 45 minutes. After incubation, the samples were extracted with 2.5 mL of ethyl acetate. The ethyl acetate layer was collected and mixed with a small aliquot of 10% ascorbic acid before drying by vacuum centrifugation. The dried residue was reconstituted in 15% acetonitrile before injecting onto the HPLC system.
For plasma green tea polyphenol concentration analysis, 200 μl of plasma was mixed with 20 μL of 0.4 M phosphate buffer (pH = 6.8) and incubated with 250 units of β-glucuronidase and 10 units of sulfatase at 37°C for 45 minutes. After incubation, the sample was extracted with 1 mL of ethyl acetate. The ethyl acetate layer was collected and mixed with a small aliquot of 10% ascorbic acid before drying by vacuum centrifugation. The dried residue was reconstituted in 15% acetonitrile before injecting onto the HPLC system.
The HPLC system consisted of an ESA HPLC system with a Coulochem electrode array detector. HPLC separation was achieved on a C18 column and a mobile phase consisted of a gradient of two buffers [11]. The eluent was monitored with potentials settings at −90,−10, 70,150, 230, 310, 400, and 480 mV. Green tea polyphenols were identified based on the elution time and peak response ratio across different potential settings. The limit of quantification for the green tea polyphenols analysis in the prostate tissue was 2 ng per sample analyzed or 1 ng injected on column. The limit of quantification for the green tea polyphenol analysis in plasma was 3.5 ng/ml or 0.35 ng injected on column.
Leukocyte Oxidative DNA Damage Biomarker
8-Hydroxy-2′-deoxyguanosine (8OHdG) to 2′-deoxyguanosine (dG) ratio in leukocyte DNA was used as a biomarker for systemic oxidative DNA damage. Isolation of DNA from buffy coat was achieved using a FlexiGene DNA kit (Qiagen, Valencia, CA). The isolated DNA was then re-precipitated by the addition of 3 M sodium acetate buffer (pH= 5.2) and 100 % cold ethanol and subsequently washed with70% ethanol. The pellet was re-suspended in water. An aliquot was used to determine the amount and purity of DNA based on the absorbance of the DNA solution at 260 and 280 nm. The remaining aliquot was mixed with one-fifth volume of 300 mM ammonium acetate w/1.2 mM zinc chloride (pH =5.2). Isolated DNA was stored at −80°C until analysis. On the day of analysis, DNA was digested to single nucleosides using a validated DNA hydrolysis procedure [12]. The hydrolysate was filtered through an Ultra-Free membrane before injection onto the HPLC-UV-mass spectrometry system. The HPLC-UV-mass spectrometry system consisted of a Surveyor HPLC system and a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron, San Jose, CA, USA). HPLC separation was achieved on a C18 column with a mobile phase gradient consisting of 10 mM ammonium formate and methanol. The eluent was monitored with in-line UV and mass spectrometry detection. UV detection was performed at 214 nm to ensure complete digestion to individual nucleosides. The mass spectrometric analysis was performed with the electrospray ionization interface operated in the positive ion mode. 8OHdG and dG were detected in the multiple reaction monitoring mode with the ion pairs of m/z 284/168 and 268/152, respectively. Results were expressed as the ratio of 8OHdG/105 dG.
IGF-1 and IGFBP-3 Measurements
The serum concentrations of IGF-1 and IGFBP-3 were measured by specific ELISA assays (DG100 and DGB300, respectively; R&D systems). For IGF-1, the assay included a simple extraction step in which IGF-1 was released from its binding proteins prior to analysis. For IGFBP-3, serum samples were first diluted 1:100 prior to analysis. Standards, controls, and extracted samples were incubated with anti-IGF-1 or anti-IGFBP-3 antibody labeled with horseradish peroxidase (HRP) in microtitration wells coated with another anti-IGF-1 or anti-IGFBP-3 antibody. After incubation and washing, the wells were incubated with the substrate tetramethylbenzidine (TMB). An acidic stopping solution was added and the degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurement at 450 and 620 nm.
Immunohistochemistry (IHC) for Tissue Biomarkers
Immunohistochemistry assays were used to assess cell proliferation, apoptosis, and angiogenesis in prostatectomy tissues. Cell proliferation was assessed by nuclear Ki67 expression. Apoptosis was assessed by positive nuclear staining for cleaved caspase 3 with a pattern of nuclear fragmentation. Angiogenesis was assessed by microvessels expressing CD34.
A precision microtome was used to prepare 3 μm sections on coated slides for each specimen. Slides were deparaffinized and conditioned (antigen retrieval with a borate-EDTA buffer for 30 minutes) on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc) using VMSI validated reagents. Slides were stained with the primary antibody online on the autostainer. Detection with biotinylated-streptavidin-HRP and diaminobenzidine (DAB), and hematoxylin counterstaining was also performed online on the autostainer. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and coverslipped with Pro-Texx mounting medium. Images were captured with a Nikon LaboPhot-2 microscope with Paxcam 3 camera and PAX-it Digital Image Management & Image Analysis. Images were standardized for light intensity. For each analysis, positive controls consisting of paraffin-embedded tissue that had been established previously to express the antigen of interest and negative controls consisting of the positive control sections processed without the primary antibody were included.
For Ki67, Dako M7240 clone MIB-1 mouse monoclonal antibody was used. The proliferation rate was expressed as % of positively stained cells within the tumor regions. For cleaved caspase 3, Cell Signaling Technologies #9661, anti-cleaved caspase 3 rabbit polyclonal antibody was used. Cleaved caspase 3 expression within the tumor regions was evaluated by % of positively stained cells that exhibited nuclear fragmentation. For CD34, Ventana Medical Systems 760–2927 CONFIRM mouse monoclonal clone QBEnd/10 was used. Any dark staining endothelial cell or cell cluster clearly separate from adjacent structures was considered a single vessel. The number of microvessels was counted in five randomly selected 40 × fields within the tumor regions.
Data Analysis
The study randomized 50 participants to receive Polyphenon E or placebo (1:1). All participants who received any study capsules were included in the report of adverse events. Of the 50, 48 (24 per group) completed the intervention and were included in the endpoint analyses. There were missing data for some of the endpoints due to the inability to collect some of the specimens or inadequate specimens for analysis. The missing rate ranged from 2% (IHC tissue markers) to 23% (8OHdG). Descriptive statistics were performed on each of the endpoints within each intervention group. The distributions for some of the endpoints were not symmetrical. Therefore, a two-sided Wilcoxon rank-sum test was used to test if the change in each of the endpoints differed by the intervention groups. In addition, the percentage of patients with positive or negative changes for each of the endpoints was compared between the intervention groups using a Fisher's exact test of proportions at a two-sided 0.05 level of significance. These secondary analyses were not corrected for multiple comparisons but the results were interpreted cautiously, given the multiple markers being explored.
Results
The study was initiated in March of 2007 and completed accrual in July, 2010. Fifty-two subjects were consented with two ultimately found to not meet inclusion criteria. Fifty subjects were randomized with 25 receiving Polyphenon E and 25 receiving placebo. One subject in each group subsequently cancelled their planned surgery, leaving 24 subjects in each group who completed intervention. A Consort flow diagram of the study is shown in Figure 1.
Figure 1.
Consort flow diagram.
The two groups were well matched for demographics with age, race, and body mass index being similar between those who received Polyphenon E and placebo. Mean age was 63.4 years versus 61.3 years respectively (p=0.25). The majority of subjects in both groups were White (96% versus 92% respectively) with 1 multiracial subject in the Polyphenon E group and 1 Native American and 1 multiracial subject in the placebo group. The mean body mass index was 26.9 and 28.1 in each group respectively (p=0.25). The time period between original diagnosis and start of intervention varied among study subjects (from 21 days to a year).
Clinical characteristics of pre-study PSA and biopsy Gleason score were similar between the groups. The mean PSA in the Polyphenon E group was 6.71 with a standard deviation of 4.04 versus 7.90 with a standard deviation of 5.54 in the placebo group (p=0.38). Most subjects in both groups had biopsy Gleason scores of 3+3=6 (70.8% versus 70.8%) while 16.7% and 20.8% had Gleason's score 7 disease on biopsy, respectively (p=1.00). Details of the demographic and clinical characteristics in each group are shown in Table 1.
Table 1.
Clinical characteristics of the study subjects who completed the intervention
| Polyphenon E (n = 24) | Placebo (n = 24) | Pa | |
|---|---|---|---|
| Age (y) | |||
| Mean ± SD | 63.4 ± 5.9 | 61.3 ± 5.7 | 0.25 |
| <65, % (n) | 50 (12) | 75 (18) | 0.14 |
| ≥65, % (n) | 50 (12) | 25 (6) | |
| Race, % (n) | |||
| White | 96 (23) | 92 (22) | 1.00 |
| Native American | 0 | 4 (1) | |
| Other (multi-racial) | 4 (1) | 4 (1) | |
| Body mass index (kg/m2) | |||
| Mean ± SD | 26.9 ± 3.4 | 28.1 ± 3.8 | 0.25 |
| < 25 (% (n)) | 29 (7) | 25 (6) | 1.00 |
| 25–29.9 (% (n)) | 50 (12) | 50 (12) | |
| ≥ 30 (% (n)) | 21 (5) | 25 (6) | |
| PSA at diagnosis (ng/ml), % (n) | |||
| Mean ± SD | 6.71 ± 4.04 | 7.90 ± 5.54 | 0.38 |
| < 4 | 12.5 (3) | 12.5 (3) | 0.71 |
| 4–10 | 75.0 (18) | 66.7 (16) | |
| 11–20 | 12.5 (3) | 12.5 (3) | |
| >20 | 0.0 (0) | 8.3 (2) | |
| Biopsy Gleason score, % (n) | |||
| 6 (3,3) | 70.8 (17) | 70.8 (17) | 1.00 |
| 7 (3,4) | 12.5 (3) | 12.5 (3) | |
| 7 (4,3) | 4.2 (1) | 8.3 (2) | |
| ≥ 8 | 12.5 (3) | 8.3 (2) |
derived from an unequal variance two-sample t-test for continuous outcome and from a Fisher's exact test for categorical outcome.
Polyphenon E was well tolerated with minimal adverse events and no withdrawals from the study secondary to adverse events. A total of 18 and 39 adverse events occurred in the Polyphenon E and placebo groups, respectively. Table 2 summarizes the adverse events occurring in greater than 4 percent of subjects treated with Polyphenon E or placebo (more than 1 subject experiencing the event in either group). Nausea was the most common event, with a similar incidence rate in each group (16% vs. 16%). Other common AEs in the Polyphenon E group include diarrhea (8% vs. 20% for Polyphenon E vs. placebo), and headache (4% vs. 8% for Polyphenon E vs. placebo). These were all Grade 1 or Grade 2 events based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. One subject in the Polyphenon E group had a mild ALT elevation (4%) while no ALT elevation was noted in the placebo group (data not shown). One subject in the placebo group had a grade 4 neutropenia in the end-of-study lab, however, this was most likely a lab error because the pre-op (3 days before the end- of-study lab) and post-op (3 days after the end-of-study lab) lab values were within normal range (data not shown).
Table 2.
Summary of adverse events occurring in greater than 4 percent of subjects treated with Polyphenon E or placebo, regardless of attribution.
| Adverse Event | Polyphenon E (N = 25) | Placebo (N = 25) |
|---|---|---|
| n (%) | n (%) | |
| Nausea | 4 (16%) | 4 (16%) |
| Diarrhea | 2 (8%) | 5 (20%) |
| Headache | 1 (4%) | 2 (8%) |
| Fever | 0 (0%) | 3 (12%) |
| Body ache | 0 (0%) | 2 (8%) |
| Muscle ache | 0 (0%) | 2 (8%) |
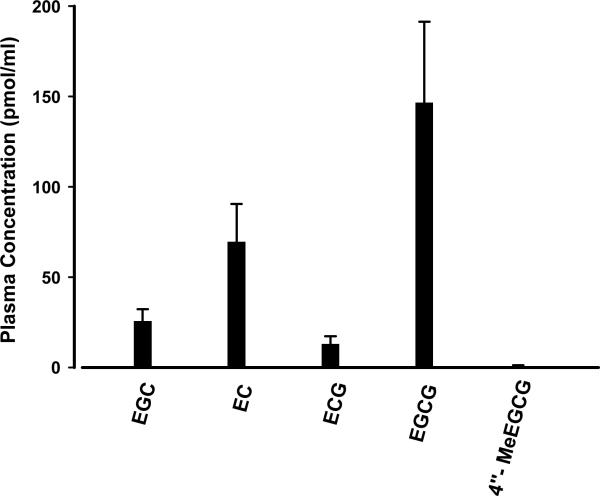
Post-intervention plasma green tea polyphenol concentrations following 3–6 weeks of Polyphenon E intervention are shown in Figure 2. EGCG, the main component catechin in Polyphenon E, reached average plasma levels of 146.6 pmol/ml in subjects given Polyphenon E while lower levels were achieved for the other catechins. The large inter-individual variation in plasma concentrations of tea polyphenols is mostly attributed to the difference in timing of blood collection in relation to the intake of the Polyphenon E. Eighteen of the twenty-hour subjects receiving Polyphenon E had measurable plasma green tea polyphenol concentrations. Five of six Polyphenon E subjects who had no detectable post-intervention plasma green tea polyphenol concentrations had their post-intervention samples collected more than 16 hours after the previous Polyphenon E dose. None of the subjects receiving placebo had detectable plasma green tea polyphenol concentrations.
Figure 2.
Post-intervention plasma green tea polyphenol concentrations following 3–6 weeks of Polyphenon E intervention. EGC: epigallocatechin; EC: epicatechin; ECG: epicatechin gallate; EGCG: epigallocatechin gallate; 4″-MeEGCG: 4″-O-methyl EGCG. Data are presented as means with standard errors (n = 24).
Fresh frozen tissue was available from 15 subjects receiving Polyphenon E and from 19 subjects receiving placebo for green tea polyphenol analysis. Two of the placebo subjects had detectable epicatechin gallate (ECG) peak in the prostate tissue, suggesting that the ECG peak identified in our system for the prostate tissue may not be specific to ECG. Alternatively, the ECG peak detected could be derived from other sources of intake. No other green tea polyphenols were detected in the placebo group. Five of the 15 Polyphenon E subjects had detectable ECG peak, ranging from 17.77 to 59.67 pmol/g. One of the 15 Polyphenon E subjects had detectable concentrations of each of the green tea polyphenols analyzed; epigallocatechin (EGC) 88.71 pmol/g, epicatechin (EC) 226.74 pmol/g, ECG 37.35 pmol/g, EGCG 36.05 pmol/g, and 4″-O-methyl-EGCG (4″-MeEGCG) 12.33 pmol/g. One subject had detectable tissue EC concentrations (83.06 pmol/g).
Systemic biomarker endpoints are summarized in Table 3. Note that PSA values collected pre-intervention were lower than those at diagnosis because of variations between values obtained from ELISA testing in our lab for pre-intervention testing and clinical lab results used for PSA values at diagnosis. PSA values demonstrated a greater decrease for those on Polyphenon E than those on placebo but this did not reach statistical significance (−0.66 ±2.56 and −0.08 ±1.28, ng/ml, p=0.26). When comparing the proportion of those who had a decrease in PSA to those that did not, 58.3% of Polyphenon E subjects versus 36.4% of placebo patients experienced a decrease after intervention (p=0.15). The 8OHdG to dG ratio, a marker of oxidative DNA damage, showed a greater mean decrease for those on Polyphenon E but this again did not reach statistical significance (−0.79 ±6.75 versus 1.81±8.37, p=0.17). The percentage of those with a decrease in 8OHdG was 65.0% versus 35.3% for those on Polyphenon E and placebo, respectively (p=0.10). Serum insulin-like growth factor-1 (IGF-1) levels, which have been correlated with increased prostate cancer risk, showed a greater decrease among those on Polyphenon E but this did not reach statistical significance (−6.90 ±20.97 versus −1.20±21.82 ng/ml, p=0.53). The proportion of subjects with a decrease in IGF-1 was likewise greater in those on Polyphenon E (54.2% versus 36.4%, p=0.25). Levels of insulin-like growth factor binding protein-3 (IGFBP-3), which modulates the bioavailability and ligand function of IGF-1, showed a greater but non-statistically significant increase in subjects on Polyphenon E intervention (20.38±289.3 versus −74.76±238.11 ng/ml, p=0.24). The proportion of those who had an increase in IGFBP-3 levels was 54.2% versus 36.4% for Polyphenon E and placebo subjects, respectively (p=0.25). The ratio of IGF-1 to IGFBP-3 similarly showed a favorable but non-significant decrease for the treatment arm (−0.003±0.011 versus 0.002±0.012, p=0.16, and 62.5% versus 45.5% showing a decrease, p=0.37).
Table 3.
Intervention induced changes in systemic biomarkers
| Polyphenon E | Placebo | P | |
|---|---|---|---|
| PSA | (n = 24) | (n = 22) | |
| Baseline, ng/ml | 5.63 ± 4.18a | 7.14 ± 6.70 | 0.43b |
| Absolute change, ng/ml | − 0.66 ± 2.56 | − 0.08 ± 1.28 | 0.26b |
| % (n) showing a decrease in PSA | 58.3 (14) | 36.4 (8) | 0.15c |
| 8OHdG/dG ratio (x105) | (n = 20) | (n = 17) | |
| Baseline | 8.89 ± 5.25 | 6.75 ± 2.75 | 0.16b |
| Absolute change | − 0.79 ± 6.75 | 1.81 ± 8.37 | 0.17b |
| % (n) showing a decrease in 8OHdG | 65.0 (13) | 35.3 (6) | 0.10c |
| IGF-1 | (n = 24) | (n = 22) | |
| Baseline, ng/ml | 109.27 ± 41.63 | 107.46 ± 36.09 | 0.96b |
| Absolute change, ng/ml | − 6.89 ± 20.97 | − 1.20 ± 21.82 | 0.53b |
| % (n) showing a decrease in IGF1 | 54.2 (13) | 36.4 (8) | 0.25c |
| IGFBP-3 | (n = 24) | (n = 22) | |
| Baseline, ng/ml | 1958.77 ± 572.30 | 2118.99 ± 417.85 | 0.31b |
| Absolute change, ng/ml | 20.38 ± 289.3 | −74.76 ± 238.11 | 0.24b |
| % (n) showing an increase in IGFBP3 | 54.2 (13) | 36.4 (8) | 0.25c |
| IGF-1/IGFBP-3 | (n = 24) | (n = 22) | |
| Baseline | 0.059 ± 0.022 | 0.052 ± 0.018 | 0.27b |
| Absolute change | − 0.003 ± 0.011 | 0.002 ± 0.013 | 0.16b |
| % (n) showing a decrease in IGF ratio | 62.5 (15) | 45.5 (10) | 0.37c |
mean ± SD
derived from a Wilcoxon rank-sum test.
derived from a Fisher's exact test.
Table 4 summarizes the IHC data on tissue biomarker endpoints determined in the prostatectomy tissue. Tissue levels of the cellular marker for proliferation, Ki-67, did not differ significantly between the Polyphenon E and placebo arms (5.65±9.47 versus 4.37±6.11 % staining, p=0.68). We measured apoptosis by determining the percentage of cells staining for cleaved caspase 3 and found no difference between the two arms (0.39±0.57 versus 0.46±0.64, p=0.29, respectively). Angiogenesis, as measured by determining microvessel density was similar between the Polyphenon E and placebo groups (22.43±9.93 versus 23.04±10.40 average number of microvessels in five random 40× fields, p=0.89).
Table 4.
Tissue biomarkers in prostatectomy specimens
| Polyphenon E | Placebo | P | |
|---|---|---|---|
| Ki67 (%) | 5.65 ± 9.47 (n = 24) | 4.37 ± 6.11 (n = 23) | 0.68a |
| Cleaved caspase 3 (%) | 0.39 ± 0.57 (n = 24) | 0.46 ± 0.64 (n = 23) | 0.29a |
| Microvessel density (average # in five random 40x field) | 22.43 ± 9.93 (n = 24) | 23.04 ± 10.40 (n = 23) | 0.89a |
derived from a Wilcoxon rank-sum test.
A greater proportion of subjects on Polyphenon E showed a decrease in Gleason score between prostate biopsy and surgical specimens but this again did not reach statistical significance (20.8% versus 8.3% showing a decrease, p=0.22 for those on Polyphenon E versus placebo). 16.7% of Polyphenon E and 37.5% of placebo subjects experienced an increase in Gleason score.
Discussion
In this randomized, double-blind, placebo controlled trial of Polyphenon E with pre-prostatectomy short duration intervention, we found that prostate tissue bioavailability of polyphenols was low and that systemic biomarkers, while showing a trend towards chemopreventative efficacy, were not significantly different between the two groups. Tissue biomarkers also did not differ between the treatment and control arms. Polyphenon E intervention in pill form was well tolerated with minimal adverse events.
Our findings suggest that green tea intervention, if effective in the chemoprevention of prostate cancer, may not act in a direct fashion on prostate tissue, as we found low to undetectable tissue bioaccumulation levels. The low tissue levels may be due to a combination of rapid systemic clearance and low bioaccumulation of polyphenols in prostate tissue. Previous studies showed that the plasma half-life of parent catechins and conjugated catechin metabolites was around 2–4 hr [11, 13]. In this study, participants took their pills in the morning, and the time between the last dose of Polyphenon E or placebo on the day prior to surgery and surgical excision of the prostate the following morning or afternoon was more than 24 hours because of restrictions on oral intake on the day of surgery. This long elapsed time would lead to undetectable tissue levels by the time of surgical excision, if minimal bioaccumulation occurs in the prostate. Serum levels in contrast were obtained while subjects were still on Polyphenon E or placebo intervention, as these were drawn on the days preceding surgery, which likely accounts for the higher levels. Unlike our study's finding of lack of bioavailability of tea polyphenols in prostate tissue, a recent randomized study did find measureable levels in the prostate and urine of men given green tea in beverage form [14]. These contrary findings may have been due to differences in the scheduling of dosing, as the published study instructed subjects to drink six cups of tea each day [14] and subjects may have therefore received catechin within 12 hours before prostatectomy, if they had drank tea up to the evening before surgery. The published study's reported low nM catechin concentrations in human prostate tissue [14] potentially still suggests indirect mechanism(s) of action for green tea polyphenols.
Importantly, because we measured tissue levels at trough rather than peak concentrations, it is possible that green tea polyphenols may achieve measurable tissue levels that cannot be assessed with our study design. If this were the case, green tea intervention may conceivably still act in a direct fashion on prostate tissue. Additionally, while our once-daily dose of Polyphenon E was equivalent to 12–16 cups of green tea a day, divided doses taken throughout the day (similar to tea infusion) may allow more constant exposure of green tea polyphenols to prostate tissue.
This study found a trend among systemic biomarkers that may suggest chemopreventive activity of Polyphenon E in prostate cancer but none of these were statistically significant. These include decreased PSA; reduced 8OHdG to dG ratio; lowered IGF-1; increased IGFBP-3, and decreased IGF-1/IGFBP-3 ratio in subjects given Polyphenon E as opposed to placebo. It is possible that the small sample size of this study limited its ability to demonstrate a statistically significant difference in systemic biomarkers. Post-hoc power analysis, based on a 2 sided two sample test with unequal variances at an alpha level of 5% demonstrated that the following sample sizes (per group) would be necessary to achieve a power of 80%: PSA: 192, IGF-1: 230, IGF-1/IGFBP3 ratio: 92, 8OHdG: 140. A repeat study may therefore need to have at least 230 subjects in each group to achieve statistical significance based on the observed differences seen in this study. In contrast to our findings, McLarty and associates in an open label, single arm study demonstrated that supplementation with Polyphenon E in pill form prior to prostatectomy significantly reduced serum prostate specific antigen, IGF axis hepatocyte growth factor, and vascular endothelial growth factor levels in their group of twenty-six men with prostate cancer [15]. The reason(s) for the discrepancy between their study and our findings remains to be defined but includes the lack of a control group in their study which it made it easier to gain significance. The published study used the same daily dose of Polyphenon E with a median duration on study (34.5 days) similar to ours (28 days), but the published study enrolled a high proportion of African Americans (62%) [15]. It is not known whether this demographic difference would impact the changes in systemic biomarkers.
Our study's finding of no statistical difference in Gleason's score change between the biopsy and surgical specimens in the treated group is not surprising given the short duration of the intervention was unlikely to cause a change in histologic characteristics. Similarly, no statistically significant treatment effects on proliferation, angiogenesis, or apoptosis was observed in the prostatectomy tumor regions. If the prostate tissue activity of tea polyphenols is mediated through indirect means, significant changes in the tissue biomarkers may only occur after sustained modulation of the systemic hormones or cytokines. Therefore, longer-term studies would likely be necessary to achieve measurable differences in tissue biomarkers and histology.
The stage of disease may play a factor in the efficacy of green tea in prostate cancer. Adhami and colleagues, in a mouse prostate cancer model, found that the effect of green tea polyphenols decreased with advancing stage [6]. Green tea may therefore be most effective in a pre-cancerous model and it effects may be too modest to meaningfully impact overt prostate cancer, as was the case with men in this study. Two other studies involving men with more advanced prostate cancer that were given green tea also did not demonstrate benefits [16, 17]. In the clinical study that did demonstrate a benefit of green tea, all subjects were in the pre-cancerous stage of having high-grade prostatic intraepithelial neoplasia on biopsy [8, 9].
The pre-prostatectomy model used in this study has several advantages including higher subject acceptability as participation did not alter a subject's chosen treatment option of surgery and the availability of serum and complete tissue specimens at the conclusion of the intervention. These attributes can facilitate the rapid evaluation of the clinical activity of potential chemopreventive agents that can guide further research. However, this model has inherent limitations, which in this study included a relatively short duration of intervention as patients who have elected surgery are faced with a possible delay of treatment, which limits the acceptable length of intervention. The duration of intervention for this study was 3–6 weeks and may, as discussed, have been inadequate to induce a significant change in systemic or tissue biomarkers.
In conclusion, the findings of this study suggest that green tea for prostate cancer chemoprevention may not be acting through direct means or is occurring without bioaccumulation. Trends seen in systemic biomarkers were suggestive of possible efficacy but may have required a larger sample size to detect. The absence of significant differences seen in this study suggests that future studies using Polyphenon E might be best directed at longer-term interventions, use of repeated doses for more constant exposure, or in a pre-cancerous model where its effects may be more demonstrable.
Acknowledgements
The authors would like to acknowledge Frances Minter, Wendy Thomas, Kathy Monroe, Catherine Cordova, Steve Rodney, Kathy McDaniel, Edward Abril, and Laura Brosh for their excellent assistance in the performance of the clinical study and endpoint assays. The authors also would like to acknowledge Drs. Larry Bans, Bruce Dalkin, Sanjay Ramakumar, and Rajesh Prasad for referring patients to the study.
Grant Support This work was supported by a contract (N01CN35158) from the National Cancer Institute and the Arizona Cancer Center Support Grant (CA023074).
The abbreviations used
- EGC
epigallocatechin
- EC
epicatechin
- ECG
epicatechin gallate
- EGCG
epigallocatechin gallate
- 4″-MeEGCG
4″-O-methyl EGCG
- PSA
prostate specific antigen
- IGF-1
insulin-like growth factor-1
- IGFBP-3
insulin-like growth factor binding protein-3
- 8OHdG
8-hydroxy-2′-deoxyguanosine
- dG
2′-deoxyguanosine
References
- 1.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton RJ, Kahwati LC, Kinsinger LS. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2164–71. doi: 10.1158/1055-9965.EPI-10-0082. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res. 2009;15:1947–53. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. Prostate. 2007;67:1576–89. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 8.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 9.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54:472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 10.Chu KO, Wang CC, Chu CY, Rogers MS, Choy KW, Pang CP. Determination of catechins and catechin gallates in tissues by liquid chromatography with coulometric array detection and selective solid phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:187–95. doi: 10.1016/j.jchromb.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 12.Huang X, Powell J, Mooney LA, Li C, Frenkel K. Importance of complete DNA digestion in minimizing variability of 8-oxo-dG analyses. Free Radic Biol Med. 2001;31:1341–51. doi: 10.1016/s0891-5849(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 13.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 14.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3:985–93. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 16.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 17.Choan E, Segal R, Jonker D, Malone S, Reaume N, Eapen L, et al. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urol Oncol. 2005;23:108–13. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]