Abstract
Animals that hunt and scavenge are often exposed to a broad array of pathogens. Theory predicts the immune systems of animals specialized for scavenging should have been molded by selective pressures associated with surviving microbial assaults from their food. Spotted hyenas (Crocuta crocuta) are capable hunters that have recently descended from carrion feeding ancestors. Hyenas have been documented to survive anthrax and rabies infections, and outbreaks of several other viral diseases that decimated populations of sympatric carnivores. In light of the extreme disease resistance manifested by spotted hyenas, our objective was to identify tools available for studying immune function in spotted hyenas and use these tools to document the hyena antibody response to immunization. Domestic cats (Felis catus) are the closest phylogenetic relatives of hyenas that have been studied in detail immunologically, and we hypothesized that anti-cat isotype-specific antibodies would cross react with hyena immunoglobulin epitopes. We used ELISA and Western blots to test isotype-specific anti-feline antibodies for specific cross-reaction to hyena Ig epitopes. Molecular weights of heavy (IgA, IgG, IgM) and light chains of hyena immunoglobulins were determined by protein electrophoresis, and as expected, they were found to be similar to feline immunoglobulins. In order to further validate the cross-reactivity of the anti-feline antibodies and document the hyena humoral response, eight spotted hyenas were immunized with dinitrophenol conjugated keyhole limpet hemocyanin (DNP-KLH) and serum anti-DNP responses were monitored by enzyme-linked immunosorbent assay (ELISA) for one year. The full array of isotype-specific antibodies identified here will allow veterinarians and other researchers to thoroughly investigate the hyena antibody response, and can be used in future studies to test hypotheses about pathogen exposure and immune function in this species.
Keywords: Hyena, Crocuta, Antibody, Isotype, Humoral, Immune
1. Introduction
Wildlife disease outbreaks can have major impacts on conservation efforts and lasting effects on ecosystem processes (Claude, 1996). For example, rabies and canine distemper virus (CDV) epizootics were associated with the extirpation of wild dogs (Lycaon pictus) in the Maasai Mara National Reserve (MMNR) in Kenya (Alexander and Appel, 1994, Kat et al., 1995, Kat et al., 1996). Additionally, a CDV outbreak in East Africa killed more than 1000 lions (Panthera leo) (Munson et al., 2008, Roelke-Parker et al., 1996).
Animals that hunt and scavenge are likely exposed to a broad array of pathogens (Schulenburg et al., 2009). Although most carnivores, including lions and wild dogs, scavenge to some extent (Houston, 1979), theory predicts that the immune systems of carnivores exhibiting morphological specializations for carrion-feeding should have been molded by selective pressures associated with surviving microbial assaults from their food (Blount et al., 2003, Mendes et al., 2006, Schulenburg et al., 2009). Spotted hyenas (Crocuta crocuta) are capable hunters that have descended within the last million years from carrion feeding ancestors (Lewis and Werdelin, 2000, Werdelin, 1989). Despite documented exposure to anthrax, rabies, CDV and several other pathogens, spotted hyenas in East Africa have exhibited extremely low mortality rates due to infectious diseases, even when epizootics decimated sympatric carnivore populations (Alexander et al., 1995, East et al., 2001, East et al., 2004, Harrison et al., 2004, Lembo et al., 2011, Watts and Holekamp, 2009). Spotted hyenas are the most abundant large carnivores in Africa, and may play a critical role in the ecology of disease in African wildlife and domestic animals throughout the continent (Hofer, 1998).
In light of the extreme disease resistance manifested by hyenas and their potential importance for overall disease dynamics in African ecosystems, we set out to identify tools available for studying immune function in the spotted hyena. The two specific aims of this study were to identify antibodies that cross-react with hyena immunoglobulins and to assess the dynamics of the hyena humoral immune response to immunization with a non-pathogenic antigen. Domestic cats (Felis catus) were the closest phylogenetic relatives of hyenas that had been studied in detail immunologically (Bininda-Emonds et al., 1999, O’Brien and Johnson, 2005), and we hypothesized that anti-cat isotype-specific antibodies would cross react with hyena immunoglobulin (Ig) epitopes.
We used ELISAs to test isotype-specific anti-feline antibodies for cross-reaction to hyena Ig epitopes and to assess temporal dynamics of hyena immunoglobulins in response to immune challenge. We used Western blots to confirm cross-reactivity and to estimate the molecular weight of hyena immunoglobulins. Reverse transcriptase polymerase chain reaction (RT-PCR), serum neutralization tests, western blots, and agglutination tests have been used previously to document pathogen exposure in spotted hyenas (East et al., 2001, East et al., 2004, Honer et al., 2006, Speck et al., 2008), but only a few studies have gone beyond documenting exposure and examined the immune response itself (East et al., 2008, Hanley et al., 2005, Honer et al., 2006, van Helden et al., 2008). The current paper represents the first report of antibodies capable of detecting spotted hyena immunoglobulins other than IgG, and the first to document the temporal dynamics of the humoral immune response of the major isotypes with a defined antigen.
2. Materials and methods
2.1. Captive spotted hyenas, sample collection, and immunization
All captive spotted hyenas were born and housed in the Field Station for Behavioral Research (FSBR) of the University of California, Berkeley (UCB). Berger et al. (1992) describe the husbandry conditions at this facility. Eight healthy adult (4 female, 4 male) spotted hyenas were subjected to an immunization protocol approved by both the University of California, Berkeley (Animal Use Protocol # R091-0609R) and Michigan State University (MSU) Institutional Animal Care and Use Committees (IACUC) (AUF # 07/08-099-00). Captive hyenas ranged from 4 to 17 years of age when immunizations began. Animals were immobilized with blow dart-delivered i.m. injections of ketamine (4–6 mg/kg) and xylazine (1 mg/kg) for immunization and blood sampling.
Blood samples for serum analysis were collected on days 0, 14, 28, 180 and 365 from the jugular vein using Vacutainer tubes (BD, Franklin Lakes, NJ, cat# 366430), allowed to clot at ambient temperature, and then centrifuged. Serum was aliquoted into cryovials and stored at −80 °C. Following blood collection on day 0, four animals were immunized with 250 μg of 2,4-dinitrophenol conjugated to keyhole limpet hemocyanin (DNP-KLH) (Calbiochem/EMD Biosciences, Germany, cat# 324121) in sterile water, and four animals were immunized with 250 μg of DNP-KLH emulsified in TiterMax Gold (TiterMax USA, Inc., Norcross, Georgia, cat# G1). Each animal received one subcutaneous 250 μl injection on each side of the neck for a total of 500 μl. A booster, consisting of 250 μg of DNP-KLH in sterile water, was administered on day 14. Blood was collected on day 14 prior to administration of booster injections.
2.2. Samples from wild spotted hyenas
Serum samples from wild spotted hyenas were collected as part of the long-term research project in the Maasai Mara National Reserve, Kenya, started in 1988. Wild spotted hyenas were immobilized using tiletamine–zolazepam (6.5 mg/kg Telazol; Fort Dodge Animal Health, Fort Dodge Iowa) in a plastic dart fired from an air rifle (Telinject Inc., Saugus, California) (Holekamp and Sisk, 2003). Whole blood samples from wild hyenas were collected from the jugular vein using Vacutainer tubes and allowed to clot at ambient temperature, then centrifuged, aliquoted into cryovials, and snap frozen in liquid nitrogen. All immobilization protocols were approved by the MSU IACUC (AUF # 07/08-099-00) and the Kenya Wildlife Service.
2.3. Purification of spotted hyena immunoglobulins
Pooled serum samples from wild spotted hyenas were used for purification of hyena IgG, IgM, and IgA. Immunoglobulins from a 25 ml serum pool were first precipitated in 50% ammonium sulphate solution (Steward and Petty, 1972), redissolved in phosphate buffered saline (PBS), and then extensively dialyzed against water.
The dialysate was centrifuged and the precipitate was redissolved in PBS as a crude IgM preparation; this was then sized over Sephacryl S-300 using a 50 cm × 2.5 cm column and the peak fraction collected as partially purified hyena IgM. Meanwhile, the clarified dialysate was passed over a DEAE Bio-Gel A column (12 cm × 2.5 cm) equilibrated with 0.1 M Tris pH 8.0, and a semi-purified IgG fraction was eluted using 0.1 M Tris pH 8.0 containing 45 mM sodium chloride; this peak was then sized over Sephacryl S-300 and the appropriate sized IgG fraction was collected. A further fraction was eluted from the DEAE column using 0.1 M Tris pH 8.0 containing 70 mM sodium chloride. This peak was then sized over Sephacryl S-300, and Ig which eluted at a position behind the IgM peak but ahead of the IgG peak, was collected as a hyena IgA enriched fraction. Protein concentrations were determined with a UV spectrophotometer reading at 280 nm. Further details about the purification of immunoglobulins are available in Grant (1995).
2.4. Western blot verification of cross-reactive antibodies and molecular weight of hyena Igs
Purified hyena and feline (F. catus) IgG, IgM, and IgA fractions were subjected to SDS-PAGE and Western blot analysis under denaturing conditions to verify cross-reactivity of anti-feline antibodies to hyena Ig epitopes and to compare the molecular weight of Ig heavy and light chains between the two species. Purified hyena immunoglobulins were diluted in Laemli sample buffer (Laemmli, 1970) with 0.1 M dithiothreitol, and were denatured by heating at 95 °C for 5 min. IgG, IgM and IgA samples were then loaded at 2 μg/well, 1 μg/well, and 2.5 μg/well, respectively, into a 10% Tris–HCl polyacrylamide gel. Prestained protein standards were added to each gel to assess molecular weight of the target proteins. Running conditions (150 V for 45 min) and running buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS) were used in accordance with manufacturer's instructions for the Bio-Rad Mini Protean II System.
Hyena and feline serum samples diluted 1:10 in Laemli sample buffer were used for analysis of cross-reactivity and molecular weight comparison of both heavy and light chains. Initial assays using serum samples exhibited migration patterns that appeared to be influenced by large quantities of albumin. AlbuminOut (GBiosciences, cat# 786–251) was therefore used to remove albumin from the serum samples, and this allowed us to estimate the molecular weight of target proteins more accurately.
Prior to transfer, the nitrocellulose membranes were soaked in transfer buffer (25 mM Tris, 192 mM glycine, 20% (v/v) methanol) for 15 min. Proteins were transferred at 100 V for 75 min at 4 °C. After transfer, membranes were place directly into 5% non-fat dry milk (NFDM) in Tris buffered saline (TBS) (5% NFDM–TBS; 420 mM Tris–HCl, 80 mM Tris, 1.5 M NaCl, 5 g/L blotting grade NFDM) and incubated overnight at 4 °C. See Table 1 for a comprehensive list of antibodies tested.
Table 1.
Antibodies tested by ELISA and Western blot (WB).
| Target | Catalog # | mAb or pAb | Assays confirmeda | Supplier |
|---|---|---|---|---|
| IgA | IgA5-3B | mono | ELISA (cat, hyena) | CMICb |
| IgA | CDA2-43 | mono | ELISA (cat, hyena) | CMIC |
| IgA | NB7264 | poly | ELISA (cat, hyena), WB (cat, hyena) | Novus Biologicals |
| IgE | E6-71 | mono | ELISA (cat, hyena) | CMIC |
| IgE | E2-19 | mono | ELISA (cat, hyena) | CMIC |
| IgG | GPB2-2 | mono | ELISA (cat, hyena), WB (cat) | CMIC |
| IgG | 04-20-02 | poly | ELISA (cat, hyena), WB (cat, hyena) | KPLc |
| IgG(H+L) | 102-065-003 | poly | ELISA (cat, hyena), WB (cat, hyena) | Jackson ImmunoResearch |
| IgG(H+L) | OB-680-05 | poly | ELISA (cat, hyena), WB (cat, hyena) | Southern Biotechnology |
| IgM | CM7 | mono | ELISA (cat, hyena), WB (cat, hyena) | CMIC |
| IgM | CM6E | mono | ELISA (cat, hyena), WB (cat, hyena) | CMIC |
| IgM | 04-20-03 | poly | ELISA (cat, hyena), WB (cat, hyena) | KPL |
| κ light chain | FIG1-7A | mono | ELISA (cat, hyena), WB (cat, hyena) | CMIC |
| λ light chain | CAG8-7C | mono | ELISA (cat, hyena), WB (cat) | CMIC |
Parentheses contain the species for which antibody binding has been confirmed.
Custom Monoclonals International Corp.
Kirkegaard & Perry Laboratories, Inc.
Staining was done using a Bio-Rad Multiscreen Apparatus. Primary monoclonal detection antibodies were added at 1 μg/ml in 1% NFDM-TBS and incubated for 90 min at ambient temperature on a shaking platform. The membrane was then washed 3 times with 0.05% Tween-20 in TBS (TBS-T). Secondary biotin-F(ab′)2 fragment goat anti-mouse IgG (H + L) was used with mAbs and was diluted 1:5000 in 1% NFDM-TBS and incubated on a shaker for 60 min. The membrane was again washed 3 times with TBS-T. Extravidin-peroxidase was diluted 1:1000 in TBS-T and incubated with the membrane for 30 min. Color change was developed using a CN/DAB substrate kit. Color change reaction was stopped by washing with distilled water after approximately 10 min. The staining process used with polyclonal HRP conjugated anti-IgG, anti-IgM, and anti-IgA involved the following process: a blocking step, wash, incubation of antibodies at 1 μg/ml, a final wash step, and followed by color development. Images were captured using the Bio-Rad VersaDoc Molecular Imaging system, and molecular weight was determined using Bio-Rad Quantity One software package.
2.5. Identification of cross-reactive anti-feline antibodies and quantification of humoral response using an enzyme-linked immunosorbent assay (ELISA)
To further examine the cross-reactivity of commercially available anti-feline immunoglobulins with spotted hyena immunoglobulins and to quantify spotted hyena humoral response to immunization, we tested spotted hyena sera using ELISAs. ELISA plates were coated with either 50 μl of bovine serum albumin (BSA) as a control or DNP-BSA at 5 μg/ml in 50 μl of carbonate buffer (0.1 M, pH 9.5) and stored overnight at 4 °C. BSA coated wells were used as a reference for background binding.
Plates were washed with PBS containing 0.05% Tween-20 (PBS-T). Plates were then blocked for 1 h with 5% NFDM–PBS. This and all subsequent incubations were run at ambient temperature on a shaking platform. Plates were then washed with PBS-T. Monoclonal anti-DNP (Silver Lakes Research, cat# CH1911) was subjected to serial two fold dilutions to generate a standard curve. Negative control wells were incubated with PBS only, and pre-immune sera (day 0) also served as an additional control. See Table 2 for dilutions of serum samples and anti-isotype detection antibodies. All serum samples were incubated for 90 min before washing and each sample was tested in duplicate. Plates were then incubated with primary anti-isotype antibodies for 90 min and washed with PBS-T. Next, 50 μl of anti-mouse IgG-HRP was diluted 1:5000 and added to each well with mAbs, including the standard wells. Plates were then incubated for 45 min and washed with PBS-T. Color change was developed using 50 μl/well of 3,3′,5,5′-tetramethylbenzidine and reaction was stopped using 50 μl of 0.5 M H2SO4 after 20–60 min, depending on the primary antibody (Ab). Absorbance was then read at 450 nm on a standard plate reader.
Table 2.
Peak anti-DNP equivalent concentration.
| Age | Adjuvant | IgA | IgE | IgG | IgM | κ light chain | λ light chain | poly IgG | poly IgM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Individual | ||||||||||
| A | 4.7 | YES | 0.464 | 0.561 | 1.623 | 2.104 | 6.2501 | 0.392 | 12.500a | 0.722 |
| B | 6.8 | NO | 0.139 | 0.138 | 0.123 | 6.361 | 4.035 | 0.077 | 5.365 | 2.502 |
| C | 11.2 | YES | 0.322 | 0.350 | 0.684 | 2.361 | 6.2501 | 0.235 | 12.500a | 1.044 |
| D | 11.3 | NO | 0.042 | 0.048 | 0.024 | 0.356 | 0.824 | 0.016 | 1.365 | 0.236 |
| E | 13.9 | YES | 0.011 | 0.024 | 0.009 | 0.192 | 0.371 | 0.006 | 0.820 | 0.101 |
| F | 15.1 | NO | 0.003 | 0.011 | 0.002 | 0.159 | 0.106 | 0.001 | 0.322 | 0.134 |
| G | 15.1 | YES | 0.034 | 0.035 | 0.018 | 0.307 | 0.608 | 0.012 | 1.183 | 0.175 |
| H | 17.0 | NO | 0.010 | 0.006 | 0.001 | 0.208 | 0.055 | 0.001 | 0.160 | 0.198 |
| Detection Ab catalog # | IgA5-3B | E6-71 | GPB2-2 | CM7 | CAG8-7C | FIG1-7A | 04-20-02 | 04-20-03 | ||
| Detection Ab conc. (μg/ml) | 5 | 10 | 10 | 5 | 10 | 5 | 0.5 | 0.5 | ||
| Serum dilution | 1:100 | 1:100 | 1:100 | 1:100 | 1:100 | 1:100 | 1:4000 | 1:1000 | ||
Age is in years. Concentrations are the mean of duplicate ELISA results. For all assays, the youngest four individuals had higher antibody responses than the four oldest individuals.
Indicates the concentration exceeded the linear range of the standard curve and was assigned the maximum value of the linear range of the standard curve.
2.6. Statistical analysis
All analyses were performed in R (R Development Core Team, 2011). Results from ELISAs are expressed as anti-DNP equivalent concentrations in order to create a relative measure that can be used for all isotypes. The Calib package in R was use to create a logistic regression based on the absorbance values of the standards in each plate. The regression was then used to calculate the relative anti-DNP concentration for each sample. The mean of duplicate anti-DNP equivalent concentrations for each time point for each individual were used in all analyses. Using serum diluted 1:1000 with polyclonal IgM detection Ab or serum diluted 1:4000 with polyclonal IgG detection Ab, we calculated a standardized percent of IgG and IgM out of the sum total of IgG and IgM (percent IgG = IgG/(IgG + IgM)).
In the ELISAs using polyclonal anti-IgG and monoclonal anti-κ, the absorbance values from the two individuals with the highest titers exceeded the linear range of the standard curve. We were unable to further optimize the assay conditions to fall within the linear range of the standard curve because doing so would render undetectable the absorbance values of the two individuals with the lowest concentrations of anti-DNP antibodies. Instead of extrapolating beyond the linear range of the standard curve for the two individuals with the highest absorbance values, we assigned to these two individuals the maximum values within the linear range of the standard curve.
3. Results
3.1. Western blot verification of cross-reactive antibodies and molecular weight of hyena immunoglobulins
Hyena γ heavy chain was slightly smaller than feline γ heavy chain based on Western blot analysis (Fig. 1 ). We estimate feline γ heavy chain to be 55–56 kilodaltons (kDa), whereas hyena γ heavy chain was 53–54 kDa (Table 3 ). Note that the estimated molecular weight for each heavy or light chain we tested varied slightly depending on whether we used purified Ig or sera, and also based on the serum dilution used; for this reason, molecular weights are presented as ranges rather than as specific values. Also, variations in sample preparation may explain the slight differences between our results and previously published results (Grant, 1995, Klotz et al., 1985, Yamada et al., 2007). Hyena μ chain, estimated at 77–82 kDa, was slightly larger than feline μ chain, which we estimated at 74–80 kDa. Feline and hyena α heavy chains were 54–60 kDa and 57–61 kDa, respectively. Neither feline nor hyena ɛ heavy chain could be detected in serum by Western blot; we were unable to obtain purified IgE.
Fig. 1.
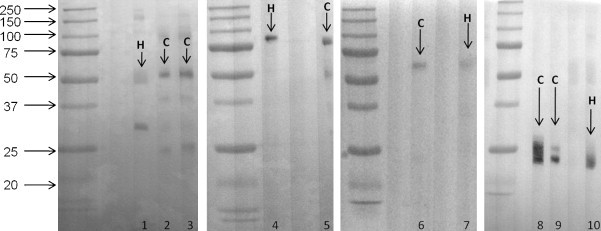
Western blot of heavy and light chains in domestic cats (C) and spotted hyenas (H). Molecular weight standards are indicated with arrows and weights are in kilodaltons. Lane 1. Purified hyena IgG. Hyena γ chain detected with polyclonal anti-IgG (04-20-02). Lane 2. Purified cat IgG. Cat γ chain detected with monoclonal anti-IgG (GPB2-2). Lane 3. Purified cat IgG. Cat γ chain detected with polyclonal anti-IgG (04-20-02). Lane 4. Purified hyena IgM. Hyena μ chain detected with monoclonal anti-IgG (CM7). Lane 5. Purified cat IgM. Cat μ chain detected with monoclonal anti-IgM (CM7). Lane 6. Purified cat IgA. Cat α chain detected with polyclonal anti-IgA (NB7264). Lane 7. Purified hyena IgA. Hyena α chain detected with polyclonal anti-IgA (NB7264). Lane 8. Cat serum with albumin removed. Cat light chain detected with monoclonal anti-λ light chain (CAG8-7C). Lane 9. Cat serum with albumin removed. Cat light chain detected with monoclonal anti- κ light chain (FIG1-7A). Lane 10. Hyena serum with albumin removed. Hyena light chain detected with monoclonal anti- κ light chain (FIG1-7A).
Table 3.
Molecular weight (kDa) of heavy and light chains.
Anti-κ light chain mAb detected a 23–25 kDa light chain protein band in cat serum samples and a 22–25 kDa band in hyena serum samples. The molecular weight and staining patterns of the anti-κ light chain mAb are consistent with the previously reported molecular weight of carnivore light chains (Grant, 1995, Klotz et al., 1985, Yamada et al., 2007). See Table 3 for a complete listing of molecular weights of feline and hyena heavy and light chains. Anti-λ light chain mAb detected a 23–28 kDa protein band in cat serum, but no bands were detectable in hyena serum or purified Ig.
3.2. Quantification of humoral response using ELISA
During the weeks after experimental immunization of captive hyenas, anti-DNP specific antibodies in serum samples increased from pre-immunization day 0 titers for all isotypes tested (Fig. 2 ). As expected, day 14 serum samples were elevated above baseline, and peak anti-DNP concentrations in serum samples were attained on day 28 post-immunization for most individuals and isotypes. Individuals with the strongest anti-DNP response still had detectable titers at one year post-immunization.
Fig. 2.
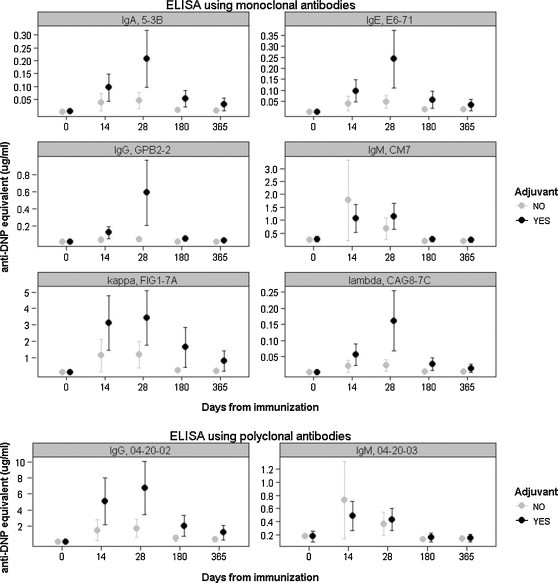
ELISA results from DNP-KLH immunization. Each point corresponds to the mean anti-DNP equivalent concentration (μg/ml) and error bars represent the SEM (n = 4). Black points are the means from individuals immunized with adjuvant and gray points are from individuals immunized without adjuvant. Day 0 represents pre-immune sera and baseline anti-DNP concentration found in serum samples.
The anti-DNP temporal dynamics for IgG and IgM were found to be similar regardless of whether we used polyclonal Abs or mAbs as anti-isotype probes. Individuals that had the highest IgG or IgM titers using mAb detection antibodies also had the highest titers using polyclonal antibodies for detection, and peak titers were reached on day 28 in most cases. Anti-IgM had the highest anti-DNP equivalent concentration for all individuals on day 0, suggesting that natural IgM antibodies to DNP were present prior to immunization (Ochsenbein and Zinkernagel, 2000). Among the anti-Ig mAbs tested, the anti-κ light chain detection antibody produced the highest anti-DNP equivalent concentrations; light chains are associated with all isotypes, so light chains are represented in higher concentrations than heavy chains.
Not surprisingly, use of adjuvant tended to elevate anti-DNP equivalent concentrations over those obtained with no adjuvant, although no statistical tests on adjuvant effect were performed due to small sample sizes. Interestingly, however, the four youngest individuals immunized attained the four highest anti-DNP titers across all isotypes tested, regardless of whether or not they received adjuvant with the initial immunization (Table 2). The oldest hyena, individual H, was 17 years old at the initial immunization and did not receive adjuvant. Individual H did not produce a detectable response at a 1:100 serum dilution using mAb detection antibodies for IgG and λ, but did produce detectable responses for all other isotypes tested.
3.3. Temporal dynamics of the IgG and IgM relationship
Using serum diluted 1:1000 with polyclonal IgM detection Ab or serum diluted 1:4000 with polyclonal IgG detection Ab, we calculated a standardized percent of IgG and IgM out of the sum total of IgG and IgM (percent IgG = IgG/(IgG + IgM)). On day 0, anti-DNP IgG accounted for roughly 10% of the sum of IgG and IgM (Fig. 3 ). However, by days 14 and 28, IgG accounted for approximately 70% and 80% of the total, respectively. At days 180 and 365, IgG continued to account for more than 50% of the total anti-DNP IgG and IgM.
Fig. 3.
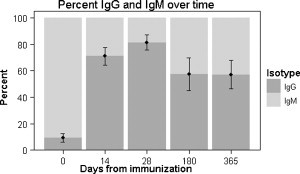
Percent of total anti-DNP IgG and anti-DNP IgM. Percent IgG = IgG/(IgG + IgM). Percent IgM = IgM/(IgG + IgM). Serum was diluted 1:4000 for IgG and 1:1000 for IgM. Polyclonal detection antibodies were used at 0.5 μg/μl for both IgG and IgM. Error bars represent the SEM of the percents for each day in the time course (n = 8). Individuals were included regardless of adjuvant status.
4. Discussion
Here we identified cross-reactive antibodies that specifically bind hyena epitopes of the four major secreted heavy chain isotypes found in carnivores, as well as two cross-reactive anti-light chain antibodies. The specific cross-reactivity of anti-cat Ig isotypes suggests a high level of homology between cats and hyenas. These antibodies can potentially be used for broad, cost effective monitoring of pathogen infections in spotted hyenas, and to establish baseline parameters of health and disease among wild members of this species throughout sub-Saharan Africa and among captive hyenas at zoos and research facilities. Additionally, these antibodies can likely be used to detect immunoglobulins in other species in the Hyaenidae family.
We confirmed binding of the antibodies to hyena immunoglobulins here with Western blots, and molecular weights were found to be close to that of cat immunoglobulins for each isotype examined. When we then used the antibodies to monitor the magnitude and temporal dynamics of humoral responses directed against the hapten-carrier complex DNP-KLH in immunized captive spotted hyenas, we observed the common pattern of an increased Ig concentration for all isotypes by day 14, with IgM concentration tapering off quickly and a gradual decrease in other isotypes over the course of one year. Thus, insofar as neither the magnitude nor temporal dynamics of the humoral response were unusual in spotted hyenas, these responses are unlikely to account for the unusual disease resistance observed in this species.
We were only able to identify one anti-IgG mAb that cross-reacted with hyena epitopes, despite identifying several cross-reacting polyclonal anti-IgG antibodies. Furthermore, this anti-IgG mAb showed either weak affinity for hyena IgG or was specific for an IgG subclass that is less abundant in hyenas than in cats. However, it will now be possible to develop anti-hyena IgG subclass-specific mAbs using the purified hyena IgG. Variation in Ab structure among species is often most pronounced in subclasses of the major isotypes (Grant, 1995), and differentiation between IgG2a and IgG1 would aid in the study of Th1 and Th2 subsets (Mosmann and Coffman, 1989, Steinman, 2007).
Interestingly, both ELISA and Western blot detected κ light chains at much higher concentrations than λ light chains in hyena sera. Generally, carnivores have approximately 90% λ and 10% κ light chains (Tizard, 2009); more specifically, cats have been reported to have a 3:1 ratio of λ:κ using the same antibodies we used in this study (Grant, 1995). It is possible that the reversed light chain ratio we observed in hyenas in this study is due to low affinity of the anti-λ light chain cat antibody for hyena immunoglobulins. However, the alternative possibility, that hyenas have a reversed light chain ratio compared to that found in most carnivores, merits further investigation.
To our knowledge, only three previous studies have reported cross-reacting antibodies that recognize immunoglobulins from any hyena species. First, anti-human IgA and IgM were found to cross-react with IgA and IgM from striped hyenas (Hyaena hyaena) using a gel diffusion assay (Neoh et al., 1973). Second, a polyclonal anti-cat IgG was used in an indirect immunofluorescence assay for diagnosis of coronavirus infection in spotted hyenas in Tanzania (East et al., 2004). Finally, polyclonal anti-cat IgG was used for Western blot detection of feline immunodeficiency virus (FIV) in spotted hyenas (Troyer et al., 2005). Two previous studies also used commercially available, Ab based test kits for confirmatory diagnosis of coronavirus and FIV infections (East et al., 2004, Harrison et al., 2004).
There are significant advantages of using hyena specific antibodies over other existing techniques for evaluating pathogen-specific responses. Most studies of pathogens infecting hyenas have relied on serum neutralization tests, agglutination assays, RT-PCR, or competitive ELISAs that do not require species-specific antibodies (Alexander et al., 1995, Cronwright-Snoeren, 2010, East et al., 2001, Harrison et al., 2004). Each of these existing techniques is limited in its usefulness by various factors, and use of the newly discovered antibodies described here would avoid many of these limitations. Serum neutralization tests rely on cell culture, which can be labor- and resource-intensive (Wellehan et al., 2009). Positive RT-PCR results for pathogens provide clear evidence of infection, but offer little information about duration of the infection or the nature of the immune response. Additionally, RT-PCR can only detect active infections. Agglutination tests are rapid and cost-effective, but are most sensitive to pentameric IgM and less sensitive to other isotypes (Cohen et al., 1967). Competitive ELISAs and serum neutralization tests requiring no species-specific antibodies are limited because they permit no analysis of specific immunoglobulin isotypes. Rapid diagnosis using ELISA techniques can aid wildlife managers and veterinarians by permitting them to quickly diagnose disease outbreaks, and allowing them to develop better-informed responses to such outbreaks.
4.1. Potential uses of the new antibodies
We found that hyena antibodies can be detected in serum at least one year after exposure, long after many pathogens would be cleared from the host. Testing for both IgG and IgM has been used previously to stage infections; this method was used to assess whether infections are in earlier or later stages for tularemia (Carlsson et al., 1979), dengue (Innis et al., 1989), Rift Valley Fever (Pepin et al., 2010), myxomatosis in rabbits (Oryctolagus cuniculs) (Kerr, 1997), and West Nile virus in equids (Durand et al., 2002). Pathogens to which wild spotted hyenas are known to be exposed include CDV, FIV, feline panleukopenia virus/canine parvovirus, feline coronavirus/feline infectious peritonitis virus, feline calicivirus, rabies, and bluetongue (Alexander et al., 1994, East et al., 2001, Harrison et al., 2004, Troyer et al., 2005). The temporal dynamics apparent in the changing Ig ratios (Fig. 3) in our hyena subjects might be carefully exploited to stage infections. However, we emphasize that samples positive for IgM, but negative for IgG, must be interpreted cautiously, as this might indicate either an early stage infection or simply the presence of natural antibodies (Ochsenbein and Zinkernagel, 2000). The results of our experimental immunizations of captive hyenas show that all individuals had readily detectable anti-DNP IgM in pre-immune sera. Pre-infection sera are seldom available in serological studies of wildlife, so natural IgM might be misinterpreted as an active infection based solely on agglutination tests. Thus, ELISA or Western blots for IgG, IgA, and/or IgE in addition to assays for IgM are more effective for serological studies than agglutination tests alone.
In addition to facilitating the monitoring of pathogen exposure in spotted hyenas, the cross-reacting antibodies identified in our study will also provide researchers with tools for assessing maternal immunoglobulins in milk. Hyenas cubs are weaned roughly 14 months after birth, a lactation interval far longer than that of most other carnivores (Hofer and East, 1995, Watts et al., 2009). Within 2–5 weeks of birth, spotted hyena cubs are transferred from an isolated natal den to a communal den where they live with up to 20 other cubs, and frequently engage in playful and aggressive interactions (Holekamp and Smale, 1998, Kruuk, 1972, Tanner et al., 2007). The frequent social interactions at communal dens create a situation that can lead to high rates of transmission of infectious pathogens (Altizer et al., 2003). Maternal IgG and IgA transferred from mother to offspring in milk could provide critical protection from pathogens during early life stages (Bourne and Curtis, 1973, Brambell, 1966, Claus et al., 2006, Mason et al., 1930).
IgG and IgM are commonly the focus of immunological studies, however, IgA is the primary Ab on mucosal surfaces, and in humans more IgA is produced than all other isotypes combined (Brandtzaeg et al., 1999, Fagarasan and Honjo, 2003, Kerr, 1990, van Egmond et al., 2001). Rabies virus has been detected in saliva of spotted hyenas expressing no clinical signs of disease (East et al., 2001); salivary IgA might play an important role in both intra- and inter-specific transmission of rabies by neutralizing the virus in saliva before the virus has a chance to infect new individuals. Furthermore, fecal samples are the most readily available and least invasive samples available in most studies of free-living carnivores, and these typically contain large quantities of IgA. Analysis of fecal IgA can thus potentially provide a glimpse into the gut and mucosal immune systems of wild hyenas.
Despite the common use of fecal parasite counts in wildlife studies (e.g. Engh et al., 2003, Gompper et al., 2003, Patton et al., 1986, Watve and Sukumar, 1995), few studies have complemented these counts with analysis of fecal IgA concentrations or serum IgE concentrations (Devalapalli et al., 2006, Gompper et al., 2003). IgE is the primary isotype involved in defense against parasitic worms. Our study has identified two cross-reacting mAb against hyena IgE in ELISAs (Table 1) that might be used to assess effects of parasites on Ig concentrations and ratios. However, the specific binding of the anti-IgE mAbs could not be confirmed here by Western blot, possibly due to the low concentration and short half-life of IgE in serum. IgE and IgG1 are associated with Th2 response (Mosmann and Coffman, 1989, Mosmann and Sad, 1996, Romagnani, 1997), a topic that is ripe for a comparative study. For example, it would be interesting to compare Th1 and Th2 markers in captive hyenas living in relatively clean environments with wild hyenas that face a continual assault from food-borne and socially transmitted pathogens.
In summary, we have identified Ig-class specific antibodies that specifically recognize hyena epitopes. The antibodies identified here can be used for monitoring the health of both wild and captive hyenas. We have shown that the cross-reacting antibodies can be used to assess specific antibody titer by ELISA; it is probable that these antibodies can also be used in Western blots for detecting antibodies against specific pathogens, which is commonly done to confirm ELISA or RT-PCR results. Finally, these basic immunology tools open the door to more advanced studies of immune function in a species that has demonstrated the remarkable ability to survive disease outbreaks that have decimated wild populations of other carnivore species living sympatrically with spotted hyenas.
Conflict of interest statement
All monoclonal antibodies were provided by Custom Monoclonals International Corp. Co-author Chris K. Grant is President of Custom Monoclonals International Corp. and he contributed in both design and performance of the study.
Acknowledgements
We would like to thank the Dr. Stephen E. Glickman and the staff of the Field Station for Behavioral Research at UC Berkeley for their assistance with maintenance and immunizations of captive hyenas, Dr. Jean Tsao for her ideas, advice and support on this project, Dr. Julia Bell for her advice on laboratory procedures, and Dr. Barry Williams for his comments on the research. This work was supported by Award No. W911NF-08-1-0310 from the Army Research Office and NSF Grant IOS0819437 to KEH, NSF Grant IOS0809914 to Stephen E. Glickman, NIH grant K26RR023080 to LSM, grants-in-aid-of research from Sigma Xi and the American Society of Mammalogists to ASF, veterinary student scholars program from the Morris Animal Foundation, and a NSF Graduate Research Fellowship to ASF.
References
- Alexander K., Appel M. African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. Journal of Wildlife Diseases. 1994;30:481. doi: 10.7589/0090-3558-30.4.481. [DOI] [PubMed] [Google Scholar]
- Alexander K.A., Kat P.W., Frank L.G., Holekamp K.E., Smale L., House C., Appel M.J.G. Evidence of canine distemper virus infection among free-ranging spotted hyenas (Crocuta crocuta) in the Masai Mara, Kenya. Journal of Zoo and Wildlife Medicine. 1995;26:201–206. [Google Scholar]
- Alexander K.A., Maclaughlin N.J., Kat P.W., House C., O’Brien S.J., Lerche N.W., Sawyer M., Frank L.G., Holekamp K.E., Smale L., McNutt J.W., Laurenson M.K., Mills M.G.L., Osburn B.I. Evidence of natural bluetongue virus infection among African and Indian carnivores. American Journal of Tropical Medicine and Hygiene. 1994:51. [PubMed] [Google Scholar]
- Altizer S., Nunn C.L., Thrall P.H., Gittleman J.L., Antonovics J., Cunningham A.A., Cunnningham A.A., Dobson A.P., Ezenwa V., Jones K.E. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology, Evolution, and Systematics. 2003;34:517–547. [Google Scholar]
- Berger D., Frank L., Glickman S. Unraveling ancient mysteries: biology, behavior, and captive management of the spotted hyena, Crocuta crocuta. Proceedings of the Joint Conference of the American Association of Zoo Veterinarians and the American Association of Wildlife Veterinarians; Oakland, CA, November 15–19; 1992. pp. 139–147. [Google Scholar]
- Bininda-Emonds O.R.P., Gittleman J.L., Purvis A. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia) Biological Reviews. 1999;74:143–175. doi: 10.1017/s0006323199005307. [DOI] [PubMed] [Google Scholar]
- Blount J., Houston D., Moller A., Wright J. Do individual branches of immune defence correlate?. A comparative case study of scavenging and non-scavenging birds. Oikos. 2003;102:340–350. [Google Scholar]
- Bourne F., Curtis J. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 1973;24:157. [PMC free article] [PubMed] [Google Scholar]
- Brambell F. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Baekkevold E.S., Farstad I.N., Jahnsen F.L., Johansen F.-E., Nilsen E.M., Yamanaka T. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunology Today. 1999;20:141–151. doi: 10.1016/s0167-5699(98)01413-3. [DOI] [PubMed] [Google Scholar]
- Carlsson H.E., Lindberg A.A., Lindberg G., Hederstedt B., Karlsson K.-A., Agell B.O. Enzyme-Linked Immunosorbent Assay for Immunological Diagnosis of Human Tularemia. Journal of Clinical Microbiology. 1979;10:615–621. doi: 10.1128/jcm.10.5.615-621.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude C. Parasites, biodiversity and ecosystem stability. Biodiversity and Conservation. 1996;5:953–962. [Google Scholar]
- Claus M.A., Levy J.K., MacDonald K., Tucker S.J., Crawford P.C. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. Journal of Feline Medicine & Surgery. 2006;8:184–191. doi: 10.1016/j.jfms.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I.R., Norins L.C., Julian A.J. Competition between, and effectiveness of, IgG and IgM antibodies in indirect fluorescent antibody and other tests. The Journal of Immunology. 1967;98:143–149. [PubMed] [Google Scholar]
- Cronwright-Snoeren A. University of Pretoria; 2010. The Prevalence of Canine Distemper Virus Antibodies in Wild Carnivores in the Kruger National Park and Marakele National Park. [Google Scholar]
- Devalapalli A.P., Lesher A., Shieh K., Solow J.S., Everett M.L., Edala A.S., Whitt P., Long R.R., Newton N., Parker W. Increased Levels of IgE and autoreactive, polyreactive IgG in wild rodents: implications for the hygiene hypothesis. Scandinavian Journal of Immunology. 2006;64:125–136. doi: 10.1111/j.1365-3083.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- Durand B., Chevalier V., Pouillot R., Labie J., Marendat I., Murgue B., Zeller H., Zientara S. West Nile virus outbreak in horses, southern France, 2000: results of a serosurvey. Emerging Infectious Diseases. 2002;8:777. doi: 10.3201/eid0808.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M., Moestl K., Benetka V., Pitra C., Höner O.P., Wachter B., Hofer H. Coronavirus infection of spotted hyenas in the Serengeti ecosystem. Veterinary Microbiology. 2004;102:1–9. doi: 10.1016/j.vetmic.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M.L., Hofer H., Cox J.H., Wulle U., Wiik H., Pitra C. Proceedings of the National Academy of Sciences of the United States of America 98. 2001. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas; pp. 15026–15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East M.L., Wibbelt G., Lieckfeldt D., Ludwig A., Goller K., Wilhelm K., Schares G., Thierer D., Hofer H. A Hepatozoon species genetically distinct from H. canis infecting spotted hyenas in the Serengeti Ecosystem, Tanzania. Journal of Wildlife Diseases. 2008;44:45–52. doi: 10.7589/0090-3558-44.1.45. [DOI] [PubMed] [Google Scholar]
- Engh A., Nelson K., Peebles R., Hernandez A., Hubbard K., Holekamp K. Coprologic survey of parasites of spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve, Kenya. Journal of Wildlife Diseases. 2003;39:224–227. doi: 10.7589/0090-3558-39.1.224. [DOI] [PubMed] [Google Scholar]
- Fagarasan S., Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nature Reviews Immunology. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- Gompper M.E., Goodman R.M., Kays R.W., Ray J.C., Fiorello C.V., Wade S.E. A survey of the parasites of coyotes (Canis latrans) in New York based on fecal analysis. Journal of Wildlife Diseases. 2003;39:712. doi: 10.7589/0090-3558-39.3.712. [DOI] [PubMed] [Google Scholar]
- Grant C.K. Purification and characterization of feline IgM and IgA isotypes and three subclasses of IgG. In: Willett B.J., Jarrett O., editors. Feline Immunology and Immunodeficiency. 1995. pp. 95–107. [Google Scholar]
- Hanley C.S., Simmons H.A., Wallace R.S., Clyde V.L. Erythema Multiforme in a spotted hyena (Crocuta crocuta) Journal of Zoo and Wildlife Medicine. 2005;36:515–519. doi: 10.1638/04-077.1. [DOI] [PubMed] [Google Scholar]
- Harrison T.M., Mazet J.K., Holekamp K.E., Dubovi E., Engh A.L., Nelson K., Van Horn R.C., Munson L. Antibidies to canine and feline viruses in spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve. Journal of Wildlife Diseases. 2004;40:1–10. doi: 10.7589/0090-3558-40.1.1. [DOI] [PubMed] [Google Scholar]
- Hofer H. Spotted hyaena (Crocuta crocuta) In: Mills M.G.L., Mills G., Hofer H., editors. Hyaenas: Status Survey and Conservation Action Plan. World Conservation Union; Gland, Switzerland: 1998. pp. 29–38. [Google Scholar]
- Hofer H., East M. Serengeti. II. Dynamics, Management, and Conservation of an Ecosystem. 1995. Population dynamics, population size, and the commuting system of Serengeti spotted hyenas. pp. 332–363. [Google Scholar]
- Holekamp K.E., Sisk C.L. Effects of dispersal status on pituitary and gonadal function in the male spotted hyena. Hormones and Behavior. 2003;44:385–394. doi: 10.1016/j.yhbeh.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Holekamp K.E., Smale L. Behavioral development in the spotted hyena. BioScience. 1998;48:997–1005. [Google Scholar]
- Honer O., Wachter B., Speck S., Wibbelt G., Ludwig A., Fyumagwa R., Wohlsein P., Lieckfeldt D., Hofer H., East M. Severe Streptococcus infection in spotted hyenas in the Ngorongoro Crater, Tanzania. Veterinary Microbiology. 2006;115:223–228. doi: 10.1016/j.vetmic.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Houston D.C. The adaptations of scavengers. In: Sinclair A.R.E., Norton-Griffiths M., editors. Serengeti: Dynamics of an Ecosystem. University of Chicago Press; Chicago: 1979. pp. 263–286. [Google Scholar]
- Innis B.L., Nisalak A., Nimmannitya S., Kusalerdchariya S., Chongswasdi V., Suntayakorn S., Puttisri P., Hoke C.H. An enzyme-linked immunosorbent assay to characterize Dengue infections where Dengue and Japanese Encephalitis co-circulate. American Journal of Tropical Medicine and Hygiene. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- Kat P.W., Alexander K.A., Smith J.S., Munson L. Rabies and African wild dogs in Kenya. Proceedings: Biological Sciences. 1995;262:229–233. doi: 10.1098/rspb.1995.0200. [DOI] [PubMed] [Google Scholar]
- Kat P.W., Alexander K.A., Smith J.S., Richardson J.D., Munson L. Rabies among African wild dogs (Lycaon pictus) in the Masai Mara, Kenya. Journal of Veterinary Diagnostic Investigation. 1996;8:420. doi: 10.1177/104063879600800403. [DOI] [PubMed] [Google Scholar]
- Kerr M. The structure and function of human IgA. Biochemical Journal. 1990;271:285. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. An ELISA for epidemiological studies of myxomatosis: persistence of antibodies to myxoma virus in European rabbits (Oryctolagus cuniculus) Wildlife Research. 1997;24:53–65. [Google Scholar]
- Klotz F., Gathings W., Cooper M. Development and distribution of B lineage cells in the domestic cat: analysis with monoclonal antibodies to cat mu-, gamma-, kappa-, and lambda-chains and heterologous anti-alpha antibodies. Journal of Immunology. 1985;134:95–100. [PubMed] [Google Scholar]
- Kruuk H. University of Chicago Press; 1972. The Spotted Hyena: A Study of Predation and Social Behaviour. [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lembo T., Hampson K., Auty H., Beesley C.A., Bessell P., Packer C., Halliday J., Fyumagwa R., Hoare R., Ernest E. Serologic surveillance of Anthrax in the Serengeti ecosystem, Tanzania, 1996–2009. Emerging Infectious Diseases. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Werdelin L. The evolution of spotted hyenas (Crocuta crocuta) IUCN Hyaena Specialist Group Newsletter. 2000;7:34–36. [Google Scholar]
- Mason J., Dalling T., Gordon W. Transmission of maternal immunity. The Journal of Pathology and Bacteriology. 1930;33:783–797. [Google Scholar]
- Mendes L., Piersma T., Hasselquist D., Matson K.D., Ricklefs R.E. Variation in the innate and acquired arms of the immune system among five shorebird species. Journal of Experimental Biology. 2006;209:284–291. doi: 10.1242/jeb.02015. [DOI] [PubMed] [Google Scholar]
- Mosmann T., Coffman R. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Munson L., Terio K.A., Kock R., Mlengeya T., Roelke M.E., Dubovi E., Summers B., Sinclair A.R.E., Packer C. Climate extremes promote fatal co-infections during canine distemper epidemics in African Lions. PLoS ONE. 2008;3:e2545. doi: 10.1371/journal.pone.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoh S.H., Jahoda D.M., Rowe D.S., Voller A. Immunoglobulin classes in mammalian species identified by cross-reactivity with antisera to human immunoglobulin. Immunochemistry. 1973;10:805–813. doi: 10.1016/0019-2791(73)90184-5. [DOI] [PubMed] [Google Scholar]
- O’Brien S.J., Johnson W.E. Big cat genomics. Annual Review of Genomics and Human Genetics. 2005;6:407–429. doi: 10.1146/annurev.genom.6.080604.162151. [DOI] [PubMed] [Google Scholar]
- Ochsenbein A., Zinkernagel R. Natural antibodies and complement link innate and acquired immunity. Trends in Immunology. 2000;21:624–629. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- Patton S., Rabinowitz A., Randolph S., Johnson S.S. A coprological survey of parasites of wild neotropical felidae. The Journal of Parasitology. 1986:517–520. [PubMed] [Google Scholar]
- Pepin M., Bouloy M., Bird B.H., Kemp A., Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Veterinary Research. 2010:41. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . In: Computing R.F.f.S., editor. R Development Core Team; Vienna, Austria: 2011. (R: A Language and Environment for Statistical Computing). [Google Scholar]
- Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S.J., Pospischil A., Hofmann-Lehmann R., Lutz H. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm. Immunology Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- Schulenburg H., Kurtz J., Moret Y., Siva-Jothy M.T. Introduction. Ecological immunology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3–14. doi: 10.1098/rstb.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S., Höner O.P., Wachter B., Fickel J. Characterization of Streptococcus equi subsp. ruminatorum isolated from spotted hyenas (Crocuta crocuta) and plains zebras (Equus burchelli), and identification of a M-like protein (SrM) encoding gene. Veterinary Microbiology. 2008;128:148–159. doi: 10.1016/j.vetmic.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Medicine. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Steward M., Petty R. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972;22:747. [PMC free article] [PubMed] [Google Scholar]
- Tanner J., Smale L., Holekamp K. Ontogenetic variation in the play behavior of spotted hyenas. Journal of Developmental Processes. 2007;2:5–30. [Google Scholar]
- Tizard I. 8th ed. Elsevier Inc; 2009. Veterinary Immunology:An Introduction. [Google Scholar]
- Troyer J., Pecon-Slattery J., Roelke M., Johnson W., VandeWoude S., Vazquez-Salat N., Brown M., Frank L., Woodroffe R., Winterbach C. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. The Journal of Virology. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Egmond M., Damen C.A., van Spriel A.B., Vidarsson G., van Garderen E., van de Winkel J.G.J. IgA and the IgA Fc receptor. Trends in Immunology. 2001;22:205–211. doi: 10.1016/s1471-4906(01)01873-7. [DOI] [PubMed] [Google Scholar]
- van Helden P.D., van Pittius N.C.G., Warren R.M., Michel A., Hlokwe T., Morar D., Godfroid J., du Plessis E.C., Bengis R. Pulmonary infection due to Mycobacterium goodii in a Spotted Hyena (Crocuta crocuta) from South Africa. Journal of Wildlife Diseases. 2008;44:151–154. doi: 10.7589/0090-3558-44.1.151. [DOI] [PubMed] [Google Scholar]
- Watts H.E., Holekamp K.E. Ecological determinants of survival and reproduction in the Spotted Hyena. Journal of Mammalogy. 2009;90:461–471. [Google Scholar]
- Watts H.E., Tanner J.B., Lundrigan B.L., Holekamp K.E. Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2291–2298. doi: 10.1098/rspb.2009.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watve M.G., Sukumar R. Parasite abundance and diversity in mammals: correlates with host ecology. Proceedings of the National Academy of Sciences. 1995;92:8945–8949. doi: 10.1073/pnas.92.19.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellehan J.F.X., Jr., Green L.G., Duke D.G., Bootorabi S., Heard D.J., Klein P.A., Jacobson E.R. Detection of specific antibody responses to vaccination in variable flying foxes (Pteropus hypomelanus) Comparative Immunology, Microbiology and Infectious Diseases. 2009;32:379–394. doi: 10.1016/j.cimid.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdelin L. Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia: Canidae) Paleobiology. 1989;15:387–401. [Google Scholar]
- Yamada O., Tamura K., Yagihara H., Isotani M., Azakami M., Sawada S., Ono K., Washizu T., Bonkobara M. Light-chain multiple myeloma in a cat. Journal of Veterinary Diagnostic Investigation. 2007;19:443–447. doi: 10.1177/104063870701900421. [DOI] [PubMed] [Google Scholar]


