Abstract
We have previously reported that lead (Pb2+) exposure results in both presynaptic and postsynaptic changes in developing neurons as a result of inhibition of the N-methyl-D-aspartate receptor (NMDAR). NMDAR inhibition by Pb2+ during synaptogenesis disrupts downstream trans-synaptic signaling of brain-derived neurotrophic factor (BDNF) and exogenous addition of BDNF can recover the effects of Pb2+ on both presynaptic protein expression and presynaptic vesicular release. NMDAR activity can modulate other trans-synaptic signaling pathways, such as nitric oxide (NO) signaling. Thus, it is possible that other trans-synaptic pathways in addition to BDNF signaling may be disrupted by Pb2+ exposure. The current study investigated whether exogenous addition of NO could recover the presynaptic vesicular proteins lost as a result of Pb2+ exposure during synaptogenesis, namely Synaptophysin (Syn) and Synaptobrevin (Syb). We observed that exogenous addition of NO during Pb2+ exposure results in complete recovery of whole-cell Syn levels and partial recovery of Syn and Syb synaptic targeting in Pb2+-exposed neurons.
Keywords: Pb2+, Nitric oxide, synaptophysin, synaptobrevin, synaptogenesis, hippocampal neurons
1. Introduction
Lead (Pb2+) is a ubiquitous environmental neurotoxicant which causes cognitive and behavioral deficits in exposed children and disrupts spatial learning tasks in animal models (Jusko et al., 2008; Lanphear et al., 2005; Toscano and Guilarte, 2005). These neurological effects of Pb2+ exposure are believed to be mediated by its interaction with the N-methyl-D-aspartate receptor (NMDAR). Pb2+ is a potent inhibitor of the NMDAR (Alkondon et al., 1990; Guilarte and Miceli, 1992; Paoletti et al., 2000; Rachline et al., 2005), which is essential for spatial memory processes in the hippocampus (Bliss and Collingridge, 1993; Lynch, 2004).
The NMDAR is a heteromultimeric ion channel composed of an obligatory NR1 subunit and accessory NR2 or NR3 subunits. In the developing hippocampus, NR2A and NR2B are the predominant accessory NMDAR subunits and exhibit differential expression during development. Early in hippocampal development, NR2B subunits predominate while NR2A subunits become increasingly incorporated as development progresses (Monyer et al., 1994). However, in rodent models of chronic developmental Pb2+ exposure, there is decreased protein and mRNA levels of NR2A (Guilarte and McGlothan, 1998; Nihei et al., 2000; Nihei and Guilarte, 1999; Zhang et al., 2002) and increased levels of NR2B-containing NMDARs (NR2B-NMDARs) (Toscano et al., 2002). Furthermore, we have recently shown that exposure to Pb2+ during synaptogenesis in primary hippocampal neurons results in decreased levels of synaptic NR2A-containing NMDARs (NR2A-NMDARs) and an increase in NR2B-NMDARs (Neal et al., 2011; Neal and Guilarte, 2010). This change in NMDAR subunit ontogeny, detected in both animal and cell culture models of Pb2+ exposure, suggests that Pb2+ exposure arrests or delays the critical developmental switch from NR2B- to NR2A-NMDARs (Neal et al., 2011; Neal and Guilarte, 2010; Toscano and Guilarte, 2005)
Disruption of normal NMDAR ontogeny can have consequences on neuronal signaling. NMDARs exhibit distinct downstream signaling based on subunit composition. NR2A-NMDARs are responsible for activating pro-survival pathways including cyclic AMP response element binding protein (CREB)-mediated signaling and increasing expression of the neurotrophin brain-derived neurotrophic factor (BDNF). Alternately, NR2B-receptors are responsible for activating pro-death pathways and CREB shutoff (Hardingham et al., 2002; Ivanov et al., 2006; Soriano et al., 2008; Vanhoutte and Bading, 2003). Thus, altered NMDAR subunit composition due to chronic Pb2+ exposure may result in changes in intracellular signaling. This hypothesis is supported by evidence of altered MAPK signaling (Cordova et al., 2004), calcium/calmodulin kinase II (CamKII) activity (Toscano et al., 2005), and CREB phosphorylation status and binding affinity in the hippocampi of animals developmentally exposed to Pb2+ (Toscano et al., 2003; Toscano et al., 2002).
NMDAR activity has been linked to the production and release of BDNF (Hartmann et al., 2001; Jiang et al., 2005; Walz et al., 2006), a trans-synaptic signaling molecule implicated in presynaptic plasticity and development (Cohen-Cory et al., 2010). Postsynaptically-derived BDNF can stimulate glutamate release and stabilize nascent synapses (Magby et al., 2006; Walz et al., 2006), and in the absence of BDNF vesicular protein levels are reduced and vesicular release is impaired (Hu et al., 2005; Pozzo-Miller et al., 1999). We have previously shown that Pb2+ exposure during synaptogenesis in rat hippocampal neurons results in a selective decrease in the levels of Synaptobrevin (Syb) and Synaptophysin (Syn) (Neal et al., 2010), two proteins involved in vesicular release and recycling (Daly and Ziff, 2002; Deak et al., 2004; Pennuto et al., 2003; Schoch et al., 2001), which is consistent with interruption of NMDAR-dependent BDNF signaling (Hu et al., 2005; Pozzo-Miller et al., 1999). The loss of these proteins results in significant impairments in vesicular release (Neal et al., 2010), and may underlie the altered neurotransmission reported in animals chronically exposed to Pb2+ (Gilbert et al., 1999). Furthermore, exogenous addition of BDNF remediated presynaptic effects of Pb2+, suggesting that disruption of NMDAR activity-dependent BDNF signaling is responsible for some of the effects of Pb2+ exposure on presynaptic plasticity (Neal et al., 2010).
Nitric oxide (NO) production has also been linked to NMDAR activity. Nitric oxide synthase (nNOS) has been shown to be associated with both the NR2A and NR2B NMDAR subunits, and is activated by NMDAR activity-dependent phosphorylation (Al Hallaq et al., 2007; Christopherson et al., 1999; Rameau et al., 2007; Rameau et al., 2004). Increased nNOS activity increases the levels of NO, which can act locally in the postsynaptic density or diffuse to the presynaptic active zone to stimulate soluable guanylyl cyclase (sGC) and increase cyclic GMP production (Arancio et al., 1996). Cyclic GMP signaling has been associated with enhanced neurotransmitter release, increased release volume (Arancio et al., 1996; O'Dell et al., 1991; Steinert et al., 2008), and increased Syn expression at glutamatergic synapses in vitro (Wang et al., 2005) and in vivo (Ota et al, 2010). Thus, disruption of NMDAR-dependent NO signaling by Pb2+ may account for some of the presynaptic changes associated with chronic Pb2+ exposure. The current studies were undertaken to determine whether exogenous addition of NO could recover presynaptic protein levels lost as a result of Pb2+ exposure during synaptogenesis. We observed that exogenous addition of NO for the final 24 hours of Pb2+ exposure in primary hippocampal neurons fully recovered Syn whole-cell levels but did not remediate the effects of Pb2+ on the synaptic targeting of Syn and Syb.
2. Results
In the current study we used a primary hippocampal culture system as described previously (Neal et al, 2011; Neal et al, 2010). Briefly, hippocampi were removed from E18 rat embryos and grown in culture for seven days (DIV7), at which point they were exposed to either vehicle- or 1.0 μM Pb2+-containing feeding media. Pb2+ exposure lasted for 5 days and cells were harvested on DIV12. The current work was originally undertaken at the same time as our previously published studies on the effect of exogenous addition of 25 ng/mL BDNF for the final 24 hours of Pb2+ exposure (Neal et al., 2010). The present work is focused on sister experiments on the effect of exogenous NO for the final 24 hours of Pb2+ exposure using the NO donor, DETA NONOate (DETA).
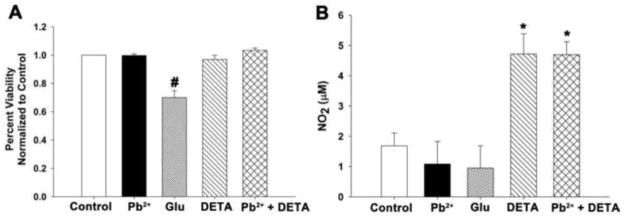
We first determined that exposure to neither 1.0 μM Pb2+ nor 10 μM DETA resulted in a loss of neuron viability (Figure 1A). Cultures treated with Pb2+ and/or DETA exhibited similar viability relative to control. We verified that DETA spontaneously released NO by assessing the levels of stable NO decomposition products with the Greiss reaction (Figure 1B), which is a colorimetric assay designed to detect the levels of nitrite in biological media (Green et al., 1982). 10 μM DETA significantly increased the levels of NO decomposition products in both control- and Pb2+-treated cultures (p<0.01). We observed that control cultures treated with 10 μM DETA for 24 hours experienced a rise in nitrite levels from 1.7 ± 0.4 μM to 4.7 ± 0.7 μM and Pb2+-exposed cultures experienced a rise from 1.1 ± 0.7 μM to 4.7 ± 0.4 μM. Thus, incubation with 10 μM DETA for the final 24 hours of Pb2+ exposure increased the levels of NO present by about 3-fold but did not cause a reduction in cell viability for either control or Pb2+-treated cultures.
Figure 1. DETA NONOate added to neuronal culture media for the final 24 hours of Pb2+ exposure spontaneously releases NO and does not affect cell viability.
(A) DETA (10 μM) did not cause a loss in cell viability as assessed by the MTS assay. Cell death was only observed with the excitotoxic concentration (100 μM) of glutamate (Glu). Data are the mean ± SEM and are the result of 4 assays. (B) During the 24 hr treatment period DETA supplementation resulted in significantly elevated levels of NO2 confirming increased NO release. Data are the mean ± SEM and are the result of 3 assays. # = significance from all other treatments, * = significance from treatments without DETA.
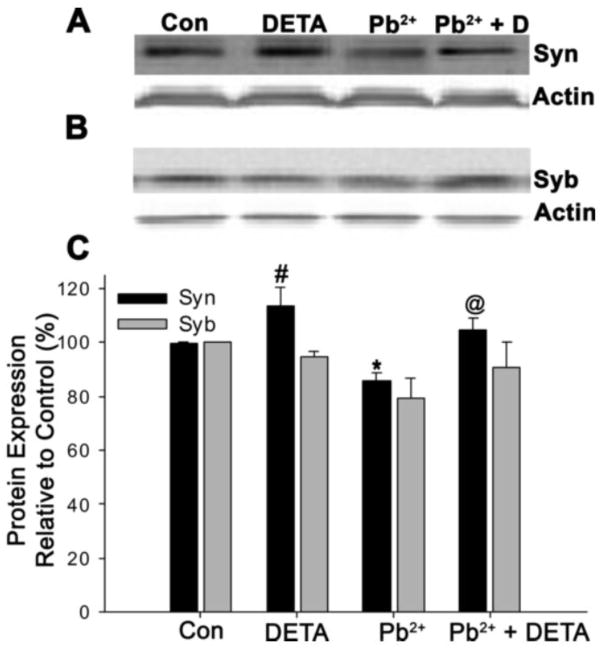
In our previous work we observed that Pb2+ reduced Syn whole-cell and presynaptic expression in a dose-dependent manner (Neal et al, 2010). Others have shown that Syn expression increases as a result of NO signaling at glutamatergic synapses (Ota et al, 2010; Wang et al, 2005). In the present study we investigated whether the decrease in Syn protein levels by Pb2+ could be remediated by incubation with 10 μM DETA for the final 24 hours of Pb2+ exposure. As shown in Figure 2, we observed a similar decrease in Syn levels during Pb2+ exposure as previously published (decrease to 85.5 ± 3.0% of control, p<0.05). This loss of Syn protein was completely recovered by exposure to DETA (recovery to 104.8 ± 4.1% of control, p<0.05). However, we also observed that exposure to DETA alone (without Pb2+ exposure) resulted in a significant elevation of Syn protein relative to control cells (elevation to 113.5 ± 6.9%, p<0.05). In contrast we did not observe any significant effect of Pb2+ or DETA on Syb whole-cell expression, although a non-significant decrease during Pb2+ exposure occurred. This would suggest that the whole-cell expression of Syn (but not Syb) is linked to NO signaling, in agreement with other work (Ota et al, 2010; Wang et al, 2005), and further shows that NO supplementation can fully recover whole-cell Syn expression in Pb2+-exposed neurons.
Figure 2. Exogenous addition of NO for the final 24 hours of Pb2+ exposure completely recovers whole-cell Syn levels.
Neuronal cultures were harvested and immunoblotted for Syn (A) or Syb (B) and Actin. Pb2+ exposure significantly reduced Syn expression relative to control but exposure to the NO donor DETA during Pb2+ exposure resulted in complete recovery of Syn levels. Exposure to DETA in absence of Pb2+ resulted in a significant elevation of Syn protein above control levels. No significant trends were observed with Syb, although a non-significant decrease in Syb expression occurred in Pb2+-treated neurons. Quantification of protein expression relative to control is shown in C. Data are mean ± SEM and are the result of 4 independent trials. For Syn expression only, * = significance from Syn control, # = significance from all other Syn treatment groups, and @ = significance from Syn treatment with Pb2+ (Fisher’s Protected LSD).
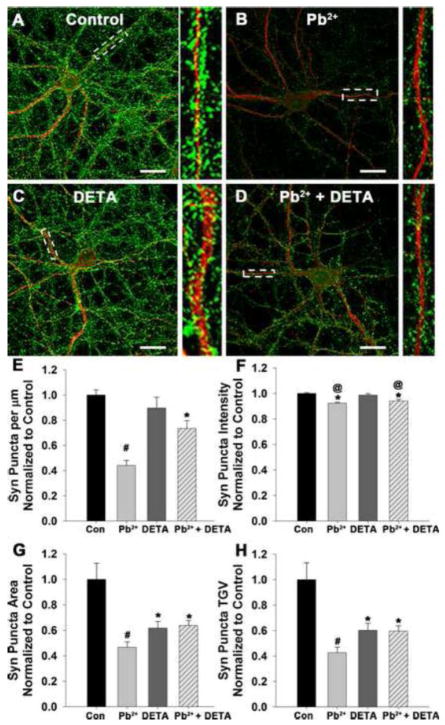
Next we determined whether incubation with 10 μM DETA for the final 24 hours of Pb2+ exposure could recover the presynaptic levels of Syn and Syb, which decrease during Pb2+ exposure (Neal et al., 2010). For these studies we measured the distinctive punctate expression of presynaptic proteins (Syn and Syb) with immunofluorescence. Using the postsynaptic marker microtubule associated protein 2 (MAP2) to visualize neuronal dendrites, we measured the characteristics of the fluorescent presynaptic proteins juxtaposed to the dendrites in terms of puncta density (number of synapses per um dendrite), puncta intensity (average grey value), puncta area, and puncta total grey value (TGV; a semi-qualitative measurement of quantity). In Figure 3 we show that incubation with DETA for the final 24 h of Pb2+ exposure resulted in partial recovery of Syn expression in the synapses of Pb2+-treated neurons. As previously published by us (Neal et al., 2010), Syn puncta density, area, intensity, and total grey value were significantly decreased after exposure to Pb2+. Here we show that treatment of Pb2+-exposed neurons with DETA resulted in partial recovery of Syn puncta density (p<0.01), area (p<0.01), and TGV (p<0.01). Syn puncta intensity was unchanged between Pb2+ and Pb2+ + DETA treatment groups. In contrast to other studies in hippocampal cultures (Wang et al., 2005), we did not observe an effect of DETA on Syn puncta density under control conditions. In our hands, control neurons treated with DETA for 24 hours exhibited a decrease in Syn puncta area, intensity, and TGV, but no difference in puncta density relative to control. Thus, supplementation with 10 μM DETA for the final 24 hours of Pb2+ exposure can partially recover the number of Syn-containing synapses in Pb2+-exposed neurons, based on partial recovery of Syn puncta density, area, and TGV. However, in control cultures DETA reduced the levels of synaptic Syn expression based on decreased puncta intensity, area, and TGV relative to control.
Figure 3. Exogenous addition of NO for the final 24 hours of Pb2+ exposure partially recovers Syn levels in Pb2+-treated neurons but reduces Syn expression under control conditions.
Representative images of neurons exposed to control medium (A), 1 μM Pb2+ (B) 10 μM DETA (C), or Pb2+ + DETA (D) stained for Syn (green) and MAP2 (red). Scale bar=20 μm. Quantification of Syn puncta density (E), intensity (F), area (G), and TGV (H) revealed that some effects of Pb2+ may be refractory to NO-mediated recovery. Data are mean ± SEM and are the result of 4 independent trials with 18–20 neurons per condition. * = significance from control, # = significance from all other treatment groups, and @ = significance from treatment with DETA alone (Fisher’s Protected LSD).
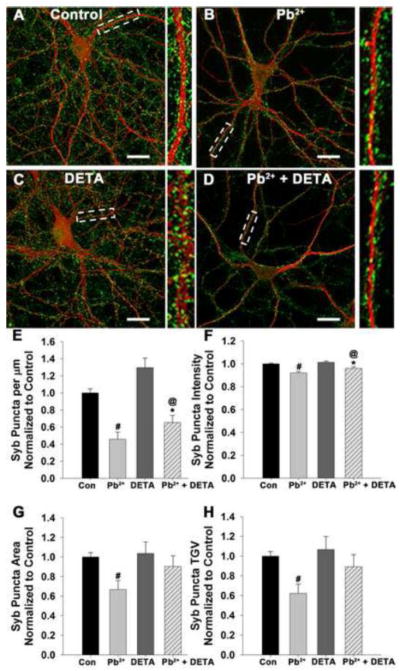
We next investigated whether DETA could recover the synaptic levels of Syb (Figure 4). Previously we have shown that the synaptic expression of Syb, a vesicular SNARE protein essential for fast neurotransmitter release, was significantly reduced by Pb2+ exposure. As previously published (Neal et al., 2010), we again observed a significant decrease in synaptic Syb levels after exposure to 1.0 μM Pb2+ (Figure 4E–H). However, in neurons exposed to Pb2+ + DETA, Syb puncta density (p<0.01) and intensity (p<0.01) exhibited a partial recovery of Syb expression while Syb area (p<0.01), and TGV (p<0.01) fully recovered to control levels. Neurons from control conditions did not exhibit any difference in Syb expression when treated with DETA for 24 hours. Thus, exposure to 10 μM DETA can partially recover Syb presynaptic expression in Pb2+-exposed neurons with no adverse effect on presynaptic Syb expression under control conditions.
Figure 4. Exogenous addition of NO for the final 24 hours of Pb2+ exposure partially recovers Syb levels in Pb2+-treated neurons with no effect on Syb levels under control conditions.
Representative images of neurons exposed to control medium (A), 1 μM Pb2+ (B) 10 μM DETA (C), or Pb2+ + DETA (D) stained for Syb (green) and MAP2 (red). Scale bar=20 μm. Quantification of Syb puncta density (E), intensity (F), area (G), and TGV (H) revealed that puncta area and TGV in Pb2+-exposed neurons were completely recovered by DETA treatment while puncta density and intensity exhibited partial recovery. Data are the mean ± SEM and are the result of 4 independent trials with 18–20 neurons per condition. * = significance from control, # = significance from all other treatment groups, and @ = significance from treatment with DETA alone.
3. Discussion
Pb2+ exposure during synaptogenesis results in changes in both pre- and postsynaptic protein expression (Neal et al, 2011; Neal et al, 2010). In particular, Pb2+ exposure results in decreased levels of whole-cell and synaptic Syn expression and decreased synaptic Syb expression (Neal et al, 2010). The changes in presynaptic protein expression are a result of inhibition of NMDARs by Pb2+ and may be responsible for impaired vesicular release in Pb2+-exposed neurons (Neal et al., 2010). These effects of Pb2+ on presynaptic structure and function can be fully remediated by supplementation with BDNF for the final 24 hours of Pb2+ exposure. Furthermore, BDNF expression and release are decreased during Pb2+ exposure, indicating that Pb2+ can disrupt trans-synaptic BDNF signaling. However, NMDARs mediate multiple trans-synaptic pathways besides BDNF-mediated signaling and these alternate pathways may also be implicated in the effects of Pb2+. One of these alternate pathways is transsynaptic NO signaling and the present study determined whether supplementation with the NO donor DETA during Pb2+ exposure was sufficient to rescue neurons from the effects of Pb2+. The results support the following conclusions. First, the decreased whole-cell expression of Syn caused by Pb2+ exposure during synaptogenesis can be fully remediated by supplementation with DETA for the final 24 hours of Pb2+ exposure. Second, the decreased synaptic expression of Syn and Syb caused by Pb2+ exposure cannot be fully remediated by DETA, but does exhibit partial recovery after NO supplementation.
We interpret the full recovery of whole-cell, but not synaptic, Syn expression in Pb2+-exposed neurons to indicate that NO signaling can stimulate the expression but not the targeting of Syn in Pb2+-exposed neurons. DETA exposure in absence of Pb2+ elevated Syn expression above control levels, an effect which was not observed with Syb. This may indicate that NO is specifically linked to Syn expression. The increased cellular expression of Syn is supported by the increase in somatic staining of Syn in Figure 3; this diffuse staining pattern, which may be indicative of Syn protein that has not yet been transported to presynaptic active zones, appears to be increased in neurons exposed to DETA with or without Pb2+ exposure (particularly evident in Figure 3D vs 3B). This hypothesis is supported by evidence in vitro that glutamatergic stimulation activates the NOS-NO-cGMP-cGK-RhoA pathway which ultimately results in increased Syn expression in hippocampal neurons (Wang et al, 2005). Inhibition of this pathway by NOS or cGMP dependent protein kinase (cGK) inhibitors prevents the glutamate-stimulated increase of Syn (Wang et al, 2005). While this study observed that stimulation of NOS resulted in increased synaptic targeting of Syn, which was not observed in our study, the increased targeting of Syn was attributed to cytoskeletal restructuring by vasodilator-stimulated phosphoprotein (VASP) and/or Ras homolog gene family member A (RhoA) activation. Both VASP and RhoA are downstream to activation of sGC by NO (Wang et al, 2005). Thus, disruption of NO signaling may affect presynaptic Syn localization; however, our work indicates that supplementation with NO is not sufficient to reverse or recover the decreased presynaptic Syn levels in Pb2+-exposed neurons.
In contrast to Syn targeting, the transcription and/or translation of Syn may involve pre- or postsynaptic activation of CaMKII and subsequent phosphorylation events, based on the results of an in vivo study which observed increased Syn levels in the lateral amygdala after fear conditioning (Ota et al, 2010). In this study, inhibition of the NMDAR or CaMKII blocked the increase in Syn expression associated with fear conditioning. CaMKIIα can be activated by protein kinase G, which in turn is stimulated by cGMP. Thus, NO is implicated in both the CamKII- and RhoA-mediated effects on Syn expression and targeting since increased cGMP levels can activate both pathways (Ota et al, 2010; Wang et al, 2005). In Pb2+-exposed cultures supplementation with NO for 24 hours was not sufficient to fully recover the deficits in Syn targeting caused by Pb2+, but could fully recover whole-cell levels of Syn.
We have previously determined that decreases in Syn and Syb synaptic expression was already evident in sister cultures after a shorter (4 days) exposure to Pb2+ (Neal et al., 2010). In those studies we observed that the number of presynaptic sites that contain Syn were reduced by roughly 25%, although no significant effect was observed on Syn puncta area, intensity or TGV at this time point. Thus, it appears that exogenous addition of NO halts the further loss of Syn from synapses during the final 24 hours of Pb2+ exposure, but does not affect the Syn puncta area, intensity, or TGV. Again, this may indicate that DETA or NO is not sufficient to recover deficits in Syn targeting. In contrast, Syb puncta area, intensity, and TGV, but not density, were significantly reduced after 4 days’ exposure to Pb2+ (Neal et al., 2010). Syb puncta area was reduced to 61 ± 7%, Syb puncta intensity decreased to 91.6 ± 2.8%, and Syb puncta TGV decreased to 59 ± 9% of control (Neal et al., 2010). These values are lower than what is observed in neurons exposed to Pb2+ for 5 days but supplemented with DETA (Figure 4). Thus, exogenous addition of DETA for the final 24 hours of Pb2+ exposure may recover Syb protein levels in regards to puncta area, intensity, and TGV, and may prevent the loss of Syb puncta density that occurs in neurons exposed to Pb2+ for longer than 4 days.
Our finding that exogenous addition of the NO-releasing chemical DETA can reduce synaptic Syn levels and yet boost whole-cell expression under control conditions is interesting and contrasts with work from other groups (Wang et al., 2005). As more is learned about NO signaling in hippocampal neurons, it becomes increasingly clear that NO-dependent signaling pathways are complex, often with contradictory cellular effects. For example, NO generation linked to NR2B-containing receptors has been implicated in cell-death pathways in response to excitotoxic conditions (Hardingham et al., 2002; Soriano et al., 2008). However, NO-dependent stimulation of sGC has been shown to be important for vesicular release and activity-dependent regulation of synaptic proteins (Arancio et al., 1996; Wang et al., 2005). Perhaps the key issue is the concentration of released NO: although we did not detect any difference in total decomposition products we would not be able to detect differences in NO levels at individual synapses. It is possible that normal synapses release tightly regulated amounts of NO, and additional NO from DETA initiates a negative feed-back loop for synaptic targeting. We have recently shown that the exogenous addition of the neurotrophic factor BDNF using the same experimental design as in the present study results in complete recovery of the Pb2+-induced decrease in synaptic Syn and Syb as well as remediate deficits in vesicular release (Neal et al., 2010). Combined, these studies suggest that Pb2+ exposure during synaptogenesis of hippocampal neurons may disrupt more than one NMDAR-dependent trans-synaptic retrograde signaling pathway.
4. Experimental Procedure
4.1 Animal Care and Use Statement
All animal studies were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee and have been carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
4.2 Cell culture
Primary hippocampal cultures were obtained from E18 Sprague-Dawley rat pups (Harlan, Frederick, MD). Low density cultures (14,000 cells/cm2) were seeded and maintained as described elsewhere (Neal et al., 2010). Feeding medium was composed of fetal bovine serum (FBS, 1% v/v, Hyclone-Thermo Scientific, Waltham, MA), 2 mM glutamax (Invitrogen, Carlsbad, CA), and penicillin/streptomycin (100 units each, Invitrogen) in neurobasal medium (Invitrogen). Pb-acetate (95% pure, Sigma Aldrich, St. Louis, MO) was added to the feeding medium on DIV7 while feeding media without Pb-acetate was added to control cultures. Neurons were harvested 5 days after dosing (DIV12), without media exchange between DIV7 and DIV12. DETA NONOate (Sigma) was added to cultures on Pb2+ exposure day 4 diluted in neurobasal medium to achieve a final concentration of 10 μM DETA. Sister plate controls received vehicle (neurobasal medium).
4.3 Pb2+ analysis
Samples of the stock solutions of Pb2+ (100 μM and 10 μM) used to dose cells were sent periodically to ESA Laboratories, Inc. (Magellan Biosciences, Chelmsford, MA) for atomic absorption spectroscopy to verify accuracy. Based on the reports from ESA labs, our 100 μM and 10 μM Pb2+ stock solutions were within the intended range (91.4 ± 4.9, n=9 samples; and 9.6 ± 0.4 μM, n=4 samples, respectively).
4.4 Immunocytochemistry
On DIV12, immunocytochemistry was performed as described elsewhere (Neal et al., 2010). Briefly neurons grown on glass coverslips were initially fixed in 4% paraformaldehyde (v/v), 4% sucrose (w/v) in phosphate buffered saline (PBS) followed by secondary fixation in ice cold methanol. Cells were permeablized in 0.2% Triton in PBS (v/v) and blocked in 10% normal goat serum in PBS (v/v). Samples were incubated in primary antibodies diluted in blocking solution overnight at 4° C using the following dilutions: 1:1000 MAP2 (Santa Cruz sc74421, Santa Cruz, CA); 1:500 Synaptophysin (Sigma S5768); 1:500 Synaptobrevin (Synaptic Systems 104202, Goettingen, German). After incubation in primary antibodies the neurons were washed in PBS and incubated in the appropriate secondary antibodies (10 μg/mL Alexafluor488 or Alexafluor594; Invitrogen-Molecular Probes, Carlsbad, CA) diluted in blocking solution (10% v/v normal goat serum in PBS) at room temperature. Following another series of washes in PBS the coverslips were mounted onto slides in ProLong Gold mounting media (Molecular Probes). Slides were coded to ensure that imaging and analyses were conducted in a blinded fashion.
4.5 Imaging and Image Analysis of Fixed Coverslips
Immunofluorescently-labeled neurons were imaged at 63× magnification using a single-point, laser scanning confocal microscope (LSM510-Meta, Zeiss, Thornwood, NY) utilizing LSM image software at the Johns Hopkins University School of Medicine Microscope Facility. All coverslips stained under the same conditions were imaged using the same scanning parameters on the same day. Four to 7 confocal stacks of single neurons were obtained for each experimental condition. Confocal stacks were projected into single images using the maximum fluorescence. Images were analyzed using Metamorph Offline (Molecular Devices, Downingtown, PA). Images obtained from the same experiment were thresholded at the same level for analysis. Several parameters of synaptic protein expression were measured using integrated morphometry analysis. They include immunofluorescent puncta density (number of puncta per μm dendrite), area (average area of puncta), intensity (average grey value of puncta), and total grey value (integration of puncta intensity relative to area). Puncta intensity thus measures the average intensity of the fluorescent puncta while puncta total grey value gives a semi-quantitative measurement of protein quantity. For colocalization (or juxtaposition) analysis, gray scale images at each wavelength for the same neuron were used to select 3 - 6 dendritic regions. Selected dendritic regions were at least 10 μm from the cell body, were clearly identifiable as single processes, and could be traced back to the imaged neuron. All dendrites which fit these criteria in a single image were sampled.
4.6 MTS Cell Viability Assay
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assays were performed per the manufacturer’s instructions (CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay, Promega). Briefly, hippocampal neurons were plated in 96-well plates at 14,000 cells/cm2. Cells were exposed to 1.0 μM Pb2+ for 5 days or to 0–10 μM DETA for 24 h. Exposure to 100 μM glutamate for 24 hours was used as a positive cytotoxic control. After the exposure period, 20 μL of the MTS reagent was added to the wells for 2 hours at 37°C. Absorbencies were read at 490 nm using a multi-channel plate reader.
4.7 Determination of Nitric Oxide Levels
Nitric oxide levels were indirectly estimated using the nitrate/nitrite Greiss reaction (Green et al., 1982). Culture media samples were taken on the 4th day of Pb2+ exposure, prior to the addition of DETA. Another set of samples were obtained 24 hours later, prior to harvesting the cells for immunocytochemistry. Standards were made from NaNO2 (0 to 50 μM) dissolved in neuron feeding media (NM1). Neither NM1 nor Pb2+ interfered with the Greiss reaction. The Greiss reagent was added in a 1:1 ratio with standards or samples in triplicate. After 15 minutes, the absorbance at 540 nm was recorded with a multiplate reader.
4.8 Whole Cell Immunoblotting
For whole cell protein levels, cells were harvested on DIV12 according to the method of Brewer et al. (2007). Western blot membranes were incubated in the appropriate primary antibodies: 1:1000 Synaptophysin (Cell Signaling 4329), 1:1000 Synaptobrevin (Synaptic Systems 104 202), 1:1000 Actin (Santa Cruz sc-1616) diluted in blocking solution overnight at 4°C. The membranes were visualized using the Odyssey imaging system (LiCor). Integrated intensity of the protein of interest was normalized to b-actin levels from the same blot.
Highlights.
Pb2+ reduces cellular synaptophysin levels and synaptic targeting of presynaptic proteins
Previous work showed that Pb2+ can disrupt NMDAR dependent trans-synaptic signaling
Supplementation with NO during Pb2+ exposure fully rescued Syn whole cell expression
NO supplementation did not recover Pb2+ effects on synaptic protein localization
Thus NO recovers deficits in cellular but not synaptic protein expression due to Pb2+
Acknowledgments
We thank Jennifer Dziedzic, Michael Chang, Jason Sheppard, and Richard Cho for their technical assistance. All confocal images were taken at the Johns Hopkins School of Medicine Microscope Facility. We would like to thank Scott Kuo, Michael Delannoy, Barbara Smith, Carol Cooke, and Loza Lee for their assistance and expertise. This work was supported by NIEHS grant ES006189 to TRG and APN was funded by NIEHS T32ES07141. This work was submitted in partial fulfillment for the doctoral degree requirements for APN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Al Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Alberto CS, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990;261:124–30. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–35. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, et al. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FM, Rodrigues LS, Giocomelli MBO, Oliveira CS, Posser T, Dunkley PR, Leal RB. Lead stimulates ERK1/2 and p38MAPK phosphorylation in the hippocampus of immature rats. Brain Res. 2004;998:65–72. doi: 10.1016/j.brainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Daly C, Ziff EB. Ca2+-dependent formation of a dynamin-synaptophysin complex: Potential role in synaptic vesicle endocytosis. J Biol Chem. 2002;277:9010–9015. doi: 10.1074/jbc.M110815200. [DOI] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure and hippocampal long-term potentiation: biphasic dose-response relationship. NeuroToxicology. 1999;20:71–82. [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of Nitrate, Nitrite and 15N Nitrate in Biological Fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Miceli RC. Age-dependent effects of lead on [3H]MK-801 binding to the NMDA receptor-gated ionophore: in vitro and in vivo studies. Neurosci Lett. 1992;148:27–30. doi: 10.1016/0304-3940(92)90796-a. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572.3:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children's intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Neal AP, Guilarte TR. Molecular Neurobiology of lead (Pb2+): Effects on synaptic function. Mol Neurobiol. 2010;42:151–160. doi: 10.1007/s12035-010-8146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR. Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: Potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci. 2010;116:249–263. doi: 10.1093/toxsci/kfq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, Worley PF, Guilarte T. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. NeuroToxicology. 2011;32:281–289. doi: 10.1016/j.neuro.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-D-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99:233–242. doi: 10.1016/s0306-4522(00)00192-5. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Guilarte TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Mol Brain Res. 1999;66:42–49. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Nat Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KT, Monsey MS, Wu MS, Schafe GE. Synaptic Plasticity and NO-CGMP-PKG Signaling Regulate Pre- and Postsynaptic Alterations at Rat Lateral Amygdala Synapses Following Fear Conditioning. PLoS One. 2010;5:e11236. doi: 10.1371/journal.pone.0011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Pennuto M, Bonanomi D, Benfenati F, Valtorta F. Synaptophysin I Controls the Targeting of VAMP2/Synaptobrevin II to Synaptic Vesicles. Mol Biol Cell. 2003;14:4909–4919. doi: 10.1091/mbc.E03-06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem. 2004;279:14307–14314. doi: 10.1074/jbc.M311103200. [DOI] [PubMed] [Google Scholar]
- Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke PGH, Wyllie DJA, Hardingham GE. Specific targeting of pro-Death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RAJ, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: From exposure to molecular effects. Brain Res Rev. 2005;49:529–555. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Hashemzadeh-Gargari H, McGlothan JL, Guilarte TR. Developmental Pb2+ exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Dev Brain Res. 2002;139:217–226. doi: 10.1016/s0165-3806(02)00569-2. [DOI] [PubMed] [Google Scholar]
- Toscano CD, McGlothan JL, Guilarte TR. Lead exposure alters cyclic-AMP response element binding protein phosphorylation and binding activity in the developing rat brain. Dev Brain Res. 2003;145:219–228. doi: 10.1016/j.devbrainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Toscano CD, O'Callaghan JP, Guilarte TR. Calcium/calmodulin-dependent protein kinase II activity and expression are altered in the hippocampus of Pb2+-exposed rats. Brain Res. 2005;1044:51–58. doi: 10.1016/j.brainres.2005.02.076. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signaling and BDNF gene regulation. Curr Op Neurobiol. 2003;13:366–371. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Walz C, Jungling KL, Gottmann K. Presynaptic plasticity in an immature neocortical network requires NMDA receptor activation and BDNF release. J Neurophysiol. 2006;96:3512–3516. doi: 10.1152/jn.00018.2006. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu AP, Ruan DY, Liu J. Effect of developmental lead exposure on the expression of specific NMDA receptor subunit mRNAs in the hippocampus of neonatal rats by digoxigenin-labeled in situ hybridization histochemistry. Neurotoxicol Teratol. 2002;24:149–160. doi: 10.1016/s0892-0362(01)00210-0. [DOI] [PubMed] [Google Scholar]