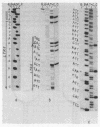
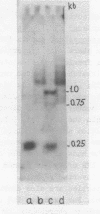
Abstract
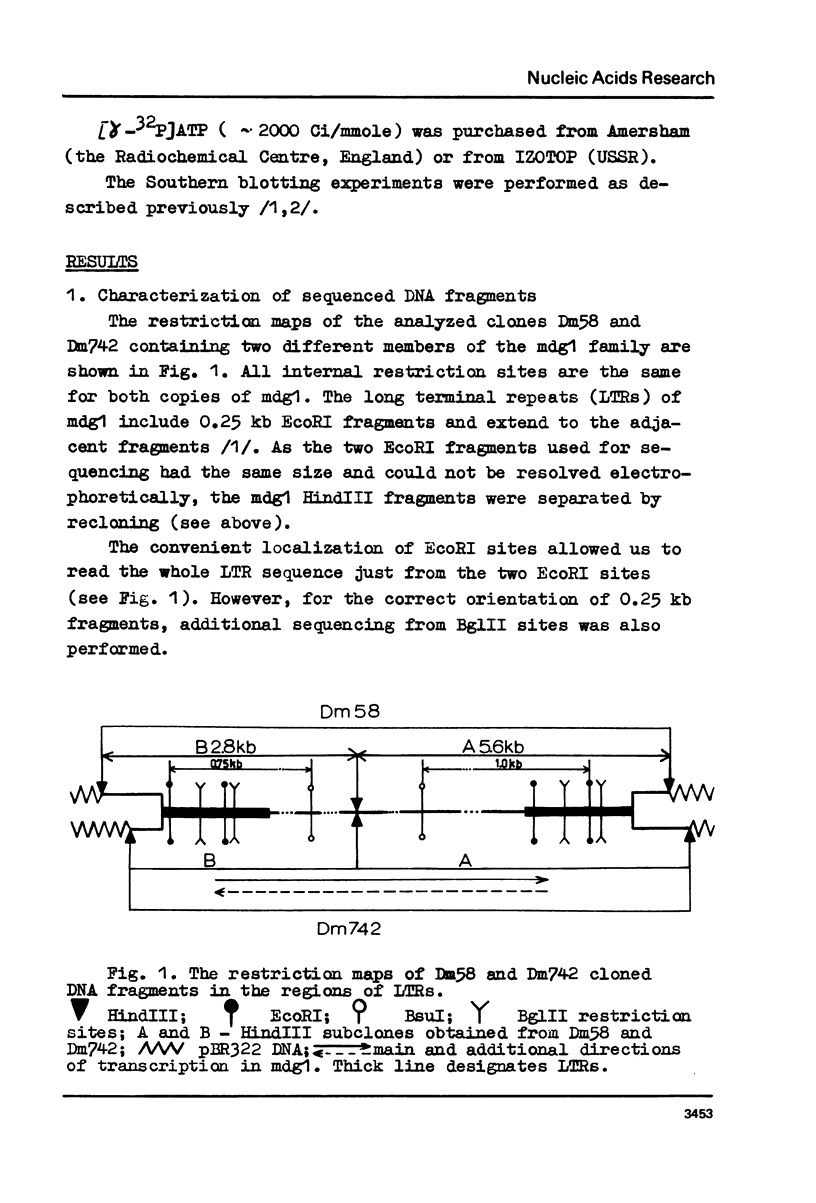
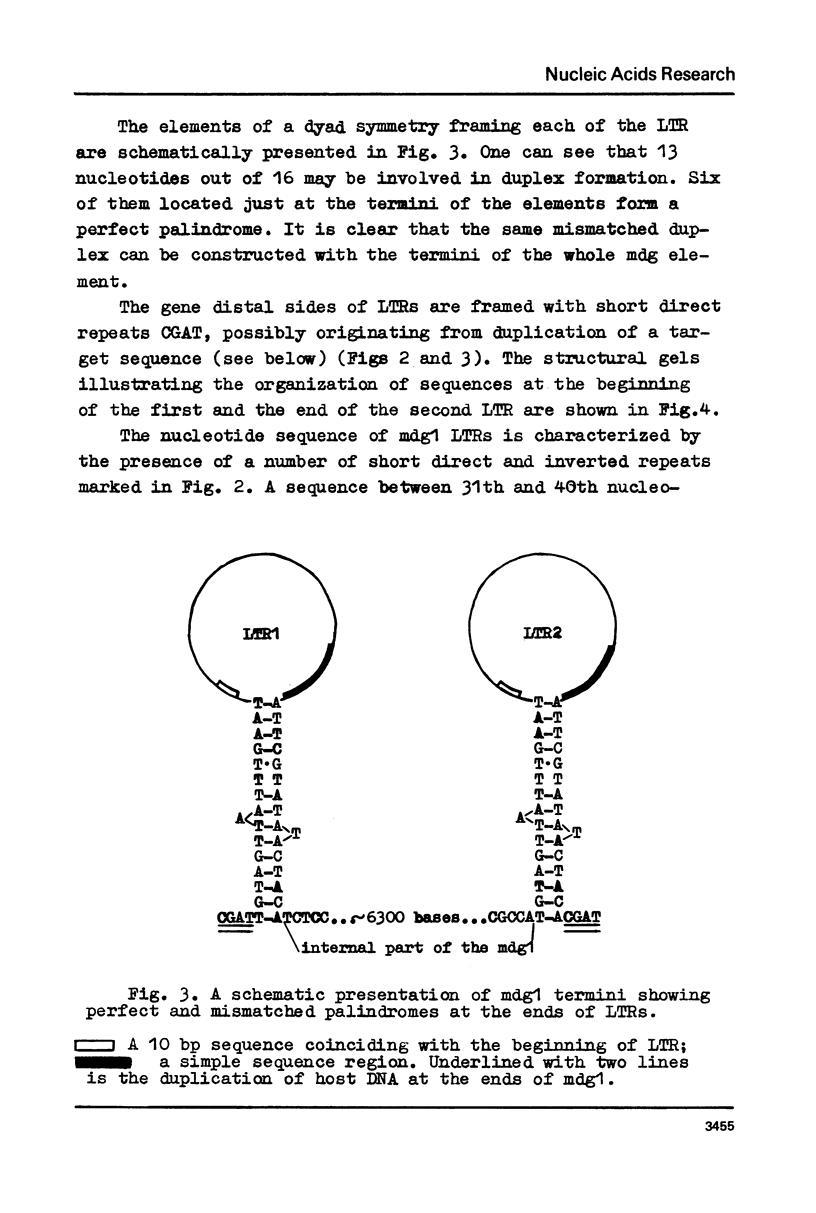
Long terminal repeats (LTRs) of two members of mdg1 family were sequenced. In the both cases, they are represented by perfect direct repeats 442 and 444 bp in length. Sixteen nucleotides in the LTRs of two different mdg1 elements are different. Each LTR contains slightly mismatched 16-nucleotide inverted repeats located at the ends of the LTR. Six base pairs closest to the termini of LTR form perfect inverted repeats. On the gene-distal sides of LTRs, short 4-nucleotide direct repeats are located, probably representing the duplication of a target DNA sequence arising from insertion of mdg. They are different in the two cases analyzed. Just as the other analyzed eukaryotic transposable elements, mdg1 starts with TGT and ends with ACA. Within the both strands of LTR, the sequences similar to Hogness box (a putative signal for RNA initiation, or a selector) and AATAAA blocks (putative polyadenylation signals) are present. The LTR of mdg1 contains many short direct and inverted repetitive sequences. These include a 10-nucleotide sequence forming a perfect direct repeat with the first ten nucleotides of the LTR. A region of LTR about 70 bp long is represented by simple repetitive sequences (TAT).
Full text
PDF


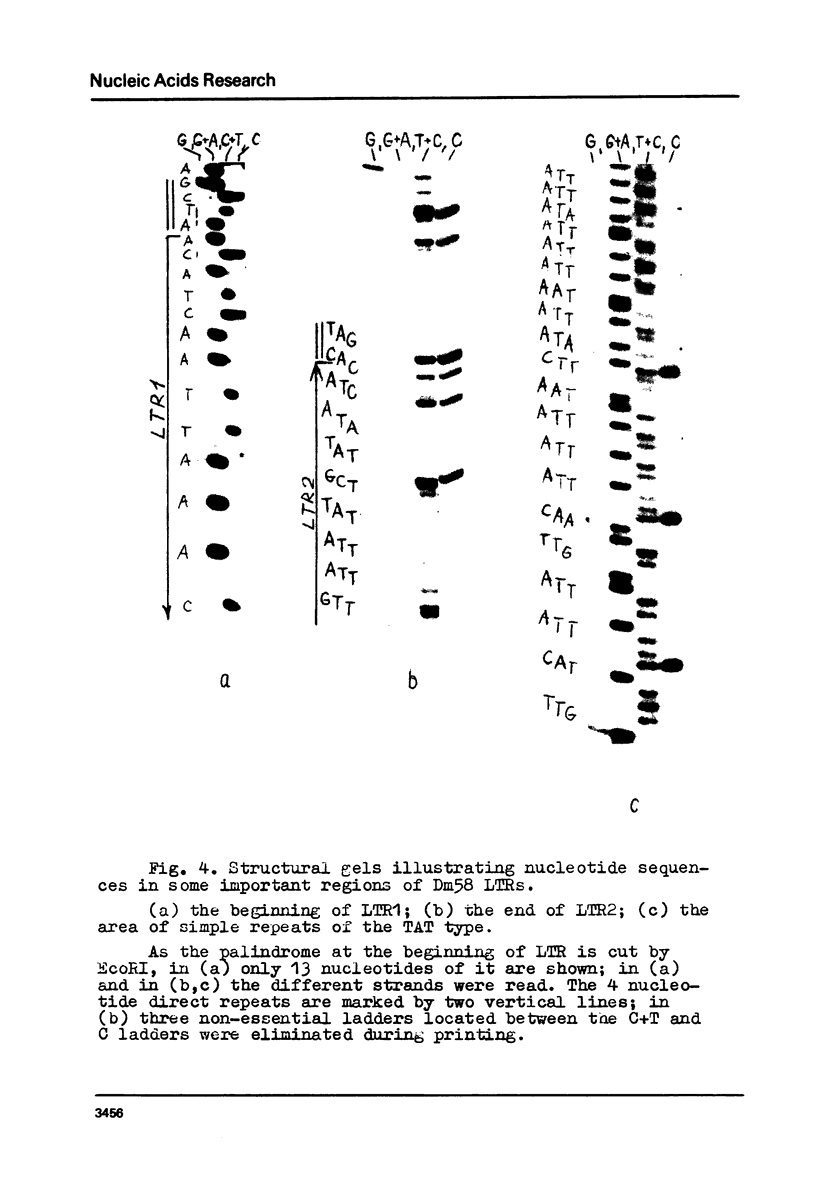




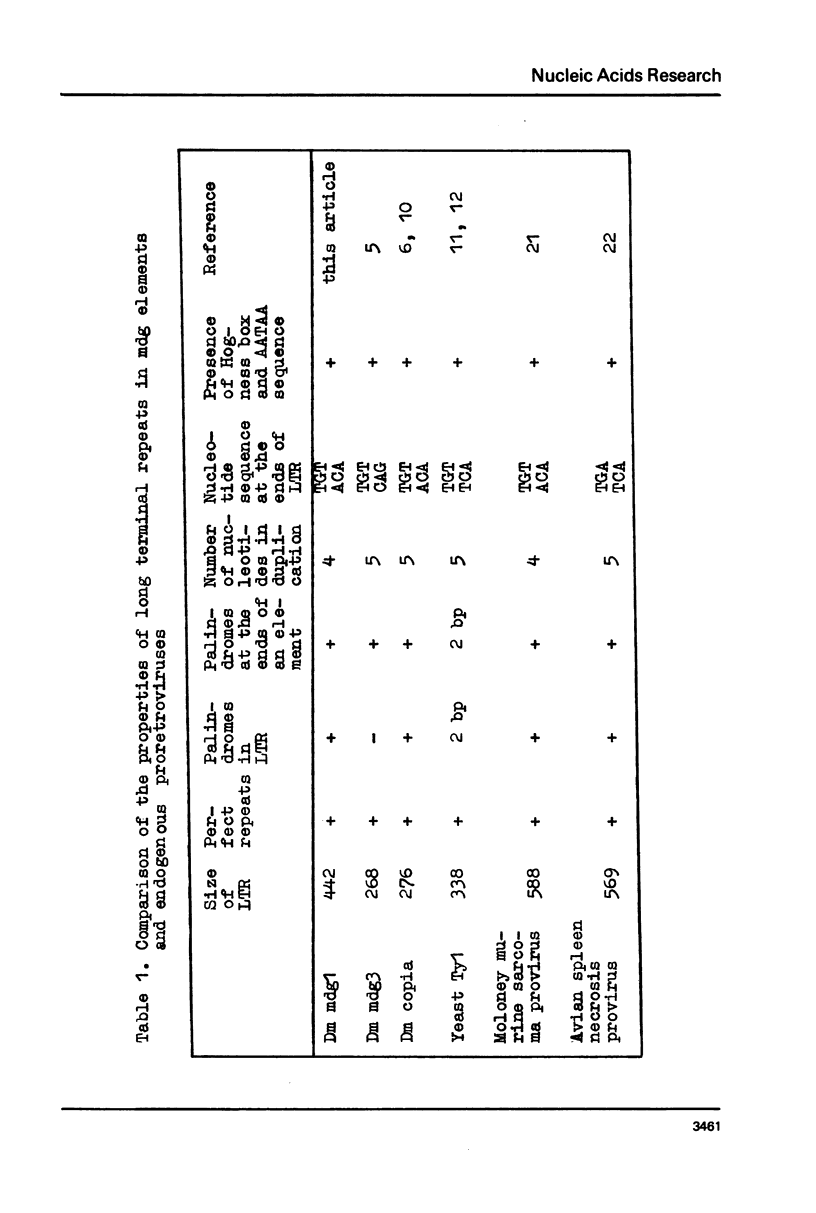



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Courtneidge S. A., Levinson A. D., Oppermann H., Quintrell N., Sheiness D. K., Weiss S. R., Varmus H. E. Origin and function of avian retrovirus transforming genes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):919–930. doi: 10.1101/sqb.1980.044.01.099. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P., Brorein W. J., Jr, Simon M. A., Rubin G. M. Insertion of the Drosophila transposable element copia generates a 5 base pair duplication. Cell. 1980 Sep;21(2):575–579. doi: 10.1016/0092-8674(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Gafner J., Philippsen P. The yeast transposon Ty1 generates duplications of target DNA on insertion. Nature. 1980 Jul 24;286(5771):414–418. doi: 10.1038/286414a0. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ilyin Y. V., Ryskov A. P., Kramerov D. A. Mobile dispersed genetic elements and their possible relation to carcinogenesis. Mol Biol Rep. 1980 Dec 31;6(4):249–254. doi: 10.1007/BF00777533. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P. On the structural organization of operon and the regulation of RNA synthesis in animal cells. J Theor Biol. 1969 Dec;25(3):473–490. doi: 10.1016/s0022-5193(69)80034-2. [DOI] [PubMed] [Google Scholar]
- Heine C. W., Kelly D. C., Avery R. J. The detection of intracellular retrovirus-like entities in Drosophila melanogaster cell cultures. J Gen Virol. 1980 Aug;49(2):385–395. doi: 10.1099/0022-1317-49-2-385. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Chmeliauskaite V. G., Ananiev E. V., Georgiev G. P. Isolation and characterization of a new family of mobile dispersed genetic elements, mdg3, in Drosophila melanogaster. Chromosoma. 1980;81(1):27–53. doi: 10.1007/BF00292421. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Chmeliauskaite V. G., Ananiev E. V., Lyubomirskaya N. V., Kulguskin V. V., Bayev A. A., Jr, Georgiev G. P. Mobile dispersed genetic element MDG1 of Drosophila melanogaster: structural organization. Nucleic Acids Res. 1980 Nov 25;8(22):5333–5346. doi: 10.1093/nar/8.22.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin Y. V., Tchurikov N. A., Ananiev E. V., Ryskov A. P., Yenikolopov G. N., Limborska S. A., Maleeva N. E., Gvozdev V. A., Georgiev G. P. Studies on the DNA fragments of mammals and Drosophila containing structural genes and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):959–969. doi: 10.1101/sqb.1978.042.01.097. [DOI] [PubMed] [Google Scholar]
- Ilyin Y., Chmeliauskaite V. G., Kulguskin V. V., Georgiev G. P. Mobile dispersed genetic element MDG1 of Drosophila melanogaster: transcription pattern. Nucleic Acids Res. 1980 Nov 25;8(22):5347–5361. doi: 10.1093/nar/8.22.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Dunsmuir P., Rubin G. M. Terminal repeats of the Drosophila transposable element copia: nucleotide sequence and genomic organization. Cell. 1980 Sep;21(2):581–588. doi: 10.1016/0092-8674(80)90496-1. [DOI] [PubMed] [Google Scholar]
- Marcoli R., Iida S., Bickle T. A. The DNA sequence of an IS/-flanked transposon coding for resistance to chloramphenicol and fusidic acid. FEBS Lett. 1980 Jan 28;110(1):11–14. doi: 10.1016/0014-5793(80)80011-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohtsubo E. Nucleotide sequence of an insertion element, IS1. Proc Natl Acad Sci U S A. 1978 Feb;75(2):615–619. doi: 10.1073/pnas.75.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Role of the C region in relative growth rates of endogenous and exogenous avian oncoviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1123–1132. doi: 10.1101/sqb.1980.044.01.121. [DOI] [PubMed] [Google Scholar]
- Young M. W. Middle repetitive DNA: a fluid component of the Drosophila genome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6274–6278. doi: 10.1073/pnas.76.12.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]