Abstract
The mammalian Golgi complex, trans Golgi network (TGN) and ER-Golgi-Intermediate Compartment (ERGIC) are comprised of membrane cisternae, coated vesicles and membrane tubules, all of which contribute to membrane trafficking and maintenance of their unique architectures. Recently, a new cast of players was discovered to regulate the Golgi and ERGIC: four unrelated cytoplasmic phospholipase A (PLA) enzymes, cPLA2α (GIVA cPLA2), PAFAH Ib (GVIII PLA2), iPLA2-β (GVIA-2 iPLA2), and iPLA1γ. These ubiquitously expressed enzymes regulate membrane trafficking from specific Golgi subcompartments, although there is evidence for some functional redundancy between PAFAH Ib and cPLA2α. Three of these enzymes, PAFAH Ib, cPLA2α, and iPLA2-β, exert effects on Golgi structure and function by inducing the formation of membrane tubules. Here, we review our current understanding of how PLA enzymes regulate Golgi and ERGIC morphology and function.
Cytoplasmic PLA enzymes associated with the Golgi complex
The functional organization of the mammalian Golgi complex is controlled by myriad proteins and enzymes that regulate the production of coated vesicles and membrane tubules for cargo export and the overall architecture of the Golgi ribbon [1]. At the heart of these processes is the Golgi complex itself, where form and function dance to the tune of secretory demand. Golgi membranes are dynamically modified to form membrane vesicles and tubules, which carry secretory cargo and maintain the morphological and functional integrity of the organelle (Box 1). For example, a sudden increase in secretory load results in the formation of intercisternal membrane tubules that facilitate anterograde trafficking through the Golgi stack [2]. Conversely, altering the ability of Golgi membranes to form vesicles and tubules dramatically changes Golgi architecture and secretory function [1, 2].
The first indication that cytoplasmic PLA activity was important for Golgi function came from a screen that showed PLA antagonists can inhibit brefeldin A (BFA)-induced membrane tubule formation from the Golgi complex and TGN [3, 4]. Subsequent studies suggested that the dynamic and continual formation of these PLA-dependent membrane tubules is important for several normal functions including assembly and maintenance of an intact Golgi ribbon [5], retrograde trafficking from the cis Golgi and ERGIC to the endoplasmic reticulum (ER) [6], and export from the TGN (Box 1) [7]. Until the identification of four Golgi-associated cytoplasmic PLA enzymes, no specific PLA enzymes had been directly linked to membrane tubule formation or Golgi function. Three of these PLA enzymes (cPLA2α, iPLA2-β and Platelet Activating Factor Acetylhydrolase Ib [PAFAH Ib]) hydrolyze at the sn-2 position of phospholipids [8, 9]. cPLA2α and iPLA2-β also possess lysophospholipase and transacylase activities in vitro and in vivo. A fourth enzyme, iPLA1γ hydrolyzes fatty acid substrates from the sn-1 position of phospholipids [10]. Each of these PLA enzymes influence Golgi function, and in at least three cases, have direct effects on the ability of the Golgi to form membrane tubules. Additional PLA enzymes are reported to associate with Golgi membranes, including GIV PLA2δ (cPLA2δ) [11] and Group V secretory PLA2 [12]. However, possible roles for these proteins in regulating Golgi structure and function are unknown. Therefore, we will restrict our discussion to the aforementioned four recently identified PLA enzymes.
PLA2 enzymes are members of a superfamily of PLAs that cleave fatty acids at the sn-2 position of glycerol phospholipids to generate a free fatty acid and a lysophospholipid [13, 14]. These enzymes are grouped (I-XV) according to sequence similarities and functions [8]. Importantly, the two products of phospholipid hydrolysis, lysophospholipids and free fatty acids, function in signal transduction, phospholipid synthesis, and membrane remodeling [15]. Thus, PLA2 enzymes are associated with functions that at first glance would not appear to have any involvement with the Golgi complex. Recently, however, studies have suggested that many phenotypes associated with loss of PLA2 function may be due to alterations in Golgi membrane trafficking capacity (Table I provides basic information on the four enzymes discussed in this review).
TABLE 1.
| Phospholipase Name | Official Gene Symbol | Aliases | PLA2 Group No. (REF) | C'some location; Protein m.w. | Features | Inhibitors |
|---|---|---|---|---|---|---|
| cPLA2α | PLA2G4A | cPLA2 GIVA cPLA2 |
IVA | 1: 85 kD | -Ca2+-dependent membrane binding -C2 domains -Ser/Asp dyad |
Pyrrolidine ONO-RS-082 MAFP AACOCF3 |
| PAFAH Ib α1 α2 β |
PAFAHIb2 PAFAHIb3 PAFAHIb1 |
Catalytic subunit 2 Catalytic subunit 3 Lis1; Regulatory subunit 1 |
VIIIA VIIIB |
11; 30 kD 19; 20 kD 17; 45 kD |
-Ca2+-independent -Specificity for PAF -Ser/His/Asp tTriad -GXSXG consensus |
BEL ONO-RS-082 |
| iPLA2-β | PLA2G6 | iPLA2-B, PNPLA9, L-iPLA2 (long transcript) GVIA-2 iPLA2 | VIA-2 | 22; 85 kD | -Ca2+-independent -ATP binding stabilizes activity -Multiple splice variants -S519/GXSXG consensus |
BEL ONO-RS-082 AACOCF3 MAFP |
| iPLA1γ | DDHD2 | KIAA0725p, Phospholipase DDHD2, DDHD domain-containing protein 2 | NA | 8; 81 kD | -GXSXG consensus -S351 active site -Preference for PA |
? |
Mechanistically, cytoplasmic PLA enzymes could influence the structure and function of the Golgi complex in several ways [3]. First, they could directly regulate membrane tubule and vesicle formation through the production of positive curvature-inducing lysophospholipids [16]. Second, PLA enzymes could generate lipid products that recruit effector proteins to Golgi membrane domains [17]. Third, they could generate lysophospholipids and fatty acids that influence downstream signal transduction and metabolic pathways. Finally, changes in membrane structure could occur via mechanisms that are independent of lipase activity, as will be discussed below. The discovery of four different cytoplasmic PLAs that regulate the Golgi complex is a surprising development, establishing new functions for these enzymes and revealing an unexpected level of complexity to Golgi structure and membrane trafficking. Here, we provide a brief summary of these enzymes, their enzymatic activities, links to previously described functions, and a more extensive discussion of recent functional studies that have uncovered new roles in regulating the functional organization of the ERGIC/Golgi complex.
cPLA2α
cPLA2α is a ubiquitously expressed cytoplasmic Group IV PLA2 (gene name PLA2G4A). It is the best studied cytoplasmic PLA2 and has been extensively reviewed elsewhere [15, 18] (Table 1). cPLA2α contains a C2 Ca2+-binding domain that is required for translocation to membranes. Upon membrane binding, phospholipid hydrolysis occurs by the PLA2α active site serine-aspartic acid dyad (Figure 1). cPLA2α is best known for its central role in mediating eicosanoid biogenesis in animal cells, catalyzing the release of arachidonic acid from membrane phospholipids [15, 18]. Arachidonic acid and its metabolites – including prostaglandins and leukotrienes that are produced by cyclooxygenases and lipoxygenases, respectively – are bioactive lipids that mediate diverse processes, including inflammation, smooth muscle contraction and cell growth [14].
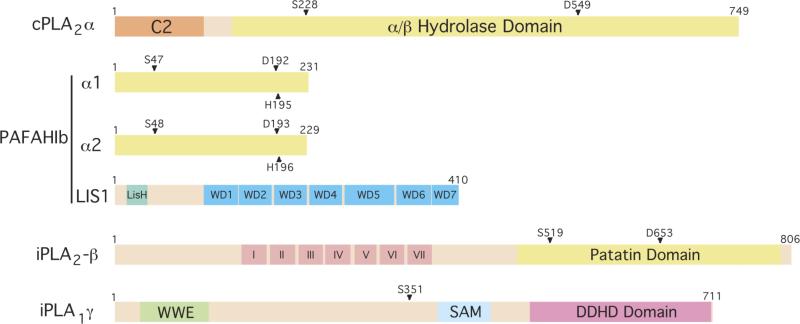
Figure 1. Domain structures of Golgi- and ERGIC-associated PLA enzymes.
cPLA2α contains a large α/β hydrolase domain with residues S228 and D549 comprising the active site dyad. It also contains a C2 Ca2+-binding domain that is necessary for translocation to Golgi membranes. PAFAH Ib is comprised of α1 and α2 homo- or heterodimers together with LIS1. The indicated residues form the active site triad. iPLA2–β contains a patatin lipase domain, with an active site at S519, and seven ankyrin repeats (I-VII). iPLA1γ contains an S351 residue, which when mutated to alanine (A) abolishes catalytic activity, a DDHD2 domain that is conserved with iPLA1β, a WWE domain predicted to mediate protein-protein interactions in ubiquitination and ADP-ribosylation systems, and a SAM domain (sterile alpha motif) that can mediate both homo- and hetero-oligomerization.
Although early work suggested a role for PLA2 activity in regulating diverse intracellular membrane trafficking events [3], the observation that cPLA2α regulates the structure and function of the Golgi complex was unanticipated. The first clues suggesting this possibility came when cPLA2α was shown to influence secretory cargo trafficking and be recruited to the Golgi complex in response to transient increases in cytoplasmic Ca2+ and other stimuli [19-22]. Recent work [23, 24] provided confirmation and further insight into this newly attributed role for cPLA2α in Golgi function.
These recent studies extended previous work, which found that cPLA2α was recruited to the Golgi complex in endothelial cells upon reaching confluency [21, 22], by showing that cPLA2α contributes to the delivery of transmembrane proteins to junction complexes in confluent endothelial cells [24]. RNAi and specific enzyme antagonists were used to show that loss of cPLA2α prevented delivery of VE-cadherin, occludin, and claudin-5 to cell-cell contacts, resulting in accumulation of these transmembrane proteins in the Golgi complex. These studies, however, did not identify the nature or location of the trafficking block.
Another study found that arrival of a secretory bolus at the Golgi complex induced the Ca2+-dependent translocation of cPLA2α from the cytoplasm to Golgi membranes [23]. Live-cell imaging and electron microscopy experiments revealed that cPLA2α mediates the formation of intercisternal tubular connections in the Golgi. Moreover, multiple approaches not only demonstrated that Golgi membrane tubulation was suppressed by loss or inactivation of cPLA2α activity, but also that anterograde intra-Golgi trafficking of several cargo proteins was blocked under these conditions. These data indicate that intercisternal tubules, formed through the activity of cPLA2α enzymes, are crucial to these trafficking events (Figure 2). Finally, the authors found that antagonists of cyclooxygenase and lipoxygenase activities had no influence on Golgi membrane tubule formation and that the addition of arachidonic acid to cells depleted of cPLA2α did not reverse the Golgi tubulation defect. Taken together, these data strongly support the conclusion that cPLA2α activity drives Golgi membrane tubulation and that this process occurs independent of the downstream biosynthesis of bioactive metabolites [18]. In addition, these studies also provide support for diffusion-based cis-to-trans intra-Golgi trafficking via tubular continuities [2, 25], at least under some circumstances (see [1] for a review of transport across the Golgi stack).
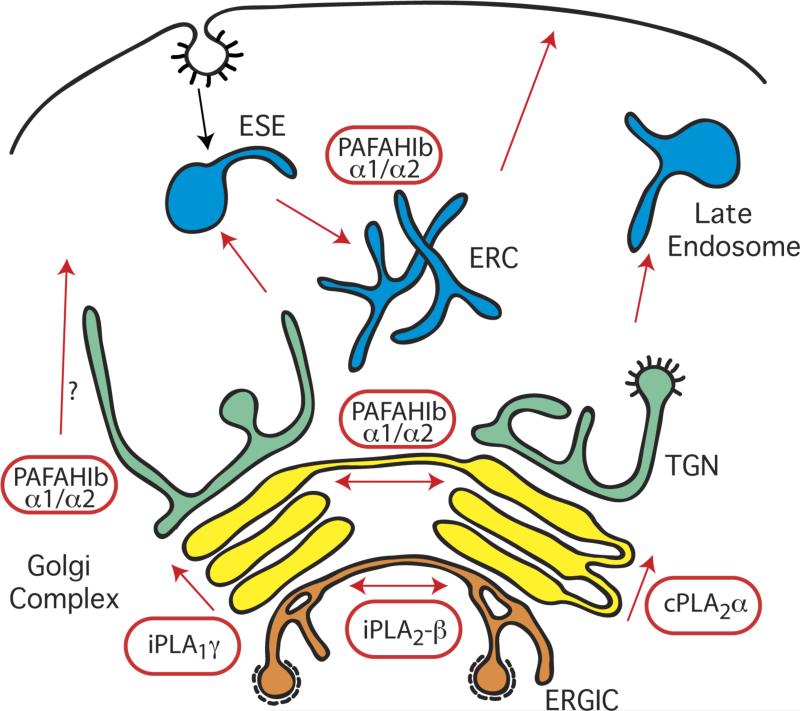
Figure 2. PLA enzymes that regulate the structure and function of the Golgi complex and endosomes.
cPLA2α localizes to the Golgi complex and mediates the formation of membrane tubules that facilitate anterograde transport through the cisternal stack. The α1 and α2 PLA2 catalytic subunits of PAFAH Ib localize to multiple Golgi cisternae, early sorting endosomes (ESEs), and the endocytic recycling compartment (ERC). These enzymes contribute to membrane tubule formation that links cisternal stacks into intact Golgi ribbons and to those that facilitate endocytic recycling of transferrin and its receptor from ESEs and the ERC. In addition, loss of α1 and α2 inhibits export from the TGN, although it is not clear if this involves membrane tubules. iPLA2–β localizes specifically to the ERGIC where it mediates the formation of membrane tubules that bridge between separate ERGIC clusters. iPLA1γ appears to localize primarily to the cis Golgi and may influence anterograde transport through the cisternal stack.
Several tantalizing questions about cPLA2α regulation of Golgi structure and function remain. cPLA2α displays a strong preference for phospholipids with sn-2 arachidonyl acyl chains suggesting that homeostasis of arachidonyl-containing phospholipids may be crucial in regulating Golgi structure and function. The molecular mechanisms that target cPLA2α specifically to Golgi membranes, as well as membranes of the ER and the nucleus, are not well established [18]. It has been proposed that the relative amount of phosphatidylcholine (PC), cholesterol, and/or ceramide-1-phosphate in Golgi membranes may dictate the affinity of cPLA2α for this organelle [26-28]. In addition, the C2 domain of cPLA2α mediates its Ca2+-dependent translocation to the Golgi complex [18]. When this domain binds Ca2+, the electrostatic potential of the surface-exposed Ca2+ binding loop is reduced and the association of the neutralized C2 domain with organelle membranes in general, and PC in particular, is promoted [29-31]. It is notable that the C2 domain of cPLA2α displays a tendency to bind the cis and medial regions of the Golgi complex, whereas the related C2 domains of protein kinase Cα and synaptotagmin display selectivity for the TGN and the plasma membrane [19]. This unique property of the cPLA2α C2 domain therefore may account for the role of cPLA2α in intra-Golgi transport, and the apparent lack of enzyme function in mediating trafficking associated with the TGN or plasma membrane. Finally, cPLA2α binding to Golgi membranes requires less cytosolic Ca2+ than binding to the ER [19]. This observation is consistent with the possibility that large increases in intracellular Ca2+ concentration alter the biological function of cPLA2α from regulating intra-Golgi transport to mediating the massive generation of arachidonic acid from ER membranes.
The recruitment of cPLA2α to Golgi membranes likely results from the transient and local release of Ca2+ from this organelle that occurs in response to increased secretory trafficking load [23, 32]. A KDEL receptor-mediated signaling cascade that initiates from the Golgi complex upon arrival of traffic from the ER may also regulate this process [33]. cPLA2α translocation to membranes is necessary but not sufficient for catalytic activity, and enzyme phosphorylation is also required for maximal catalytic activity in vitro and in vivo [34]. The mechanisms by which Ca2+ transients and protein phosphorylation coordinately regulate cPLA2α activity on Golgi membranes in vivo remain to be determined.
Finally, the cPLA2α knockout mouse does not exhibit profound defects in growth or viability [35, 36]. Moreover, a human patient with inherited cPLA2α deficiency carrying loss-of-function mutations in both cPLA2α alleles has globally decreased eicosanoid production and suffers from intestinal ulcers as well as platelet dysfunction [37-39]. These data demonstrate that cPLA2α activity itself is not essential for organismal viability, and suggest that other PLA2 activities compensate for the loss of activity or ablation of this enzyme in humans and whole animal model systems, respectively [18]. In support of this, an siRNA screen in which the expression of PLA2s of the Group IV, VI, VII and VIII PLA2 families were knocked down in cPLA2α-deficient fibroblasts found that the Group VIIIA enzyme, PAFAHIB, also contributes to anterograde transport of secreted cargo through the Golgi complex [23]. These data establish that redundant PLA2 pathways can mediate intra-Golgi trafficking.
PAFAH Ib
The discovery that PAFAH Ib (Platelet Activating Factor Acetylhydrolase Ib) is involved in Golgi trafficking was equally unexpected. PAFAH Ib is a protein complex comprised of two PLA2 subunits, α1 (gene name PAFAHIB2) and α2 (gene name PAFAHIB3), and a dimer of a third non-catalytic subunit, the dynein regulator Lis1 (gene name PAFAH1B1) (Figure 1; Table 1). The α1 and α2 subunits, which are 63% identical at the amino acid level, can form catalytically active, Ca2+-independent homo- and heterodimers, with modest differences in rates of hydrolysis and substrate preference [40-42]. The active site contains a catalytic triad of serine, aspartic acid and histidine [43, 44]. PAFAH Ib was originally purified based on its ability to hydrolyze the extracellular, inflammatory signaling lipid, PAF, which has an acetyl group in its sn-2 position [45, 46]. Subsequent studies, however, indicated that cytoplasmic PAFAH Ib is not involved in global downregulation of PAF signaling [47, 48]. Instead, both α1 and α2 are partially localized on Golgi membranes and purified catalytically active, but not inactive, subunits induce Golgi membrane tubule formation in a cell-free reconstitution system [49]. Knockdown and overexpression experiments revealed that both PLA2 activity and Lis1 binding are important for the assembly and maintenance of the Golgi complex. RNAi knockdown and rescue experiments demonstrated that re-expression of wild type but not catalytically inactive α1 restored export from the TGN to the cell surface, revealing that the PLA2 activity of PAFAH Ib contributes to secretory efficiency. Reduction in both α1 and α2 levels resulted in Golgi, ERGIC and TGN fragmentation into numerous ‘mini-stacks’, indicating that PAFAH Ib plays a role in the dynamic formation of membrane tubules that link Golgi stacks into an intact ribbon (Figure 2). Imaging of live cells treated with PLA2 and dynein antagonists confirmed the importance of these proteins in membrane tubule-dependent Golgi assembly into an intact ribbon [50].
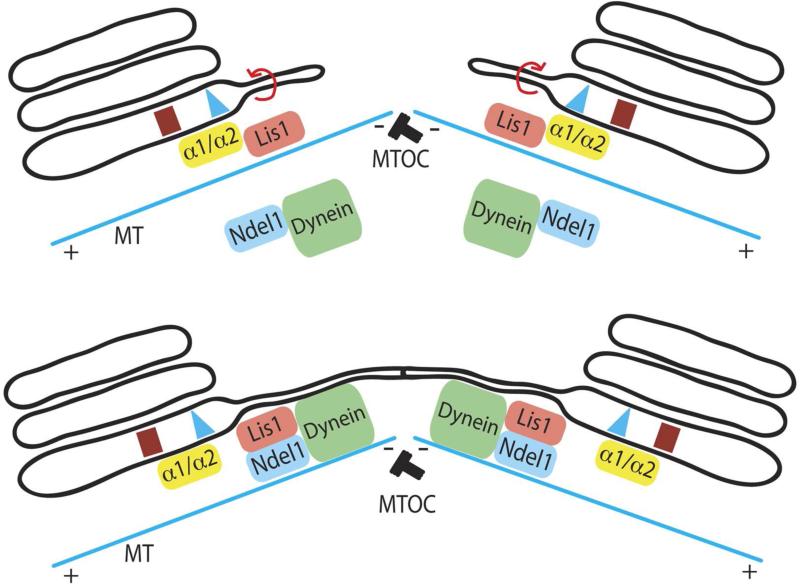
These results suggest that PAFAH Ib can link PLA2-mediated membrane tubule formation with subsequent movement along microtubules (Figure 3). Recent studies have shown that Lis1, the causative agent of human lissencephaly [51], recruits dynein to the plus ends of microtubules [52]. Once at the plus end, dynein activity is stimulated by Ndel1, a known Lis1 and dynein binding partner [53]. This activation would allow the Lis1-dynein-Ndel1 complex to transport cargo to the minus ends of microtubules [54]. We propose that the catalytic dimers, α1 and α2, when bound to Lis1, initiate PLA2-dependent membrane curvature, thus forming membrane tubules. Subsequently, Lis1 binds to a Lis1-Ndel1-dynein complex, facilitating the minus end movement of membrane tubules along microtubules towards the microtubule organizing center. This mechanism couples the formation of Golgi membrane tubules to dynein motors for the assembly and maintenance of a centrally located, intact Golgi ribbon. Currently, it is not known if any lissencephaly phenotypes result from functional changes at the Golgi complex.
Figure 3. Model integrating membrane curvature produced by the PLA2 activity of PAFAH Ib α1 and α2 with Lis1-mediated dynein transport along microtubules.
PAFAHIB initiates outward membrane curvature to generate a membrane tubule, which can be pulled/extended along microtubules (MT) by Lis1 and Ndel interactions with dynein. Dynein may be able to carry the membrane tubule towards the minus end of microtubules, facilitating the convergence of the Golgi stack and positioning at the microtubule organizing center (MTOC, minus end of the microtubules).
In addition to the ERGIC, Golgi and TGN, various endosomal compartments also exhibit membrane tubular extensions, which were originally shown to be involved in receptor segregation, sorting and trafficking [55, 56] and inhibited by PLA2 antagonists [57, 58]. Recently, PAFAH Ib α1 and α2 subunits were also found to localize to early sorting endosomes and the endocytic recycling compartment [59] (Figure 2). RNAi knockdown and overexpression experiments demonstrated that α1 and α2 are required for endosome membrane tubule formation and the recycling of transferrin and transferrin receptors from endosomes, functions that are dependent on PLA2 activity but independent of Lis1. These results show that PAFAH Ib α1 and α2 can function at different compartments by controlling the formation of membrane tubules that contribute to intracellular trafficking.
These results raise several issues. First, PAFAH Ib has a strict in vitro substrate preference for PAF and its analogs, which have an acetyl group in the sn-2 position and are generally associated with extracellular signaling during inflammation [60]. It is unclear if PAF also serves a function in Golgi and endosome membranes or if PAFAH Ib hydrolyzes other substrates in vivo. Second, α1-/-/α2-/- mice are reasonably normal except for severe male spermatogenesis defects [47, 48], indicating that other cytoplasmic PLA2 enzymes compensate for their loss. Third, although α1 and α2 can form functional homo- and heterodimers, a variety of studies show that they are not completely redundant. For example, α1-/- mice exhibit no detectable phenotypes, whereas α2-/- mice have mild spermatogenesis defects [47, 48]. In addition, α1 and α2 have different phospholipid head group preferences, catalytic rates when bound to Lis1, developmental expression patterns [61], and neurological phenotypes in mutant backgrounds [62]. Consistent with these studies, the knockdown of individual α1 or α2 results in noticeably different effects on Golgi morphology and secretory trafficking (M. Bechler and W. Brown, unpublished data). Therefore, it may be more appropriate to conclude that α1 homodimers and α2 homodimers have both overlapping and distinct functions.
iPLA2-β
iPLA2–β belongs to the Group VI family of cytoplasmic, Ca2+-independent PLA2 enzymes (also called iPLA2B, GVIA-2 iPLA2 and PNPLA9) (Table 1) [15]. Human iPLA2–β is a ubiquitously expressed 85 kD protein with 7 ankryin repeats and an active site serine within a consensus lipase motif (GXSXG) in the C-terminal patatin domain (Figure 1). iPLA2–β can yield several splice variants. The full-length protein (also called L-iPLA2) is catalytically active but with relatively little fatty acid substrate preference [63], whereas the two smaller alternatively spliced variants, ankryin-iPLA2-1 and ankryin-iPLA2-2, are missing the lipase active site. Interestingly, these catalytically inactive variants appear to modulate the activity of L-iPLA2 and the ratio of L-iPLA2 to ankryin-iPLA2 determines overall iPLA2-β activity, likely as a result of forming oligomers [64]. iPLA2-β is associated with a variety of functions and pathologies including adipocyte differentiation [65], glucose-mediated insulin secretion [66] and neurodegenerative disease [67, 68], and has been reviewed elsewhere [8, 69]. Recent studies, however, show that iPLA2-β plays a role in regulating the ERGIC through the formation of membrane tubules [70].
The ERGIC is very closely associated with the cis Golgi but is considered an independent compartment from the Golgi proper [71, 72]. It is comprised of a complex array of tubulovesicular membranes that serve as an intermediate compartment in bidirectional trafficking between the ER and Golgi complex. The ERGIC is an active site of COPI vesicle formation for retrograde trafficking back to the ER [71]. Recruitment of COPI vesicle proteins to ERGIC and Golgi membrane requires activation of the Arf family of GTP binding proteins by their cognate guanine nucleotide exchanges factors (GEFs) [73]. Double knockdown of Arf1 and Arf4 causes dispersal of COPI proteins and stimulation of ERGIC membrane tubules [74] COPI vesicle budding is inhibited by brefeldin A (BFA), which forms abortive BFA:Arf-GEF complexes thereby preventing Arf activation [75]. As a consequence of BFA treatment, and siRNA-mediated knockdown of Arf GEFs, the ERGIC and Golgi generate membrane tubules instead of vesicles [76, 77].
These recent studies provide evidence that ERGIC membrane tubules are dependent on iPLA2-β in conditions of Arf1 and Arf4 knockdown. First, using live cell imaging, the authors found that ERGIC membrane tubules continuously grow and shrink in Arf1 and Arf4 knockdown cells, but that the overall morphology of the ERGIC was largely unaffected. Second, the formation of ERGIC membrane tubules was inhibited by antagonists of Ca2+-independent PLA2 enzymes, but not those of Ca2+-dependent enzymes. These observations therefore ruled out cPLA2α. More definitive evidence came from siRNA knockdown experiments, which showed that decreased levels of L-iPLA2 inhibited ERGIC membrane tubules formed in Arf1/4-deficient cells. Moreover, iPLA2-β translocates from the cytoplasm to the ERGIC membranes upon Arf1/4 knockdown. Importantly, the effects of iPLA2-β knockdown on ERGIC tubules were not limited to the special circumstance of Arf1/4-deficient cells, as the ERGIC membrane tubules that formed following temperature shift experiments were similarly reduced. Although the exact function of these membrane tubules is not fully established, secretory cargo can move between ERGIC clusters via membrane tubules.
These results strongly implicate iPLA2-β in the formation of ERGIC membrane tubules (Figure 2). It is suggested that Arf1/4 may negatively regulate the activity of iPLA2-β, which was shown by pull-down assays to interact with Arf1. Thus, loss of Arf1/4 would activate iPLA2-β and enhance the formation of ERGIC membrane tubules. Consistent with a role in membrane trafficking, iPLA2-B is recruited to the juxtanuclear Golgi region in response to secretory stimuli in pancreatic β–cells [78], and insulin secretion is reduced in PLA2G6 (gene symbol for iPLA2-β) null mice [79].
Interestingly, mutations in the human PLA2G6 gene result in several childhood neurological disorders including infantile neuroaxonal dystrophy (INAD) and idiopathic neurodegeneration with brain iron accumulation (NBIA) [80], which have been recapitulated in mouse models [81, 82]. These autosomal recessive neurodegenerative disorders, now referred to as PLA2G6-associated neurodegeneration (PLAN), cause severe cognitive and motor regression [83]. PLA2G6 knockout mice also display other defects including impaired hypercontractility in endothelial cells [84]. Currently, the connection between iPLA2-β function at the ERGIC and disease manifestations in PLAN is not understood, but it is tempting to speculate that neuronal abnormalities could arise from defects in secretory trafficking.
iPLA1γ
iPLA1γ (gene name DDH2) is a phosphatidic acid (PA)-specific PLA1 enzyme [85, 86] and member of the iPLA1 family of proteins that also includes PA-iPLA1 (iPLA1α) and p125 (iPLA1β) [87] (Table 1). iPLA1 enzymes hydrolyze the sn-1 bond of phospholipids to produce lysophospholipids and fatty acids and are involved in a variety of functions including membrane trafficking (Figure 1). For example, mammalian iPLA1β is implicated in regulating ER-Golgi function and interacts with the COPII vesicle component, Sec23p [88, 89].
Although iPLA1γ localizes to the Golgi complex , its role in regulating membrane trafficking is less clear than the other PLAs. Overexpression of iPLA1γ causes the ERGIC and Golgi to become dispersed. Based on results from siRNA knockdown, iPLA1γ was suggested to be involved in BFA-induced retrograde trafficking from the Golgi complex to the ER, and in maintaining the morphological integrity of the Golgi [85]. Subsequent studies, however, found that the siRNA used in these studies also caused an off-target loss of Rab6 [86], a key GTPase involved in retrograde trafficking [90], which could be responsible for the retrograde trafficking and Golgi loss phenotype. Using other siRNAs that did not result in reduction of Rab6, loss of iPLA1γ did not inhibit BFA-induced membrane tubules or disrupt Golgi structure [86]. Instead, loss of iPLA1γ had a modest kinetic effect on trafficking of the temperature-sensitive vesicular stomatitus virus G protein (VSV-G) from the Golgi to the plasma membrane but not from the ER to the Golgi. Interestingly, iPLA1γ appears to be more closely associated with the cis Golgi, and it rapidly cycles on and off Golgi membranes as shown by fluorescent recovery after photobleaching experiments. Unlike catalytically inactive PAFAH Ib [49], mutating the catalytic serine (S351A) of iPLA1γ reduced its ability to localize to Golgi membranes [85].
Exactly how iPLA1γ contributes to Golgi trafficking is unclear. If it is primarily localized to the cis Golgi, then we might expect that it functions early in the Golgi and is not directly involved in export from the TGN. One possibility is that iPLA1γ contributes to anterograde transport through the Golgi stack, exerting its effect at an early stage (Figure 2). It is also uncertain whether the phospholipase activity of iPLA1γ is required for its Golgi function, which will be important for understanding its potential role in trafficking.
Concluding remarks
Cytoplasmic PLA enzymes historically have been associated with signal transduction pathways; however, the reports reviewed here show that these enzymes have important functions in directly regulating membrane structure and function. Indeed, membrane tubule formation via the activity of cytoplasmic PLA enzymes has been understudied for many years, and we have just begun to reveal a new layer of complexity in regulation of trafficking through the Golgi complex. Although many unresolved questions remain or have arisen (Box 2), recent studies have shed light on a long-standing puzzle: what is the molecular relationship between membrane tubules and COPI coated vesicles? Using in vitro reconstitution assays, a recent study revealed an intimate relationship between COPI coated vesicles and membrane tubules, which is mediated by the opposing activities of cPLA2α and an integral membrane lysophospholipid acyltransferase, LPAAT3(γ) [91]. Their studies suggest that COPI proteins first drive bud formation from Golgi membranes, and then tubules or vesicles can arise depending on the relative strength of cPLA2α and LPAAT3(γ) activities. Following COPI bud formation, cPLA2γ promotes the formation of COP1 membrane tubules, whereas LPAAT3(γ) promotes the fission of vesicles. These results are consistent with previous studies which found that LPAAT3(γ), a lysophosphatidic acid specific acyltransferase, negatively regulates membrane tubule formation [92]. Thus, under normal conditions, the formation of COPI vesicles would be dictated by a balance of cPLA2α and LPAAT3(γ) activities. PLA2-driven membrane tubules, however, are not dependent on COPI proteins to initiate membrane bending, as clearly demonstrated by in vitro reconstruction experiments [49] and BFA treatment in vivo [76, 93-95].
Another important question to address is how are PLA enzymes targeted to the Golgi complex? With the exception of cPLA2α, where insights into enzyme targeting have begun to emerge, the mechanisms by which other PLAs selectively target organelle membranes remain unclear. One possible mechanism was recently suggested by the observation that PAFAH Ib α1 selectively binds to phosphatidylinositol 3- and 4-phosphate (PI3P and PI4P) [59], which are specific markers for endosomes and the Golgi complex, respectively [96, 97]. In addition, bioinformatic analyses of molecular interaction or co-expression database resources for molecules that interact with PLA enzymes may also provide starting points for interrogating this question.
The exact mechanism(s) by which PLA enzymes produce membrane curvature to generate tubules is not clear and will require reconstitution systems to definitively address this issue. The two hydrolysis products, lysophospholipids and fatty acid, may directly initiate membrane shape change by altering curve-inducing properties in one leaflet of the membrane [98]. Inhibiting the metabolism of arachidonic acid to downstream products does not prevent PLA2-dependent membrane tubulation, at least in some circumstances, thus arguing that accumulation of positive curve-inducing lysophospholipids is important [3, 4, 23]. Lysophospholipids and fatty acids may also aid in recruiting effector proteins to drive tubule and vesicle formation [98]. In addition, they may indirectly serve as secondary messengers to induce longer term effects or be funneled into other metabolic pathways. Finally, hydrolysis-independent mechanisms could also influence tubule formation. The enzymes themselves could contribute to membrane bending by virtue of their interaction with the cytoplasmic leaflet. For example, the C2 domain of cPLA2α inserts into membranes when bound to Ca2+ [18], and C2 domains of other proteins such as synaptotagmin and Doc2 also insert into membranes and induce tubule formation [99-101]. Thus, the C2 domain of cPLA2α could play a similar role. In addition, Golgi membrane tubule formation in vivo is often facilitated by microtubules and their associated motor proteins [102]. PLA2 enzymes and microtubule motors may intereact to coordinate membrane tubule formation, as evidenced by the connection between PAFAH Ib, Lis1 and dynein [49]. However, the details of these interactions and whether other PLA2 enzymes have cytoskeletal associations remain unclear. Future studies will no doubt focus on the molecular mechanisms by which PLA enzymes are integrated into the extensive machinery that controls the functional organization of the Golgi complex.
Box 1 Membrane Trafficking in the Secretory and Endocytic Pathways.
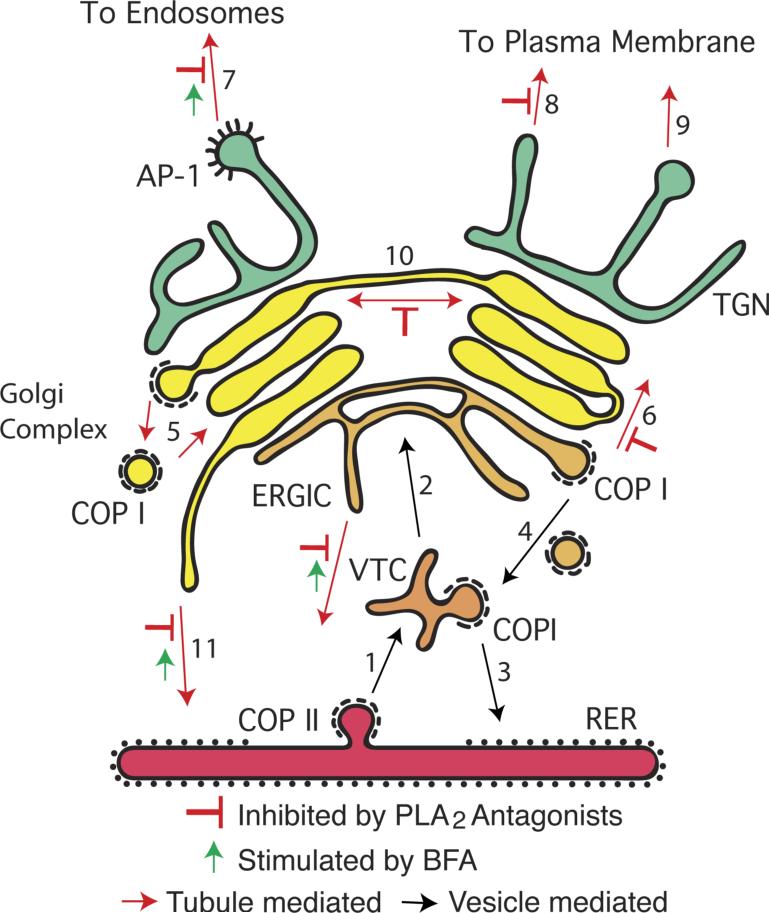
Trafficking through the secretory pathway involves coated vesicles and membrane tubules that sort, package, and transport material between organelles. These pathways usually define cycles of vectoral movement of cargoes for secretion, which are coupled to recycling pathways for bulk membrane return and conservation of trafficking machinery. Soluble and membrane-bounded cargo moving in the anterograde direction are first packaged into COPII coated vesicles at ER exit sites (Figure I, step 1). After shedding their coats, COPII vesicles rapidly fuse to form a pleiomorphic Vesicular Tubular Cluster (VTC), which is transported to the Golgi complex for possible maturation into the ER-Golgi-Intermediate Compartment (ERGIC) (step 2). The VTC can also recycle membranes to the ER via COPI coated vesicles (step 3) or receive COPI-dependent retrograde traffic from the Golgi that is destined for the ER (step 4). Anterograde cargo reaches the ERGIC, which may also constitute a biosynthetic precursor of the first Golgi cisterna via a maturation process. The mammalian Golgi complex is composed of a stack of cisternal membranes, with the cis-most receiving traffic from the ER and the trans-most exporting cargo to other destinations. The mechanism by which cargo moves from the cis to the trans Golgi remains under investigation [1]. Currently, three mechanisms are in vogue: i) cisternal maturation where individual cisternae are remodeled by COPI-dependent recycling of resident proteins (step 5), such that cis proteins become medial and then trans; ii) formation of tubular connections between adjacent cisternae [2, 23]; and iii) rapid partitioning via a two-phase membrane system [25]. These mechanisms are not mutually exclusive and their relative contributions could change to accommodate physiological demand. For example, when a bolus of secretory protein is released from the ER and reaches the Golgi, cisternae respond by elaborating membrane tubules, which form intercisternal bridges that facilitate anterograde trafficking through the cisternal stack (step 6). At the trans-most aspect is the TGN, which serves as the major depot for vesicle- and tubule-mediated export to other parts of the cell (steps 7-9). PLA2 inhibitors first revealed a role for phospholipase-dependent membrane tubules in Golgi transport (red inhibitor symbols) [4, 5], the biogenesis of cisternae into large ribbon-like structures (step 10), retrograde trafficking to the ER (step 11) [6], and export from the TGN (step 8) [7]. Many of these steps are stimulated by brefeldin A (BFA) [76, 93-95, 103].
Figure I.
A simplified diagram of the Golgi complex as a hub of membrane trafficking.
Box 2. Outstanding questions.
Do PLA2 enzymes modify the shape of membranes by producing positive curve-inducing lysophospholipids and/or by recruiting other factors that also contribute to tubule formation?
What are the molecular mechanisms used to recruit cytoplasmic PLA enzymes specifically to Golgi subcompartments and not other organelles?
How are the activities of these enzymes spatially and temporally regulated in response to secretory load?
Have all of the PLA enzymes directly involved in regulating Golgi (and endosome) function been identified?
Why do the Golgi complex, ERGIC and TGN respond to BFA by inducing massive membrane tubular networks?
Acknowledgments
The authors would like to thank Ben Clarke, Kevin Ha, Danielle Kalkofen, and Sricharan Murugesan for carefully reading this manuscript. This work was supported by NIH grant DK51596 to WJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emr S, et al. Journeys through the Golgi--taking stock in a new era. J Cell Biol. 2009;187:449–453. doi: 10.1083/jcb.200909011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trucco A, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 3.Brown WJ, et al. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.de Figueiredo P, et al. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci U S A. 1998;95:8642–8647. doi: 10.1073/pnas.95.15.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Figueiredo P, et al. Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol Biol Cell. 1999;10:1763–1782. doi: 10.1091/mbc.10.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Figueiredo P, et al. Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic. 2000;1:504–511. doi: 10.1034/j.1600-0854.2000.010608.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt JA, et al. A role for phospholipase A2 activity in membrane tubule formation and TGN trafficking. Traffic. 2010;11:1530–1536. doi: 10.1111/j.1600-0854.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima K, et al. A novel phospholipase A1 with sequence homology to a mammalian Sec23p-interacting protein, p125. J Biol Chem. 2002;277:11329–11335. doi: 10.1074/jbc.M111092200. [DOI] [PubMed] [Google Scholar]
- 11.Ohto T, et al. Identification of novel cytosolic phospholipase A2s, murine cPLA2δ, ε, and ζ, which form a gene cluster with cPLA2β. J Biol Chem. 2005;280:24576–24583. doi: 10.1074/jbc.M413711200. [DOI] [PubMed] [Google Scholar]
- 12.Balestrieri B, et al. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J Biol Chem. 2006;281:6691–6698. doi: 10.1074/jbc.M508314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 14.Balsinde J, et al. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 15.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 17.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 18.Leslie CC, et al. Localization and function of cytosolic phospholipase A2α at the Golgi. Biochimie. 2010;92:620–626. doi: 10.1016/j.biochi.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JH, et al. The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells. Mol Biol Cell. 2004;15:371–383. doi: 10.1091/mbc.E03-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal S, et al. Cytosolic phospholipase A2-α and cyclooxygenase-2 localize to intracellular membranes of EA.hy.926 endothelial cells that are distinct from the endoplasmic reticulum and the Golgi apparatus. Febs J. 2005;272:1278–1290. doi: 10.1111/j.1742-4658.2005.04565.x. [DOI] [PubMed] [Google Scholar]
- 21.Herbert SP, et al. Cytosolic phospholipase A2-α mediates endothelial cell proliferation and is inactivated by association with the Golgi apparatus. Mol Biol Cell. 2005;16:3800–3809. doi: 10.1091/mbc.E05-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbert SP, Walker JH. Group VIA calcium-independent phospholipase A2 mediates endothelial cell S phase progression. J Biol Chem. 2006;281:35709–35716. doi: 10.1074/jbc.M600699200. [DOI] [PubMed] [Google Scholar]
- 23.San Pietro E, et al. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan-Klapisz E, et al. Golgi-associated cPLA2α regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol Biol Cell. 2009;20:4225–4234. doi: 10.1091/mbc.E08-02-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson GH, et al. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamour NF, et al. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J Biol Chem. 2009;284:26897–26907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura H, et al. Modulation of the activity of cytosolic phospholipase A2α (cPLA2α) by cellular sphingolipids and inhibition of cPLA2α by sphingomyelin. J Lipid Res. 2010;51:720–728. doi: 10.1194/jlr.M002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu M, et al. Effects of ceramide, ceramidase inhibition and expression of ceramide kinase on cytosolic phospholipase A2α; additional role of ceramide-1-phosphate in phosphorylation and Ca2+ signaling. Cell Signal. 2009;21:440–447. doi: 10.1016/j.cellsig.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Ball A, et al. Interfacial membrane docking of cytosolic phospholipase A2 C2 domain using electrostatic potential-modulated spin relaxation magnetic resonance. Proc Natl Acad Sci U S A. 1999;96:6637–6642. doi: 10.1073/pnas.96.12.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. J Biol Chem. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- 31.Wooten RE, et al. Novel translocation responses of cytosolic phospholipase A2α fluorescent proteins. Biochim Biophys Acta. 2008;1783:1544–1550. doi: 10.1016/j.bbamcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micaroni M, et al. Synchronous intra-Golgi transport induces the release of Ca2+ from the Golgi apparatus. Exp Cell Res. 2010;316:2071–2086. doi: 10.1016/j.yexcr.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Pulvirenti T, et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008;10:912–922. doi: 10.1038/ncb1751. [DOI] [PubMed] [Google Scholar]
- 34.Das S, et al. Mechanism of group IVA cytosolic phospholipase A2 activation by phosphorylation. J Biol Chem. 2003;278:41431–41442. doi: 10.1074/jbc.M304897200. [DOI] [PubMed] [Google Scholar]
- 35.Bonventre JV, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 36.Uozumi N, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 37.Adler DH, et al. Inherited human cPLA2α deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed KA, et al. Functional Characterization of Mutations in Inherited Human cPLA2 Deficiency. Biochemistry. 2011;50:1731–1738. doi: 10.1021/bi101877n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler DH, et al. The enteropathy of prostaglandin deficiency. J Gastroenterol. 2009;44(Suppl 19):1–7. doi: 10.1007/s00535-008-2253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattori M, et al. The catalytic subunit of bovine brain platelet-activating factor acetylhydrolase is a novel type of serine esterase. J Biol Chem. 1994;269:23150–23155. [PubMed] [Google Scholar]
- 41.Hattori M, et al. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1993;268:18748–18753. [PubMed] [Google Scholar]
- 42.Ho YS, et al. Probing the substrate specificity of the intracellular brain platelet-activating factor acetylhydrolase. Protein Eng. 1999;12:693–700. doi: 10.1093/protein/12.8.693. [DOI] [PubMed] [Google Scholar]
- 43.Ho YS, et al. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature. 1997;385:89–93. doi: 10.1038/385089a0. [DOI] [PubMed] [Google Scholar]
- 44.Tarricone C, et al. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–821. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Arai H, et al. Platelet-activating factor acetylhydrolase (PAF-AH). J Biochem (Tokyo) 2002;131:635–640. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre TM, et al. The emerging roles of PAF acetylhydrolase. J Lipid Res. 2009;50(Suppl):S255–259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koizumi H, et al. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J Biol Chem. 2003;278:12489–12494. doi: 10.1074/jbc.M211836200. [DOI] [PubMed] [Google Scholar]
- 48.Yan W, et al. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bechler ME, et al. The phospholipase complex PAFAH Ib regulates the functional organization of the Golgi complex. J Cell Biol. 2010;190:45–53. doi: 10.1083/jcb.200908105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Judson BL, Brown WJ. Assembly of an intact Golgi complex requires phospholipase A2 (PLA2) activity, membrane tubules, and dynein-mediated microtubule transport. Biochem Biophys Res Commun. 2009;389:473–477. doi: 10.1016/j.bbrc.2009.08.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato M, Dobyns WB. Lissencephaly and the molecular basis of neuronal migration. Hum Mol Genet. 2003;12:R89–96. doi: 10.1093/hmg/ddg086. [DOI] [PubMed] [Google Scholar]
- 52.Yamada M, et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. Embo J. 2008;27:2471–2483. doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 54.Wynshaw-Boris A, et al. Lissencephaly: mechanistic insights from animal models and potential therapeutic strategies. Semin Cell Dev Biol. 2010;21:823–830. doi: 10.1016/j.semcdb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geuze HJ, et al. Membranes of sorting organelles display lateral heterogeneity in receptor distribution. J Cell Biol. 1987;104:1715–1723. doi: 10.1083/jcb.104.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geuze HJ, et al. Intracellular site of asiaologlycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 57.de Figueiredo P, et al. Inhibition of transferrin recycling and endosome tubulation by phospholipase A2 antagonists. J. Biol. Chem. 2001;276:47361–47370. doi: 10.1074/jbc.M108508200. [DOI] [PubMed] [Google Scholar]
- 58.Doody AM, et al. Cytoplasmic phospholipase A2 antagonists inhibit multiple endocytic membrane trafficking pathways. Biochem Biophys Res Commun. 2009;388:695–699. doi: 10.1016/j.bbrc.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bechler ME, et al. The phospholipase A2 enzyme complex PAFAH Ib mediates endosomal membrane tubule formation and trafficking. Mol Biol Cell. 2011;22:2348–2359. doi: 10.1091/mbc.E09-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manya H, et al. Biochemical characterization of various catalytic complexes of the brain platelet-activating factor acetylhydrolase. J Biol Chem. 1999;274:31827–31832. doi: 10.1074/jbc.274.45.31827. [DOI] [PubMed] [Google Scholar]
- 61.Manya H, et al. Switching of platelet-activating factor acetylhydrolase catalytic subunits in developing rat brain. J. Biol. Chem. 1998;273:18567–18572. doi: 10.1074/jbc.273.29.18567. [DOI] [PubMed] [Google Scholar]
- 62.Zhang G, et al. The Pafah1b complex interacts with the Reelin receptor VLDLR. PLoS ONE. 2007;2:e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsson PK, et al. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 64.Ackermann EJ, et al. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 65.Su X, et al. Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2004;279:21740–21748. doi: 10.1074/jbc.M314166200. [DOI] [PubMed] [Google Scholar]
- 66.Ramanadham S, et al. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J Biol Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- 67.Gregory A, et al. Neurodegeneration associated with genetic defects in phospholipase A2. Neurology. 2008;71:1402–1409. doi: 10.1212/01.wnl.0000327094.67726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan NV, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nature genetics. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hooks SB, Cummings BS. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol. 2008;76:1059–1067. doi: 10.1016/j.bcp.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-Tekaya H, et al. ADP Ribosylation Factors 1 and 4 and Group VIA Phospholipase A2 Regulate Morphology and Intraorganellar Traffic in the Endoplasmic Reticulum-Golgi Intermediate Compartment. Mol. Biol. Cell. 2010;21:4130–4140. doi: 10.1091/mbc.E10-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 72.Sannerud R, et al. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr Opin Cell Biol. 2003;15:438–445. doi: 10.1016/s0955-0674(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 73.Bui QT, et al. Large Arf1 guanine nucleotide exchange factors: evolution, domain structure, and roles in membrane trafficking and human disease. Mol Genet Genomics. 2009;282:329–350. doi: 10.1007/s00438-009-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volpicelli-Daley LA, et al. Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol Biol Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peyroche A, et al. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- 76.Lippincott-Schwartz J, et al. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 77.Szul T, et al. Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J Cell Sci. 2007;120:3929–3940. doi: 10.1242/jcs.010769. [DOI] [PubMed] [Google Scholar]
- 78.Bao S, et al. Beta-cell calcium-independent group VIA phospholipase A2 (iPLA2β): tracking iPLA2β movements in response to stimulation with insulin secretagogues in INS-1 cells. Diabetes. 2004;53(Suppl 1):S186–189. doi: 10.2337/diabetes.53.2007.s186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao S, et al. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic beta-cells and in iPLA2β-null mice. Am J Physiol Endocrinol Metab. 2008;294:E217–229. doi: 10.1152/ajpendo.00474.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gregory A, Hayflick SJ. Genetics of neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2011;11:254–261. doi: 10.1007/s11910-011-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malik I, et al. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shinzawa K, et al. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurian MA, et al. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN). Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 84.Dietrich HH, et al. Genetic ablation of calcium-independent phospholipase A2β causes hypercontractility and markedly attenuates endothelium-dependent relaxation to acetylcholine. Am J Physiol Heart Circ Physiol. 2010;298:H2208–2220. doi: 10.1152/ajpheart.00839.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morikawa RK, et al. Intracellular phospholipase A1γ (iPLA1γ) is a novel factor involved in coat protein complex I- and Rab6-independent retrograde transport between the endoplasmic reticulum and the Golgi complex. J Biol Chem. 2009;284:26620–26630. doi: 10.1074/jbc.M109.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato S, et al. Golgi-localized KIAA0725p regulates membrane trafficking from the Golgi apparatus to the plasma membrane in mammalian cells. FEBS Lett. 2010;584:4389–4395. doi: 10.1016/j.febslet.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 87.Inoue A, Aoki A. Phospholipase A1: structure, location and function. Future Lipidol. 2006;1:687–700. [Google Scholar]
- 88.Shimoi W, et al. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- 89.Tani K, et al. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J Biol Chem. 1999;274:20505–20512. doi: 10.1074/jbc.274.29.20505. [DOI] [PubMed] [Google Scholar]
- 90.Darchen F, Goud B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie. 2000;82:375–384. doi: 10.1016/s0300-9084(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 91.Yang JS, et al. COPI acts in both vesicular and tubular transport. Nat Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lippincott-Schwartz J, et al. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 94.Wood SA, Brown WJ. The morphology but not the function of endosomes and lysosomes is altered by brefeldin-A. J Cell Biol. 1992;119:273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood SA, et al. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 96.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 97.Sato TK, et al. Location, location, location: membrane targeting directed by PX domains. Science. 2001;294:1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- 98.Bankaitis VA. The Cirque du Soleil of Golgi membrane dynamics. J Cell Biol. 2009;186:169–171. doi: 10.1083/jcb.200907008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Groffen AJ, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martens S, et al. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 101.McMahon HT, et al. Membrane curvature in synaptic vesicle fusion and beyond. Cell. 2010;140:601–605. doi: 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 102.Lippincott-Schwartz J. Cytoskeletal proteins and Golgi dynamics. Curr Opin Cell Biol. 1998;10:52–59. doi: 10.1016/s0955-0674(98)80086-0. [DOI] [PubMed] [Google Scholar]
- 103.Lippincott-Schwartz J, et al. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]