Abstract
Sphingosine-1-phosphate-induced α1B-adrenergic receptor desensitization and phosphorylation was studied in rat-1 fibroblasts stably expressing enhanced green fluorescent protein-tagged adrenoceptors. Sphingosine-1-phosphate induced adrenoceptor desensitization and phosphorylation through a signaling cascade that involved phosphoinositide 3-kinase and protein kinase C activities. The autocrine/paracrine role of sphingosine-1-phosphate was also studied. It was observed that activation of receptor tyrosine kinases, such as insulin growth factor-1 (IGF-I) and epidermal growth factor (EGF) receptors increased sphingosine kinase activity. Such activation and consequent production of sphingosine-1-phosphate appears to be functionally relevant in IGF-I- and EGF-induced α1B-adrenoceptor phosphorylation and desensitization as evidenced by the following facts: a) expression of a catalytically inactive (dominant-negative) mutant of sphingosine kinase 1 or b) S1P1 receptor knockdown markedly reduced this growth factor action. This action of sphingosine-1-phosphate involves EGF receptor transactivation. In addition, taking advantage of the presence of the eGFP tag in the receptor construction, we showed that S1P was capable of inducing α1B-adrenergic receptor internalization and that its autocrine/paracrine generation was relevant for internalization induced by IGF-I. Four distinct hormone receptors and two autocrine/paracrine mediators participate in IGF-I receptor- α1B-adrenergic receptor crosstalk.
Keywords: α1B-adrenoceptor, α1B-adrenergic receptor, sphingosine-1-phosphate, EGF receptor, transactivation
1. Introduction
The actions of adrenaline and noradrenaline (NA) are mediated through three families of receptors with three members each, i. e., the α1- family (comprising theα1A-, α1B- and α1D-adrenergic receptors (ARs); the α2- family (α2A-, α2B- and α2C-ARs) and the β-adrenergic family(β1-, β2- and β3-ARs) [1].
The α1- family participates in many of the physiological effects of these catecholamines and is also involved in the pathogenesis of some diseases [2–4]. α1B-ARs were the first receptors to be cloned of this family [5] and have been studied in much greater detail. Evidence indicates that the function of these receptors is regulated through cycles of phosphorylation-dephosphorylation [2] and that protein kinases such as G protein coupled receptor kinases 2 and 3 [6, 7] and protein kinase C (PKC) play the central roles [8]. G protein coupled receptor kinases phosphorylate agonist-occupied receptors, being mainly responsible for homologous desensitization [6, 7] whereas PKC mediates α1B-AR phosphorylation induced by phorbol esters and many non-adrenergic hormones and neurotransmitters participating in the heterologous desensitization of these ARs [9–18]. α1B-AR phosphorylation sites have been determined at Ser404, Ser408, and Ser410 (for G protein receptor kinases) and at Ser394 and Ser400 (for PKC)[8]. Another key enzyme is the protein and phospholipid kinase, phosphoinositide 3-kinase (PI3K), which appears to act upstream of PKC [2]. Much less is known concerning the role of protein phosphatases in regulating α1B-AR phosphorylation; but evidence indicates that inhibition of these enzymes increases the phosphorylation state of α1B-ARs [19].
It is now known that G-protein coupled receptor-induced epidermal growth factor (EGF) receptor transactivation involves stimulation of metalloproteinases. These proteases cause shedding of HB-EGF from the plasma membrane and subsequent activation of EGF receptor intrinsic tyrosine kinase, resulting in the triggering of intracellular signaling [20, 21]. Surprisingly, this process is much more general than we could have anticipated and is involved in both homologous and heterologous desensitization and phosphorylation of α1B-ARs [9, 10, 15, 16].
In our study on IGF-I-induced α1B-AR desensitization and phosphorylation we observed that EGF receptor transactivation as well as pertussis toxin-sensitive G proteins were involved [16]; in fact, the action of other hormones/growth factors that act through receptor tyrosine kinase receptors also share these characteristics [16]. These data suggest the possibility that receptor tyrosine kinases could couple to pertussis toxin-sensitive G proteins and in this way activate metalloproteinases and the EGF receptor transactivation process [16]. G protein involvement in receptor tyrosine kinase action has been frequently observed, but the mechanisms have been elusive [22]. However, the possibility that receptor tyrosine kinases might couple to G proteins has been difficult to prove. At least one other possibility exists. It has been elegantly shown that many growth factors, hormones and neurotransmitters can activate sphingosine kinase-1 (SPHK-1), which generates sphingosine-1-phosphate (S1P) [23–26]; this phospholipid acts in an autocrine loop, activating cells through its own G protein-coupled receptors [27]. We have suggested the possibility that the connection between the IGF-I receptor and metalloproteinases could involve the generation and action of this sphingophospholipid [15, 28]. This possibility was tested and the results are presented here. Our results indicate that IGF-I-induced α1B-AR desensitization and phosphorylation involves two autocrine/paracrine mediators (S1P and HB-EGF) and four receptor types (IGF-I receptors, S1P1, EGF receptors and α1B-ARs). Additionally, receptor phosphorylation is associated with internalization. Here we show that S1P and IGF-I induce α1B-AR internalization and that their effects are interconnected.
2. Materials and Methods
2.1. Materials
Sphingosine-1-phosphate, (-)-noradrenaline, IGF-I, endothelin-1, tetradecanoyl phorbol acetate, staurosporine, wortmannin, bis-indolyl-maleimide I and protease inhibitors were purchased from Sigma Chemical Co. LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one) and AG1478 were obtained from Calbiochem. Dulbecco’s modified Eagle’s medium, fetal bovine serum, trypsin, antibiotics, and other reagents used for cell culture were from Life Technologies. [γ-32P]ATP (3000 Ci/mmol) and [32P]Pi (8500–9120 Ci/mmol) were obtained from Perkin Elmer Life Sciences, while agarose-coupled protein A was from Upstate Biotechnology. DNA purification kits were obtained from Qiagen. Anti-HB-EGF neutralizing antibodies and chemiluminescence’s kits were purchased from Pierce. The pDrive cloning system was purchased from QIAGEN and the pEGFP-N1 vector from Clontech. FM4-64 and Fura 2AM were obtained from Molecular Probes. Plasmids for expression of wild-type SPHK-1 and the catalytically inactive (dominant-negative) mutant were kindly provided to us by Dr. Stuart M. Pitson (The University of Adelaide, Australia)[29]. Primary antibodies against SPHK-1, β-actin and S1P1 receptor were from Santa Cruz Biotechnology whereas those against Flag were from Sigma Chemical Co. BB94 was generously provided to us by Dr. S. Mobashery (University of Notre Dame, Notre Dame, IN, USA).
2.2. Cell line
The cell line used was described previously [15]. Briefly, a line of rat-1 fibroblast (American Type Culture Collection) stably expressing human α1B-ARs tagged with enhanced green fluorescent protein (eGFP) was generated. Cells were cultured in a selection media: glutamine-containing high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 300 μg/ml of the neomycin analog, G-418 sulfate, 100 μg/ml streptomycin, 100 U/ml penicillin, and 0.25 μg/ml amphotericin B at 37°C under a 95% air/5% CO2 atmosphere. These cells express α1B-ARs with a density of 900–1600 fmol/mg membrane cell protein (in the range observed in rodent hepatocytes [30]), with high affinity for [3H]prazosin (Kd 0.2-0.3 nM). Photolabeled receptors were identified as a band of Mr ≈ 115–120 kDa and can be immunoprecipitated with an anti-eGFP antiserum generated in our laboratory with reasonably high efficacy (≈ 20%, as evidenced by radioactive photo-labeled receptor immunoprecipitation); these receptors are fully functional and have essentially the same pharmacological characteristics than wild-type receptors [15].
2.3. Intracellular calcium determinations
Cells were loaded with 2.5 μM of the fluorescent Ca2+ indicator, Fura-2/AM, in Krebs-Ringer-HEPES containing 0.05% bovine serum albumin, pH 7.4 for 1 h at 37°C and then washed three times to eliminate unincorporated dye. Fluorescence measurements were carried out at 340 and 380 nm excitation wavelengths and at 510 nm emission wavelength, with a chopper interval set at 0.5 sec, utilizing an AMINCO-Bowman Series 2 luminescence spectrometer (Rochester, NY, USA). Intracellular calcium ([Ca2+]i) was calculated according to Grynkiewicz et. al. [31].
2.4. Phosphorylation of α1B-adrenoceptors
The procedure employed to study α1B-AR phosphorylation, has been previously described in detail [15, 16, 18]. In brief, cells were maintained overnight in phosphate-free Dulbecco’s modified Eagle’s medium without serum. The following day, cells were incubated in 1 ml of the same medium containing [32P]Pi (50 μCi/ml) for 3 h at 37°C. Labeled cells were stimulated as indicated, washed with ice-cold phosphate-buffered saline, and solubilized with 0.5 ml of ice-cold buffer containing 50 mM Tris-HCl pH 8.0, 50 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 10 mM NaF, 1 mM Na3VO4, 10 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 1 mM p-serine, 1 mM p-threonine, 1 mM p-tyrosine, and protease inhibitors [15]. Cell lysates were centrifuged at 12,700 × g for 15 min at 4°C and supernatants were incubated overnight at 4°C with an anti-eGFP antiserum generated in our laboratory [15, 32] and protein A-Sepharose. After two washes with 50 mM Hepes, 50 mM NaH2PO4, 100 mM NaCl, pH 7.2, 1% Triton X-100, 0.1% SDS, and 100 mM NaF, pellets containing the immune complexes were boiled for 5 min in SDS-sample buffer containing 5% β-mercaptoethanol, and subjected to SDS-polyacrylamide gel electrophoresis. Gels were dried and exposed for 18–24 h and level of receptor phosphorylation was assessed with a Molecular Dynamics PhosphorImager using the Imagequant software (Amersham Biosciences). Data fell within the linear range of detection of the apparatus and were plotted using Prism 4 from GraphPad software.
2.5. Transfection for transient expression
Cells were transfected utilizing Lipofectamine 2000 following the manufacturer’s instructions and were cultured as described previously. Repetition of transfection (2–3 times) increased efficiency as shown by Yamamoto et. al. [33] (from 20% to 40–50% in our experiments); cells were employed 3–4 days after transfection. Using this transfection repetition protocol the effect of the SPHK catalytically inactive (dominant-negative) mutant was evident for up to 7 days, decreasing afterward.
2.6. Construction of human/rat S1P1 receptor short hairpin RNA
For gene knockdown we used the short hairpin RNA oligonucleotide 5′-TGCTGTTGACAGTGAGCGAGCTCTACCACAAGCACTATATTAGTGAAGCCAC AGATGTAATATAGTGCTTGTGGTAGAGCGTGCCTACTGCCTCGGA-3′ containing the sense/antisense target sequence against human/rat S1PR1. This oligonucleotide was employedas template for cloning the short hairpin RNA into the pSHAG MAGIC2 (pSM2) vector (Openbiosystems, Huntsville, AL) as reported by Paddison et al. [34]. In brief, we PCR amplified the oligonucleotide utilizing universal primers containing XhoI (5′-CAGAAGGCTCGAGAAGGTATATTGCTGTTGACAGTGAGCG-3′) and EcoRI (5′-CTAAAGTAGCCCCTTGAATTCCGAGGCAGTAGGCA-3′) sites. These PCR fragments were digested, cloned into the hairpin cloning site of pSM2 vector and transformed into PIR1-competent bacteria. After growth selection with chloramphenicol and kanamycin, we obtained the pSM2 vector containing the shRNA against S1P1, which was tested for gene knockdown by transient transfection as described previously.
2.7 Detection of S1P1 receptor expression by RT-PCR
Total RNA was isolated using TRIzol® reagent (Invitrogen) according to the manufacturer’s instructions. For reverse transcription–PCR we used, Promega Access RT-PCR System A1250, Primers were as follows: to amplify S1P1 receptor, forward primer 5′-GCTGCTTGATCATCCTAGAG and reverse primer 5′-GAAAGGAGCGCGAGCTGTTG-3′ [35] and to amplify GAPDH, forward primer 5′-GGTGTGAACCACGAGAAATATGAC-3′ and reverse primer 5′-CTCCAGGCGGCATGTCAGATCCAC-3′ [36] were synthesized at the Molecular Biology Unit of our Institute.
2.8. Western blot assays
Cells were washed with ice-cold phosphate-buffered saline and lysed for 1 h in buffer containing NaCl 150 mM, Tris 50 mM (pH 7.4), EDTA 1 mM and 1 % Nonidet P40 on ice. Lysates were centrifuged at 12,700 × g for 15 min and proteins in supernatants were separated by electrophoresis on 10% SDS-PAGE. Proteins were electrotransferred to nitrocellulose membranes and immunoblottings were performed using the same membranes. Incubation with primary selective antibodies was conducted for 12 hs at 4 °C and with the secondary antibody for 30 min at room temperature. Super signal-enhanced chemiluminescence’s kits were employed exposing the membranes to X-Omat X-ray films. Signals were quantified by densitometric analysis utilizing the Scion Image software from Scion Corporation (Frederick, MD, USA).
2.9. Sphingosine Kinase (SPHK) activity
Activity was assayed in cell extracts essentially as described by Olivera and Spiegel [37]. In brief cells were incubated in the presence of vehicle or the agents indicated for 15 min and extracts were obtained. SPHK activity was determined using D-sphingosine and [γ-32P]ATP as substrates, reactions were initiated by addition of the cellular extracts and terminated with chloroform. Lipids were extracted and separated by thin-layer chromatography as described [37]. Reaction was linear with respect to time and amount of extract under the conditions employed.
2.10. Confocal microscopy
Confocal images were obtained using a Flowview FV 1000 laser confocal system (Olympus) attached/interfaced to an OLYMPUS IX81 inverted light microscope with a 40× glycerol-immersion objective; eGFP was excited using the 488-nm line of a krypton/argon laser and the emitted fluorescence detected with a 515–540-nm band pass filter. Operating the laser at a low power setting (97–99% attenuation) substantially reduced photobleaching and photodamage. Confocal images were viewed and processed using the FV10-ASW 1.6 software (Olympus). FM4-64 was used as a plasma membrane marker. To estimate receptor densities the area corresponding to the cell membrane (as guided by membrane marker FM4-64) was selected in each image and the amount of green fluorescence was quantified using ImageJ with Plugging Analyze Particles software [38].
2.11. Statistical Analysis
Statistical analysis between comparable groups was performed using ANOVA with Bonferroni’s post-test and was performed with the software included in the GraphPad Prism program.
3. Results
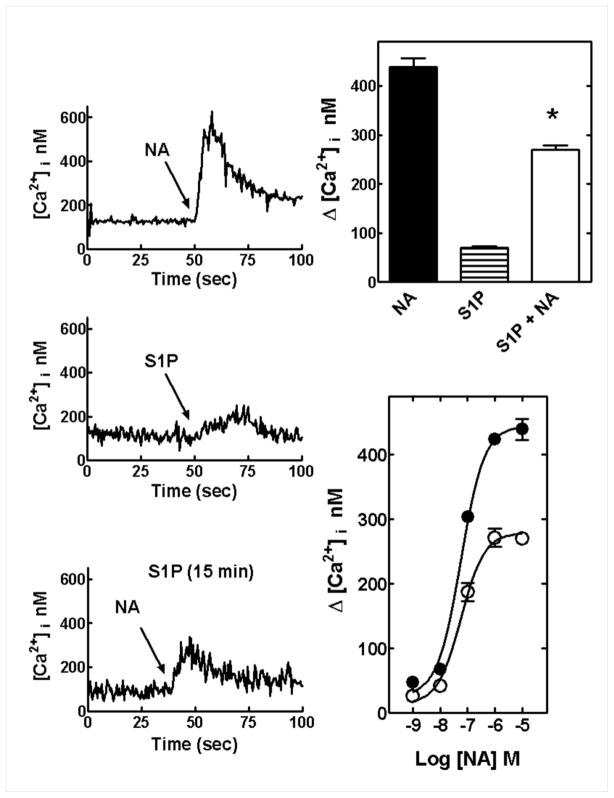
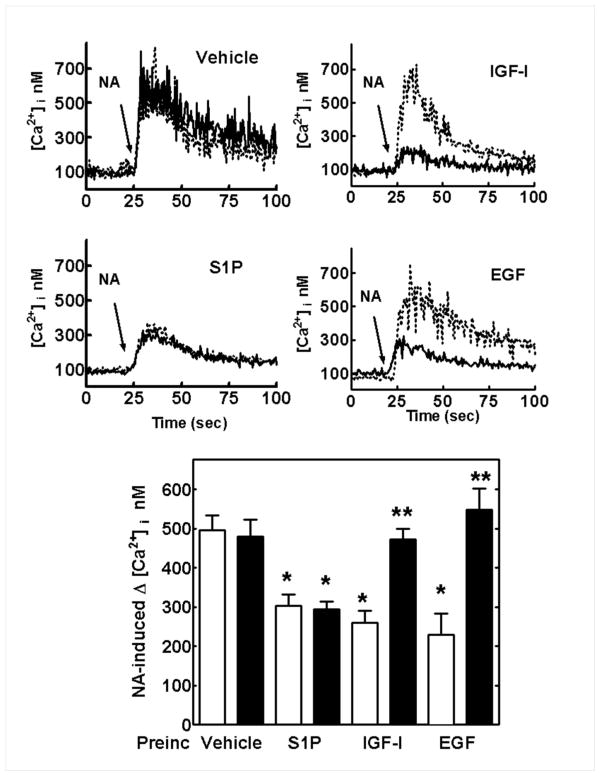
We first wanted to determine wether S1P plays a role in α1B-AR desensitization and phosphorylation. As shown in Fig. 1, 10 μM noradrenaline (NA) induced an immediate and robust ≈ 4-fold increase in intracellular calcium, which remained clearly above basal levels for more than 50 sec (upper left panel). In contrast, S1P (1 μM) induced a much smaller response (≈ 50% over basal values) that developed slowly and vanished after 30–40 seconds. When cells were incubated with S1P, for 15 min, and then challenged with NA the response to the adrenergic agent was markedly reduced (Fig. 1, representative tracings are shown in the left panel). Concentration-response curves in cells treated for 15 min with vehicle or S1P showed that the phospholipid treatment markedly reduced (by 40–50%) the maximal effect of NA without affecting its EC50 value (50–75 nM). The possibility of some calcium-storage depletion cannot be ruled out. However, 100 nM endothelin-1 induced a more robust effect than NA on this parameter and it was not decreased in cells treated with 1 μM S1P for 15 min, as compared with untreated cells (Supplementary Fig. S1).
Fig. 1.
Effect of sphingosine-1-phosphate (S1P) on α1B-adrenergic receptor (α1B-AR) action. Left panels: representative tracings of intracellular free-calcium concentration ([Ca2+]i); upper tracing stimulation with 10 μM noradrenaline (NA), middle tracing with 1 μM S1P and lower tracing of cells preincubated with 1 μM S1P and challenged with 10 μM noradrenaline (NA). Upper right panel shows quantitative levels of intracellular calcium observed in cells challenged with 10 μM noradrenaline (NA), 1 μM S1P or 10 μM noradrenaline (NA) after a 15 min preincubation with 1 μM S1P (S1P + NA). Means are plotted with vertical lines representing the S.E.M. of 6 to 8 determinations using different cells preparations. Lower right panel: concentration- response curves of cells to noradrenaline (NA) preincubated for 15 min in the absence of any agent (solid circles) or presence of 1 μM S1P (open circles). Means are plotted with vertical lines representing the S.E.M. of 4 to 6 determinations using different cell preparations. * p < 0.001 vs. the other groups.
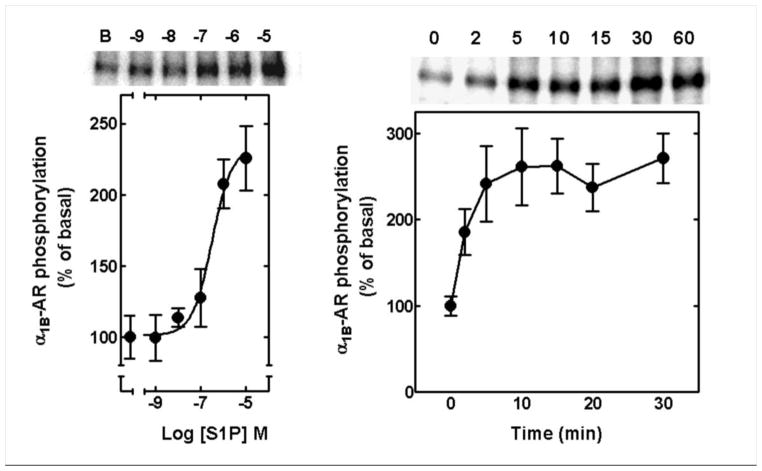
Next we explored the possibility that functional α1B-AR desensitization could be associated with adrenoceptor phosphorylation. As expected, S1P induced a concentration-dependent increase in the phosphorylation state of α1B-ARs; the maximal increase was ≈ 2–2.5-fold with an EC50 of ≈ 0.5–1 μM (Fig. 2, left panel). S1P action was rapid inducing clearly detectable α1B-AR phosphorylation 2 min after its addition, reaching a maximum at ≈ h10 min and remaining at a plateau for 60 min (Fig. 2, right panel).
Fig. 2.
Effect of sphingosine-1-phosphate (S1P) on α1B-adrenergic receptor (α1B-AR) phosphorylation. Left panel: cells were incubated with the indicated concentration of SIP for 15 min. Right panel: cells were incubated in the presence of 1 μM S1P for the indicated times. Means are plotted with vertical lines representing the S.E.M. of 4 to 6 determinations using different cells preparations. Representative autoradiograms are shown. B, basal.
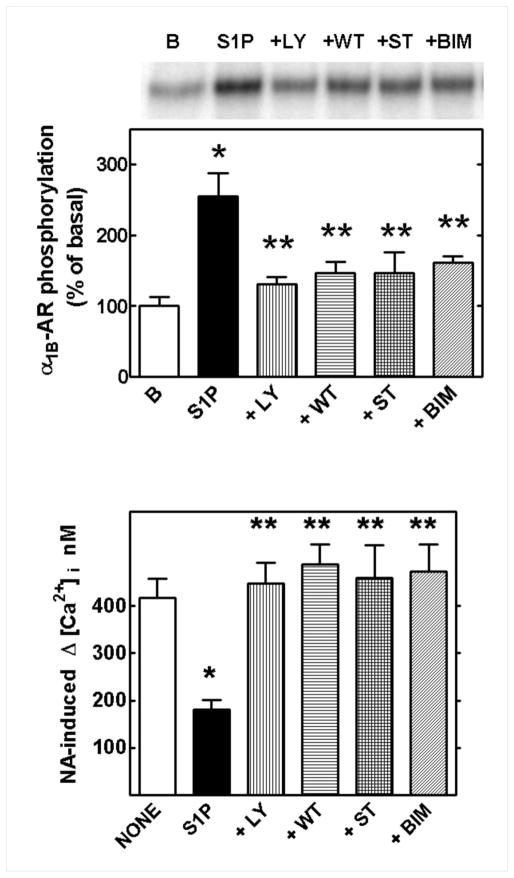
The signaling pathways involved in S1P-induced α1B-AR phosphorylation and desensitization were next explored. As anticipated, based on previous findings from our laboratory with other agents [2, 9–18], inhibitors of PI3K, such as wortmannin and LY294002, staurosporine, a general protein kinase inhibitor, and bis-indolyl-maleimide I, a selective PKC inhibitor, did not alter basal receptor phosphorylation but were able to block S1P-induced α1B-AR phosphorylation (Fig. 3, upper panel). In addition, S1P-induced functional desensitization of these ARs (as reflected by the increase in intracellular calcium) was also clearly blocked by these inhibitors of protein and phospholipids kinases (Fig. 3, lower panel). Besides clarifying the signaling pathway involved in the action of S1P, the effect of the inhibitors also indicates that calcium-depletion was not a major player in the functional effect.
Fig. 3.
Effect of protein kinase C and phosphoinositide 3-kinase inhibitors on α1B-adrenergic receptor (α1B-AR) phosphorylation and action. Cells were preincubated in the absence or presence of the following inhibitors for 30 min 1μM LY294002 (+LY), 100 nM wortmannin (+WT), 100 nM staurosporine (+ST) or 1μM bis-indolyl-maleimide I (+BIM). To study (α1B-AR) phosphorylation (upper panel), cells were incubated in the absence of any agent (B, basal) or challenged with 1 μM S1P (all of the remaining conditions) for 15 min. A representative autoradiogram is shown. To study the functional repercussion (lower panel): cells were preincubated with the inhibitors as indicated above and then no agent (NONE) or 1 μM S1P was added. The increase in intracellular calcium induced by 10 μM noradrenaline (NA) was recorded and the maximal effect observed is presented. Means are plotted with vertical lines representing the S.E.M. of 5 to 7 (receptor phosphorylation) or 12 to 15 (intracellular calcium) determinations using different cell preparations. *p < 0.001 vs basal (B, NONE), **p < 0.001 vs S1P.
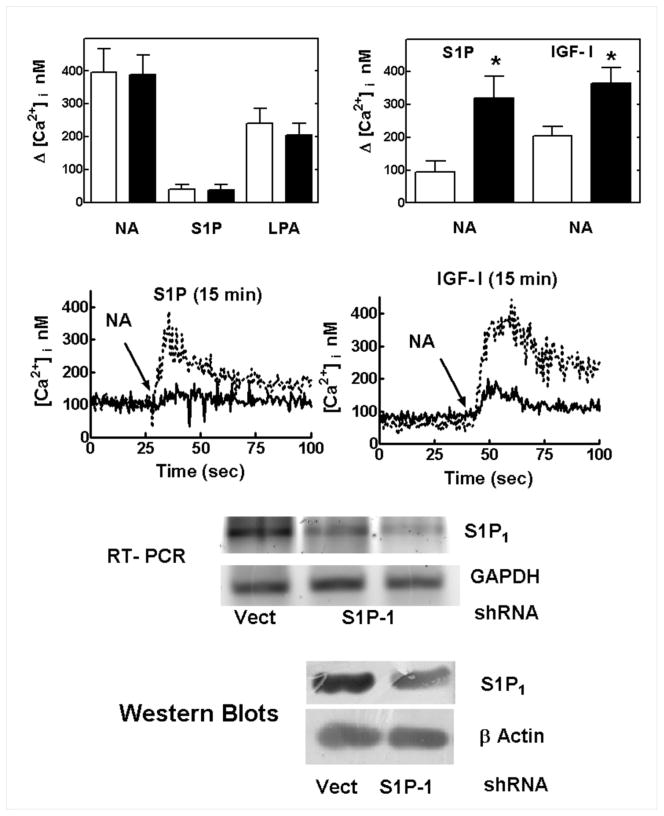
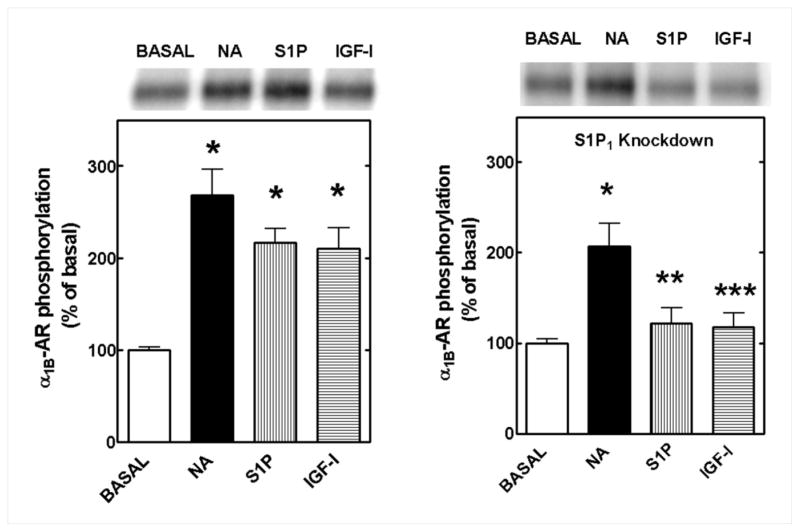
As mentioned previously, the effects of S1P can be mediated through a family of S1P receptors [27]. Interestingly, however, S1P1 receptors are widely distributed among cells and tissues and appear to mediate the paracrine/autocrine action of S1P [23–26]. We tested the possibility that these receptors could be involved in S1P actions by knocking down S1P1 expression using shRNA technology. Expression of S1P1 at the level of mRNA and protein was decreased 30–40% as shown by both RT-PCR and Western blotting; GAPDH (RT-PCR) and β-actin (Western blots) were used as controls and no changes were observed (Fig. 4, bottom panel). The pSM2 empty vector did not alter S1P1 receptor expression. None of these treatments changed the calcium response to 10 μM NA, 1 μM S1P or 100 ng/ml IGF-I (Fig. 4, upper left panel). Both S1P-mediated and IGF-mediated α1B-AR desensitization were clearly observed in cells transfected with the pSM2 vector as shown by the diminished NA-mediated intracellular calcium increases (Fig. 4, upper right panel, white columns). In contrast, the response to NA was much less affected by preincubation with S1P or IGF-I in cells in which S1P1 was knocked down (Fig. 4, upper right panel, solid columns). This was very clear in the calcium tracings depicted in Fig. 4 (middle panel).
Fig. 4.
Effect of knocking down S1P1 receptors on the α1B-adrenergic receptor desensitization induced by S1P and IGF-I. Upper left panel: Cells transfected with the empty vector (open bars) or with S1P1 shRNA (solid bars) were challenged with 10 μM noradrenaline (NA), 1 μM S1P or 1 μM lysophosphatidic acid (LPA). Upper right panel: Cells transfected with the vector (open bars) or with S1P1 shRNA (solid bars) were preincubated for 15 min with 1 μM S1P or 100 ng/ml IGF-I and then challenged with 10 μM noradrenaline (NA). Means are plotted with vertical lines representing the S.E.M. of 5 to 10 determinations using different cell preparations. Middle panel: representative calcium tracings of intracellular free-calcium obtained from cells transfected with the empty vector (solid lines) or with S1P1 shRNA (dotted lines); cells were preincubated with 1 μM S1P or 100 ng/ml IGF-I and then challenged with 10 μM noradrenaline (NA). Representative Reverse transcription (RT)-PCR determinations and Western blots are shown in the bottom panel.
Next, we studied α1B-AR phosphorylation in control transfected and S1P1-knocked down cells. As shown in Fig. 5 (left panel) 10 μM NA, 1 μM S1P and 100 ng/ml IGF-I increased α1B-AR phosphorylation 2 to 3-fold in control transfected cells. In contrast, in S1P1 knocked down cells, the effect of NA was slightly decreased but those of S1P and IGF-I were essentially abolished (Fig. 5, right panel). The effect of NA was studied at 5 min whereas those of the other agents (S1P and IGF-I) were examined at 15 min due to the different time-course of their effects [16, 18, 39].
Fig. 5.
Effect of knocking down S1P1 receptors on α1B-adrenergic receptor (α1B-AR) phosphorylation. Cells transfected with the empty vector (left panel) or with S1P1 shRNA (right panel) were challenged with 10 μM noradrenaline (NA), 1 μM S1P or 100 ng/ml IGF-I. Means are plotted with vertical lines representing the S.E.M. of 6 to 9 determinations using different cell preparations. Representative autoradiograms are shown. *p <0.001 vs. basal; **p < 0.01 vs. S1P of vector-transfected cells; ***p <0.05 vs. IGF-I of vector-transfected cells.
The effect of hormonal stimulation on SPHK activity was assayed in cell extracts by in vitro phosphorylation of sphingosine. As shown in Fig. 6, extracts from cells treated with 1 μM S1P and 10 μM NA induced a consistently small (40%) and statistically insignificant increase in the enzyme’s activity, while tetradecanoyl phorbol acetate (that was used as a positive control), IGF-I and EGF induced much greater, statistically significant 2-fold activity increases. The data are consistent with an autocrine/paracrine role of S1P in α1B-AR phosphorylation.
Fig. 6.
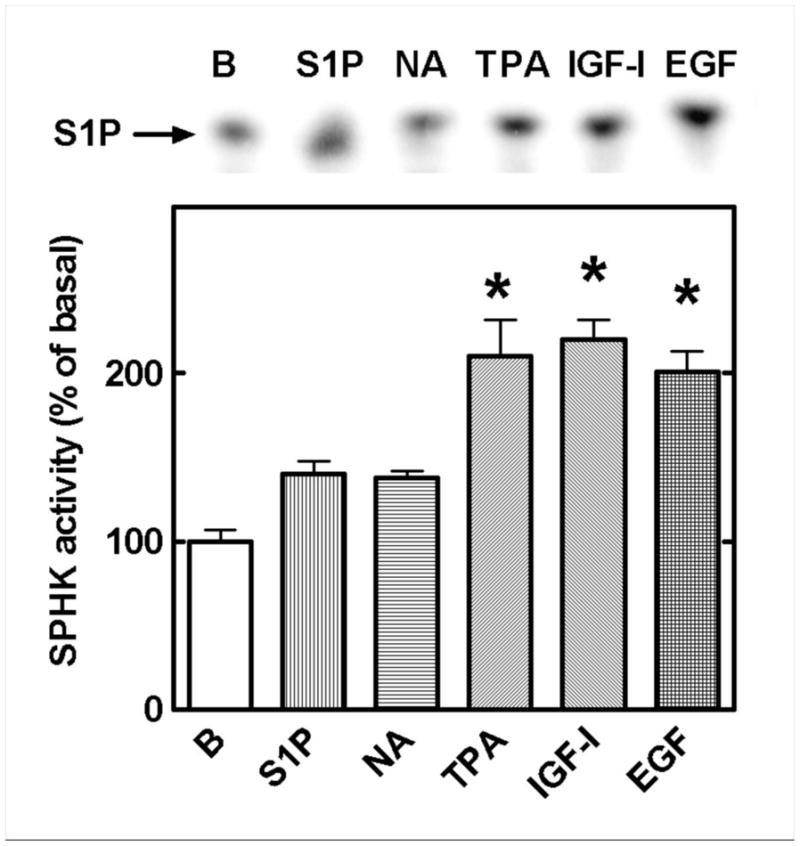
Sphingosine kinase (SPHK) activity in cell extracts. Cells were incubated for 30 min in the absence (B, basal) or presence of 1 μM S1P, 10 μM noradrenaline (NA), 1 μM tetradecanoyl phorbol acetate (TPA), 100 ng/ml IGF-I or 100 ng/ml EGF, extracts were prepared and enzyme activity assayed by determining sphingosine phosphorylation. Means are plotted with vertical lines representing the S.E.M. of 6 to 10 determinations using different cell preparations. A representative autoradiogram is presented. p <0.001 vs basal (B).
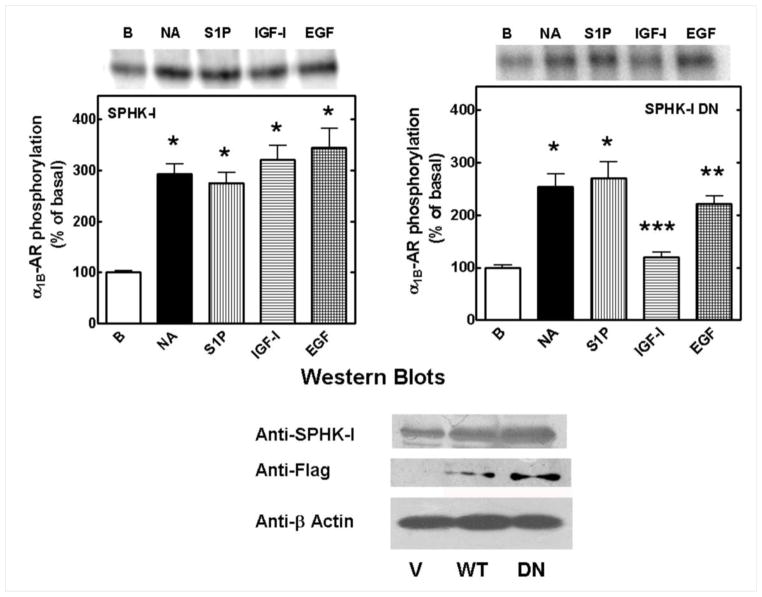
In order to test this model further, we blocked S1P generation with an SPHK-1 catalytically inactive (dominant-negative) mutant and assayed for α1B-AR desensitization by measuring intracellular calcium concentration responses [29]; expression of the wild type SPHK-1 was used as a control. Expression of wild-type enzyme or of the dominant-negative mutant did not alter the effect of 10 μM NA on intracellular calcium (Fig. 7, upper left panel and bottom panel). As expected, neither expression of the wild-type enzyme nor that of the dominant-negative mutant altered α1B-AR desensitization induced by a 15 min preincubation with 1 μM S1P (Fig. 7, left middle and bottom panels); but in agreement with previous findings, expression of the dominant- negative SPHK-1 mutant markedly inhibited IGF-I- and EGF-induced α1B-AR desensitization (Fig 7, upper and middle right panels and bottom panel). The effect of the SPHK-1 dominant-negative mutant on α1B-AR phosphorylation was also examined. In cells expressing wild-type SPHK-1, 10 μM NA, 1 μM S1P, 100 ng/ml IGF-I or 100 ng/ml EGF clearly increased (≈ 3-fold) α1B-AR phosphorylation (Fig. 8, upper left panel); i. e., expression of the wild type kinase did not alter receptor phosphorylation induced by these agents. In contrast, in cells expressing the dominant-negative sphingolipid kinase, EGF-mediated α1B-AR phosphorylation was markedly reduced, and that induced by IGF-I was nearly abolished (Fig. 8, upper right panel). Western blotting of cell extracts, with anti SPHK-1 and anti-flag antibodies, evidenced expression of the wild type enzyme and the dominant-negative mutant.(Fig 8, lower panel).
Fig. 7.
Effect of sphingosine kinase-1 (SPHK-1) dominant-negative expression on α1B-adrenergic receptor desensitization induced by S1P and IGF-I. Upper and middle panels: representative calcium tracings of intracellular free-calcium obtained from cells transfected with wild type SPHK-1 (solid lines) or with the dominant-negative mutant (dotted lines); cells were preincubated for 15 min with vehicle (upper left panel) 1 μM S1P (middle left panel) 100 ng/ml IGF-I (upper right panel) or 100 ng/ml EGF (middle right panel) and then challenged with 10 μM noradrenaline (NA). Lower panel: Noradrenaline (10 μM)-induced increase in intracellular calcium of cells treated as described above; cells transfected with wild type SPHK-1 (open bars) or with the dominant-negative mutant (solid bars). Means are plotted with vertical lines representing the S.E.M. of 10 to 15 determinations using different cell preparations. *p < 0.001 vs. respective vehicle group; **p < 0.001 vs its respective EGF or IGF-I (wild type SPHK-1) group.
Fig. 8.
Effect of sphingosine kinase-1 (SPHK-1) dominant-negative expression on α1B-adrenergic receptor (α1B-AR) phosphorylation. Cells transfected with wild type SPHK-1 (left panel) or with the dominant-negative mutant (right panel) were challenged with vehicle (B, basal), 10 μM noradrenaline (NA), 1 μM S1P, 100 ng/ml IGF-I μM or 100 ng/ml EGF. Means are plotted with vertical lines representing the S.E.M. of 5 to 6 determinations using different cell preparations. Representative autoradiograms are shown. *p <0.001 vs. basal; **p < 0.01 vs. basal. p< 0.05 vs. EGF (wild type SPHK-1) group ***p < 0.01 vs IGF-I (wild type SPHK-1) group. Representative Western blots are shown in the bottom panel
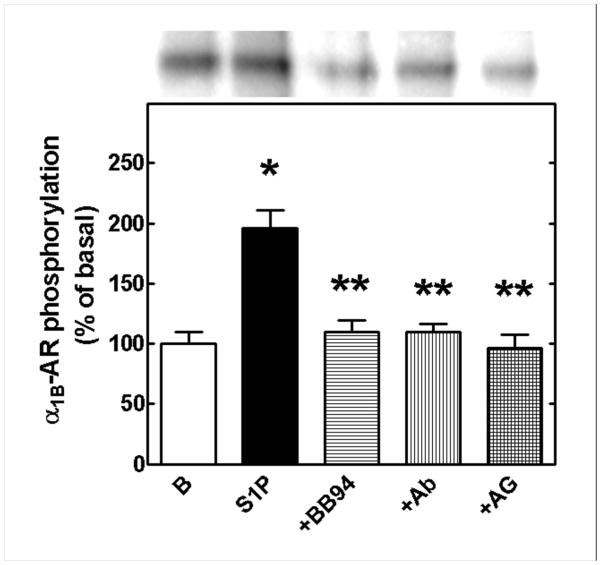
The possibility that S1P-induced α1B-AR phosphorylation might involve EGF receptor transactivation was experimentally tested; to do this, the general metalloproteinase inhibitor BB94, a neutralizing anti-HB-EGF antibody and AG1478, an inhibitor of EGF receptor tyrosine kinase activity, were used. None of these agents alter basal α1B-AR phosphorylation (data not shown, see [9, 10, 15, 16]). However, they essentially abolish S1P-indced adrenoceptor phosphorylation as shown in Fig. 9.
Fig. 9.
Effect of inhibitors of EGF receptor transactivation on sphingosine-1-phosphate (S1P)-mediated α1B-adrenergic receptor (α1B-AR) phosphorylation. Cells were preincubated for 30 min in the absence or presence of 10 μM BB94, 5 μg/ml of neutralizing anti HB-EGF antibody or 5 μM AG1478 and then further incubated for 15 min in the presence of 1 μM S1P. Means are plotted with vertical lines representing the S.E.M. of 6 determinations, using different cell preparations. * p< 0.001 vs. basal; ** p< 0.001 vs S1P alone. A representative autoradiograph is presented.
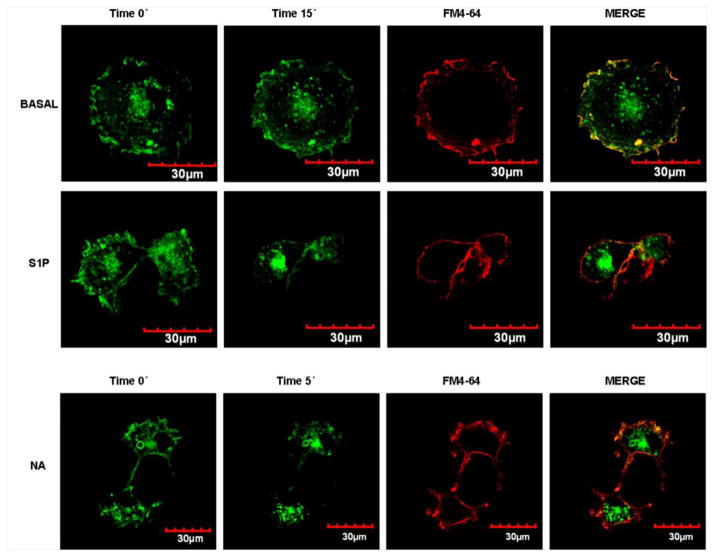
As indicated previously, the use of the human α1B-ARs tagged with the enhanced green fluorescent protein (eGFP) allows in cellulo receptor visualization. As depicted in Fig. 10, α1B-AR-eGFP fluorescence was localized to the plasma membrane and in intracellular vesicles; the membrane marker FM4-64 (red) allowed precise membrane localization (merge, yellow). Addition of 10 μM NA or 1 μM S1P induces receptor internalization as evidenced by a marked decrease of α1B-AR-eGFP/FM4-64 colocalization (Fig. 10) (quantitative analysis is presented as Supplementary Fig. S2). A video is also included, as supplementary material (V1) showing the effect of S1P on receptor location. S1P induced marked changes in cell shape and receptor mobilization from the plasma membrane to intracellular vesicles. It is interesting that, at some time points, the receptors appear to be located in “lanes” and clusters.
Fig. 10.
Confocal microscopy images of cells expressing α1B-adrenergic receptor-enhanced green fluorescent protein construction. Images were taken at the beginning of incubation (Time 0′) and at the end of the incubation with the reagents (Time 5′ or Time 15′); immediately thereafter, membrane marker FM4-64 (red) was added and images were taken. Merged images (end of incubation-FM4-64) are presented for colocalization (yellow). Cells were incubated in the absence of any agent (BASAL) or presence of 1 μM S1P or 10 μM noradrenaline (NA). Images are representative of 3 to 4 observations using different cell preparations.
Cells transfected with a shRNA vector exhibited receptor internalization in response to S1P whereas those transfected with an S1P1 receptor shRNA did not (Supplementary Fig. S3). In cells transfected with wild-type SPHK-1, both 1 μM S1P and 100 ng/ml IGF-I induced α1B-AR internalization (Supplementary Fig. S4). In contrast, in cells transfected with the dominant-negative SPHK-1 only S1P, but not IGF-I, induced internalization (Supplementary Fig. S5).
4. Discussion
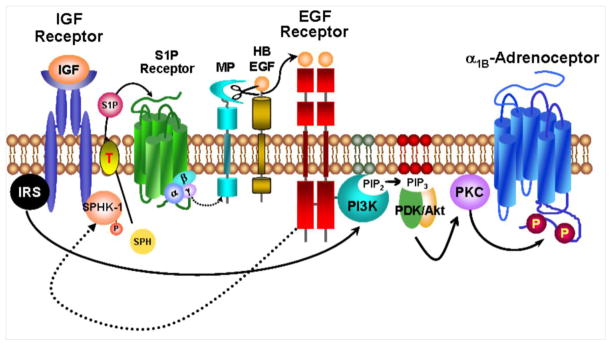
In this manuscript we show that activation of S1P receptors induced α1B-AR desensitization and phosphorylation. PI3K and PKC, are key enzymes involved in this action. We also demonstrate that activation of receptor tyrosine kinases, such as IGF-I and EGF receptors increased SPHK activity. Such activation and the consequent production of S1P appear to be functionally relevant in IGF-I- and EGF-induced α1B-AR phosphorylation and desensitization as evidenced by the following facts: a) expression of a dominant-negative mutant of SPHK or b) S1P1 receptor knockdown, markedly reduced this growth factor action. These aspects are diagrammatically presented in Fig.11. In addition, taking advantage of the presence of the eGFP tag added in receptor construction, we showed that S1P was capable of inducing α1B-AR internalization and that its autocrine/paracrine generation was relevant for the receptor internalization induced by IGF-I.
Fig. 11.
Model for IGF-I receptor-mediated α1B-adrenergic receptor (α1B-AR) phosphorylation. IGF, insulin-like growth factor I; IRS, insulin receptor substrate; SPHK1, sphingosine kinase 1; SPH, sphingosine; S1P, sphingosine-1-phosphate; MP, metalloproteinase; αβγ, G protein subunits; HB-EGF, heparin-binding EGF; EGF, epidermal growth factor; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol (4,5)- bisphosphate; PIP3, phosphatidylinositol (3,4,5)- trisphosphate; PDK, phosphoinositide-dependent kinase; Akt, protein kinase B; PKC, protein kinase C; T, transporter.
IGF-I is a relatively small (70 amino acid) peptide that is mainly produced in the liver in response to growth hormone and that mediates the majority of its actions, including those on anabolism, growth and development [40]. IGF-I signals through type 1 transmembrane tyrosine kinase receptors similar to the insulin receptors [41]. These receptors phosphorylate members of the insulin receptor substrate family of proteins that bind and activate PI3K and this enzyme can lead to activation of PKC through formation of 3′-phosphorylated phosphoinositides which activate PDK-1 and Akt/PKB [42]. As mentioned in the introduction, IGF-I-induced α1B-AR phosphorylation involves EGF receptor transactivation and also pertussis toxin-sensitive G proteins [16]. The present work clarifies that the action of IGF-I including the autocrine/paracrine generation of S1P and its action through the G protein-coupled receptor, S1P1.
S1P induced a small increase in intracellular calcium that was not observed when cells were stimulated with IGF-I [15, 16]. These data indicate that differences exist between direct agonist addition to the media and accumulation through autocrine/paracrine generation. Calcium signaling does not appear to be required for the actions studied here, but we can not discount the possibility that there was a very small effect of IGF-I on basal intracellular calcium concentration that was not detected.
Our current model shows that in order to induce α1B-AR desensitization and phosphorylation, S1P1 receptor stimulation leads to G protein-mediated EGF receptor transactivation, i. e., metalloproteinase activation, HB-EGF shedding from the plasma membrane and stimulation of EGF receptors triggering. This was experimentally shown to be the case, as it has been observed for the actions of other G protein-coupled receptors, such as those for LPA and endothelin-1 and NA [9–11]. It is note worthy that there is already evidence indicating that S1P can induce EGF receptor transactivation (for example see [43–46]). Our data knocking down S1P1 receptors suggest that these receptors play a major role. However, we are unable to discard the possibility that other S1P receptor subtypes could be involved (although to a lesser extent). In this regard, it is important to mention that it has been shown that S1P induced EGF transactivation through S1P3 receptors [46].
The ability to observe the eGFP-tagged receptor in cellulo and in real-time allowed us to define the importance of S1P action in α1B-AR internalization. We previously showed that IGF-I induced α1B-AR internalization, which was blocked by hypertonic sucrose and concanavalin I [15]; our present results showed that S1P generation and action on S1P1 receptors play a central role. In addition, a correlation was observed among the functional sensitivity of ARs, their phosphorylation state and the receptor internalization process.
We were surprised by the intense action of S1P on cell morphology, receptor internalization and vesicular traffic; it was stronger in magnitude than that of IGF-I. The formation of “lanes” and “clusters” of receptors was noticeable. This observation suggests that internalized vesicles containing α1B-ARs interact with cytoskeletal elements. There is already evidence indicating that such interactions might take place. G protein-coupled estrogen receptor 1 (GPR30) traffics intracellularly on cytokeratin intermediate filaments [47] and the transport of α2B-ARs from the endoplasmic reticulum to the cell surface is controlled through their association with tubulin [48]. Certainly much more additional work will be required to define how α1B-ARs internalize and the molecular elements that participate in this.
In summary, our present work adds significantly to the mechanisms that participate in IGF-I receptor-α1B-AR crosstalk. Taking into account the present information and that previously published [16] it is clear that four different receptors (IGF-I receptors, α1B-ARs, S1P1 receptors and EGF receptors) and two autocrine/paracrine mediators (S1P and HB-EGF) participate in this crosstalk. How general this process is remains to be determined. These two paracrine mediators appear to play very general roles, allowing crosstalks of receptor tyrosine kinases and G protein-coupled receptors, and in this manner taking part in the fine tuning of cell responsiveness. It is important to remember that the final target, i. e. α1B-ARs, is phosphorylated by a second messenger-activated kinase, i. e., PKC. In principle, other receptors (or channels, or transporters) with functionally relevant consensus sequences for PKC-mediated phosphorylation, could be subjected to similar processes. Identification of the molecular elements that participate in regulatory processes provide by default potential sites for therapeutic intervention. It is worth remembering that all of these receptors and autocrine/paracrine messengers take part in health maintenance and that their dysfunction might be relevant for the development of diseases [23, 40, 49–52].
Supplementary Material
Acknowledgments
This research was partially supported by Grants from CONACyT [79908] and DGAPA-UNAM [IN212609]. JC is supported by the NIH (MH51699). JAC-B is a student of Programa de Doctorado en Ciencias Bioquímicas-UNAM and recipient of a PhD fellowship from CONACyT. We express our gratitude to Dr. Stuart Pitson for kindly donating plasmids for expression of wild type SPHK-1 and the dominant-negative mutant of this enzyme, and to Dr. Shahriar Mobashery for his generous gift of BB94. We thank Dr. Rocío Alcántara-Hernández, Dr. Araceli Patrón, Gabriel Orozco, Dr. Claudia Rivera, Dr. Héctor Malagón, Aurey Galván and Manuel Ortínez for their technical help and advice.
Abbreviations used
- NA
noradrenaline
- AR
adrenergic receptor
- PKC
protein kinase C
- PI3K
phosphoinositide 3-kinase
- S1P
sphingosine 1-phosphate
- S1P
sphingosine 1-phosphate
- SPHK-1
sphingosine kinase-1
- eGFP
enhanced green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR., Jr International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- 2.García-Sáinz JA, Vázquez-Prado J, Medina LC. Alpha 1-adrenoceptors: function and phosphorylation. Eur J Pharmacol. 2000;389:1–12. doi: 10.1016/s0014-2999(99)00896-1. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Koshimizu TA, Yamauchi J, Hirasawa A, Tanoue A, Tsujimoto G. Recent progress in alpha 1-adrenoceptor pharmacology. Biol Pharm Bull. 2002;25:401–408. doi: 10.1248/bpb.25.401. [DOI] [PubMed] [Google Scholar]
- 5.Cotecchia S, Schwinn DA, Randall RR, Lefkowitz RJ, Caron MG, Kobilka BK. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor. J Biol Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 7.Iacovelli L, Franchetti R, Grisolia D, De Blasi A. Selective regulation of G protein-coupled receptor-mediated signaling by G protein-coupled receptor kinase 2 in FRTL-5 cells: analysis of thyrotropin, alpha(1B)-adrenergic, and A(1) adenosine receptor-mediated responses. Mol Pharmacol. 1999;56:316–324. doi: 10.1124/mol.56.2.316. [DOI] [PubMed] [Google Scholar]
- 8.Diviani D, Lattion AL, Cotecchia S. Characterization of the phosphorylation sites involved in G protein-coupled receptor kinase- and protein kinase C-mediated desensitization of the alpha1B-adrenergic receptor. J Biol Chem. 1997;272:28712–28719. doi: 10.1074/jbc.272.45.28712. [DOI] [PubMed] [Google Scholar]
- 9.Casas-González P, García-Sáinz JA. Role of epidermal growth factor receptor transactivation in alpha1B-adrenoceptor phosphorylation. Eur J Pharmacol. 2006;542:31–36. doi: 10.1016/j.ejphar.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Casas-González P, Ruiz-Martínez A, García-Sáinz JA. Lysophosphatidic acid induces alpha-1b-adrenergic receptor phosphorylation through G-beta-gamma, phosphoinositide 3-kinase, protein kinase C and epidermal growth factor receptor transactivation. Biochim Biophys Acta. 2003;1633:75–83. [PubMed] [Google Scholar]
- 11.Casas-González P, Vázquez-Prado J, García-Sáinz JA. Lysophosphatidic acid modulates alpha(1b)-adrenoceptor phosphorylation and function: roles of Gi and phosphoinositide 3-kinase. Mol Pharmacol. 2000;57:1027–1033. [PubMed] [Google Scholar]
- 12.González-Arenas A, Aguilar-Maldonado B, Avendaño-Vázquez SE, García-Sáinz JA. Estrogens cross-talk to alpha1b-adrenergic receptors. Mol Pharmacol. 2006;70:154–162. doi: 10.1124/mol.106.025064. [DOI] [PubMed] [Google Scholar]
- 13.Medina LC, Vázquez-Prado J, García-Sáinz JA. Cross-talk between receptors with intrinsic tyrosine kinase activity and alpha1b-adrenoceptors. Biochem J. 2000;350(Pt 2):413–419. doi: 10.1042/0264-6021:3500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina LC, Vázquez-Prado J, Torres-Padilla ME, Mendoza-Mendoza A, Cruz Muñoz ME, García-Sáinz JA. Crosstalk: phosphorylation of alpha1b-adrenoceptors induced through activation of bradykinin B2 receptors. FEBS Lett. 1998;422:141–145. doi: 10.1016/s0014-5793(97)01615-3. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Muñoz T, Romero-Ávila MT, Avendaño-Vázquez SE, García-Sáinz JA. Phosphorylation, desensitization and internalization of human alpha(1B)-adrenoceptors induced by insulin-like growth factor-I. Eur J Pharmacol. 2008;578:1–10. doi: 10.1016/j.ejphar.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Molina-Muñoz T, Romero-Ávila MT, García-Sáinz JA. Insulin-like Growth Factor-I induces {alpha}1B-Adrenergic Receptor Phosphorylation Through G{beta}{gamma} and Epidermal Growth Factor Receptor Transactivation. Mol Endocrinol. 2006;20:2773–2783. doi: 10.1210/me.2006-0090. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Ávila MT, Flores-Jasso CF, García-Sáinz JA. alpha1B-Adrenergic receptor phosphorylation and desensitization induced by transforming growth factor-beta. Biochem J. 2002;368:581–587. doi: 10.1042/BJ20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vázquez-Prado J, Medina LC, García-Sáinz JA. Activation of endothelin ETA receptors induces phosphorylation of alpha1b-adrenoreceptors in Rat-1 fibroblasts. J Biol Chem. 1997;272:27330–27337. doi: 10.1074/jbc.272.43.27330. [DOI] [PubMed] [Google Scholar]
- 19.Alcántara-Hernández R, Vázquez-Prado J, García-Sáinz JA. Protein phosphatase-protein kinase interplay modulates alpha 1b-adrenoceptor phosphorylation: effects of okadaic acid. Br J Pharmacol. 2000;129:724–730. doi: 10.1038/sj.bjp.0703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 21.Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 22.Patel TB. Single transmembrane spanning heterotrimeric g protein-coupled receptors and their signaling cascades. Pharmacol Rev. 2004;56:371–385. doi: 10.1124/pr.56.3.4. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 25.Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life. 2006;58:467–472. doi: 10.1080/15216540600871126. [DOI] [PubMed] [Google Scholar]
- 26.Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal. 2009;21:14–21. doi: 10.1016/j.cellsig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 28.García-Sáinz JA, Romero-Ávila MT, Medina LC. Dissecting how receptor tyrosine kinases modulate G protein-coupled receptor function. Eur J Pharmacol. 2010;648:1–5. doi: 10.1016/j.ejphar.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 29.Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, D’Andrea RJ, Wattenberg BW. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J Biol Chem. 2000;275:33945–33950. doi: 10.1074/jbc.M006176200. [DOI] [PubMed] [Google Scholar]
- 30.García-Sáinz JA, Casas-González P, Romero-Ávila MT, González-Espinosa C. Characterization of the hepatic alpha 1B-adrenoceptors of rats, mice and hamsters. Life Sci. 1994;54:1995–2003. doi: 10.1016/0024-3205(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 31.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 32.Avendaño-Vázquez SE, García-Caballero A, García-Sáinz JA. Phosphorylation and desensitization of the lysophosphatidic acid receptor LPA1. Biochem J. 2005;385:677–684. doi: 10.1042/BJ20040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto M, Okumura S, Schwencke C, Sadoshima J, Ishikawa Y. High efficiency gene transfer by multiple transfection protocol. Histochem J. 1999;31:241–243. doi: 10.1023/a:1003598614323. [DOI] [PubMed] [Google Scholar]
- 34.Paddison PJ, Cleary M, Silva JM, Chang K, Sheth N, Sachidanandam R, Hannon GJ. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Meth. 2004;1:163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 35.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King KE, Iyemere VP, Weissberg PL, Shanahan CM. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem. 2003;278:11661–11669. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- 37.Olivera A, Spiegel S. Sphingosine kinase. Assay and product analysis. Methods Mol Biol. 1998;105:233–242. doi: 10.1385/0-89603-491-7:233. [DOI] [PubMed] [Google Scholar]
- 38.Rasband WS. ImageJ. National Institutes of Health; 1997–2004. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 39.Vázquez-Prado J, Medina LC, Romero-Ávila MT, González-Espinosa C, García-Sáinz JA. Norepinephrine- and phorbol ester-induced phosphorylation of alpha(1a)-adrenergic receptors. Functional aspects. J Biol Chem. 2000;275:6553–6559. doi: 10.1074/jbc.275.9.6553. [DOI] [PubMed] [Google Scholar]
- 40.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abarca B, Ballesteros R, Bielsa P, Moragues J, D’Ocon P, García-Zaragoza E, Noguera MA. Opposite vascular activity of (R)-apomorphine and its oxidised derivatives. Endothelium-dependent vasoconstriction induced by the auto-oxidation metabolite. Eur J Med Chem. 2003;38:501–511. doi: 10.1016/s0223-5234(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 42.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 43.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Yatomi Y, Nagawa H. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004;577:333–338. doi: 10.1016/j.febslet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Yogi A, Callera GE, Aranha AB, Antunes TT, Graham D, McBride M, Dominiczak A, Touyz RM. Sphingosine-1-phosphate-induced inflammation involves receptor tyrosine kinase transactivation in vascular cells: upregulation in hypertension. Hypertension. 2011;57:809–818. doi: 10.1161/HYPERTENSIONAHA.110.162719. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Song WK, Chun JS. Sphingosine 1-phosphate activates Erk-1/-2 by transactivating epidermal growth factor receptor in rat-2 cells. IUBMB Life. 2000;50:119–124. doi: 10.1080/713803698. [DOI] [PubMed] [Google Scholar]
- 46.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanden C, Broselid S, Cornmark L, Andersson K, Daszkiewicz-Nilsson J, Martensson UE, Olde B, Leeb-Lundberg LM. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol Pharmacol. 2011;79:400–410. doi: 10.1124/mol.110.069500. [DOI] [PubMed] [Google Scholar]
- 48.Duvernay MT, Wang H, Dong C, Guidry JJ, Sackett DL, Wu G. {alpha}2B-Adrenergic Receptor Interaction with Tubulin Controls Its Transport from the Endoplasmic Reticulum to the Cell Surface. J Biol Chem. 2011;286:14080–14089. doi: 10.1074/jbc.M111.222323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 50.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 51.Leserer M, Gschwind A, Ullrich A. Epidermal growth factor receptor signal transactivation. IUBMB Life. 2000;49:405–409. doi: 10.1080/152165400410254. [DOI] [PubMed] [Google Scholar]
- 52.García-Sáinz JA, Vázquez-Prado J, Villalobos-Molina R. Alpha 1-adrenoceptors: subtypes, signaling, and roles in health and disease. Arch Med Res. 1999;30:449–458. doi: 10.1016/s0188-0128(99)00059-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.