Abstract
Background
Therapeutic hypothermia has been shown to reduce neurological morbidity and mortality in the setting of out-of-hospital cardiac arrest and may be beneficial following brain injury and cardiopulmonary bypass. We conducted a systematic review to ascertain the effect of therapeutic hypothermia on development of acute kidney injury (AKI) and mortality.
Methods
We searched for randomized controlled trials in MEDLINE through February 2011. We included trials comparing hypothermia to normothermia that reported kidney-related outcomes including, development of AKI, dialysis requirement, changes in serum creatinine, and mortality. We performed Peto fixed-effect and random-effects model meta-analyses, and meta-regressions.
Results
Nineteen trials reporting on 2,218 patients were included; in the normothermia group, the weighted rate of AKI was 4.2%, dialysis requirement 3.7%, and mortality 10.8%. By meta-analysis, hypothermia was not associated with a lower odds of AKI (odds ratio [OR] 1.01, 95% confidence interval [CI] 0.68, 1.51; P = 0.95) or dialysis requirement (OR 0.81; 95% CI 0.30, 2.19; P = 0.68); however, by meta-regression, a lower target cooling temperature was associated with a lower odds of AKI (P = 0.01). Hypothermia was associated with lower mortality (OR 0.69; 95% CI 0.51, 0.92; P = 0.01).
Conclusions
In trials that ascertained kidney endpoints, therapeutic hypothermia prevented neither the development of AKI nor dialysis requirement, but was associated with lower mortality. Different definitions and rates of AKI, differences in mortality rates, and concerns about the optimal target cooling temperature preclude definitive conclusions.
Keywords: critical illness (or critically ill), hypothermia, acute kidney injury, mortality, meta-analysis
Introduction
On the basis of the published evidence, the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation has adopted therapeutic hypothermia into its guidelines for the treatment of unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest 1. These recommendations are based on a clear demonstrable benefit of therapeutic hypothermia on neurological morbidity and mortality following out-of hospital cardiac arrest 2, 3, and some potential benefit in the setting of traumatic brain injury 4, 5 and cardiopulmonary bypass for major cardiovascular surgery6. As a result, therapeutic hypothermia has been widely used in critically ill adults in the intensive care unit7, 8.
Acute kidney injury (AKI), as defined by a wide range of serum creatinine increments 9, is a consistent and powerful predictor of in-hospital mortality, and is associated with an increase in hospital length of stay, hospital costs, and resource utilization 10, 11. Acute kidney injury is commonly observed in patients who have undergone cardiopulmonary bypass 12, 13, and following resuscitation from spontaneous cardiac arrest 14, 15, and carries an increased mortality risk 15. Previously published trials comparing the effect of therapeutic hypothermia vs. normothermia on kidney endpoints have yielded conflicting results16 due in part to the small sample size and low study quality. To shed further light on this question, we conducted a systematic review and meta-analysis of the existing randomized controlled trials (RCTs) comparing the effect of therapeutic hypothermia vs. normothermia in adults on the development of AKI (primary outcome) and all-cause mortality (secondary outcome).
Methods
Data Sources and Search Strategy
We searched Medline (1965 - February 2011) using the following MeSH database search terms: “Hypothermia”, “Hypothermia Induced”, and “Deep Hypothermia Induced”. The search was limited to human RCTs with no language restrictions. We also searched http://www.ClinicalTrials.gov for completed trials using similar search terms, reviewed abstracts from the annual scientific meetings of the American Society of Nephrology (2000-2010), and performed a manual search of references in narrative and systematic reviews on therapeutic hypothermia.
Study Selection
We included all RCTs that examined primary or secondary kidney endpoints (as defined below) in adults undergoing therapeutic hypothermia vs. normothermia. We excluded trials of newborns and children, as well as duplicate publications. If authors published more than one manuscript on the same study, data from the most inclusive report were used.
The primary outcome of interest was AKI, as defined by the authors of individual trials. We also assessed other kidney endpoints including continuous changes in serum creatinine, creatinine clearance and dialysis requirement. The secondary outcome of interest was all-cause mortality, which was only evaluated in the trials reporting kidney endpoints.
Data Extraction and Quality Assessment
Two of the authors independently reviewed and screened the titles and abstracts of all the MEDLINE citations (PS and MA), and the scientific abstracts of the annual meetings of the American Society of Nephrology (ACB and PS). The full-text articles were retrieved for comprehensive review and re-screened, and the data were extracted and tabulated. The following variables were extracted: country of origin, year of publication, study design, population setting, (e.g., out-of hospital cardiac arrest, brain injury, and cardiopulmonary bypass for major cardiovascular surgery), total number of patients, sex, mean age, mean duration of cardiac arrest, mean aortic cross clamp time (for on-pump cardiovascular surgery), cooling target temperature, control arm temperature, cooling technique (including infusate type), duration of cooling, development of AKI, definition of AKI, mean baseline serum creatinine, mean baseline creatinine clearance, mean follow-up serum creatinine, mean follow up creatinine clearance, duration of follow-up, and mortality rate. Disagreements were resolved through consensus and arbitration by a third author (BLJ). In the case of trials with more than 2 groups, separate analyses were performed comparing each therapeutic hypothermia intervention group with the normothermia control group. Corresponding authors of 4 trials were contacted by e-mail for data clarification, and 2 provided additional information.
Study quality was assessed using a modified Jadad scale 17, which is based on the adequacy of randomization, blinding and attrition 18. A score of 0-1, 2-3, and 4-5 corresponds to a study of poor, fair, and good quality, respectively.
Data Synthesis and Analysis
For our primary analysis, due to the low number of events in most studies (often zero in one study group) 19, we performed a Peto fixed-effect meta-analysis to assess the odds ratio (OR) (with 95% confidence interval [CI]) for the development of AKI, dialysis requirement, and mortality in the therapeutic hypothermia group relative to the normothermia group. We also performed a random-effects model meta-analysis as a sensitivity analysis 20. Trials with no events in both groups were excluded from the analyses. A random-effects model meta-analysis was also performed to assess the net change in serum creatinine and creatinine clearance in the therapeutic hypothermia group relative to the normothermia group.
Existence of heterogeneity among effect sizes estimated by individual trials was tested using the I2 index, and chi-squared P value. Heterogeneity was explored by subgroup analyses based on the 3 population settings, mainly traumatic brain injury, out-of hospital cardiac arrest, and cardiopulmonary bypass, as well as the cooling technique, infusion type, study quality and sample size (≤ vs. >100 patients). The Student t-test was used to compare subgroups. Meta-regression analyses were also performed to explore heterogeneity including the cooling target temperature, cardiac arrest duration, and duration of cooling against the odds of AKI and mortality rate. Finally, publication bias was formally assessed using funnel plots. The meta-analyses were performed using Comprehensive Meta-Analysis version 2.0 and MetaAnalyst beta version 3.1 (Tufts University, Boston, MA)
Results
Characteristics and Quality of the Studies
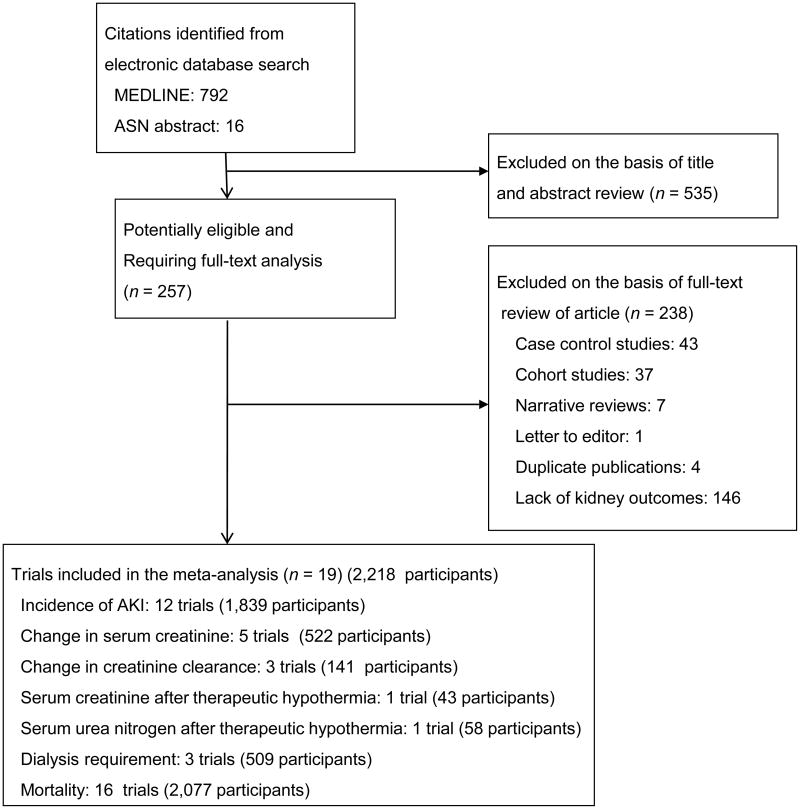
A total of 792 potentially relevant citations were identified and screened; 257 articles were retrieved for detailed evaluation, of which 19 fulfilled eligibility criteria (Figure 1) 21-39. Three trials tested 2 therapeutic hypothermia interventions 22, 25, 26, which were each compared with the control group. One parent study providing data on rate of AKI 32 had a subsequent report on a subset of patients where changes in serum creatinine were examined 34; the latter report was used for the meta-analysis of continuous change in serum creatinine.
Figure 1.
Study selection flow diagram.
Characteristics of the individual trials are displayed in Table 1. The trials spanned more than 10 years, varied in sample size (23-291 patients) and involved the three population settings. All trials had mostly men (range of 53-95%) with a mean age ranging from 29 to 69 years.
Table 1. Characteristics of the randomized controlled trials included in the meta-analysis.
| Author | Year | Country | Population setting |
Total number of patients |
Mean age (years) |
Men (%) |
Mean cardiac arrest duration (min) |
Hypothermia group target temperature (°C) |
Normothermia group temperature (°C) |
Cooling technique | Infusion type | Duration of cooling (min) |
Study Quality |
Definition of AKI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clifton | 1993 | USA | Traumatic brain injury | 46 | 29 | NR | NA | 32-33 | 37 | Cooling blanket | NA | 2880 | 1 | Not specified |
| Lajos | 1993 | USA | CABG-CPB | 163 | 63 | 71 | 73 | 30 | 37 | Fluid infusion | Crystalloid | 91 | 1 | Not specified |
| 30 | 37 | Fluid infusion | Blood | 98 | ||||||||||
| Ip-Yam | 1994 | UK | CABG-CPB | 23 | 60 | 83 | 62 | 28 | 37 | Fluid infusion | Hartmann's solution | 112 | 1 | Change in Cr clearance |
| Kaukoranta | 1995 | Finland | CABG-CPB | 101 | 59 | 81 | 103 | 32-33 | 37 | Fluid infusion | Blood | 126 | 1 | Not specified |
| Regragui | 1995 | UK | CABG-CPB | 30 | 59 | 57 | 35 | 28 | 37 | Fluid infusion | Crystalloid | 75 | 2 | Change in sCr and |
| 32 | 37 | Fluid infusion | Crystalloid | 72 | Cr clearance | |||||||||
| Engelman | 1999 | USA | CABG-CPB | 291 | 63 | 77 | 86 | 23-28 | 35-36 | Fluid infusion | (Blood:crystall oid 4:1) | 135 | 2 | Not specified |
| 32-33 | 35-36 | Fluid infusion | (Blood:crystall oid 4:1) | 139 | ||||||||||
| Jacquet | 1999 | Belgium | CABG-CPB | 200 | 65 | 73 | 65 | 30 | 37 | Fluid infusion | Crystalloid | 115 | 1 | Change in sCr not specified; need for dialysis |
| Kuhn-Regnier | 1999 | Germany | CABG-CPB | 60 | 65 | 77 | 55 | NR | NR | Fluid infusion | Blood | 89 | 1 | Change in sCr |
| Bernard | 2002 | Australia | Out-of hospital cardiac arrest | 77 | 59 | 67 | 26 | 33 | 37 | Ice packs | NA | 720 | 3 | Change in sCr |
| Gaudino | 2002 | Italy | CABG-CPB | 113 | NA | 93 | 62 | 26 | 37 | Fluid infusion | Blood | 88 | 2 | Not specified |
| Koksoy | 2002 | USA | AAA-CPB | 34 | 64 | 53 | 62 | 15a | 37 | Fluid infusion | Ringer' solution | 23 | 3 | > 50% increase in sCr within first 10 postoperative days; need for dialysis |
| HACA | 2002 | European countries | Out-of hospital cardiac arrest | 275 | 59 | 76 | 22 | 32-34 | 37 | Mattress & ice packs | NA | 1440 | 3 | Not specified |
| Baron | 2003 | France | CABG-CPB | 69 | 64 | 87 | 49 | 15b | 37 | Fluid infusion | Blood+ crystalloid | 98 | 1 | Not specified |
| Zeiner | 2004 | Austria | Out-of hospital cardiac arrest | 88 | 54 | 73 | 22 | 32-34 | 37 | Mattress & ice pack | NA | 1440 | 3 | Change in sCr and Cr clearance, need for dialysis |
| Qiu | 2007 | China | Traumatic brain injury | 80 | 41 | 65 | NA | 34.5-36 | 37 | Cooling blanket, and cooling cap ice pack | NA | 5760 | 4 | Not specified |
| Boodhwani | 2009 | Canada | CABG-CPB | 267 | 69 | 88 | 46 | 34 | 37 | Thermal pads connected to thermal control system | NA | 77.3 | 2 | >25% increase in sCr or decrease in Cr clearance |
| Kamarainen | 2009 | Finland | Out-of hospital cardiac arrest | 43 | 61 | 95 | 23 | 33 | 37 | Fluid infusion | Ringer' solution | 37 | 3 | Single sCr measurement (in emergency room) |
| Castrén | 2010 | European countries | Out-of hospital cardiac arrest | 200 | 65 | 75 | 31 | 34 | 37 | Intranasal cooling | NA | 32 | 3 | Not specified |
| Hemmen | 2010 | USA | Ischemic stroke | 58 | 66 | 55 | NA | 33 | 37 | Intravascular cooling with thermal control system | NA | 1440 | 3 | sCr & urea nitrogen measurement at day 2 & 7 |
AAA denotes abdominal aortic aneurysm; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass pump; HACA, Hypothermia after Cardiac Arrest Study Group; NR, not reported; Cr, creatinine; sCr, serum creatinine;
renal temperature
temperature of solution. A study quality score of 0-1, 2-3, and 4-5 corresponds to a study of poor, fair, and good quality, respectively.
Overall study quality was mostly poor 21-24, 27, 28, 33 to fair 25, 26, 29-32, 34, 36-39, with only one study being rated as good 35. Reported randomization methods were adequate in only 12 of the 19 trials 25, 26, 29-32, 34-39, and 7 trials described the number of withdrawals or dropouts, and used an intention-to-treat analysis 29, 31, 32, 34, 37-39.
Effect of Therapeutic Hypothermia on Development of AKI
Twelve RCTs reported on the development of AKI in a total of 1,839 analyzable patients; however 2 of these studies 22,33 had no patients with AKI in either group and thus did not contribute to the meta-analysis. The overall weighted rate of AKI in the normothermia group was 4.2% (range of 0 to 59%). By meta-analysis, therapeutic hypothermia was not associated with a lowed odds of AKI (OR 1.01, 95% CI 0.68, 1.51; P = 0.95; Figure 2). Although the trials differed considerably in their size, quality score, and population setting, the test for heterogeneity was not significant (I2 = 0%; P = 0.46); however, this is largely due to the small number of AKI events in most studies, and thus the very wide confidence intervals.
Figure 2.
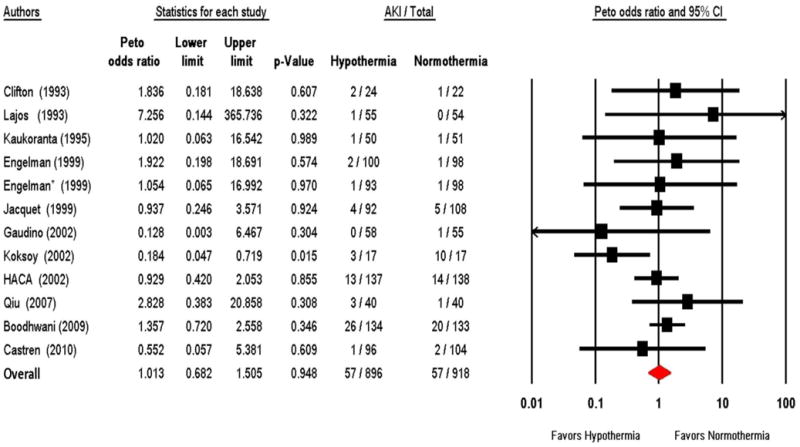
Forest plot of therapeutic hypothermia vs. normothermia on the development of AKI. HACA denotes Hypothermia after Cardiac Arrest Study Group. * Refers to the second therapeutic hypothermia intervention tested in the same trial.
By meta-regression, a lower target cooling temperature was associated with a lower odds of AKI (P = 0.01, Figure 3), whereas the duration of therapeutic hypothermia (P = 0.31), and the duration of cardiac arrest were not (P = 0.49). Of note, however, after the removal of an influential trial that delivered intra-renal arterial cooling of 15°C 31, the target cooling temperature was no longer associated with a lower odds of AKI (P = 0.68).
Figure 3.
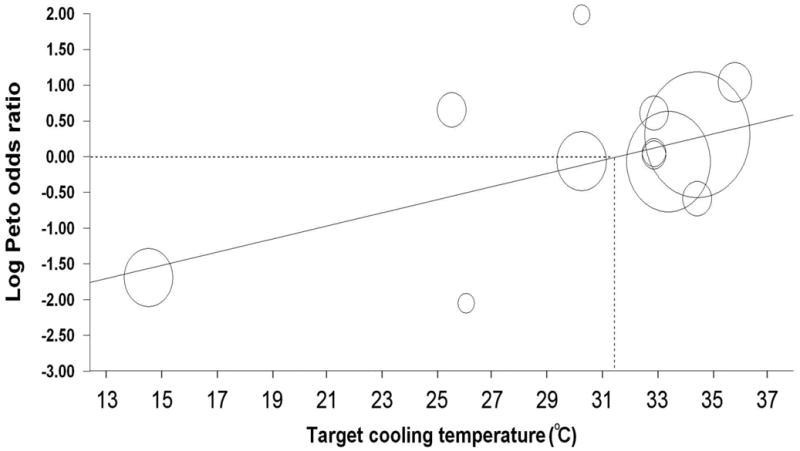
Meta-regression plot examining the relationship between the target cooling temperature and the log of the Peto odds ratio for the development of AKI (P = 0.011). The meta-regression equation is as follows: Log Peto odds ratio = −3.004 + 0.096(°C).
Effect of Therapeutic Hypothermia on Serum Creatinine and Creatinine Clearance
Five trials reported changes in serum creatinine 25, 28, 29, 34, 36 in a total of 522 analyzable patients. By meta-analysis, therapeutic hypothermia resulted in a net decrease in serum creatinine of 0.5 mg/dL (95% CI -1.8, 0.7 mg/dL), which did not reach statistical significance (P = 0.40). Three trials reported changes in creatinine clearance 23, 25, 34, with a total of 141 analyzable patients. By meta-analysis, therapeutic hypothermia resulted in a net increase in creatinine clearance of 0.4 mL/min (95% CI -2.2, 3.0 mL/min), which was also not significant (P = 0.76).
In one study, compared to normothermia, therapeutic hypothermia resulted in higher serum urea nitrogen at day 2 (23 vs. 13 mg/dL) but not at day 7 (16 vs. 15 mg/dL), but there was no significant difference in serum creatinine at the same time points (no reported values) 39. In another study, there was no significant difference in the serum creatinine between the normothermia and therapeutic hypothermia group (1.06 vs. 1.05 mg/dL) 37.
Effect of Therapeutic Hypothermia on Dialysis Requirement
Three RCTs reported on dialysis requirement totaling 509 analyzable patients 27, 31, 32. One study had no patients who required dialysis in either group and thus did not contribute to the meta-analysis 31. The overall weighted incidence of dialysis requirement in the normothermia group was 3.7% (range of 0 to 4.3%). By meta-analysis, therapeutic hypothermia was not associated with a significantly lower odds for dialysis requirement (OR 0.81; 95% CI 0.30, 2.19; P = 0.68).
Effect of Therapeutic Hypothermia on Mortality
This analysis was restricted to the 16 RCTs that reported kidney and mortality endpoints, totaling 2,077 analyzable patients. Mortality was ascertained post-operatively in 2 trials 26, 33, in-hospital in 5 trials 28, 32, 36-38, at 15 days in 3 trials 24, 27, 30, at 30 days in 2 trials 29, 31, and at 90 days in one trial 39. The duration of follow up was not documented in the 3 remaining studies 21, 22, 35. There was no death in either group in one study, which did not contribute to the meta-analysis 28. The overall weighted mortality rate in the normothermia group was 10.8% (range of 0 to 87.5%). By meta-analysis, therapeutic hypothermia was associated with a significant 31% lower odds for mortality (OR 0.69; 95% CI 0.51, 0.92; P = 0.01; Figure 4). Although the trials differed considerably in their size, quality score, and population setting, the test for heterogeneity was not significant (I2 = 0%; P = 0.63). By meta-regression, the duration of cardiac arrest, the cooling target temperature, and the duration of hypothermia were not associated with mortality.
Figure 4.
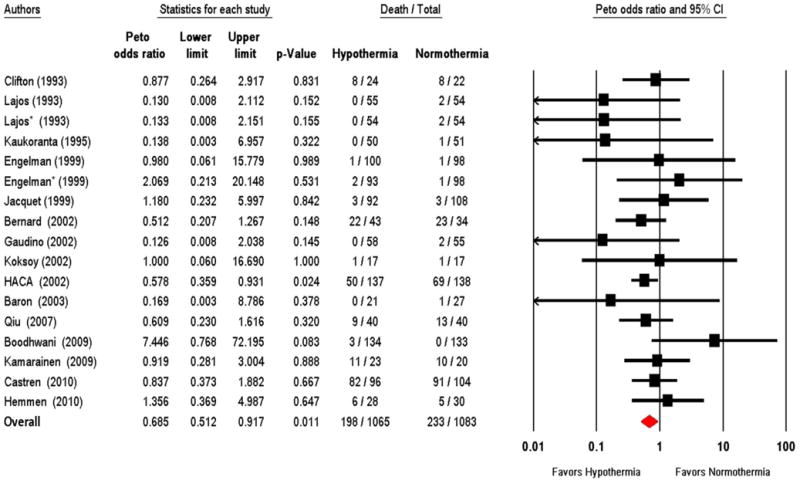
Forest plot of therapeutic hypothermia vs. normothermia on mortality. HACA denotes Hypothermia after Cardiac Arrest Study Group. * Refers to the second therapeutic hypothermia intervention tested in the same trial.
Sensitivity and Subgroup Analyses
Despite the absence of significant heterogeneity among the trials, we performed random-effects meta-analyses, which generated similar results (data not shown). Subgroup analyses by population settings, cooling technique, type of fluid, study quality, and sample size did not influence the odds of AKI (data not shown). However, although therapeutic hypothermia was associated with lower odds for mortality in patients suffering from out-of-hospital cardiac arrest (OR 0.64; 95% CI 0.45, 0.91), compared to those with brain injury (OR 0.83; 95% CI 0.43, 1.60) or those undergoing cardiopulmonary bypass surgery (OR 0.75; 95% CI 0.33, 1.68), there was no significant difference between these clinical settings. Subgroup analyses stratified according to the cooling technique, type of fluid, and sample size (>100 patients) did not significantly influence the mortality analysis (data not shown). However, low-quality studies did not demonstrate a mortality benefit of therapeutic hypothermia (OR 0.58; 95% CI 0.25, 1.33) whereas in fair-to-good-quality studies, there was a demonstrable mortality benefit of hypothermia (OR 0.70; 95% CI 0.51, 0.96, P=0.03). In addition, compared to studies that excluded patients with pre-existing chronic kidney disease 26, 30, 36, those that included patients with chronic kidney disease 24, 27, 31 displayed lower odd ratio for development of AKI (OR 1.30, 95% CI 0.72-2.35; vs. OR 0.46, 95% CI 0.19-1.14), but this did not reach statistical significance (P = 0.42).
Although funnel plots were slightly asymmetric for the development of AKI with 2 unpublished studies favoring hypothermia, they were symmetric for the outcome of dialysis requirement and mortality.
Discussion
The present meta-analysis suggests that therapeutic hypothermia applied in different population settings including out-of-hospital cardiac arrest, major cardiovascular surgery with cardiopulmonary bypass, and brain injury is associated with a reduction in mortality, but has no impact on the prevention of AKI and dialysis requirement.
Acute kidney injury is a common occurrence following out-of-hospital cardiac arrest with spontaneous return of circulation, and on-pump cardiovascular surgery 12, 14, and is associated with an increased risk for mortality, dialysis requirement, and prolonged hospital length of stay 15, 40. With an incidence of out-of-hospital cardiac arrest estimated at 38 per 100,000 person-years 41, and greater than 163,000 cardiac surgeries performed annually in the US 42, several strategies have been explored to prevent AKI including minimization of ischemic time, use of an off-pump technique, and adoption of therapeutic hypothermia 18, 23, 25, 31, 34.
Induction of moderate hypothermia has been successfully used since the 1950s to protect the brain against global ischemia after cardiac arrest 43, but was subsequently abandoned due to uncertain benefit and difficulties with its use. In more recent years, this strategy has regained recognition following the completion of several RCTs demonstrating a clear benefit on neurological morbidity and mortality following out-of hospital cardiac arrest 2, 3, 29, 32. Although therapeutic hypothermia has been adopted into the treatment guidelines of adults with spontaneous circulation after out-of-hospital cardiac arrest 1, there is scant data on the potential protective benefit of this strategy on kidney endpoints.
Unfortunately, in the present meta-analysis, we were unable to demonstrate a kidney-related benefit of therapeutic hypothermia. That may be due in part to the induction of renal vasoconstriction by systemic therapeutic hypothermia, resulting in possible kidney injury 44, whereas locally applied therapeutic hypothermia may decrease the metabolic demand of the kidneys 45 and oxygen consumption 46, 47. These effects correlate with the temperature of the kidney. Indeed, following temperature reduction to 30°C, 20°C, and 10°C, the kidney oxygen consumption is reduced to 40%, 15% and less than 5%, respectively 47.
In the present meta-analysis, we identified only one study that measured kidney temperature, demonstrating a protective effect of hypothermia against AKI 31. Although our meta-regression analysis suggests that a target cooling temperature cut-off point of 31°C might confer kidney protection, this hypothesis requires formal testing, as this association was highly influenced by a single study 31. Moreover, therapeutic hypothermia appeared to be associated with a lower risk of AKI in studies that included patients with pre-existing chronic kidney disease, but this still did not reach statistical significance.
Strengths of our synthesis include the demonstrable survival benefit of therapeutic hypothermia especially in studies of fair-to-good quality, which is in agreement with previously published meta-analyses 4. Mild hypothermia is thought to suppress many of the biological responses associated with ischemia reperfusion injury. These include the generation of free radicals, the release of amino acids, and transcellular calcium shifts, which can lead to mitochondrial injury and apoptosis 48-50, especially following out-of hospital cardiac arrest. The main limitation of our meta-analysis is the variable or lack of definition of AKI reported in the individual studies. The small sample size of most of the trials is also an important limitation of the evidence. The analysis was restricted to adults and excluded newborns and children. Furthermore, the trials included in this analysis were not originally designed to examine the effect of therapeutic hypothermia on kidney endpoints as their primary endpoint. In addition, we were unable to address the safety of therapeutic hypothermia including, the potential risk for the development of arrhythmias, infections, and coagulopathy 2, 4. Our analysis of mortality is limited by the lack of inclusion of trials that did not report kidney outcomes.
In conclusion, the currently available trials indicate that therapeutic hypothermia does not prevent AKI including dialysis requirement, but is associated with lower mortality following out-of-hospital cardiac arrest, major cardiovascular surgery and brain injury. The present analysis however, calls for the design of future studies to formally test whether therapeutic hypothermia prevents AKI in the setting of major cardiovascular surgery and out-of-hospital cardiac arrest, two clinical settings known to be associated with this complication. Such studies would need to explore a range of cooling temperatures with the hope of identifying the optimal kidney protective hypothermic strategy, and monitor for adverse effects.
Acknowledgments
This work has been made possible in part through Dr. Susantitaphong's International Society of Nephrology funded Fellowship. This work was supported in part by Grant number UL1 RR025752 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Authors' Contributions: Conception and design: M. Alfayez, B.L. Jaber
Analysis and interpretation of the data: P. Susantitaphong, B.L. Jaber, E.M. Balk
Drafting of the article: P. Susantitaphong, B.L. Jaber
Critical revision of the article for important intellectual content: P. Susantitaphong, E.M. Balk B.L. Jaber
Final approval of the article: P. Susantitaphong, M. Alfayez, A.C. Bucay, E.M. Balk, B.L. Jaber
Provision of study materials or patients: not applicable
Statistical expertise: E.M. Balk, B.L. Jaber
Administrative, technical, or logistic support: not applicable
Collection and assembly of data: P. Susantitaphong, M. Alfayez, A.C. Bucay
Statement of Competing Financial Interests: The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nolan JP, Morley PT, Hoek TL, Hickey RW. Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation. 2003 Jun;57(3):231–5. doi: 10.1016/s0300-9572(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 2.Arrich J, Holzer M, Herkner H, Mullner M. Cochrane corner: hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Anesth Analg. 2009 Apr 1;110(4):1239. doi: 10.1213/ANE.0b013e3181ce8d34. [DOI] [PubMed] [Google Scholar]
- 3.Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005 Feb;33(2):414–8. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- 4.Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008 Jan;25(1):62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- 5.Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009;(2):CD001048. doi: 10.1002/14651858.CD001048.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Rees K, Beranek-Stanley M, Burke M, Ebrahim S. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database Syst Rev. 2001;(1):CD002138. doi: 10.1002/14651858.CD002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006 Jul;34(7):1865–73. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PubMed] [Google Scholar]
- 8.Bouwes A, Kuiper MA, Hijdra A, Horn J. Induced hypothermia and determination of neurological outcome after CPR in ICUs in the Netherlands: results of a survey. Resuscitation. 2010 Apr;81(4):393–7. doi: 10.1016/j.resuscitation.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 11.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006 Jan;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998 Apr;104(4):343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 13.Zanardo G, Michielon P, Paccagnella A, Rosi P, Calo M, Salandin V, et al. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994 Jun;107(6):1489–95. [PubMed] [Google Scholar]
- 14.Domanovits H, Mullner M, Sterz F, Schillinger M, Klosch C, Paulis M, et al. Impairment of renal function in patients resuscitated from cardiac arrest: frequency, determinants and impact on outcome. Wien Klin Wochenschr. 2000 Feb 25;112(4):157–61. [PubMed] [Google Scholar]
- 15.Mattana J, Singhal PC. Prevalence and determinants of acute renal failure following cardiopulmonary resuscitation. Arch Intern Med. 1993 Jan 25;153(2):235–9. [PubMed] [Google Scholar]
- 16.Swaminathan M, East C, Phillips-Bute B, Newman MF, Reves JG, Smith PK, et al. Report of a substudy on warm versus cold cardiopulmonary bypass: changes in creatinine clearance. Ann Thorac Surg. 2001 Nov;72(5):1603–9. doi: 10.1016/s0003-4975(01)03223-4. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996 Feb;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2010 Oct;5(10):1734–44. doi: 10.2215/CJN.02800310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rucker G, Schwarzer G, Carpenter J, Olkin I. Why add anything to nothing? The arcsine difference as a measure of treatment effect in meta-analysis with zero cells. Stat Med. 2009 Feb 28;28(5):721–38. doi: 10.1002/sim.3511. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Clifton GL, Allen S, Barrodale P, Plenger P, Berry J, Koch S, et al. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993 Fall;10(3):263–71. doi: 10.1089/neu.1993.10.263. discussion 73. [DOI] [PubMed] [Google Scholar]
- 22.Lajos TZ, Espersen CC, Lajos PS, Fiedler RC, Bergsland J, Joyce LT. Comparison of cold versus warm cardioplegia. Crystalloid antegrade or retrograde blood. Circulation. 1993 Nov;88(5 Pt 2):II344–9. [PubMed] [Google Scholar]
- 23.Ip-Yam PC, Murphy S, Baines M, Fox MA, Desmond MJ, Innes PA. Renal function and proteinuria after cardiopulmonary bypass: the effects of temperature and mannitol. Anesth Analg. 1994 May;78(5):842–7. doi: 10.1213/00000539-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kaukoranta P, Lepojarvi M, Nissinen J, Raatikainen P, Peuhkurinen KJ. Normothermic versus mild hypothermic retrograde blood cardioplegia: a prospective, randomized study. Ann Thorac Surg. 1995 Oct;60(4):1087–93. doi: 10.1016/0003-4975(95)00671-7. [DOI] [PubMed] [Google Scholar]
- 25.Regragui IA, Izzat MB, Birdi I, Lapsley M, Bryan AJ, Angelini GD. Cardiopulmonary bypass perfusion temperature does not influence perioperative renal function. Ann Thorac Surg. 1995 Jul;60(1):160–4. [PubMed] [Google Scholar]
- 26.Engelman RM, Pleet AB, Rousou JA, Flack JE, 3rd, Deaton DW, Pekow PS, et al. Influence of cardiopulmonary bypass perfusion temperature on neurologic and hematologic function after coronary artery bypass grafting. Ann Thorac Surg. 1999 Jun;67(6):1547–55. doi: 10.1016/s0003-4975(99)00360-4. discussion 56. [DOI] [PubMed] [Google Scholar]
- 27.Jacquet LM, Noirhomme PH, Van Dyck MJ, El Khoury GA, Matta AJ, Goenen MJ, et al. Randomized trial of intermittent antegrade warm blood versus cold crystalloid cardioplegia. Ann Thorac Surg. 1999 Feb;67(2):471–7. doi: 10.1016/s0003-4975(98)01198-9. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn-Regnier F, Natour E, Dhein S, Dapunt O, Geissler HJ, LaRose K, et al. Beta-blockade versus Buckberg blood-cardioplegia in coronary bypass operation. Eur J Cardiothorac Surg. 1999 Jan;15(1):67–74. doi: 10.1016/s1010-7940(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 29.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002 Feb 21;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 30.Gaudino M, Zamparelli R, Andreotti F, Burzotta F, Iacoviello L, Glieca F, et al. Normothermia does not improve postoperative hemostasis nor does it reduce inflammatory activation in patients undergoing primary isolated coronary artery bypass. J Thorac Cardiovasc Surg. 2002 Jun;123(6):1092–100. doi: 10.1067/mtc.2002.120709. [DOI] [PubMed] [Google Scholar]
- 31.Koksoy C, LeMaire SA, Curling PE, Raskin SA, Schmittling ZC, Conklin LD, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg. 2002 Mar;73(3):730–8. doi: 10.1016/s0003-4975(01)03575-5. [DOI] [PubMed] [Google Scholar]
- 32.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 Feb 21;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 33.Baron O, Roussel JC, Delaroche O, Peron S, Duveau D. Prospective clinical and biological comparison of three blood cardioplegia techniques in low-risk CABG patients: better is worse than good enough. Cardiovasc Surg. 2003 Dec;11(6):489–95. doi: 10.1016/S0967-2109(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 34.Zeiner A, Sunder-Plassmann G, Sterz F, Holzer M, Losert H, Laggner AN, et al. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation. 2004 Mar;60(3):253–61. doi: 10.1016/j.resuscitation.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007 Sep;22(3):229–35. doi: 10.1016/j.jcrc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Boodhwani M, Rubens FD, Wozny D, Nathan HJ. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg. 2009 Feb;87(2):489–95. doi: 10.1016/j.athoracsur.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 37.Kamarainen A, Virkkunen I, Tenhunen J, Yli-Hankala A, Silfvast T. Prehospital therapeutic hypothermia for comatose survivors of cardiac arrest: a randomized controlled trial. Acta Anaesthesiol Scand. 2009 Aug;53(7):900–7. doi: 10.1111/j.1399-6576.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 38.Castren M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness) Circulation. 2010 Aug 17;122(7):729–36. doi: 10.1161/CIRCULATIONAHA.109.931691. [DOI] [PubMed] [Google Scholar]
- 39.Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010 Oct;41(10):2265–70. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antunes PE, Prieto D, Ferrao de Oliveira J, Antunes MJ. Renal dysfunction after myocardial revascularization. Eur J Cardiothorac Surg. 2004 Apr;25(4):597–604. doi: 10.1016/j.ejcts.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation. 2005 Oct;67(1):75–80. doi: 10.1016/j.resuscitation.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams GR, Jr, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958 Sep;148(3):462–8. doi: 10.1097/00000658-195809000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broman M, Kallskog O. The effects of hypothermia on renal function and haemodynamics in the rat. Acta Physiol Scand. 1995 Feb;153(2):179–84. doi: 10.1111/j.1748-1716.1995.tb09849.x. [DOI] [PubMed] [Google Scholar]
- 45.Semb G, Krog J, Johansen K. Renal metabolism and blood flow during local hypothermia, studied by means of renal perfusion in situ. Acta Chir Scand Suppl. 1960;253(Suppl):196–202. [PubMed] [Google Scholar]
- 46.Harvey RB. Effect of temperature on function of isolated dog kidney. Am J Physiol. 1959 Jul;197(1):181–6. doi: 10.1152/ajplegacy.1959.197.1.181. [DOI] [PubMed] [Google Scholar]
- 47.Levy MN. Oxygen consumption and blood flow in the hypothermic, perfused kidney. Am J Physiol. 1959 Nov;197:1111–4. doi: 10.1152/ajplegacy.1959.197.5.1111. [DOI] [PubMed] [Google Scholar]
- 48.Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997 Jun;14(3):171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- 49.Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. 1992 Fall;4(3):189–225. [PubMed] [Google Scholar]
- 50.Safar PJ, Kochanek PM. Therapeutic hypothermia after cardiac arrest. N Engl J Med. 2002 Feb 21;346(8):612–3. doi: 10.1056/NEJM200202213460811. [DOI] [PubMed] [Google Scholar]