Abstract
A mechanistic understanding of gene regulatory network dynamics requires quantitative single-cell data of multiple network components in response to well-defined perturbations. Recent advances in the development of fluorescent biomarkers for proteins, detection of RNA and interactions, microfluidic technology, and high-resolution imaging have set the stage for a host of new studies that elucidate the important roles of stochasticity and cell-cell variability in response to external perturbations. In this review, we briefly describe methods for high-resolution visualization and the control of gene expression, along with application of these novel methods to recent studies involving gene networks.
Keywords: single-cell imaging, microfluidics, gene networks
Introduction
Genes, mRNAs, and proteins form a large and intricate network that defines cell phenotype and response to external stimuli. Much is known about the structure of this complex network through extensive genomic, proteomic, and interactomic studies [1, 2]. However, a complete understanding of the systems biology of the cell can only be gained through studies of the dynamics that occur on these networks. This presents experimental challenges for a number of reasons. First, dynamical studies require time-resolved measurements of many network components simultaneously. Second, and importantly for this review, there is considerable variability in the responses of genetically identical cells. Cells respond differently to the same external stimuli either because of differences in cell parameters (cell size, cell cycle stage, chemical concentrations of metabolites, etc.) or because of the intrinsic stochasticity of the underlying biochemical reactions [3, 4]. In order to account for the role of such variability in the dynamics, it is often insufficient to measure distributions of relevant observables within a population, as can be achieved by flow cytometry [5]. In order to avoid ambiguity in interpreting dynamical data, one has to track the fates of individual cells in the population over times greater than the characteristic time of the process of interest. Often, this time spans many cell division cycles, which poses challenges in collecting and interpreting data.
Recent years have seen remarkable progress in single-cell measurements of dynamical processes at the intra- and intercellular levels. In this review, we will highlight several technological advances and describe several representative studies that have taken advantage of these advances to obtain novel insights into the dynamics of gene regulation. We will focus on methods of high-resolution visualization of gene expression that are based on the development of novel biomarkers of single molecules and methods of registering their activity. Significant recent advances have involved imaging of mRNA activity using a variety of techniques such as FISH, MS2-GFP, and molecular beacons. We then describe imaging techniques involving fluorescent microscopy beyond that diffraction limit, which allows for the tracking of intra-cell processes at single-molecule resolution. Progress has also been made in our ability to control and manipulate cell cultures. It is well known that intrinsic cellular processes or their responses to external cues depend strongly on environmental conditions. Furthermore, an important line of research in studying cell response to external perturbations has been the role of intrinsic and extrinsic noise. Here too it is paramount to control the cellular environment over long periods of time and to vary it in a prescribed manner. We will describe novel approaches involving high-throughput microfluidics for the simultaneous tracking of hundreds of cells under identical or diverging conditions, and also advances in optogenetics which allow for precise spatially and temporally resolved perturbations within a population of cells. Finally, we will describe applications of these novel methods to the NF-κB pathway [6, 7] and Notch-Delta signaling[8].
Using single molecule biomarkers for spatiotemporal analysis of gene expression
Fluorescent proteins are by now a gold standard for real-time reporters of in vivo gene activity [9]. Initially, quantitative techniques were developed for population-averaged measurements of fluorescence (flow cytometry [5]). While extremely valuable, this approach originally lacked the capability to assay the detailed stochastic patterns of transient gene activity, for which following the gene expression in the same cell for a period of time is needed. In recent years advances in microscopy and microfluidics have led to the emergence of quantitative single-cell fluorescence measurements [10, 11] that permit the characterization of the dynamics and stochasticity of gene expression, as well as cell-cell variability under identical external conditions [3, 12, 13, 14]. Still, these measurements often have insufficient temporal and spatial resolution to study complex sub-cellular processes related to gene expression and regulation. In particular, there are many highly dynamic steps between gene activation and the formation of a fully active protein that are not easily resolved with the standard techniques. In this section, we describe several methods that have been recently developed to investigate structure, dynamics, and function of single mRNA and protein molecules, and to obtain information about their spatial location within a cell.
The first step in gene expression involves transcription initiation in which RNA polymerase (RNAP) binds to the DNA at the promoter region and its subsequent elongation that results in formation of the nascent RNA molecule. This process has been visualized in vivo using scanning force microscopy and optical trapping [15, 16, 17, 18]. These studies revealed intricacies of the complex proofreading mechanism accomplished during transcription by RNAP pausing and backtracking. The number of RNA molecules in the cell is small (often several orders of magnitude less than the number of proteins), so measurements of RNA concentration are difficult. One of the earliest effective methods for quantifying RNAs in individual cell was single-molecule RNA fluorescence in situ hybridization (FISH) [19, 20, 21]. The technique is based on specific binding of fluorescently labeled oligonucleotide probes to target RNA sequences in fixed and permeabilized cells [22]. Improvements to the probe design and fluorophores have allowed for single RNA recognition and tracking of up to five different transcripts at a time [20, 23, 24]. Still, for some applications, the inability to track RNA in real-time can be a significant shortcoming of FISH.
Over the past decade, real-time measurement of RNA has been achieved though several novel techniques. The main difficulty in using of single fused fluorescent proteins is the difficulty of their detection above the cellular autofluorescence. Therefore, several groups have developed the MS2 tagging system, with one plasmid containing fluorophore sequence fused to coding sequence for bacteriophage MS2 single-standed RNA capsid protein and the other, RNA reporter plasmid containing the RNA of interest along with multiple stem loop MS2-binding sites. Upon expression of both plasmids in live cells, multiple fluorophores fused MS2 capsid proteins are able to bind to the MS2-binding sites in the untranslated region of RNA of interest [12, 25, 26, 13]. This technique can generate a strong enough fluorescent signal to allow for the visualization of an individual RNA molecule (Figure 1E). The bulkiness of the MS2 system, however, can have detrimental effects on the activity of the RNA, causing clumping and impeding trafficking of mRNA [27, 25]. Furthermore, unbound fluorescent protein generates high background fluorescence and thus limits the sensitivity of the method. In vivo hybridization with molecular beacons is another method that allows for real-time tracking of target RNAs. Molecular beacons are single stranded nucleic acid probes containing a fluorophore and a quencher, which separate only upon binding to target RNA sequence [28]. This approach removes background noise, allowing for single fluorophore labeling and easier image analysis. The downside of molecular beacons is the need to disturb the cell through microinjection or listiolysin-O for delivery[27]. Yet another method of RNA visualization is based on the protein complementation assay [29]. The method is based on the interaction of a split RNA-binding protein, eIF4A, fused with two inactive fragments of the marker protein EGFP, with its corresponding RNA aptamer. This method has the advantage of low background fluorescence and great dynamic range. In addition to the above techniques, a number of other approaches have been developed, which are not described here but can be found in [27, 30, 31, 32, 33, 34].
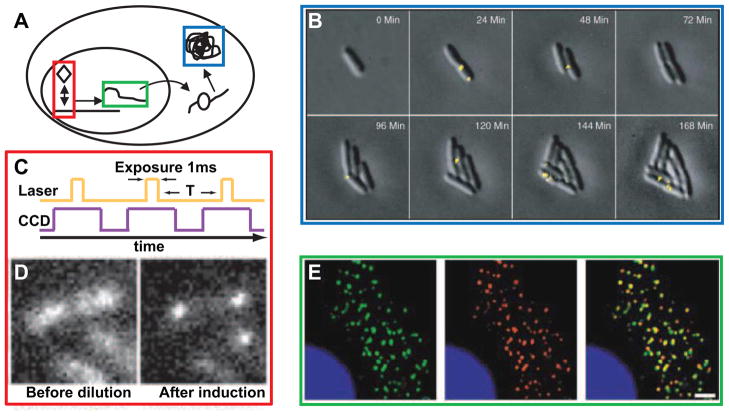
Figure 1. Single-molecule in vivo visualization techniques.
(A) Schematic of cellular components: transcription factors (red), RNAs (green), and proteins (blue). (B) Real-time monitoring of the expression of Tsr-Venus under the control of repressed lac promoter. An 1100-ms exposure was applied after each image collection to photobleach the Venus fluorophores. Reprinted from [37] with permission from AAAS. (C) Timing diagram for stroboscopic illumination. Each laser pulse is synchronized to a CCD frame. (D) lac repressor dynamics in live cells. Dilution of IPTG results in lac repressor biding to the promoter region. Reprinted from [36] with permission from AAAS. (E) Using MS2-GFP fusion to visualize single mRNA proteins in live cells. Colocalization (yellow) of MS2-GFP proteins (green) and in situ mRNA reporter probes (red). The scale bar represents 2 μm. Reprinted from [13] with permission from Elsevier.
Visualization of protein dynamics
Using genetic engineering, scientists have fused Green Fluorescent Protein (GFP) and its various derivatives to proteins of interest, thus allowing for real-time visualization of those proteins. Using multi-color fluorescent microscopy, simultaneous measurement of multiple protein concentrations is feasible. As but one example of this widely used technique, it has been used to access the relative roles of intrinsic and extrinsic noise in gene expression [3, 4]. However, due to the relatively fast diffusion of fluorescent proteins, this approach does not allow for the visualization of the location of individual proteins[35, 36]. In some cases, when the diffusion of proteins is reduced, such as in the case of membrane bound proteins, one can achieve single protein visualization with fast-maturing bright fluorescent proteins (Figure 1B) [37, 38]. Similarly, one can track the DNA binding dynamics of transcription factors due to the slow diffusion rate of DNA bound proteins (Figure 1D) [36].
Fluorescence resonance energy transfer [FRET] is a recent technique that utilizes a donor and an acceptor fluorophore to visualize conformational changes in individual molecules. When the two fluorephores come close to one another, the donor fluorophore transfers energy to the acceptor fluorophore, therefore changing the wavelength of the signal fluorescence. This approach has been instrumental in studies of protein folding [39], RNA polymerase dynamics in E. coli [16], and enzyme dynamics such as cyclic AMP [40]. Bimolecular fluorescence complementation (BiFC) is another method that has been used to visualize interaction between proteins and conformational changes in molecules [41, 42, 43]. This technique breaks apart a yellow fluorescent protein (YFP) into two inactive fragments and fuses them to independent targets of interest. When the two targets come close to one another, the fused YFP fragments form an active fluorophore allowing for visualization of target interaction. There are several reviews that detail these and other protein visualization techniques [27, 33, 41]. Typically, there is a tradeoff between signal detection and molecular perturbation, with most of the methods requiring careful controls that demonstrate that the applied fluorescent tags do not significantly disturb the system.
Single molecule microscopy
Once target molecules are tagged with fluorophores, the goal is to detect their number and localization in vivo. Fluorescence microscopy is a common method for assaying the mean fluorescence of tagged proteins and, in some cases, determining the location of single molecules (e.g. with the mRNA-tagged MS2 system). However, generally the detection of single fluorophores requires more advanced technology. Typically the high diffusion rate of single molecules inside a living cell renders their imaging extremely difficult. One of the approaches to overcome this problem is to reduce the diffusion rate by localizing the molecules to the membrane where the diffusion rate is much slower than in the cytoplasm (Figure 1B) [37]. Another approach is stroboscopic excitation, which allows one to study dynamical properties of individual molecules without manipulation of the sample (Figure 1C ). The Xie group has been able to achieve single molecule detection using a confocal microscope and a focused laser beam [33]. This technique does not involve adjustment of the shutter speed of the camera, but instead uses short laser pulses that allow the shutter to remain open for longer periods of time. This prevents significant diffusion of the fluorescent reporter, but the high laser intensity can be phototoxic to the cells or damage the fluorophores.
The highest resolution that has been achieved with the above techniques is about 200nm due to the diffraction limit of visible light. In recent years, microscopy techniques that achieve much higher resolution have been developed. Near-field scanning optical microscopy, photoactivated localization microscopy, stochastic optical reconstruction microscopy, and stimulated emission depletion are some of the methods that have been developed for sub-diffraction microscopy [44]. The shortcoming of these methods is that they require scanning of a large area with a small window, which is often too slow for characterizing live-cell dynamics. Interestingly, FRET is a technique that can allow for resolution in the range of 1nm, or the distance between two fluorophores that results in energy transfer, but the applications of this technique are currently limited to the study of protein-protein interactions and conformational changes [44].
Dynamic manipulation of cell environment
In studies of cell dynamics it is often important to control the environment and possibly perturb the input stimuli in a pre-described manner. There are many signaling and regulatory systems can be better characterized by observing their transient behavior after various specific perturbations. Advances in microfluidic technology allow for precise control and treatment of cells and their environment through small volume manipulations. Microfluidic devices can be very complicated and contain multiple mixing chambers, with computer-controlled injection pumps and valves that allow for high-throughput studies [45, 46, 47]. Simpler devices that are more pedestrian have been instrumental in studies of genetic circuits [48, 49]. The sustainability of cell populations for long periods of time sets the stage for lineage and evolution studies.
The spatial and temporal resolution of the environmental control in microfluidic devices is somewhat limited by the diffusion of chemical signals. This leads to an inability to target individual cells on a typical chip. Optogenetics is a newly emerging field that combines genetic and optical methods to achieve very precise control of specific events inside the cell [50]. In early applications, optical trapping systems were used to study such activities as RNA polymerase dynamics [13, 17]. Using light-switchable molecules adapted from Arabidopsis thaliana, Levskaya et al. were able to control translocation of target proteins to the membrane [5]. In another study, the Voigt group engineered light-sensing bacteria that could respond to the light background by activating genes in congruence with the original gradient [51], or use small genetically engineered circuits to detect edges of light and dark mask [52].
Quantifying Gene Network Dynamics
The new technical advances have led to a wealth of quantitative information about individual cellular processes. Concurrent tracking of mRNA using the MS2 system and fluorescently tagged proteins has shown that mRNA-protein correlations in E. coli are weaker early in cell cycle, following cell division when mRNA partitioning is approximately binomial [12]. Taniguchi et al. [53] expanded on this study by looking at a library of YFP-tagged proteins and their corresponding mRNA in E. coli, using FISH to show that mRNA and proteins are highly uncorrelated. In another study, Bosisio et al. [54] used fluorescence recovery after photobleaching (FRAP) analysis to quantify NF-κB binding-dissociation dynamics and show that they are fast, such that the transcription factors are in equilibrium with their promoters. A study of a developmental gene network in nematodes using FISH showed incomplete penetrance of a skn-1 mutant phenotype due to large variations in gene expression, which experience a threshold during development [55]. Elf et al. [36] used transcription factor labeling along with stroboscopic excitation to study the binding characteristics and derive a model for lac repressor diffusion dynamics in the nucleus.
The combination of these techniques within a single experiment opens further possibilities for characterizing the dynamics of intracellular regulation on several levels. High-throughput microfluidic cell culture and fluorescence microscopy have been used to quantify the fluctuations and variability in the dynamics of the transcription factor NF-κB in response to the signaling molecule tumor-necrosis factor (TNFα) [35, 6]. Using fluorescently tagged NF-κB molecules allowed the authors to investigate the nuclear localization dynamics at the single cell level under different TNFα excitation conditions. By tracking the dynamics of NF-κB movement between the nucleus and cytoplasm in over 400 cells, Tay et al. showed that cells respond to TNFα in a stochastic manner, with the fraction of responding cells dependent on the strength of TNFα signal. At the same time, the NF-κB amplitude of responding cells remains high, even with decreasing TNFα signal. Sung et al. [7] further looked at NF-κB mobility using FRAP, examining its chromatin residence time, to elucidate how sustained NF-kB cycles could guide gene expression patterns. These studies represent canonical examples of the importance of single-cell, high resolution data for advancing our understanding of the function of biological networks.
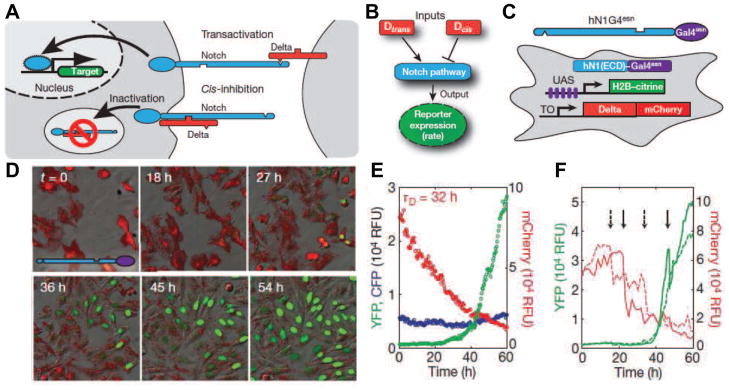
Recently, simultaneous visualization of multiple components of the network led to a greater quantitative understanding Notch-Delta. In this study [8], Sprinzak et al. used tetracycline-inducible mCherry-Delta fusion trans-membrane protein and a Notch activated fluorescent reporter to measure the cir-trans input-output relationship in the network. The Delta transmembrane protein on one cell can bind to Notch transmembrane protein on a nearby cell, resulting in proteolytic release of Notch intracellular domain, which then translocates and activates target genes in the nucleus (trans). The Delta protein can also prevent Notch activation by binding Notch transmembrane proteins in its own cell (cis) (Figure 2A). By measuring and analyzing the Notch reporter expression rate under digital expression levels of mCherry-Delta and different cell concentrations, Sprinzak et al. were able to characterize the signaling dynamics of this system (Figure 2B-F ). Using a mathematical model of mutual inactivation, Spinzak et al. showed the existence of switch-like behavior in the dynamics of the system associated with different concentrations of Delta and Notch transmembrane proteins. This signaling switch could optimize the Notch-Delta pathway to allow for faster dynamics and directional signaling, which elucidates its involvement in developmental pathways.
Figure 2. Quantitative analysis of Notch-Delta system.
(A) Notch (blue) and Delta (red) interactions are indicated schematically. (B) Notch activity integrates cis-and trans-Delta. (C) The cell line incorporated a variant of human NOTCH1 with activator Gal4esn replacing the Notch intracellular domain and genes for histone 2B (H2B) -citrine (YFP) reporter controlled by an upstream activating sequence (UAS) promoter and a tetracycline-inducible (TO) Delta-mCHerry fusion protein. (D) Filmstrip showing Delta-mCHerry fluorescence (red) and concomitant activation of Notch reporter (green) at the indicated times. (E) Population average (median) response shows a slow decay of Delta-mCherry fluorescence (red), but sharp response of reporter expression (green). Constitutively expressed pCMV-H2B-cerulean (blue) remains constant (control). (F) Single-cell response for two individual cells (solid and dashed lines, colors as in (E)). Adapted from [8] by permission from Macmillan Publishers Ltd.
Conclusions
In this short review we described novel approaches to the quantitative analysis of gene regulatory circuits in living cells. A complete mechanistic understanding of these networks, which addresses not just the average properties of cell cultures but the sources of fluctuations and intercellular variability, requires not only high spatial and temporal resolution of fluorescent imaging, but concurrent measurement of the expression of multiple network components. We have described the novel fluorescent marker techniques that allow one to simultaneously track multiple species of RNA and proteins in vivo with sub-cellular resolution, and methods for precise environmental control and manipulation using microfluidics and optogenetics. So far, there are relatively few studies where these diverse tools have been combined in network analysis. However, we expect that as our ability to simultaneously visualize multiple transcripts and proteins improves, we will see continued rapid progress in the understanding of the dynamics of gene regulation and cellular signaling.
Highlights.
We review methods for high-resolution visualization and the control of gene expression
The methods allow for simultaneous tracking of multiple species of RNA and protein in vivo
We present several key studies applying these methods to study network dynamics
Improvements in these methods will lead to understanding of gene regulation and cell signaling
Acknowledgments
This work was supported by the National Institutes of Health and General Medicine through grants P50-GM085764 (JS) RO1-GM69811 (JH), and RO1-GM089976 (LT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blackstock WP, Weir MP. Proteomics: quantitative and physical mapping of cellular proteins. Trends in biotechnology. 1999;17(3):121–127. doi: 10.1016/s0167-7799(98)01245-1. [DOI] [PubMed] [Google Scholar]
- 2.Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. Metabolomics by numbers: acquiring and understanding global metabolite data. TRENDS in Biotechnology. 2004;22(5):245–252. doi: 10.1016/j.tibtech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Swain PS, Elowitz MB, Siggia ED. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proceedings of the National Academy of Sciences. 2002;99(20):12,795. doi: 10.1073/pnas.162041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raser JM, O’Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304(5678):1811. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke EJ, Voigt CA. Characterization of combinatorial patterns generated by multiple two-component sensors in e. coli that respond to many stimuli. Biotechnology and bioengineering. 2011;108(3):666–675. doi: 10.1002/bit.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TK, Covert MW. High-throughput, single-cell nf-[kappa] b dynamics. Current opinion in genetics & development. 2010;20:1–7. doi: 10.1016/j.gde.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Sung MH, Salvatore L, De Lorenzi R, Indrawan A, Pasparakis M, Hager GL, Bianchi ME, Agresti A. Sustained oscillations of nf-κb produce distinct genome scanning and gene expression profiles. PLoS One. 2009;4(9):e7163. doi: 10.1371/journal.pone.0007163. In this article, the authors look at NF-κB dynamics and describe the function of sustained NF-κB oscillations in single cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Sprinzak D, Lakhanpal A, LeBon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB. Cis-interactions between notch and delta generate mutually exclusive signalling states. Nature. 2010;465(7294):86–90. doi: 10.1038/nature08959. This article describes use of quantitative multifluorescent time-lapse microscopy to analyze Notch-Delta signaling dynamics in individual mammalian cells. Results of the study help explain the difference in response of Notch to trans and cis-Delta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox RS, III, Dunlop MJ, Elowitz MB. A synthetic three-color scaffold for monitoring genetic regulation and noise. Journal of Biological Engineering. 2010;4:10. doi: 10.1186/1754-1611-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JCW, Elowitz MB. Using movies to analyse gene circuit dynamics in single cells. Nature reviews Microbiology. 2009;7(5):383. doi: 10.1038/nrmicro2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grilly C, Stricker J, Pang WL, Bennett MR, Hasty J. A synthetic gene network for tuning protein degradation in saccharomyces cerevisiae. Molecular systems biology. 2007;3(1) doi: 10.1038/msb4100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123(6):1025–1036. doi: 10.1016/j.cell.2005.09.031. This article looks at single E.Coli cell’s mRNA (MS2) and protein (fused-GFP) correlation. Results show that mRNA-protein correlation is weaker earlier in cell cycle, following cell division where mRNA partitioning is approximately binomial. [DOI] [PubMed] [Google Scholar]
- 13**.Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, Bertrand E. Single mrna molecules demonstrate probabilistic movement in living mammalian cells. Current Biology. 2003;13(2):161–167. doi: 10.1016/s0960-9822(02)01436-7. In this article, the authors used MS2 method to study mRNA movement inside the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotman B. Measurement of activity of single molecules of β-d-galactosidase. Proceedings of the National Academy of Sciences of the United States of America. 1961;47(12):1981. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustamante C, Bryant Z, Smith SB. Ten years of tension: single-molecule dna mechanics. Nature. 2003;421:423–426. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 16*.Schwartz JJ, Quake SR. Single molecule measurement of the speed limit of dna polymerase. Proceedings of the National Academy of Sciences. 2009;106(48):20,294. doi: 10.1073/pnas.0907404106. This article focuses on RNA polymerase dynamics in E.Coli measured using FRET. The “speed limit” of DNA polymerase I was found to be faster than bulk measurements had suggested, but also highly stochastic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Adelman K, La Porta A, Santangelo TJ, Lis JT, Roberts JW, Wang MD. Single molecule analysis of rna polymerase elongation reveals uniform kinetic behavior. Proceedings of the National Academy of Sciences. 2002;99(21):13,538. doi: 10.1073/pnas.212358999. Measurements of E.Coli RNA polymerase dynamics using optical trapping approach. Results show that variable timing and duration of pauses is responsible for the variable rate of RNA polymerase elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Shaevitz JW, Abbondanzieri EA, Landick R, Block SM. Backtracking by single rna polymerase molecules observed at near-base-pair resolution. Nature. 2003;426(6967):684. doi: 10.1038/nature02191. In this article, the authors use optical trapping with novel two-bead assay to study transcriptional elongation, observing backtracking and recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single rna transcripts in situ. Science. 1998;280(5363):585. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 20.Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of rna expression in drosophila embryos. Science. 2004;305(5685):846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- 21.Zenklusen D, Singer RH. Analyzing mrna expression using single mrna resolution fluorescent in situ hybridization. Methods in enzymology. 2010;470:641–659. doi: 10.1016/S0076-6879(10)70026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. Journal of Cell Science. 2003;116(14):2833. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- 23*.Lauter G, Soll I, Hauptmann G. Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural development. 2011;6:10. doi: 10.1186/1749-8104-6-10. This article describes a multicolor FISH procedure which allows visualization of up to three unique transcripts in whole zebrafish embryos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mrna molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HY, Buxbaum AR, Singer RH. Single mrna tracking in live cells. Methods in enzymology. 2010;472:387–406. doi: 10.1016/S0076-6879(10)72003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ash1 mrna particles in living yeast. Molecular cell. 1998;2(4):437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 27.Raj A, van Oudenaarden A. Single-molecule approaches to stochastic gene expression. Annual review of biophysics. 2009;38:255–270. doi: 10.1146/annurev.biophys.37.032807.125928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyagi S, Kramer FR, et al. Molecular beacons: probes that fluoresce upon hybridization. Nature biotechnology. 1996;14(3):303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 29*.Valencia-Burton M, McCullough RM, Cantor CR, Broude NE. Rna visualization in live bacterial cells using fluorescent protein complementation. Nature methods. 2007;4(5):421–427. doi: 10.1038/nmeth1023. In this article, the authors describe fluorescent-protein complementation appoach to detect RNA transcripts. EGFP protein is split into two RNA-binding protein-fused fragments whose fluorescence is restored in the presence of target transcript. [DOI] [PubMed] [Google Scholar]
- 30.Itzkovitz S, van Oudenaarden A. Validating transcripts with probes and imaging technology. Nature. 2011;201:1. doi: 10.1038/nmeth.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie XS. Enzymology and life at the single molecule level. Single Molecule Spectroscopy in Chemistry, Physics and Biology. 2009:435–448. [Google Scholar]
- 32**.Ozawa T, Natori Y, Sato M, Umezawa Y. Imaging dynamics of endogenous mitochondrial rna in single living cells. Nature methods. 2007;4(5):413–419. doi: 10.1038/nmeth1030. In this article, authors describe a method for visualizing endogenous mRNA, where two proteins with parts of GFP bind to target mRNA leading to formation of active fluorophore. [DOI] [PubMed] [Google Scholar]
- 33.Xie XS, Choi PJ, Li GW, Lee NK, Lia G. Single-molecule approach to molecular biology in living bacterial cells. Annu Rev Biophys. 2008;37:417–444. doi: 10.1146/annurev.biophys.37.092607.174640. [DOI] [PubMed] [Google Scholar]
- 34.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nature Methods. 2005;2(12):905. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 35**.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell nf-κb dynamics reveal digital activation and analogue information processing. Nature. 2010;466(7303):267–271. doi: 10.1038/nature09145. The authors use microfluidics platform, fluorescent mircoscopy, and sinlge cell imaging to analyze NF-κB dynamics. Results show digital activation and analogue information processing by the NF-κB network in response to TNFα signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Elf J, Li GW, Xie XS. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007;316(5828):1191. doi: 10.1126/science.1141967. In this article, authors looked at binding and dissociation dynamics of lac repressor in response to metabolic signals observed using fluorescent labeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Xiao J, Ren X, Lao K, Xie XS. Probing gene expression in live cells, one protein molecule at a time. Science. 2006;311(5767):1600. doi: 10.1126/science.1119623. [DOI] [PubMed] [Google Scholar]
- 38*.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Genetics. 1989;122:19–27. doi: 10.1038/nbt0102-87. This article describes the fast maturing yellow fluorescent protein (venus) [DOI] [PubMed] [Google Scholar]
- 39*.Kahra D, Kovermann M, Low C, Hirschfeld V, Haupt C, Balbach J, Hübner CG. Conformational plasticity and dynamics in the generic protein folding catalyst slyd unraveled by single-molecule fret. Journal of Molecular Biology. 2011;411:781–790. doi: 10.1016/j.jmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 40*.Gesellchen F, Stangherlin A, Surdo N, Terrin A, Zoccarato A, Zaccolo M. Measuring spatiotemporal dynamics of cyclic amp signaling in real-time using fret-based biosensors. Methods in molecular biology (Clifton, NJ) 2011;746:297. doi: 10.1007/978-1-61779-126-0_16. This article describes a method for using FRET to study cyclic AMP dynamics. [DOI] [PubMed] [Google Scholar]
- 41.Kerppola TK. Visualization of molecular interactions by fluorescence complementation. Nature reviews Molecular cell biology. 2006;7(6):449. doi: 10.1038/nrm1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Hu CD, Kerppola TK. Simultaneous visualization of multiple protein interactions in living cells using multicolor 3uorescence complementation analysis. Nature biotechnology. 2003;21(5):539. doi: 10.1038/nbt816. In this article, the authors describe how Bifc approach can be used to visualize multiple protein interactions and study competitive binding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Lalonde S, Ehrhardt DW, Frommer WB. Shining light on signaling and metabolic networks by genetically encoded biosensors. Current opinion in plant biology. 2005;8(6):574–581. doi: 10.1016/j.pbi.2005.09.015. In this article, the authors discuss how FRET can be used to study protein interactions and enzyme conformations as well as be used as fluorescent biosensors for metabolites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmolze DB, Standley C, Fogarty KE, Fischer AH. Advances in microscopy techniques. Archives of Pathology & Laboratory Medicine. 2011;135(2):255–263. doi: 10.5858/135.2.255. [DOI] [PubMed] [Google Scholar]
- 45*.Bao XR, I, Fraser DC, Wall EA, Quake SR, Simon MI. Variability in g-protein-coupled signaling studied with microfluidic devices. Biophysical journal. 2010;99(8):2414–2422. doi: 10.1016/j.bpj.2010.08.043. The authors use microfluidics and calcium dye to study calcium dynamics associated with G-protein-activated calcium release in macrophages. Results show various sources of variability in release dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fordyce PM, Gerber D, Tran D, Zheng J, Li H, DeRisi JL, Quake SR. De novo identification and biophysical characterization of transcription-factor binding sites with microfluidic affinity analysis. Nature biotechnology. 2010;28:970975. doi: 10.1038/nbt.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S, Streets AM, Lin RR, Quake SR, Weiss S, Majumdar DS. High-throughput single-molecule optofluidic analysis. Nature methods. 2011;8(3):242. doi: 10.1038/nmeth.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett MR, Hasty J. Microfluidic devices for measuring gene network dynamics in single cells. Nature Reviews Genetics. 2009;10(9):628–638. doi: 10.1038/nrg2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferry MS, Razinkov IA, Hasty J. Microfluidics for synthetic biology from design to execution. Methods in enzymology. 2011;497:295. doi: 10.1016/B978-0-12-385075-1.00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nature methods. 2010;8(1):35–38. doi: 10.1038/nmeth.f.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levskaya A, Chevalier AA, Tabor JJ, Simpson ZB, Lavery LA, Levy M, Davidson EA, Scouras A, Ellington AD, Marcotte EM, et al. Synthetic biology: engineering escherichia coli to see light. Nature. 2005;438(7067):441–442. doi: 10.1038/nature04405. [DOI] [PubMed] [Google Scholar]
- 52**.Tabor JJ, Salis HM, Simpson ZB, Chevalier AA, Levskaya A, Marcotte EM, Voigt CA, Ellington AD. A synthetic genetic edge detection program. Cell. 2009;137(7):1272–1281. doi: 10.1016/j.cell.2009.04.048. In this article the authors describe application of engineered genetic circuits in E.Coli to detect light and dark regions and communicate that information in the form of diffusible signal to the neighboring cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying e. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329(5991):533. doi: 10.1126/science.1188308. Authors look at correlation between mRNA and protein levels using simultaneous FISH and fluorescently tagged protein measurements. Results show little correlation between the two species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls nf-κb-dependent gene activity. The EMBO Journal. 2006;25(4):798–810. doi: 10.1038/sj.emboj.7600977. FRAP was used to study DNA binding dynamics of NF-κB. Results show that the transcription factor is in equilibrium with its promoter in the nucleus of the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Raj A, Rifkin SA, Andersen E, Van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463(7283):913. doi: 10.1038/nature08781. A study of a developmental gene network in a nematode using FISH. Results show incomplete penetrance of skn-1 mutant phenotype due to large variations in gene expression, which experience a threshold during development. [DOI] [PMC free article] [PubMed] [Google Scholar]