Abstract
Cre-mediated apoptosis has been observed in many contexts in mice expressing Cre-recombinase, and can confound the analysis of genetically engineered conditional mutant or transgenic alleles. Several mechanisms have been proposed to explain this phenomenon. We find that the degree of apoptosis induced correlates roughly with the copy number of loxP sites present in the genome and that some level of increased apoptosis accompanies the presence of even only a few loxP sites, as occurs in conditional floxed alleles. Cre-induced apoptosis in this context is completely p53-dependent, suggesting that the apoptosis is stimulated by p53 activation in response to DNA damage incurred during the process of Cre-mediated recombination.
Keywords: transgenic mice, loxP sites, Cre recombinase, apoptosis
Introduction
Cre recombinase, encoded by the coliphage P1, recognizes a specific 34-bp sequence (loxP site) to catalyze site-specific concerted recombination, resulting in either excision of DNA sequences between two directly repeated loxP sites, or inversion of DNA sequences between two inverted sites (Hoess and Abremski, 1985; Abremski et al, 1986; rev. by Lewandoski, 2001 and Schmidt-Supprian and Rajewsky, 2007). Since Cre-lox technology was first developed for use in engineered mouse mutants, there have been several reports of cell death ensuing from Cre-mediated recombination in mouse embryos (Lewandoski and Martin, 1997; Schmidt et al, 2000; Loonstra et al, 2001; Naiche and Papaioannou, 2007; Gregoire and Kmita, 2008; Huh et al, 2010; rev. by Schmidt-Supprian and Rajewsky, 2007). The mechanisms that have been proposed to explain this phenomenon include deleterious gene deletions and genomic rearrangements resulting from recombination at cryptic, endogenous loxP-like sites, or genomic rearrangements with chromosome loss resulting from recombination of inverted loxP sequences in engineered transgenic mice. Recombination between directly repeated, perfect loxP sequences has been presumed to be without cytoxic consequences, being normally a concerted reaction.
Recombination at endogenous genomic loxP-like sequences provides an explanation for apoptosis observed in several Cre lines independent of introduced engineered loxP consensus sequences, which may be particularly problematic at very early embryonic stages with high levels of proliferation (Schmidt et al, 2000; Loonstra et al, 2001; Naiche and Papaioannou, 2007). However, we have surveyed a number of Cre lines (AP2Cre, Hoxb6Cre, Prx1Cre, Col2CreER, Hoxb6CreER) that do not display appreciable apoptosis at sites of Cre expression in the absence of introduced loxP sites (unpubl. results), suggesting that recombination at cryptic endogenous sites is an inefficient and low probability event, perhaps most evident in the context of very high level, early embryonic, and/or sustained Cre expression. Inverted loxP sites can clearly cause cell death via chromosomal rearrangements and loss (Lewandoski and Martin, 1997), and this feature has in fact been exploited as a strategy to target the ablation of specific cell types in mice (Gregoire and Kmita, 2008). However, the accidental creation of inverted loxP sites in tandemly repeated transgenes due to the inclusion of palindromically inverted transgene copies is expected to be an infrequent event. Tandem arrays are usually integrated into the genome as direct repeats; palindromic inverted repeated sequences (ie. long hairpins) are inherently less stable during DNA replication, and occur infrequently on a random basis both in prokaryotes and eukaryotes including mammals (Gordenin et al, 1993; Waldman et al, 1999; Voineagu et al., 2008 and references therein). We recently noticed cell death associated with the presence of single copy, floxed allele in the absence of inverted loxP sites (Zhu et al, 2008), raising the question of how recombination of low copy number, directly repeated, perfect loxP consensus sites can lead to apoptosis.
In the present study, we surveyed several independently generated conditional transgenic lines containing tandemly repeated loxP sites and found that some degree of recombination-induced apoptosis occurs with high frequency in loxP site-containing transgenic lines, and the amount of apoptosis correlates roughly with loxP site frequency. Furthermore, whereas Cre-induced apoptosis in the context of loxP-containing lines not expected to contain inverted loxP sites occurred over a limited time interval following the onset of Cre expression, presumably because of loss of loxP substrate, sustained apoptosis was observed in the case of a transgenic line known to contain inverted loxP sites and capable of undergoing multiple rounds of recombination. A low, but detectable level of apoptosis was also observed in certain cases with engineered loxP sites known not to have an inverted orientation, suggesting that failed recombination can indeed occur even at perfect loxP consensus sequences, albeit with low frequency. Low level, incomplete recombination could result in apoptosis in a small percentage of cells due to DNA damage-induced signals following the rare occurrence of nicks or double strand breaks introduced into the DNA by Cre recombinase (Hoess and Abremski, 1985; Abremski et al, 1986). Consistent with this explanation, we found that removal of p53, the major regulator and effector of the apoptotic cascade induced in response to DNA damage (Vogelstein et al, 2000; Vousden and Lu, 2002), can rescue cell survival in the context of Cre-driven recombination.
Results and Discussion
Cre-induced apoptosis correlates directly with loxP copy number
To evaluate the frequency and severity of Cre-induced apoptosis, we focused on the embryonic limb bud as a model, since apoptosis and subsequent phenotypic consequences can be readily observed without affecting embryo viability in utero per se, and the early limb bud is also a highly proliferative tissue with a short cell cycle time (~12 hrs). A number of Cre drivers that target recombination to the early limb bud have been developed and characterized (Lowe et al, 2000; Sun et al, 2000; Ahn et al, 2001; Logan et al, 2002; Nelson and Williams, 2004; Nakamura et al, 2006; Grieshammer et al, 2008; Nguyen et al, 2009;). We surveyed several of these Cre lines with strong constitutive or conditional (tamoxifen-dependent) Cre expression (AP2Cre, Hoxb6Cre, Prx1Cre, Hoxb6CreER, Col2CreER), but did not observe significant Cre-induced apoptosis or abnormal phenotypes in any of these lines without the introduction of genetically engineered loxP sites (data not shown; see also eg. in Fig 3A–B). Consequently, the occurrence of Cre-associated apoptosis cannot always be attributed to activity at cryptic loxP-like sites in the presence of high level and/or early timing of Cre expression, since the lines we evaluated include some that have both very robust and very early onset of Cre expression (eg. E8 for Hoxb6 promoter), yet have no appreciable apoptosis. To assess how frequently Cre–induced apoptosis occurs in transgenic lines that contain engineered loxP sites, we surveyed a number of different independently generated transgenic lines using a single Cre line, AP2Cre (MGI name Tg(TFAP2A-cre)1Will; Nelson and Williams, 2004), which expresses Cre recombinase uniformly in limb mesenchyme beginning at about the time limb buds first appear as swellings in the lateral plate mesoderm. This AP2Cre line was crossed with α-ACrys-loxP-TAg (Lakso et al, 1992), Col2-loxP-Hoxd12 (E. Nakamura and S. Mackem, unpubl.), and CMV-loxP-NT2 transgenic lines (K. Torigoe and Y. Yamada, unpubl.) containing different copy numbers of tandemly repeated loxP sites (probably ranging from several to ~250, see below). At E10.5, about 24 hours after onset of AP2Cre expression in the limb bud, varying levels of apoptosis were observed in the limb buds of different transgenic lines (Fig.1), but some apoptosis above background levels was observed frequently in transgenic lines. Although the amount of apoptosis was highly variable between the different lines, the level observed within a given line was very consistent. Notably, some of the loxP-containing transgenes surveyed are driven by promoters that are not appreciably expressed in early stage limb buds (Col2 promoter, see eg. Nakamura et al, 2006), or that are not expressed at all in the limb (αAcrystallin promoter, see Lakso et al, 1992), making it unlikely that the apoptosis is related to transgene expression per se. No obvious apoptosis was seen in transgenic embryos negative for the AP2Cre allele (data not shown), consequently the observed apoptosis was also unrelated to integration site effects of the transgene.
Figure 3.
Cre-mediated apoptosis in engineered conditional lines containing from 2 to 6 loxP sites. Lysotracker Red staining for apoptosis (Zucker et al, 1999) in E10.5-E11 limb buds. Rosa26LacZ-hom;AP2Cre+ embryos (C) show no increase in apoptosis compared to either wild-type (A) or AP2Cre+ embryos (B). ShhFl/+;AP2Cre+ embryos with 2 loxP sites (D) display an increased level of apoptosis compared to Shh+/−;AP2Cre+ (C) and control embryos (A,B), whereas Rosa26LacZ-hom;AP2Cre+ embryos (E) with 4 loxP sites have similar levels of apoptosis compared to either wild-type (A) or AP2Cre+ embryos (B). However, Rosa26LacZ-hom; ShhFl/+;AP2Cre+ embryos (F), having 4 additional loxP sites more than ShhFl/+, display an increase in the level of apoptosis compared to ShhFl/+;AP2Cre+ embryos (compare panel F to panels D, E).
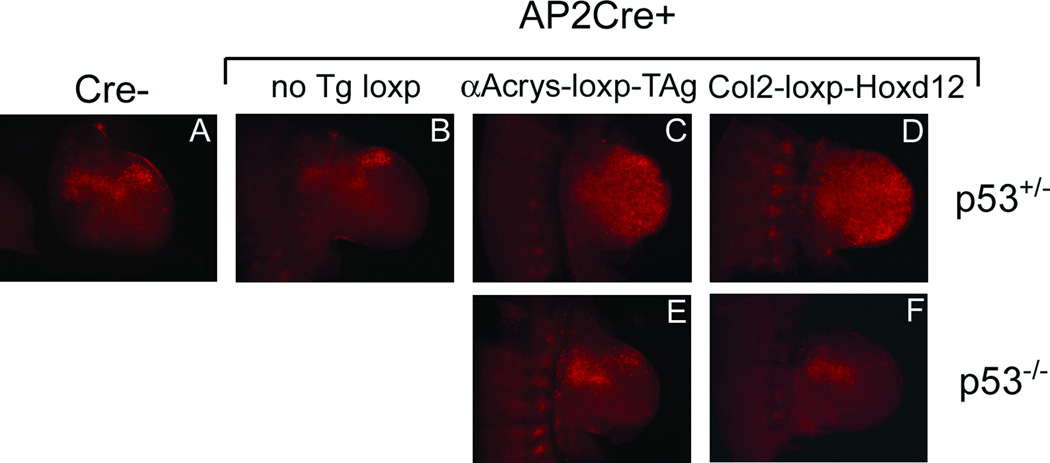
Figure 1.
Varying levels of apoptosis in the limb buds of several conditional transgenic lines crossed to the AP2Cre driver line. Embryos were harvested at E10.5 and stained with Nile Blue Sulfate. Forelimb buds are shown in all the panels. The transgenic lines used are indicated at the top of each panel.
To determine whether the severity of apoptosis correlated directly with transgene copy number (loxP site frequency), QPCR was used to estimate the transgene copy number for αAcrys-loxP-TAg and Col2-loxP-Hoxd12 lines relative to a control single copy allele containing the same amplicon used (see Materials and Methods). Both the Col2-loxP-Hoxd12 and αAcrys-loxP-TAg lines had a very significant amount of apoptosis above endogenous levels (Fig 2), and above that observed in single copy floxed alleles (see Fig. 3), with a somewhat higher level of apoptosis apparent in the Col2-loxP-Hoxd12 (Fig 2D) than the αAcrys-loxP-TAg line (Fig 2C). We estimated the αAcrys-loxP-TAg transgenic line contains 32 transgene copies (64 loxP sites) in the hemizygote (comparable to a previous estimate of ~50; Lakso et al, 1992), and the Col2-loxP-Hoxd12 transgenic line contains 128 transgene copies (256 loxP sites) in the hemizygote. Hence apoptosis was most prominent in transgenic lines with high copy number loxP sites, and correlated roughly with loxP copy number. Some apoptosis, albeit at a much lower level, was also observed above endogenous apoptosis even when only 2 loxP sites are present, for at least certain floxed alleles. Increased apoptosis was not obvious for the widely used Rosa26LacZ reporter alone (Soriano, 1999), when either heterozygous (data not shown) or homozygous (Fig. 3E). However, we have consistently observed that recombination of a single Shh-floxed allele using either a conditional tamoxifen-dependent Cre driver (see Zhu et al, 2008), or the non-conditional AP2Cre (Fig. 3D,F), results in apoptosis above that present in control or in Cre+;Shh+/− embryos (Fig. 3A–C), which can only be attributed to recombination at the engineered loxP sites. Interestingly, compound mutant embryos positive for both the Shh-floxed allele and homozygous for the Rosa26LacZ reporter (Fig. 3F) have a somewhat higher level of apoptosis in the presence of Cre than do either those positive for the Shh-floxed allele or Rosa26LacZ alone, again suggesting that the level of apoptosis observed depends on the total loxP substrate dosage. These results indicate that Cre-induced apoptosis is not solely a consequence of recombination at inverted loxP sites, or cryptic endogenous loxP sites. Although the possibility of an inverted loxP sequence embedded in the tandemly repeated transgene arrays we analyzed cannot be categorically excluded, we consider this a very unlikely general explanation for the observed apoptosis, which was present in most (5/6) of the transgenic lines that we checked, and which was also observed in previously well-characterized floxed knock-in alleles, at a reduced level. If apoptosis is directly related to the loxP dosage and the number of recombination events, then one would also predict that alleles containing inverted loxP sites, capable of undergoing multiple rounds of recombination efficiently (since no product is excised), should exhibit sustained apoptosis in the presence of Cre. We tested this using a modified Brainbow-type fluorescent reporter containing inverted loxP sites (Livet et al., 2007) under the control of a ubiquitous promoter (see Fig. 4A). In fact, this allele showed sustained apoptosis over several days (E10.5-E13.5, Fig. 4), in contrast to the other multi-copy loxP containing transgenic lines tested, in which apoptosis subsides within about 24 hrs of the onset of Cre expression (eg. CMV-loxP-NT2, Col2-loxP-Hoxd12, αAcrys-loxP-TAg, data not shown). By E13.5, apoptosis in the presence of AP2Cre has lessened (Fig. 4K–M), possibly owing to the decline in AP2 promoter expression by this stage (Zhang and Williams, 2003). Notably, the level of sustained apoptosis is high enough to begin producing digit phenotypes by E13.5 (Fig. 4M).
Figure 2.
Apoptosis in conditional transgenic lines crossed to AP2Cre in a p53+/− or p53−/− background. Lysotracker Red staining for apoptosis (Zucker et al, 1999) in E10.5 forelimb buds. Extensive apoptosis in both αACrys-loxP-TAg;AP2Cre;p53+/− (C) and Col2-loxP-Hoxd12;AP2cre;p53+/− embryos (D) was blocked in a p53 null background (E,F), whereas the endogenous apoptosis normally seen in the limb bud at this stage (Fernandez-Teran et al, 2006) was not affected (compare panels A–B with panels E–F).
Figure 4. Sustained apoptosis in Cre+ embryos carrying transgenes containing inverted loxP sites.
A: Diagram of CAGG-Brainbow-2.1 transgene, modified from CMV-Brainbow-2.1 (Livet et al, 2007), containing two pairs of inverted loxP sites flanking fluorescent protein genes. B-M: Lysotracker Red staining for apoptosis in E10.5-E13.5 hindlimb buds. CAGG-Brainbow-2.1;AP2Cre+ embryos (D, G, J, M) display a sustained, increased level of apoptosis compared to either wild–type (B, E, H, K) or AP2Cre+ embryos (C, F, I, L). Note that apoptosis lessens by E13.5 (compare panel M to panels D, G, J), while a consequent digit phenotype is apparent by this stage (M).
If Cre-induced apoptosis is solely a consequence of cryptic, endogenous, poor consensus loxP sequences, as has been proposed (Schmidt et al, 2000; Loonstra et al, 2001; Naiche and Papaioannou, 2007), then the apoptosis should depend only on the presence of Cre activity and not exogenously introduced transgenic loxP sequences, as observed here. In addition, the severity of apoptosis would correlate only with the expression level of Cre in a particular line, rather than the number of bona fide loxP sites introduced transgenically. The high frequency with which apoptosis is detected in loxP containing transgenic lines, and the variable level of apoptosis seen between different transgenic lines is also not readily explained by Cre-induced apoptosis owing to the inadvertent introduction of an inverted loxP site in tandemly repeated transgenes, which appears to result in a uniformly high level of apoptosis when inverted repeats are present, based both on our results, and other published work (Gregoire and Kmita, 2008).
Cre-induced apoptosis associated with high copy number loxP sites leads to phenotypes secondary to extensive cell death
We noticed that Cre-mediated recombination at the early blastocyst stage (using EIIa-Cre expressed ubiquitously in the inner cell mass (Lakso et al, 1996) was associated with a high level of prenatal mortality in the case of transgenic lines (such as αAcrys-loxP-TAg) containing multiple loxP sites (about 35% survival of Cre+; transgene+ offspring relative to the expected frequency, p < 0.005, n = 80, data not shown). This reduced viability suggested phenotypic consequences related to apoptosis in very early embryos, as did the apparent digit phenotypes following AP2Cre exposure of a Brainbow reporter allele with known inverted loxP sequences (Fig. 4M).
To further assess the phenotypic consequences of apoptosis induced by Cre expression at a later organogenesis stage, we turned again to the developing limb and analyzed the effect of AP2Cre-induced apoptosis on limb skeletal development. αAcrys-loxP-TAg;AP2cre embryos and Col2-loxP-Hoxd12;AP2cre embryos were harvested at E16.5-E17.5 to evaluate the limb skeleton. αAcrys-loxP-TAg;AP2cre embryos invariably had mild to moderately shorter limbs, affecting all proximodistal components, although the stylopod was often most severely affected (Fig. 5A). Since the αAcrystallin promoter driving the T-Antigen transgene is not expressed at all in the limb, the observed shortening of skeletal elements can be attributed entirely to the apoptosis induced by Cre-mediated recombination. Col2-loxP-Hoxd12;AP2cre embryos harvested at a late skeletal stage had a more severe phenotype including complete loss of long bone elements as well as severe shortening, and variable loss of anterior digits (Fig. 5B). Since the Col2 transgenic promoter drives expression in chondrocytes, part of this phenotype could be related to enforced transgene expression. However, we have also analyzed the phenotypes of multiple primary founder embryos using the same transgene, but without conditional expression requiring Cre (i.e. no loxP-stop sequence present), and found that Hoxd12 misexpression in cartilage resulted in a consistent, mild phenotype of shortened cartilage elements without any loss of digit or long bone elements (A. Bao, M-T. Nguyen, E. Nakamura and S. Mackem unpublished results; see also Fig. 6). The severe shortening and selective loss of anterior skeletal elements were clearly related to Cre-induced apoptosis. The limb reduction phenotypes observed following Cre-induced apoptosis are overall similar to those observed in mutants in which cell survival is drastically affected in the limb mesoderm (e.g. Shh, Gremlin, or Fgf4,8 limb knockouts; Chiang et al, 2001; Michos et al, 2004; Sun et al, 2000).
Figure 5.
Skeletal phenotypes of αACrys-loxP-TAg;AP2cre (E16.5) and Col2-loxP-Hoxd12;AP2cre embryos (E17.5). All three limb skeletal components are shortened in αAcrys-loxP-TAg;AP2cre forelimbs compared to cre negative control littermates (left panels). More severe phenotypes, including marked long bone shortening, complete loss of radius and the loss of anterior digits, are invariably seen in Col2-loxP-Hoxd12;AP2cre forelimbs (right panels). Note that AP2Cre is not expressed in scapula progenitors, which are spared.
Figure 6.
Skeletal phenotypes of Col2-loxP-Hoxd12;AP2cre embryos generated in a p53+/− or p53−/− background. E17.5 forelimbs are shown in all the panels. Note that severe phenotypes such as missing long bone elements and loss of anterior digits (lower panel) are absent from p53−/− embryos, although a milder long bone shortening phenotype remains (middle panel).
Apoptosis triggered by Cre-mediated recombination is p53 dependent
Since Cre-related apoptosis is observed consistently in association with the presence of bona fide loxP consensus sites, and in this context appears to be dependent on the total loxP dosage, we speculated whether this might be a consequence of incomplete recombination by Cre recombinase (i.e. leaving single strand nicks or double strand breaks in the target DNA). Cre recombination is normally a highly concerted reaction, but in the context of poor loxP consensus matches, incompletely recombined intermediates with nicks or breaks can be detected readily (Abremski et al, 1986). A similar incomplete reaction has been observed even between recombination of two perfect consensus loxP sites ((Hoess and Abremski, 1985), albeit at a much lower frequency. p53 is situated at the crossroads of a network of signaling pathways that are essential for cell growth regulation and apoptosis induced by genotoxic and non-genotoxic stresses (Vogelstein et al, 2000; Vousden and Lu, 2002). DNA damage such as nicks and breaks normally stimulate a pathway leading to apoptosis mediated by p53 activity, and in fact p53 expression has been observed to increase in Cre-expressing cells (Huh et al, 2010). If a response to DNA damage is indeed the mechanism leading to apoptosis, then inactivation of p53 should rescue cell survival in the context of Cre-induced apoptosis.
To test this possibility, we generated αAcrys-loxP-TAg;AP2cre and Col2-loxP-Hoxd12;AP2cre embryos in a p53 null background, and found that apoptosis induced by Cre-mediated recombination in early limb buds in both of these lines is completely blocked when p53 is absent (Fig. 2C–F), suggesting that Cre-induced apoptosis results from p53 pathway activation. To determine whether the rescue of cell survival by p53 deletion was sufficient to restore normal organogenesis (in this case, normal skeletal development), we also examined the limb skeleton of Col2-loxP-Hoxd12;AP2cre embryos (which had the highest level of apoptosis at early stages). p53 null mutants do have some modest alterations in osteogenesis, which affects primarily late differentiation of osteoblasts, and does not significantly alter the formation of the cartilage model of the limb skeleton (Lengner et al, 2006; Wang et al, 2006). In the Col2-loxP-Hoxd12;AP2cre;p53−/− embryos (18/18 embryos from 10 litters), both the formation of all long bone elements and normal digit numbers were restored (Fig. 6). Mild long bone shortening, compared to non-transgenic controls, was still present. This residual phenotypic abnormality in cartilage size was consistent with effects of Hoxd12 misexpression in cartilage observed in non-conditional, transgenic Col2-Hoxd12 founder embryos, as discussed above. Hence, the phenotypes attributable entirely to Cre-induced apoptosis (eg. loss of skeletal elements) were rescued by removal of p53.
Based on the ability of p53 removal to rescue both cell survival and normal development, we propose that the rare creation of nicks and double-stranded breaks in genomic DNA generated during Cre-mediated recombination (even between perfect loxP consensus sites) are recognized through activation of the p53 pathway, in turn leading to apoptosis. If the frequency of failed ligation is relatively constant, the frequency of apoptotic cells should correlate with the total number of recombination events/cell (i.e. the more loxP sites present, the higher the frequency of apoptosis). The observed dependency of the loxP-related apoptosis on the number of loxP sequences suggests a mechanism related to the recombination of bona fide loxP recognition sites by Cre. Although Cre-mediated recombination is normally a highly concerted process, creation of nicks or double-stranded breaks have been demonstrated as a rare occurrence between such bona fide loxP sites (Hoess and Abremski, 1985), which can then act as signals for the repair mechanisms that also trigger apoptosis. Alternatively, the presence of a perfect loxP site may stimulate recombination with an endogenous, cryptic-poor consensus partner, resulting in a higher frequency of abortive recombination and production of DNA nicks and breaks (Hoess and Abremski, 1986). Depending on the proximity of cryptic loxP sites, this might explain the context dependence observed for the induction of apoptosis by recombination of certain floxed alleles, but not others (eg. Shh-floxed vs. Rosa26LacZ alone, see Fig. 3).
This study adds to our understanding of the mechanisms of apoptosis frequently associated with use of the Cre-loxP system. Since conditional targeting is such a widespread and important tool to control gene expression spatially and temporally, as previously cautioned (Loonstra et al, 2001; Naiche and Papaioannou, 2007; rev. by Schmidt-Supprian and Rajewsky, 2007), it is important to be aware that Cre-mediated apoptosis might contribute to phenotypes. Consequently, appropriate controls are essential to carefully assess the contribution of Cre-related ‘background’ apoptosis to cell death and phenotypes in conditional knockout lines. p53 deletion may in some instances also be a useful strategy for inhibiting the DNA-damage related apoptosis and assist in revealing underlying phenotypes using conditional knockout alleles. The potential problems associated with Cre-induced apoptosis in engineered and conditionally expressed transgenes can be most easily circumvented by the inclusion of a single FRT site for Flp-mediated recombination (rev. by Lewandoski, 2001), which will reduce a tandemly repeated array to a single copy transgene integrant. Alternatively, ES cell transfection or lentiviral vector strategies also produce single or low copy transgene integrants (rev. by Park, 2007).
Materials and Methods
Mouse strains
The αAcrys-loxP-TAg (Lakso et al, 1992), EIIACre (Lakso et al, 1996), AP2Cre (MGI name Tg(TFAP2A-cre)1Will; Nelson and Williams, 2004), Hoxb6CreER (Nguyen et al, 2009), p53 null mutant (Donehower et al, 1992), and Shh-Floxed (Lewis et al, 2001) mouse lines used have all been previously described. A Col2-loxP-Hoxd12 transgene was generated by introducing the Hoxd12 coding sequence (Knezevic et al, 1997) into a Col2-promoter/enhancer vector (see Nakamura et al, 2006), and inserting a 1.2kbp ‘stop sequence’ flanked by loxP sites (Lakso et al, 1992) between the Col2 promoter and the Hoxd12 coding sequence to prevent expression prior to recombination. A modified CAGG-Brainbow-2.1 reporter construct was generated by replacing the CMV promoter in Brainbow-2.1 (Livet et al, 2007) with a ubiquitous CAGG promoter (Niwa et al, 1991). The Col2-loxP-Hoxd12 and CAGG-Brainbow-2.1 transgenic lines were generated by pronuclear microinjection of DNA into FVB/n zygotes as described by Hogan et al. (1994). CMV-loxP-NT2;AP2Cre embryos were kindly provided by Y. Yamada and K. Torigoe. All alleles were maintained on a predominantly FVB/n background. Noon on the date of the vaginal plug was defined as E0.5.
Detection of cell death
Apoptosis was detected using either Nile Blue Sulfate or Lysotracker Red staining (see Zucker et al, 1999). Embryos were dissected in phosphate buffered saline (PBS) and immediately transferred into either 0.1% Nile Blue sulfate (Sigma) solution PBS for staining and bright-field photography, or into 5µM Lysotracker Red (Invitrogen L-7528) in PBS. Lysotracker Red stained embryos were dehydrated in methanol and cleared in Murray's clear (BABB) (Sigma), prior to fluorescence photography.
Skeletal staining
Embryos (E16.5-E17.5) were stained in alcian blue/alizarin red, cleared in glycerol/KOH and photographed as previously described (Zhu et al, 2008).
Determination of transgene (loxP) copy number
The transgene copy number in the αAcrys-loxP-TAg and Col2-loxP-Hoxd12 lines were determined by qPCR of an amplicon within the SV40 polyA additional signal that was present in all of the lines used, and compared to the RosaLacZ reporter line, which is known to contain 4 SV40 polyA addition sites in the single copy heterozygous knock-in allele (Soriano, 1999). To control for differences in DNA quantity and quality, an endogenous wild type Gli3 gene amplicon was used as an internal control. qPCR was performed with a Roche Lightcycler 480. Reactions were performed in duplicate on genomic DNA from 2 different mice for each of the lines. From the qPCR results, normalized against the Gli3 amplicon, the αAcrys-loxP-TAg transgenic line contains 32 transgene copies and the Col2-loxP-Hoxd12 transgenic line contains 128 transgene copies per hemizygous allele. The sequences of primers used are given below.
SV40 Late orientation polyA signal (includes 5’ linker sequences, italicized):
Forward: 5’-GGGAAGCTTCATAATCAGCCATACCAC-3’
Reverse: 5’-GGGGTCGACTGATAAGATACATTGATG-3’
Gli3:
Forward: 5’-GGCCCAAACATCTACCAACACATAG-3’
Reverse: 5’-GTTGGCTGCTGCATGAAGACTGAC-3’
Acknowledgements
We are grateful to Yoshi Yamada and Kiyoyuki Torigoe for providing transgenic CMV-loxP-NT2;AP2Cre embryos for analysis, to Heiner Westphal for AdenoEIIA-Cre and aA-crys-loxP-TAg mouse lines, and to Trevor Williams and Danielle Nelson for sharing the AP2Cre mouse line prior to publication. We also thank Naiche Adler for helpful discussions and Trevor Williams for comments on the manuscript. This research was supported by the Center for Cancer Research, National Cancer Institute, NIH.
References
- Abremski K, Wierzbicki A, Frommer B, Hoess RH. Bacteriophage P1 Cre-loxP site-specific recombination. J Biol Chem. 1986;261:391–396. [PubMed] [Google Scholar]
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran MA, Hinchliffe JR, Ros MA. Birth and Death of Cells in Limb Development: A Mapping Study. Dev Dynamics. 2006;235:2521–2537. doi: 10.1002/dvdy.20916. [DOI] [PubMed] [Google Scholar]
- Gordenin DA, Lobachev KS, Degtyareva NP, Malkova AL, Perkins E, Resnick MA. Inverted DNA repeats: a source of eukaryotic genomic instability. Mol Cell Biol. 1993;13:5315–5322. doi: 10.1128/mcb.13.9.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire D, Kmita M. Recombination between invertedloxP sites is cytotoxic for proliferating cells and provides a simple tool for conditional cell ablation. Proc Nati Acad Sci USA. 2008;105:14492–14496. doi: 10.1073/pnas.0807484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshammer U, Agarwal P, Martin GR. A Cre transgene active in developing endodermal organs, heart, limb, and extra-ocular muscle. Genesis. 2008;46:69–73. doi: 10.1002/dvg.20366. [DOI] [PubMed] [Google Scholar]
- Hoess RH, Abremski K. Mechanism of strand cleavage and exchange in the Cre-loxsite-specific recombination system. J Mol Biol. 1985;181:351–362. doi: 10.1016/0022-2836(85)90224-4. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: a Laboratory Manual. 2nd edition. Cold Spring Harbor: CSH Laboratory Press; 1994. [Google Scholar]
- Huh WJ, Mysorekar IU, Mills JC. Am J Physiol Gastrointest Liver Physiol. Vol. 299. 2010. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of“floxed” alleles; pp. G368–G380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic V, De Santo R, Schughart K, Huffstadt U, Chiang C, Mahon KA, Mackem S. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Sauer B, Mosinger B, Jr, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Martin G. Cre-mediated chromosome loss in mice. Nat Genet. 1997;17:223–225. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Mouse genomic technologies: Conditional control of gene expression in the mouse. Nature Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin C. Expression of cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Nati Acad Sci USA. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. HoxB6-Cre transgenic mice express Cre recombinase in extra-embryonic mesoderm, in lateral plate and limb mesoderm and at the midbrain/hindbrain junction. Genesis. 2000;26:118–120. doi: 10.1002/(sici)1526-968x(200002)26:2<118::aid-gene5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Nelson DK, Williams T. Frontonasal process-specific disruption of AP-2alpha results in postnatal midfacial hypoplasia, vascular anomalies, and nasal cavity defects. Dev Biol. 2004;267:72–92. doi: 10.1016/j.ydbio.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Zhu J, Nakamura E, Bao X, Mackem S. Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud. Dev Dyn. 2009;238:467–474. doi: 10.1002/dvdy.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Park F. Lentiviral vectors: are they the future of animal transgenesis? Physiol Genomics. 2007;31:159–173. doi: 10.1152/physiolgenomics.00069.2007. [DOI] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Nati Acad Sci USA. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE, Jr, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Narayanan V, Lobachev KS, Mirkin SM. Replication stalling at unstable inverted repeats: interplay between DNA hairpins and fork stabilizing proteins. Proc Natl Acad Sci U S A. 2008;105:9936–9941. doi: 10.1073/pnas.0804510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Waldman AS, Tran H, Goldsmith EC, Resnick MA. Long inverted repeats are an at-risk motif for recombination in mammalian cells. Genetics. 1999;153:1873–1883. doi: 10.1093/genetics/153.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol. 2006;172:115–125. doi: 10.1083/jcb.200507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Williams T. Identification and regulation of tissue-specific cis-acting elements associated with the human AP-2alpha gene. Dev Dyn. 2003;228:194–207. doi: 10.1002/dvdy.10365. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen M-T, Bao X, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RM, Hunter ES, Rogers JM. Apoptosis and morphology in mouse embryos by confocal laser scanning microscopy. Methods. 1999;18:473–480. doi: 10.1006/meth.1999.0815. [DOI] [PubMed] [Google Scholar]