Abstract
Potent glucocorticoids (GC) administered early in life has improved premature infant survival dramatically. However, these agents may increase the risk for physical, neurological and behavior alterations. Anxiety, depression and attention difficulties are commonly described in adolescent and young adult survivors of prematurity. In the present study we administered vehicle, dexamethasone, or hydrocortisone to Sprague-Dawley rat pups on postnatal days 5 and 6, mimicking a short term clinical protocol commonly used in human infants. Two systems that are implicated in the regulation of stress and behavior were assessed: the limbic-hypothalamic-pituitary-adrenal axis [LHPA, glucocorticoid and mineralocorticoid receptors within] and the Serotonin (5-HT) system. We found that as adults, male Sprague-Dawley pups treated with GC showed agent specific altered growth, anxiety-related behavior, changes in corticoid response to novelty and gene expression changes within LHPA and 5-HT–related circuitry. The data suggest that prolonged GC-receptor stimulation during the early neonatal period can contribute to the development of individual differences in stress response and anxiety-related behavior later in life.
Keywords: Dexamethasone/*administration & dosage/*adverse effects; Hydrocortisone/ *administration & dosage/*adverse effects; Body Weight/drug effects; Animals, Newborn/growth & development; Anxiety Behavior/Attention; Stress response; Adult; Rat
Introduction
Premature birth defined as birth prior to 37th week of gestation, accounts for approximately 11% of all births in the USA (Centers for Disease Control and Prevention 2007). In 2007 this was equivalent to over 500,000 newborns (Centers for Disease Control and Prevention 2007). Over the last two decades the survival of the most premature infants in this group has increased to approximately 40% at 24 weeks of gestation and 95% at 28 weeks (Hack and Fanaroff 1999; Lorenz 2001). Improvements in survival have created a population of extremely low birth weight premature infant survivors with unique physical and behavioral challenges (Hack and Fanaroff 1999; Hack et al. 2000). A significant aspect of prematurity is the fact that brain maturation after birth is very complex, corresponding to that which is occurring in utero during the late second and entire third trimester of human pregnancy. This “ex-utero” brain development remains the least understood in terms of its role in long-term neurodevelopmental outcome. Complicating this process, the brains of critically ill premature infants must develop while simultaneously experiencing multiple stressors (e.g. cold, light, noise, pain), as well as exposure to neuroactive agents, such as glucocorticoids (GC), and opiates.
The glucocorticoids dexamethasone (DEX) and hydrocortisone (HC) are likely the most frequently used neuroactive agents in the neonatal intensive care setting for multiple reasons, the most prominent of which are lung disease (Cummings et al. 1989; O'Shea et al. 1999) and refractory hypotension (Martens et al. 2003). For the past two decades, DEX has been the glucocorticoid agent most often used in this clinical setting. Unfortunately, DEX administration has been associated with neurodevelopmental impairments among preterm infants (The American Academy of Pediatrics 2002). Neurodevelopmental difficulties described in the premature infant survivors who are not neurologically impaired vary depending on the age of the child. At pre-school age (2–3 y), several reports indicate delayed psychomotor development and anxious/depressed and/or withdrawn behavior (Sajaniemi et al. 2001; Stoelhorst et al. 2003; Stoelhorst et al. 2003). At school age and adolescence, increased incidences of social competence problems, attention disorders and hyperactivity have been described (Pharoah et al. 1994; Stjernqvist and Svenningsen 1995; Schothorst and van Engeland 1996; Botting et al. 1997; Saigal 2000; Hille et al. 2001). A systematic review of the DEX experience in neonates (Doyle and Davis 2000; Barrington 2001) led to a consensus statement from The American Academy of Pediatrics which recommended limited use of DEX in premature infants (American Academy of Pediatrics 2002). During the past decade, hydrocortisone (HC), a less potent glucocorticoid anti-inflammatory agent that has the same chemical structure as the endogenous glucocorticoid (cortisol) in humans, has become a preferred agent in neonatal medicine for the treatment of hypotension and chronic lung disease (van der Heide-Jalving et al. 2003). Premature infant survivors treated with HC during their neonatal period are at early school age now so less is known regarding the neurodevelopmental effects of hydrocortisone use in this population, compared to those children who are now adolescent and young adults that received DEX early in life. Immediate effects on neurodevelopment appear to be benign (Benders et al. 2009), but dosing regimens with HC are markedly variable and there are mixed reports of the long term consequences (Lodygensky et al. 2005; Thompson et al. 2008).
The brain is clearly a target for GC action in the neonate (De Kloet et al. 1988; Vazquez 1998) and specific brain systems expressing receptor molecules that are under organization at the time of GC exposure may be at the root of the long term consequences observed in survivors of premature birth. Pre-clinical animal models have been developed to study the long term effects of early life GC exposure on neurodevelopment, eliminating confounding perinatal and postnatal factors found in human studies (Kurosawa et al. 1980; Slotkin et al. 1982; Kauffman et al. 1994; Felszeghy et al. 1996; Ferguson and Holson 1999; Felszeghy et al. 2000; Heine et al. 2009). However, few animal studies have administered GC agents in the same manner as performed in neonatal intensive units across the nation. Most recently, our laboratory reported neonatal DEX effects on neonatal neurodevelopment, adolescent rat behavior, and adult limbic neurochemistry using a pre-clinical animal model that mimics the prolonged DEX exposure that until 2002 was common in neonatal intensive care settings (Flagel et al. 2002; Neal et al. 2004; Neal et al. 2004; Bhatt-Mehta et al. 2010). Long-term effects of neonatal treatments in any of these developmental animal models are interpreted under the assumption that, although timing differs significantly between species, the general sequence of brain growth is similar (Dobbing 1981; Flagel et al. 2002). While much caution is necessary when extrapolating from animal models to the human condition, one can still take advantage of similarities in sequence and timing of brain development between species. In humans, excluding the cerebellum and hippocampus, neuronal proliferation is essentially completed before 24 wks of gestation (Dobbing 1974). Beyond 24 wks of gestation, glia continues to proliferate and oligodendroglias maintain ongoing myelination, with a peak in brain growth occurring near term. In contrast to humans, rodents experience their brain growth spurt after birth. It is estimated that on postnatal day 10 the rodent brain is roughly equivalent to that of the full term human brain of 38 to 40 weeks post-conception (Dobbing 1974; Dobbing 1981; Hagberg et al. 1997). Extrapolating from this model, the brain of a rodent pup at birth (postnatal day 1 or PD 1) corresponds to that of a human fetal brain at or near 19–21 weeks of gestation (Dobbing 1981; Whitelaw and Thoresen 2000). The PD 3 corresponds with that of a 24–26 week human, and PD 5–6 approximates a 28–32 week human. Given these parallels, the neonatal rodent is an ideal model in which to investigate effects of a tapering course of neonatal GC exposure on the developing brain.
In the present study, our objective was to compare the effects of the two leading GC agents under clinical use in premature neonates over the last two decades (DEX and HC) on the developing central nervous system. We chose to focus on a short course of GC treatment given on PD 5 and PD 6 for several reasons. First, the majority of infants receiving GC for chronic lung disease will be those born at 23–25 weeks of gestation (Hack and Fanaroff 1999; Lorenz 2001). These infants will receive steroids for lung disease usually around 3–4 weeks of life. In addition, HC is often given to infants who are born at 24–28 weeks of gestation for refractory hypotension. Consequently, a relatively short course of HC treatment is used extensively in neonatal intensive units for non-pulmonary reasons. Second, the timing of GC treatment is critical, because normal development of the human limbic-hypothalamic-pituitary axis (LHPA) provides a low GC milieu during a period when elevated GC levels may negatively impact neuronal circuits (Watterberg et al. 2001). Third, we wished to focus on specific structures of the developing limbic system and brainstem, locations where there is a high density of corticoid and serotonin (5-HT) receptors and 5-HT producing cells, that have roles in the development of stress and emotional regulation (McEwen 1987; Benesova and Pavlik 1989; Weinstock et al. 1992; Gunnar and Vázquez 2006). Thus, in the present study our objectives were three fold: 1) to provide GC during a postnatal age in the rat that corresponds to the neurodevelopmental time point at which human premature infants commonly receive GC therapy, 2) to provide tapering doses for both agents at a particular time after birth (from PD 5 to PD 6) with the specific goal to more closely mimic and compare GC protocols provided in the neonatal intensive care setting, where 3–7 day courses are administered (Halliday et al. 2003), and 3) to ascertain the long term effect of each of these agents on: a) growth, b) hormonal response to stress, c) anxiogenic behavior and d) molecules relevant to GC action and the serotonin (5-HT) system. We hypothesized that somatic effects and emotional instability will be present in animals treated early in life with both glucocorticoid agents, with DEX having the greatest effect. In addition, we hypothesized that the behavioral phenotype will be associated with developmental adaptations of both the limbic-corticoid and serotonin receptor systems, to early life exposure of GC.
Material and Methods
Animals
The subjects in this study are adult male rats born and raised at the University of Michigan Animal Facility. The University Committee on Use and Care of Animal (UCUCA) approved and monitored compliance with applicable standards, policies and regulation for all procedures and animal room facilities. Litter management and animal handling of neonatal rats in this study was similar to that reported from our laboratories previously (Flagel et al. 2002). Adult Sprague-Dawley rats (Charles Rivers, Wilmington, MA) were housed and treated according to Guide for the Care and Use of Laboratory Animals. All animals were kept under constant temperature (25 + 2°C) and photoperiodicity (14:10h light-dark cycle) and provided with food and water ad libitum. Twelve female rats were mated using one to one mating system (1F:1M). Assuming a 21-day gestation, pregnant females were housed separately, starting on day 18. They were then checked twice daily until pups were born. The day of the birth of the pups was designated as postnatal day one (PD1). On PD2, each litter was sexed and culled to 10–12 pups (5–6M:5–6F) that were randomly selected in male: female pairs from at least 3 different dams giving birth on the same day. This ensured both genetic diversity, equality in nutrition and maternal care within litters. On PD3, 3–4 pups representing both sexes were assigned to one of three treatment groups within each litter (3–4 pups × 3 treatment groups= 10–12 pups /litter): 1) Vehicle Controls (VEH), 2) Dexamethasone (DEX) and 3) Hydrocortisone (HC).
Drug Treatment
On the day of drug treatments began, cages with the dam and pups were brought into a room adjacent to the animal colony and placed in a warm pad (temperature 30–35° C). Mothers were removed, placed in a holding cage and moved to a separate room sheltered from the noise that may have been present in the treatment room. Pups were sexed, weigh and treated. Animals in the DEX group received an intramuscular (IM) injection of DEX in a tapering dose of 0.5 mg/kg on PD 5 and 0.1 mg/kg on PD 6. Animals in the HC group also received an IM injection of HC in a tapering dose of 5.0 mg/kg on PD 5 and 1.0 mg/kg PD 6. Animals in the vehicle (VEH) group received equivalent volume of IM sterile normal saline as the DEX and HC animals. The dam was then reunited with the pups. The procedure time was 5 minutes and these were performed between the hours of 1100 and 1300.
Housing
Cohort 1 Weaning was performed at PD 21. The seventy two male animals generated (Cohort 1) were grouped housed 6 animals per cage from PD 21 to PD 45. At post-natal day 45, these animals were separated to single housing in plastic “shoe box” cages due to their weight at PD 45 until the end of the experiments. This was done following the “Guidelines for the Care and Use of Laboratory Animals”, which bases the number of animals in a cage on the size of the animals and expected growth without food restriction. Also following the “Guidelines for the Care and Use of Laboratory Animals” in our institution, animals were handled as part of the procedure for cage and bedding change done weekly.
Housing
Cohort 2 Because single housing separation may be construed as a stressor leading to anxiety-related behavior, a second cohort of animals was generated from ten additional litters (Cohort 2). Mating, birthing and drug treatment protocols were done as described above with four pups per litter (2 male:2 female) assigned to one of three treatment groups at PD3. Once weaned at PD21, males pups were grouped housed 6 per cage from PD 21 to PD 45, as was done for Cohort 1. At PD45, animals were randomized to single housing or grouped housing. This design generated the following groups, each with 20 animals: 1) Grouped Housed (GH) Vehicle (VEH), 2) GH Dexamethasone (DEX), 3) GH Hydrocortisone (HC), 4) Single Housed (SH) VEH, 5) SH DEX and 6) SH HC. The 61 animals assigned to grouped housing were housed 3–4 animals per cage until PD 60, then 2–3 per cage until PD 70. At PD 70–76, these male animals were subjected to behavioral testing on the Light: Dark Preference box following the protocol described below. Animals were not subjected to any other test or measurement.
Somatic Growth (Cohort 1 and 2)
Weight was measured for each pup in each treatment group before treatments on PD 5 and 6 and weekly until weaning. Male animals were then weighed at regular time frames until animals were 140 days old (post-natal days 5, 6, 14, 20, 28, 33, 40, 50, 60, 70–76, and 140). Food was also weighed daily to calculate individual food consumption weekly. Animals were measured to obtain linear growth in Cohort 1 only (at 5, 6, 7, 14, 20, 33 and 60 days). Measurements in Cohort 1 were missed beyond 60 days of life due to laboratory error. Length was not obtained in Cohort 2. When performed, length was measured from the nose to the base of the tail (head-rump length).
Behavioral Testing
Light-dark Preference Box (Cohort 1 and 2)- The light-dark preference procedure was used to evaluate the locomotion and investigatory behavior of the animals when 70–76 days old. Preference for darkness and decreased activity are gross measurements of anxiety-related behavior (File 1990; Bourin and Hascoet 2003; Ramos 2008). Three days prior to testing (PD 67), animals were acclimated to the handling needed before the start of the procedure. Handling consisted in transporting the cage to the test room and removal from the cage at the expected time of testing. This was done for three consecutive days. The testing apparatus was a covered 30 × 60 × 30-cm Plexiglas shuttle-box with a computerized monitor. It has two equal sized compartments (light and dark) with a 12-cm wide opening and stainless steel grid floor suspended above the corncob bedding. The light compartment was constructed of white Plexiglas and brightly illuminated. The dark compartment was constructed of black Plexiglas and minimally illuminated. Fluorescent lights above the box provided the illumination. Half of the boxes had the light compartment on the right side, half on the left side; this controlled for lateralization across the groups. To start the session, each animal was placed in the dark compartment and the timer set to start. Each animal's locomotor activity, the time spent in each compartment, the number of transitions and the latency to leave the dark were scored. Locomotor activity as well as time spent in each compartment was monitored by photocells located on the wall of each box, with the number of photocell beams interrupted per unit time recorded with microprocessor. The number of transitions was recorded manually. Total testing time was 5 minutes (Flagel et al. 2002).
Adrenocortical Response to Novelty Stress (Cohort 1)
Immediately after light-dark preference testing was completed on PD 70, ten animals per group underwent a tail nick procedure to collect blood. Blood was collected from the tail vein at 15, 30 and 60 min after placing the rat in the dark compartment of the light-dark box. The last blood sample was collected 120 min after the start of the test at which time the animals were decapitated. The time of testing was equally randomized across the groups. A pre-stress blood sample was obtained the day prior to the light-dark box as the animal was acclimated to handling. The time of the pre-stress sample corresponded to the same time of the start of the procedure on the following day. Blood samples were collected in pre chilled tubes containing EDTA, placed on ice and subsequently spun at 2000 rpm for 7 min. The plasma was separated and stored at −20°C until assayed for corticosterone hormone concentrations.
Hormonal Assay
Corticosterone (CORT) levels were measured using a commercially available corticosterone I125 radioimmunoassay kit (Cat. #07-120102, MP Biomedicals LLC, Diagnostic Division, Orangeburg, New York). Un-extracted plasma samples were diluted to 1:200 in Phosphosaline gelatin buffer (pH 7.0). The intra- and inter assay CVs for corticosterone were 4.4% and 6.5%, respectively.
Brain Sectioning (Cohort 1)
Six animals per group were randomly selected for brain sectioning and anatomical analyses that followed. For this purpose, the blocks between Bregma +5.9mm to −2mm (“forebrain”) and between Bregma −2mm to Bregma −7mm (“raphe”, with DR cell groups typically between Bregma −4.1mm and −5.2mm) were frozen in dry ice-isopentane at −20°C followed by storage at −80°C. We generated two brain blocks per animal. The raphe block was sectioned at 12 mu and collected in sets of 5 deep (sections 1–5 on slides 1–5, respectively; sections 6–10 on slides on 1–5 again, etc, repeated until full, then on subsequent groups of 5 slides). This generates 5 “sets” of slides, each of which surveys the region at ~60 μm intervals. The forebrain block (containing dorsal hippocampus and amygdala in the same coronal plane) were acquired 8 sections deep. This sampling scheme generated approximately 250 sections per animal for the rostral raphe group, generating 9 slides per animal per set (× 5 sets), with each slide containing six sections. Three set of slides surveying raphe were analyzed separately by in situ hybridization (ISH) for 1) neuronal tryptophan hydroxylase (nTPH- also known as tryptophan hydroxylase 2-riboprobe derived from 589bp, 2036–2624nt), 2) serotonin transporter (5-HTt-riboprobe derived from 659bp, 772–1431nt), 3) 5-HT1a receptor mRNA (riboprobe derived from 910bp, transmembrane domains II,VI&VII). Sections through the third ventricle captured the peraventricular nucleus of the hypothalamus (PVN) which was processed for corticotrophin releasing hormone (CRH) mRNA (riboprobe derived from 353 bp, exon2). Sections through the hippocampus were processed for 1) 5-HT1a mRNA, 2) mineralocorticoid receptor (MR) mRNA (riboprobe derived from 347bp, protein binding domain & 3'-UTP), and 3) glucocorticoid receptor (GR) mRNA (riboprobe derived from 456bp, 3'-UTP).
Hybridization
Sections were removed from storage at −80°C and placed directly into 4% buffered paraformaldehyde at room temperature. After 60 min, slides were rinsed in isotonic phosphate-buffered saline and treated with proteinase K (1 μg/mL in 100 mmol/L TRIS/HCl, pH 8.0) for 10 min at 37°C. Subsequently, sections underwent successive washes in water (1 min), 0.1 mol/L triethanolamine (pH 8.0, plus 0.25% acetic anhydride) for 10 min and 2× SSC (0.3 mmol/L NaCl, 0.03 mmol/L sodium citrate, pH 7.2) for 5 min. Sections were then dehydrated through graded alcohols and air dried.
Postfixed sections were hybridized with 1.0 × 106 dpm [35S]UTP-labeled riboprobes in hybridization buffer containing 50% formamide, 10% dextran sulphate, 3× SSC, 50 mmol/L sodium phosphate buffer (pH 7.4), 1× Denhardt's solution, 0.1 mg/mL yeast transfer RNA (tRNA), and 10 mmol/L dithiothreitol in a total volume of 25 μL. The probe was applied to sections on a glass coverslip and hybridized overnight at 55°C. Next day the sections were washed in 2× SSC for 5 min and then treated with RNase A (200 μg/mL in 10 mmol/L TRIS/HCl, pH 8.0, containing 0.5 mol/L NaCl) for 60 min at 37°C. Subsequently, sections were washed in 2× SSC for 5 min, 1× SSC for 5 min, and 0.5× SSC for 60 min at hybridization temperature, and 0.5× SSC at room temperature for 5 min, and then dehydrated in graded alcohols and air dried. For signal detection, sections were placed on Kodak XAR-5 X-ray film and exposed for 2 days at room temperature.
Microdensitometric Analysis
Autoradiograms generated from the ISH were analyzed using an automated image analysis system (Dage camera, Scion Image Beta 4.03; Scion Corporation). Anatomical regions of interest were interactively selected and mean optical density measurements for each region were determined from at least six coronal sections in a single animal. This single data point was utilized in the statistical analyses. Hippocampus subfields were determined with reference to Nissl-stained sections and the anatomical atlas of Paxinos and Watson (Paxinos and Watson 1986). Nonspecific labeling of [35S]-riboprobes was determined from an area of section exhibiting apparent lack of hybridization signal.
Statistical Analyses
Analyses of data were performed on data obtained from adult male rats born and raised in our animal facility. Statistical differences were determined by analysis of variance (ANOVA). Significance was indicated by a p value p<0.05. Once significance was observed by ANOVA, the Fisher's least significant difference (Fisher's PLSD) method was utilized for further pair wise comparisons. To analyze the longitudinal weight and length data obtained we utilized general linear models for repeated measures. Significance was again designated by a p value p<0.05.
Results
Somatic Growth
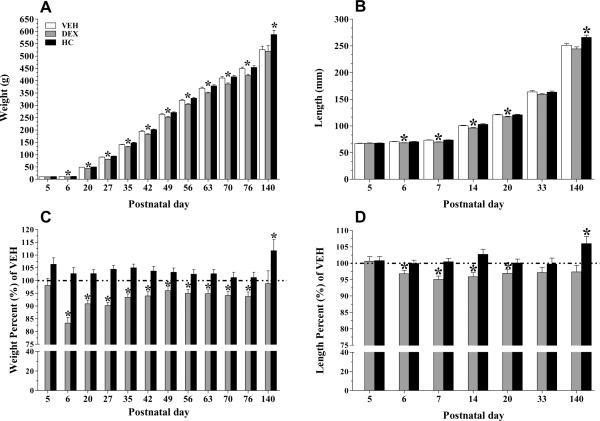
We employed the most flexible general linear model to analyze the mean progression of weight and length over time. The initial model included terms of treatment, housing, day (postnatal day), day*day (time progression), and their interactions. The objective was to test the hypothesis that the treatment effect is associated with the pattern of change of the weights and lengths. Once, the covariance structure was chosen based on this model, an analysis using parametric trend followed. The final model selected included terms of treatment, day, day*day and the interaction treatment*day. On Panels A and B, of Figure 1 we show the mean weight and length progression in male animals. To highlight the individual day post-hoc significance, Panels C and D depict the data expressed as percent of VEH control.
Figure 1.
Effects of tapering doses of DEX or HC given at post-natal day 5 and 6 on body weight and length. Growth progression of males is observed in Panels A (weight) and C (length), while data expressed as % of the reference group (VEH) are depicted in Panels B (weight) and D (length). Note that the Y axis is truncated for ease of comparison. Hydrocortisone treatment resulted in an increase growth in males at adulthood. Dexamethasone treated male animals had growth failure early, but catch-up growth by young adulthood. Data are expressed as means ± SEM; Weight, n= 37 – 44 per group, at every age; Length, n = 11 – 18 per group, at every age. *p < 0.05 DEX vs HC and VEH; † p<0.05 vs VEH.
Weight Progression
Consistent with the randomization design of the study, we did not observe a treatment effect on the baseline weight measures (see Treatment, Table 1). In addition, we did not detect a significant housing effect on the weight increase trajectory (Housing, Table 1). While controlling for housing condition, a significant treatment effect was detected on the average rate of change of weight across time. Specifically, VEH –treated animals were significantly different from DEX and HC – treated animals (see Table 1, day*treatment; VEH vs DEX: p= 0.05 and VEH vs HC: p=0.04). A computation of the average weight gain for VEH and HC –treated animals was possible by adding the estimate for the interaction term (Day*Treatment, Table 1) and the estimate for the term for the daily rate of weight change (Day, Table 1). VEH -treated animals had an average rate change of 3.9 grams each day, while HC -treatment animals had an average rate of weight change, 4.1 grams each day. DEX – treated animals had an average weight increase of 3.66 grams each day. This was computed by adding the estimate for the quadratic effect of time term (day*day) and the estimate for the daily rate of weight change seen in Table 1(day). Of interest, a calculation of the weight increase observed on the last 64 days of life (PD76 to PD140), indicates that the HC-treated animals had a high rate of gain compared to the other groups (VEH=1.1, DEX= 1.6 and HC= 2.08 g/day). Thus, comparing the rate of weight change between groups, DEX –treated animals had a slower weight increase overtime, while HC –treated animals had the highest.
Table 1.
Somatic Growth Statistical Analyses Derived from the General Linear Mixed Model
| Effects | Weight | Length | ||||
|---|---|---|---|---|---|---|
| Estimate (β) | SE (β) | p value | Estimate (β) | SE (β) | p value | |
| Treatment | ||||||
| At Baseline (VEH vs DEX) | −2.0564 | 2.8615 | 0.4725 | −2.0009 | 0.8809 | 0.0244 |
| At Baseline (VEH vs HC) | 0.9358 | 2.8251 | 0.7405 | −0.1402 | 0.8807 | 0.8737 |
| At Baseline (HC vs DEX) | −1.1206 | 2.8060 | 0.6897 | 1.8607 | 0.8809 | 0.0362 |
| housing | 0.0256 | 0.6100 | 0.9666 | |||
| day (of life) | 3.5887 | 0.1496 | <0.0001 | 4.0523 | 0.0508 | <0.0001 |
| day*day | 0.0343 | 0.0012 | <0.0001 | −0.0184 | 0.0003 | <0.0001 |
| day*treatment (VEH vs DEX) | 0.3340 | 0.1669 | 0.0457 | −0.0432 | 0.0394 | 0.2754 |
| day*treatment (VEH vs HC) | 0.3136 | 0.1567 | 0.0429 | 0.0879 | 0.0394 | 0.0270 |
| day*treatment (HC vs DEX) | 0.4645 | 0.1634 | 0.0046 | 0.1310 | 0.0394 | 0.0011 |
Length Progression
Lengths were done only in animals that were ultimately singled housed beyond day 45 of life, thus a housing term was not included in this analysis. The general linear model derived detected a significant length difference at baseline. Specifically, pups assigned to DEX treatment were significantly smaller than pups to be treated with VEH and HC at postnatal day 5 (see Treatment, Length column in Table 1; VEH vs DEX and HC vs DEX, p=0.02 & 0.04, respectively). The progression analyses showed a treatment effect overtime. The average rate of length change was found to be significantly different in HC –treated animals (see Table 1, Day*Treatment: VEH vs HC, p=0.03 and HC vs DEX, p=0.001). Following the computation pattern described above we found that in VEH –treated animals, the length increased by 4.02 millimeters (mm) each day (Day, Table 1, p<0.0001). For HC – treated animals, the average rate of length change was 4.15 mm daily. In contrast, DEX –treated animals averaged a length increase of 3.97 mm each day, the lowest rate calculated.
Food Intake
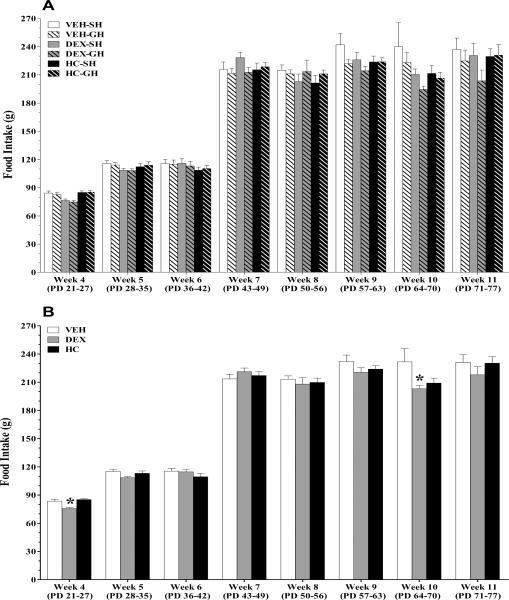
Food intake was measured daily and averaged weekly in a total of 115 animals during the course of the experiments, sixty of which were grouped housed. This was done because of the disparity in body size observed in the DEX –treated animals during the immediate postnatal period and the increased weight observed in the HC-treated animals at adulthood. The weekly average intake was computed per animal and submitted to analyses (see results in Table 2). . A repeated measure (RM) ANOVA performed on data obtained showed a food intake effect (p<0.0001). No food*treatment (p=0.1105), food*housing (p=1166) or food*treatment*housing (p=0.9728) interactions were detected. The repeated measure ANOVA was followed by a factorial analyses to determine the age range at which significant differences were detected. A treatment effect was observed immediately after weaning, on the 4th week of life (22–28 days old; Treatment, p < 0.0001, Housing, p=0.5588; treatment*housing interaction, p=0.8465) and a marginal effect at 10th wk of life (70–76 days old; treatment, p= 0.0556, housing, p= 0.2135; treatment*housing, p= 0.8626). In view of the lack of housing effect the analysis was collapsed across this variable. DEX –treated pups consumed less food when compared to VEH and HC –treated animals at 21–28 days of age (VEH- 83.4 ± 1.7; DEX- 75.6 ± 1.2; HC- 85.1 ± 1.2- [mean grams ± SEM ]; treatment, p <0.0001). At 70 to 76 days old, DEX animals also had decreased intake (VEH- 231.7 ± 14.0; DEX- 203 ± 3.6; HC- 209.1 ± 5.2; p= 0.0543).
Table 2.
Food Intake and Housing Condition After Weaning from Maternal Care
| Food Intake | |
|---|---|
| ANOVA Repeated Measure | p-values |
| Treatment | 0.1090 |
| Housing | 0.1693 |
| Treatment*Housing | 0.3057 |
| Food Intake | <0.0001 |
| Food Intake*Treatment | 0.1105 |
| Food Intake*Housing | 0.1166 |
| Food Intake*Treatment*Housing | 0.9728 |
| Factorial ANOVA Two-Way | Week 4 (PD21–27) p-value | Week 5 (PD28 –35) p-value | Week 6 (PD36 – 42) p-value | Week 7 (PD43 – 49) p-value | Week 8 (PD50 –56) p-value | Week 9 (PD57 –63) p-value | Week 10 (PD64 –70) p-value | Week 11 (PD71–77) p-value |
|---|---|---|---|---|---|---|---|---|
| Treatment | <0.0001 | 0.0668 | 0.3494 | 0.5104 | 0.8188 | 0.2627 | 0.0556 | 0.3917 |
| Housing | 0.5588 | 0.9238 | 0.8701 | 0.2733 | 0.2010 | 0.0739 | 0.2135 | 0.1727 |
| Treatment*Housing | 0.8465 | 0.8379 | 0.8766 | 0.3086 | 0.3685 | 0.3685 | 0.8626 | 0.4542 |
| Factorial ANOVA One-Way | Week 4 (PD21–27) p-value | Week 5 (PD28 –35) p-value | Week 6 (PD36 – 42) p-value | Week 7 (PD43 – 49) p-value | Week 8 (PD50 –56) p-value | Week 9 (PD57 –63) p-value | Week 10 (PD64 –70) p-value | Week 11 (PD71–77) p-value |
|---|---|---|---|---|---|---|---|---|
| Treatment | <0.0001 | 0.0616 | 0.3355 | 0.4777 | 0.7989 | 0.2662 | 0.0543 | 0.4266 |
Anxiety-Related Behaviors
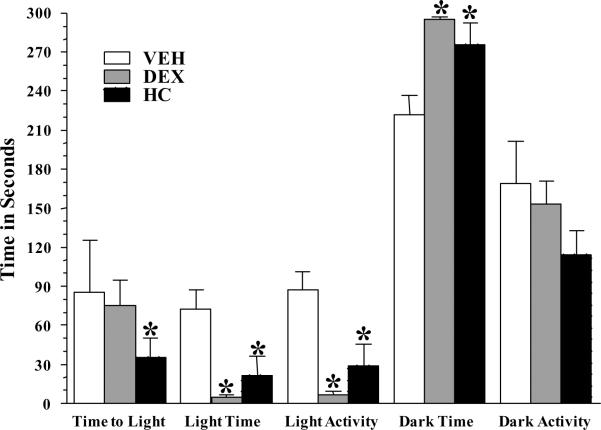
A two-way ANOVA which considered treatment and housing as independent variables revealed a treatment effect on several of the parameters assessed with the L-D box test, but no housing effect (see Table 3). Thus, the data was collapsed across the “housing” variable revealing a significant effect of treatment (p < 0.0001) on the time spent and locomotion activity in the light compartment, and the time spent in dark. Post hoc analyses revealed that as young adult, DEX- and HC- treated male animals show altered behaviors in the L-D box that were similar. Compared to VEH controls, both the DEX and HC -treated animals showed significantly decreased time and exploratory behavior in the light compartment. HC and DEX-treated animals also spent an increased amount of time in the dark compartment when compared to VEH. Both DEX and HC –treated animals moved significantly less in the light compartment when compared to VEH control animals.
Table 3.
Behavioral Response in the Light: Dark Preference Box: Comparison of Housing Conditions
| Light: Dark Box Mean seconds ± SEM (number of animals) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Time in Light | Activity in Light | Time in Dark | Activity in Dark | Latency to Light | |||||
| Single Housed | Grouped Housed | Single Housed | Grouped Housed | Single Housed | Grouped Housed | Single Housed | Grouped Housed | Single Housed | Grouped Housed | |
| Vehicle | 74.0 ± 8.4 (21) | 81.3 ± 11.4 (20) | 79.4 ± 7.2 (21) | 74.2 ± 7.9 (20) | 221.3 ± 8.6 (21) | 212.3 ± 11.7 (20) | 163.5 ± 12.9 (21) | 170.9 ± 5.5 (20) | 89.3 ± 18.2 (22) | 103.3 ± 17.7 (20) |
| Dexamethasone | 25.8 ± 5.8 (23) | 51.5 ± 4.9 (20) | 26.7 ± 5.8 (23) | 48.3 ± 6.1 (20) | 272.5 ± 6.2 (23) | 244.6 ± 5.2 (20) | 159.7 ± 7.5 (23) | 150.3 ± 11.3 (20) | 98.5 ± 19.0 (24) | 66.6 ± 16.2 (20) |
| Hydrocortisone | 39.7 ± 6.9 (21) | 40.6 ± 5.5 (20) | 48.7 ± 10.0 (21) | 53.2 ± 7.4 (21) | 256.0 ± 7.8 (21) | 255.2 ± 6.0 (20) | 157.6 ± 12.0 (21) | 176.2 ± 9.7 (20) | 88.2 ± 18.5 (24) | 120.4 ± 24.3 (22) |
| ANOVA Two-Way | Time in Light p-values | Activity in Light p-values | Time in Dark p-values | Activity in Dark p-values | Latency to Light p-values |
|---|---|---|---|---|---|
| Treatment | <0.0001 | 0.0003 | <0.0001 | 0.5673 | 0.6612 |
| Housing | 0.1349 | 0.3736 | 0.1157 | 0.6047 | 0.8097 |
| Treat*Housing | 0.3743 | 0.3648 | 0.3605 | 0.5568 | 0.3973 |
| ANOVA One-Way | p-values | p-values | p-values | p-values | p-values |
|---|---|---|---|---|---|
| Treatment | <0.0001 | <0.0001 | <0.0001 | 0.7363 | 0.9164 |
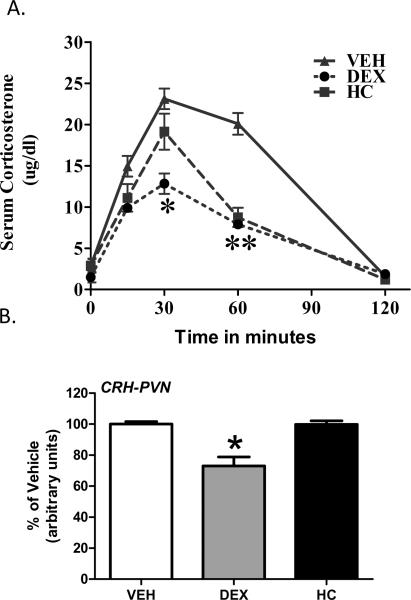
Stress Response (Figure 4)
Figure 4.
Adrenocortical response to 5 minutes of novelty stress in adult animals treated with tapering doses of DEX or HC on post-natal day 5 and 6 (Panel A) and CRH gene expression in the paraventricular nucleus (PVN) of the hypothalamus. Eight to ten animals are included in each time point obtained to determine the stress response pattern. Six to seven brains obtained from each treatment group were used for the CRH mRNA determination. VEH treated animals serve as the comparison group. Error bars missing are because these are too small to be visualized. *p<0.05 vs VEH, **p<0.05 VEH vs DEX and VEH vs HC; by Fisher's least significant difference.
A significant effect for treatment and time, with a treatment by time interaction was observed in the ANOVA analyses (treatment p < 0.0001; time p<0.0001; treatment:time, p<0.001). DEX-treated adult rats had a blunted adrenocortical response (CORT) to novelty stress compared to all groups. HC-treated animals were also different from VEH-treated animals. HC -treated animals had a lower peak response and faster CORT inhibition when compared to VEH.
Analysis of CRH gene expression in the PVN revealed a treatment effect (p= 0.05). CRH expression was significantly decreased in the adult animals treated with DEX on PD5 and 6 (see Figure 4, Panel B),
Corticoid Receptors [glucocorticoid receptor (GR) and mineralocorticoid receptor (MR)) and Serotonin (5-HT] Molecules (Figure 5 and 6)
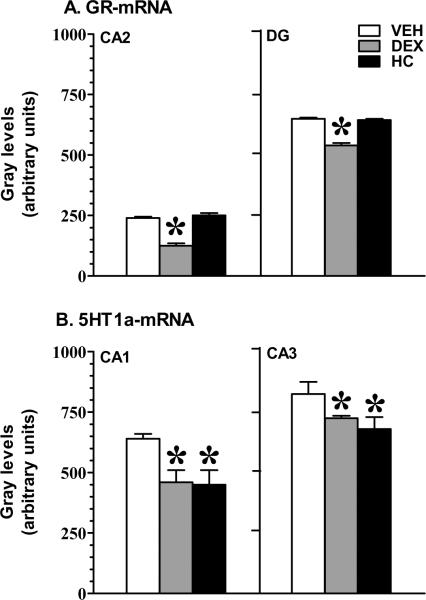
Figure 5.
Hippocampal GR (Panel A) and 5HT1a (Panel B) mRNA gene expression in adult animals treated with tapering doses of DEX or HC on postnatal day 5 and 6. The specific areas of regional significance are presented here. Error bars missing are because these are too small to be visualized. Mean ± SEM; *p<0.05 vs VEH n=6–7/group by Fisher's least significant difference.
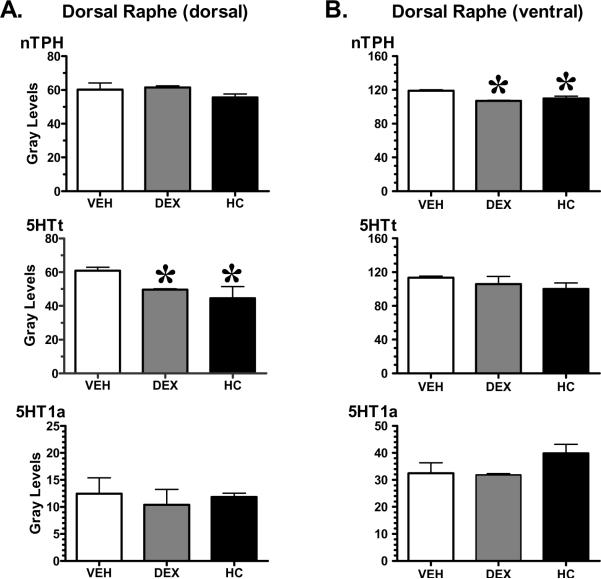
Figure 6.
Densitometric analyses of 5-HT related molecules in Dorsal Raphe (dorsal and ventral portions). The specific areas of regional significance are presented here. Mean ± SEM; *p<0.05 vs VEH n=6–7/group by Fisher's least significant difference. Error bars missing are because these are too small to be visualized. nTPH= neuronal tryptophan hydroxylase or neuronal tryptophan hydroxylase 2, 5-HTt= serotonin transporter, 5-HT1a= serotonin 1a receptor.
Hippocampus
ANOVA revealed a treatment (p<0.01), and region (p<0.001) effect on GR gene expression. Since a significant region effect was observed for GR mRNA, the ANOVA analysis was split by region. We found that adult DEX-treated rats had significantly decreased GR mRNA expression in the CA2 area of Ammon's horn, and in dentate gyrus (DG) when compared to HC and VEH animals (CA2 p<0.001; CA3, p=0.13; DG, p=0.03). Post hoc analysis demonstrated that DEX treated animals had down-regulation when compared to VEH control. No effect of DEX was found on the MR gene expression (ANOVA p=0.345) and no GR or MR mRNA changes were detected in the HC-treated animals compared to VEH controls.
The analysis of variance (ANOVA) for the 5-HT1a receptor mRNA levels in the hippocampus revealed a treatment (p<0.0001) and region effect (p=0.001). When split by region the effect was in CA1 and CA3 (CA1 p=0.006; CA2 p=0.62; CA3 p=0.05; DG p=0.63). Post hoc analysis using Fisher protected least significance difference (Fisher PLSD) showed significant down-regulation in the DEX and HC treated animals when compared to vehicle (VEH) [see Figure 5].
Raphe
ANOVA revealed no treatment (p=0.84) or region effects for 5-HT1a receptor gene expression (dorsal raphe dorsal, p=0.8 and dorsal raphe ventral, p=0.93; see Figure 6, Panels A and B, third row). Treatment and regional effects were observed in this structure for nTPH and 5-HTt gene expression measurements (ANOVA nTPH treatment= p< 0.0001; region= p< 0.05; ANOVA 5-HTt treatment= p< 0.0001; region= p< 0.005). In the DEX and HC -treated animals nTPH mRNA was significantly decreased in the ventral part of the Dorsal Raphe nucleus (DRN). HC -treated animals showed a trend towards down-regulation in the dorsal part of the DRN (p=0.06). Both DEX and HC treated animals also showed a modest but significant down regulation of the 5HTt mRNA levels in the dorsal area of the DRN when compared to VEH controls. Notice that compared to VEH-treated animals the 5-HT1a auto-receptor gene expression was found to be unchanged in the DRN of the DEX and HC treated animals.
Discussion
In the present study, we have investigated long-term effects of a DEX and HC treatment regimen given during the first week of life in the infant rat. We administered tapering doses of these agents on postnatal days 5 and 6 in an attempt to mimic short treatment regimens of DEX and HC that are commonly used in the neonatal intensive care setting in premature infants. The timing of drug administration is critical in our rat model, since it corresponds to late third trimester of human pregnancy (28 to 32 weeks of gestation), a period of growth and development that renders the brain highly vulnerable to insult in the human neonate (Dobbing 1981). We found that our treatments had significant effects on somatic growth, affecting both length and weight in the developing organism. Beyond these somatic observations, we found that a short course of either DEX or HC administration in the postnatal period affects stress response, and results in anxiogenic behavior in the adult animal. Changes in the gene expression of corticoid receptors and serotonin related molecules were also observed in hippocampus and raphe of those animals experiencing anxiety-related behavior. These findings suggest that early short-term exposure to either DEX or HC may have long-lasting consequences when a tapering dose regimen is implemented.
Dexamethasone or HC administered during early in life have a lasting impact on somatic growth. The effects were specific for each of the agents used, both in terms of time at which the growth was affected and the persistence of the change. Consistent with previous reports, we found that DEX treatment induced significant decreases in length and weight gain in the rat pup that persisted for up to two weeks after treatment (Flagel et al. 2002; Neal et al. 2004; Kanagawa et al. 2006; Kreider et al. 2006; Slotkin et al. 2006). Catch up growth was consistently achieved by adulthood. In contrast, HC-treated animals were not affected by this regimen during infancy, but were significantly larger, for both weight and length, than VEH and DEX treated animals at adulthood. We observed that although we randomized the animals to their treatment group at birth, DEX –treated pups were smaller in length (but not weight) when compared to the other two treatment groups on PD5. We do not have an explanation for this finding. Though the decreased length in the DEX –treated animals may have influenced their initial linear growth pattern, the overall decreased somatic growth observed in the DEX-treated pups may be the result of inadequate nutritional intake during the postnatal period and first week after weaning. It is known that beyond nutrition, maternal care activities are also important for the pups to thrive and grow (Schanberg et al. 1984; Levine 1994; Meaney 2010). Thus, during the immediate post-natal period the decrease in weight and length progression I the DEX –treated animals may be due to inability of the pup to attach to the mother's nipples, or poor suckling. In the immediate post weaning week the somatic growth progression is dependent on the agility/ability of the pup to climb and sustain an inverse position for seeking food. DEX animals may have had decreased muscle mass to sustain these activities. Unfortunately, our study did not include post-natal observation of maternal care or observation after weaning to objectively identify these factors that may have influenced the developmental progression. However, though decreased nutrition and maternal care may explain our results, it has been shown that the dam spends more time providing nutrition, stimulation, and warmth to a litter that is perceived to have poor health (Lynch 1976; Wiener et al. 1977; Wiener and Levine 1978; Brunelli et al. 1994; Stern 1997). A direct effect of DEX on protein catabolism that results in reduced growth and lean body mass may explain our observations (Weiler et al. 1997; Leret et al. 2004). The adverse effects of early life DEX treatment on weight gain during infancy likely contribute to long-term risks of cardiovascular and metabolic abnormalities documented in both animal and human literature (Barrington 2001; Seckl 2008). The increased risk is despite the eventual recovery of body weight, as seen in our study.
Animals treated with postnatal HC have a unique pattern of growth when compared to DEX-treated animals: these do not have altered growth early in life, but show significant weight gain and linear growth in adulthood. Several factors may contribute to these findings. It is possible that the potency of HC at the given doses was significantly less than that of DEX. Alternatively, insights into the mechanisms of corticoid receptor action can offer additional explanations (Kino 2007). There are numerous factors subject to regulatory mechanisms that contribute to the GC action. These include ligand availability, ligand affinity, receptor isoform expression, intracellular circulation, promoter association, attraction of cofactors, and clearance of the receptor from the target genes. Ligand availability is influenced by the presence of transport proteins that prevent receptor activation in specific tissues. The ATP-binding multidrug resistance cassette protein- P-glycoprotein (P-gp), is one of such proteins that exclude diverse toxic, endogenous, and pharmacological molecules, including HC and DEX, from cells in which it is expressed (Uhr et al. 2002). The P-gp is expressed predominantly in pancreas, renal proximal tubules, in biliary membrane of hepatocytes, in the apical membrane of mucosal cells in the intestine, in capillary endothelial cells of brain and testis, in adrenal gland and in placental trophoblasts (Thiebaut et al. 1987; Schinkel et al. 1995). This distribution protects the organism by excreting detrimental compounds into urine, bile, and the intestinal lumen, thus preventing their access to critical organs such as brain and liver. Though investigation of tissue specific developmental progression of P-gp is very limited (Gaillard et al. 2001; Kalabis et al. 2009), the decrease of its expression in placenta in late gestation (Kalabis et al. 2005; Petropoulos et al. 2007; Kalabis et al. 2009) and its limited expression in many tissues, including muscle, fat and bone, in the adult animal and human (Thiebaut et al. 1987), suggests that there may be critical developmental periods in which a full GC effect may not be prevented. Therefore, it is possible that differential exclusion of DEX over HC may contribute to the differential growth effect found in the DEX and HC – treated animals. The ability to bind to glucocorticoid vs mineralocorticoid receptors in specific tissues may also be another explanation for the differential effect of these two agents. DEX binds exclusively to GR, whereas HC has a greater binding affinity for MR when compared to GR (Oitzl et al. 2010). Thus, it follows that it is conceivable that the known negative effects of GCs on metabolism and bone growth plates (Zhang et al. 2007) was readily achieved in DEX –treated animals, whereas, the preferential affinity of HC for MR and the MR's ability to affect adipose tissue differentiation and function (Zennaro et al. 2009) may explain the long term effects observed on weight gain observed in the HC –treated animals. The enhanced growth and weight gain observed later in life in the HC –treated animals may also be linked to a change in GR sensitivity associated with weight gain and increased visceral obesity -related insulin resistance (Kino et al. 2003; Pivonello et al. 2010). Of particular importance is the fact that GC levels achieved by early life treatments can induce an insulin resistant state that promote adiposity later in life (Grino 2005) and elevated insulin can act as a growth factor increasing linear growth because of its structural similarity to insulin-like-growth factors directed by growth hormone (Johnson et al. 1996; Dupont and LeRoith 2001). It has been speculated that post-translational modification of the GR, such as phosphorylation, nitrosylation, acetylation, and sumoylation, that alters the transcriptional activities of GR are linked to epigenetic mechanisms associated to protracted manifestation of long term GC effects. The fact that the catch-up growth observed in the HC -treated animals in our study was not related to increased food intake suggests that the weight increase may be linked to altered metabolic endpoints that perpetuate the weight gain cycle, increase the risk for insulin resistance and subsequent diabetes later in life (Eriksson et al. 2003; Langley-Evans 2006). A thorough review of the postnatal HC literature in humans or animals treated with HC early in life revealed that this phenomenon of increased weight gain in adulthood following early life HC treatment has received little attention (Macri et al. 2009; Peltoniemi et al. 2009). Further research on the metabolic effects of early life exogenous HC exposure on is warranted.
Similar to the rodent, in the premature human infant the ability to mount a cortisol response to stress is limited (but present) (Davis et al. 2004). In the human the long term effect of early glucocorticoid exposure has received limited attention. The research in the human has focused on the regulation of the LHPA in preemies that received antenatal glucocorticoid treatment. These studies indicate that baseline cortisol levels are profoundly suppressed for 2 to 7 days after birth, subsequently return to normal levels (Kauppila et al. 1978; Ballard et al. 1980; Dorr et al. 1989; Wittekind et al. 1993). Antenatal glucocorticoid administration results in an impaired response to stress that lasts beyond the first week after treatment (Davis et al. 2004). The duration of the suppression is not known. The human literature warns that future research is needed to explore whether the effects of prenatal corticosteroids persist and alter the developmental trajectory of the LHPA as well as related cognitive and emotion systems (Davis et al. 2004). In the present study, the adult adrenocortical stress response was altered by early life GC treatments. Basal corticosterone levels were not different between groups. In response to novelty stress, the male animals exposed to HC or to DEX-early in life had a blunted adrenocortical response, with an adequate termination of the stress response. These patterns of stress response are consistent with other animal and human reports that indicate lifetime altered responses with early life DEX treatments (Felszeghy et al. 2000; Flagel et al. 2002; Karemaker et al. 2008; Ng et al. 2008). Such limited adrenocortical response to stress could be a beneficial adaptation since elevated endogenous GC levels have been associated with atherosclerosis, immunosuppression, depression and cognitive impairment, in addition to raised cholesterol levels and increased incidence of diabetes (Matthews 2002; Seckl 2008). In contrast, studies in which pups have been treated with controlled low doses of HC post-natally indicate a decreased response to stress, beneficial cognitive effects and protection from brain ischemic injury at adulthood (Catalani et al. 1993; Casolini et al. 2007; Macri et al. 2009).
Modulation of glucocorticoid receptors in hippocampus are linked to baseline corticosterone levels and the quality of the adrenocortical response [reviewed in (De Kloet et al. 1998)]. Our findings of unchanged MR mRNA expression in the hippocampus are consistent with the basal corticosterone levels observed in both treatment groups. However, decreased GR mRNA expression but no change in MR mRNA expression in adult rat hippocampus would predict a delayed return to baseline in serum corticosterone after stress (reviewed in (De Kloet et al. 1998)). This combination of corticoid receptor imbalance was found in our DEX-treated (but not the HC-treated) animals; however an adequate inhibition of corticosterone secretion was present. One possible explanation is that a much stronger stressor is needed to challenge the DEX treated animals in order for this to result in a loss of regulatory feedback inhibition of corticosterone secretion. Neal and co-workers showed that peak corticosterone response to crowding stress in adult animals exposed to tapering doses of DEX from postnatal day 3 to 6 is no different from VEH control animals, but delayed return to baseline is noted in the DEX-exposed cohort (Neal et al. 2004). Taken together, these data indicate either that the type of stressor is important for the adrenocortical response observed or that the timing of GC exposure early in life is an important factor modulating long-term effects of neonatal exposure.
Increased anxiety-related behaviors were observed in both DEX and HC –treated animals when tested in the light-dark test. Single housing, linked to the development of an “isolation syndrome” (Ader et al. 1960; Stern et al. 1960; Hatch et al. 1965) is not the source of the anxiety-related behaviors observed in the DEX and HC –treated animals. Anxiety –related behaviors were detected in DEX and HC –treated adult animals irrespective of the single housing or the grouped housing condition that was imposed from adolescence to adulthood. This is in agreement with studies from Holson and colleagues who concluded that the timid, or fearful behavior associated with the “isolation syndrome” is dependent on environmental factors and not the isolation itself (Holson et al. 1991). Animals reared isolated in plastic cages but with human contact as part of the usual animal care are completely protected against the “isolation stress syndrome”. Our animals were isolated in plastic cages and received care as described in Holson's study.
Early life DEX and HC treatment results in an animal with lower corticosterone response to novelty stress and anxiety –related behaviors. The dissociation is reminiscent of the individual difference in behavioral responses to a novel environment observed in high responder (HR) and low responder (LR) rats (Dellu et al. 1996; Kabbaj et al. 2000). HR rats that exhibit high rates of locomotor activity and sustained exploration in a novel environment also exhibit high concentrations of stress-induced plasma corticosterone. In contrast, their LR -counterparts, are timid and fearful and have a blunted, low plasma corticosterone profile in a novel environment. The HR rats have a greater propensity to self-administer drugs when compared to the LR, thus though intuitively one would consider the combination of anxiety – related behavior and low stress response as maladaptive, it is possible that these attributes may be beneficial for specific unhealthy behaviors.
Both DEX and HC early life treatments are associated with long-term repercussions in behavior and change in the serotonin (5-HT) system. Animals treated with our GC tapering paradigm exhibited, as adults [but not pre-adolescence(Bhatt-Mehta et al. 2010)], anxiety-related behavior in the Light-Dark test. When compared to VEH control, both DEX and HC treated animals also showed a down-regulation of the expression of the rate limiting enzyme for 5-HT synthesis, nTPH and the molecule that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons, 5-HTt. A down-regulation of the postsynaptic (but not the pre-synaptic) 5HT1a receptor was also observed when compared to VEH control. Since nTPH is the rate-limiting enzyme in 5-HT biosynthesis, a decrease in nTPH expression, with a consequent decrease in 5-HT synthesis, may be responsible for the fear-related behavior we observed in adult animals exposed to neonatal GC treatments. The observed down-regulation of 5-HTt gene expression in these animals is also consistent with this hypothesis. Decreasing the molecules responsible for the reuptake of 5-HT may represent an adaptive neuronal mechanism to compensate for lower 5-HT levels. Interestingly, this “compensatory” down-regulation of the 5-HTt has been observed in the brains of depressed subjects as well as in suicide victims (Mann et al. 2000), and it is also thought to be a response to lower 5-HT levels. Compounding this problem is the simultaneous down-regulation of hippocampal 5HT1a, a receptor implicated in anxiety, as well as in depression. We suggest that GC treatment within that specific developmental window may trigger unique adaptations of the 5-HT system that may have repercussions in behavior later in life. In adult animals these adaptations may be consistent with anxiety-related behavior (Blier and de Montigny 1994; Zhuang et al. 1999; Schmidt 2010).
Hydrocortisone treated animals did not exhibit a down-regulation of GR. Nevertheless, these animals showed behavioral and 5HT abnormalities similar to the DEX treated group. It is likely that the HC treatment favored MR stimulation in brain (Fischer et al. 2002; Macleod et al. 2003; Crochemore et al. 2005) particularly since, HC has the highest affinity to MR (when compared to GR); MR's highest hippocampal MR signal intensity is seen in the first week of life (Vázquez et al. 1993) and the brain does not acquire the limited adult-like MR distribution until close to adolescence (Herman et al. 1989; Vázquez et al. 1993; Oitzl et al. 2010). Of importance is the fact that MR activation has been shown to be neuroprotective (Fischer et al. 2002; Macleod et al. 2003; Crochemore et al. 2005; Oitzl et al. 2010). If this is true in these animals, it would indicate that even if HC is relatively neuroprotective compared to DEX, it can still cause long term behavioral and neurochemical changes. Pharmacological studies are currently being designed in our laboratory to address both the issue of DEX/HC dose equivalence and the possible mechanisms involved in the HC behavioral effects.
In conclusion, the present study suggests that GC exposure during a specific time in the early postnatal period of the rat leads to critical changes in the hypothalamic-pituitary-adrenal axis and 5-HT circuitry in the neonatal animal that set up the stage for vulnerability later in life. The notion that the external environmental milieu influences permanent hardwiring in the stress responsive limbic-hypothalamic-pituitary-adrenal axis and serotonin brain system is not a novel concept (Lauder 1990; Walker et al. 2001; Gaspar et al. 2002; Ansorge et al. 2004). These systems are highly plastic during development and adverse levels of either stress or GC can have a profound impact on development (McEwen et al. 1987; McEwen et al. 1987; Gould 1994; McEwen 1994; Macri et al. 2009; Cirulli et al. 2010) and plasticity in adults (Smythe et al. 1997; McCullers and Herman 1998; Spencer et al. 1998; McEwen 2000; Macri et al. 2009; Sullivan and Holman 2010). Our findings raise concerns about routine use of hydrocortisone in premature infants even when the glucocorticoid anti-inflammatory potency of this agent is considerably reduced compared to dexamethasone, an agent associated with significant neurodevelopmental effects when used routinely for prolonged periods. Although to-date the immediate and short term effects of early hydrocortisone treatment in premature infants who are now in school age appears to be benign, subtle maladaptive behavioral strategies may not be recognizable until later in development. Such effects may have important implications on learning, mood and ultimately quality of life in survivors of prematurity. Both DEX and HC should continue to be used with caution in this vulnerable population.
Figure 2.
Food intakes during the post-natal period. Food was measured daily, and weekly average intake was calculated per animal per group, per treatment group. Panel A shows the data obtained from a cohort of animals that were randomly assigned to single housed or group housed conditions. Data are expressed as means ± SEM; n = 20–23 per group, at every age. Panel B depicts the data collapsed across the housing variable since the initial two-way ANOVA did not reveal a significant housing effect. Data are expressed as means ± SEM; n = 31–37 per group, at every age. *p < 0.005, vs VEH by Fisher's least significant difference.
Figure 3.
Adult behavior in Light:Dark preference box evaluated the locomotion and investigatory behavior of the animals in adulthood. Preference for darkness, delay to move to light compartment and decreased activity within the space chosen are gross measurements of anxiety-related behavior. Data are expressed as means ± SEM; n = 20–23 per group, at every age. *p < 0.001, vs age-matched VEH by Fisher's least significant difference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Beels CC, Tatum R. Social factors affecting emotinality and resistance to disease in animals: II. Susceptibility to gastric ulceration as a function of interruptions in social interactions and the time at which they occur. J Comp Physiol Psychol. 1960;53:455–8. doi: 10.1037/h0048003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=13681474. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. F. T. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109(2):330–8. doi: 10.1542/peds.109.2.330. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11826218. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-Life Blockade of the 5-HT Transporter Alters Emotional Behavior in Adult Mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Gluckman PD, Liggins GC, Kaplan SL, Grumbach MM. Steroid and growth hormone levels in premature infants after prenatal betamethasone therapy to prevent respiratory distress syndrome. Pediatr Res. 1980;14(2):122–7. doi: 10.1203/00006450-198002000-00011. http://www.ncbi.nlm.nih.gov/pubmed/6444710. [DOI] [PubMed] [Google Scholar]
- Barrington KJ. Postnatal steroids and neurodevelopmental outcomes: a problem in the making. Pediatrics. 2001;107(6):1425–6. doi: 10.1542/peds.107.6.1425. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11389269. [DOI] [PubMed] [Google Scholar]
- Benders MJ, Groenendaal F, van Bel F, Ha Vinh R, Dubois J, Lazeyras F, Warfield SK, Huppi PS, de Vries LS. Title: Brain development of the preterm neonate after neonatal hydrocortisone treatment for chronic lung disease. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181b3aec5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19581837. [DOI] [PMC free article] [PubMed]
- Benesova O, Pavlik A. Perinatal treatment with glucocorticoids and the risk of maldevelopment of the brain. Neuropharmacology. 1989;28(1):89–97. doi: 10.1016/0028-3908(89)90073-7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2927582. [DOI] [PubMed] [Google Scholar]
- Bhatt-Mehta V, Huang LS, Neal CR, Pawar M, Vazquez DM. Stress. 2010. Tapering Dose of Hydrocortisone and Dexamethasone in Neonatal Rats: Effect on the Growth, Neurological Development and Hypothalamic-Pituitary-Adrenal Response to Stress at Pre-Adolescence. Submitted. [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15(7):220–6. doi: 10.1016/0165-6147(94)90315-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7940983. [DOI] [PubMed] [Google Scholar]
- Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38(8):931–41. doi: 10.1111/j.1469-7610.1997.tb01612.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9413793. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65. doi: 10.1016/s0014-2999(03)01274-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12600702. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol. 1994;108(3):298–303. doi: 10.1037/0735-7036.108.3.298. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7924260. [DOI] [PubMed] [Google Scholar]
- Casolini P, Domenici MR, Cinque C, Alema GS, Chiodi V, Galluzzo M, Musumeci M, Mairesse J, Zuena AR, Matteucci P, Marano G, Maccari S, Nicoletti F, Catalani A. Maternal exposure to low levels of corticosterone during lactation protects the adult offspring against ischemic brain damage. J Neurosci. 2007;27(26):7041–6. doi: 10.1523/JNEUROSCI.1074-07.2007. http://www.ncbi.nlm.nih.gov/pubmed/17596453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624(1–2):209–15. doi: 10.1016/0006-8993(93)90079-3. http://www.ncbi.nlm.nih.gov/pubmed/8252393. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention QuickStats: Distribution of Births, by Gestational Age – United States. MMWR. 2007;(14):344. [Google Scholar]
- Cirulli F, Berry A, Bonsignore LT, Capone F, D'Andrea I, Aloe L, Branchi I, Alleva E. Early life influences on emotional reactivity: evidence that social enrichment has greater effects than handling on anxiety-like behaviors, neuroendocrine responses to stress and central BDNF levels. Neurosci Biobehav Rev. 2010;34(6):808–20. doi: 10.1016/j.neubiorev.2010.02.008. http://www.ncbi.nlm.nih.gov/pubmed/20171244. [DOI] [PubMed] [Google Scholar]
- Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry. 2005;10(8):790–8. doi: 10.1038/sj.mp.4001679. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15940303. [DOI] [PubMed] [Google Scholar]
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. 1989;320(23):1505–10. doi: 10.1056/NEJM198906083202301. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2657423. [DOI] [PubMed] [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Ciffuentes RF, Lussky RC. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology. 2004;29(8):1028–36. doi: 10.1016/j.psyneuen.2003.10.005. http://www.ncbi.nlm.nih.gov/pubmed/15219654. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rosenfeld P, Van Eekelen JA, Sutanto W, Levine S. Stress, glucocorticoids and development. Prog Brain Res. 1988;73:101–20. doi: 10.1016/S0079-6123(08)60500-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3047791. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9626555. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–45. doi: 10.1159/000119305. http://www.ncbi.nlm.nih.gov/pubmed/8916071. [DOI] [PubMed] [Google Scholar]
- Dobbing J. The later development of the brain and its vulnerability. Heinemann Medical Books; London: 1974. [Google Scholar]
- Dobbing J. The later development of the brain and its vulnerability. Heinemann Medical Books; London: 1981. [Google Scholar]
- Dorr HG, Heller A, Versmold HT, Sippell WG, Herrmann M, Bidlingmaier F, Knorr D. Longitudinal study of progestins, mineralocorticoids and glucocorticoids throughout human pregnancy. J Clin Endocrinol Metab. 1989;68(5):863–8. doi: 10.1210/jcem-68-5-863. http://www.ncbi.nlm.nih.gov/pubmed/2715289. [DOI] [PubMed] [Google Scholar]
- Doyle L, Davis P. Postnatal corticosteroids in preterm infants: systematic review of effects on mortality and motor function. J Paediatr Child Health. 2000;36(2):101–7. doi: 10.1046/j.1440-1754.2000.00481.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10760004. [DOI] [PubMed] [Google Scholar]
- Dupont J, LeRoith D. Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm Res. 2001;55(Suppl 2):22–6. doi: 10.1159/000063469. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11684871. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46(2):190–4. doi: 10.1007/s00125-002-1012-5. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12627317. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Bagdy G, Nyakas C. Blunted pituitary-adrenocortical stress response in adult rats following neonatal dexamethasone treatment. J Neuroendocrinol. 2000;12(10):1014–21. doi: 10.1046/j.1365-2826.2000.00551.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11012843. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Gaspar E, Nyakas C. Long-term selective down-regulation of brain glucocorticoid receptors after neonatal dexamethasone treatment in rats. J Neuroendocrinol. 1996;8(7):493–9. doi: 10.1046/j.1365-2826.1996.04822.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8843017. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Holson RR. Neonatal dexamethasone on day 7 causes mild hyperactivity and cerebellar stunting. Neurotoxicol Teratol. 1999;21(1):71–6. doi: 10.1016/s0892-0362(98)00029-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10023803. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5(3):195–201. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1688319. [PubMed] [Google Scholar]
- Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czeh B. The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat. Brain Res. 2002;947(2):290–3. doi: 10.1016/s0006-8993(02)03042-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12176172. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Neal JCR, Watson JSJ, Vázquez DM. Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment and stress response. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R55–63. doi: 10.1152/ajpregu.2002.282.1.R55. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11742823. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Vazquez DM, Watson SJ, Jr., Neal CR., Jr. Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment and stress response. Am J Physiol Regul Integr Comp Physiol. 2002;282(1):R55–63. doi: 10.1152/ajpregu.2002.282.1.R55. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11742823. [DOI] [PubMed] [Google Scholar]
- Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12(3):215–22. doi: 10.1016/s0928-0987(00)00123-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11113640. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience. 2002;4(12):1002–12. doi: 10.1038/nrn1256. [Review] [142 refs] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14618156. [DOI] [PubMed] [Google Scholar]
- Gould E. The effects of adrenal steroids and excitatory input on neuronal birth and survival. Ann N Y Acad Sci. 1994;743:73–92. doi: 10.1111/j.1749-6632.1994.tb55788.x. discussion 92-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7802420. [DOI] [PubMed] [Google Scholar]
- Grino M. Prenatal nutritional programming of central obesity and the metabolic syndrome: role of adipose tissue glucocorticoid metabolism. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1233–5. doi: 10.1152/ajpregu.00542.2005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16221979. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vázquez DM. Stress neurobiology and developmental psychopathology. Developmental Neuroscience. 2006;2:533–577. D. Cicchetti and D. Cohen. New York Wiley. [Google Scholar]
- Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990's. Early Hum Dev. 1999;53(3):193–218. doi: 10.1016/s0378-3782(98)00052-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10088988. [DOI] [PubMed] [Google Scholar]
- Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med. 2000;154(7):725–31. doi: 10.1001/archpedi.154.7.725. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10891026. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Bona E, Gilland E, Puka-Sundvall M. Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl. 1997;422:85–8. doi: 10.1111/j.1651-2227.1997.tb18353.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9298801. [DOI] [PubMed] [Google Scholar]
- Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7–14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003;(1):CD001144. doi: 10.1002/14651858.CD001144. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12535400. [DOI] [PubMed] [Google Scholar]
- Hatch AM, Wiberg GS, Zawidzka Z, Cann M, Airth JM, Grice HC. Isolation syndrome in the rat. Toxicol Appl Pharmacol. 1965;7(5):737–45. doi: 10.1016/0041-008x(65)90132-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=4286670. [DOI] [PubMed] [Google Scholar]
- Heine VM, Rowitch DH, Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. Journal of Clinical Investigation. 2009;119(2):267–77. doi: 10.1172/JCI36376. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3(11):1886–94. doi: 10.1210/mend-3-11-1886. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2558306. [DOI] [PubMed] [Google Scholar]
- Hille ET, den Ouden AL, Saigal S, Wolke D, Lambert M, Whitaker A, Pinto-Martin JA, Hoult L, Meyer R, Feldman JF, Verloove-Vanhorick SP, Paneth N. Behavioural problems in children who weigh 1000 g or less at birth in four countries. Lancet. 2001;357(9269):1641–3. doi: 10.1016/S0140-6736(00)04818-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11425366. [DOI] [PubMed] [Google Scholar]
- Holson RR, Scallet AC, Ali SF, Turner BB. “Isolation stress” revisited: isolation-rearing effects depend on animal care methods. Physiol Behav. 1991;49(6):1107–18. doi: 10.1016/0031-9384(91)90338-o. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1896492. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Calogero AE, Gold PW, Chrousos GP. Effects of early parenting on growth and development in a small primate. Pediatr Res. 1996;39(6):999–1005. doi: 10.1203/00006450-199606000-00012. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8725261. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10995843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod. 2005;73(4):591–7. doi: 10.1095/biolreprod.105.042242. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15917342. [DOI] [PubMed] [Google Scholar]
- Kalabis GM, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can J Physiol Pharmacol. 2009;87(11):973–8. doi: 10.1139/y09-087. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19935905. [DOI] [PubMed] [Google Scholar]
- Kanagawa T, Tomimatsu T, Hayashi S, Shioji M, Fukuda H, Shimoya K, Murata Y. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am J Obstet Gynecol. 2006;194(1):231–8. doi: 10.1016/j.ajog.2005.06.015. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16389037. [DOI] [PubMed] [Google Scholar]
- Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg-Kamperman M, Baerts W, Veen S, Samsom JF, Visser GH, van Bel F, Heijnen CJ, Karemaker R, Kavelaars A, ter Wolbeek M, Tersteeg-Kamperman M, Baerts W, Veen S, Samsom JF, Visser GHA, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121(4):e870–8. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- Kauffman KS, Seidler FJ, Slotkin TA. Prenatal dexamethasone exposure causes loss of neonatal hypoxia tolerance: cellular mechanisms. Pediatr Res. 1994;35(5):515–22. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8065832. [PubMed] [Google Scholar]
- Kauppila A, Tuimala R, Ylikorkala O, Haapalahti J, Karppanen H, Viinikka L. Effects of ritodrine and isoxsuprine with and without dexamethasone during late pregnancy. Obstet Gynecol. 1978;51(3):288–92. doi: 10.1097/00006250-197803000-00007. http://www.ncbi.nlm.nih.gov/pubmed/203880. [DOI] [PubMed] [Google Scholar]
- Kino T. Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res. 2007;39(6):420–4. doi: 10.1055/s-2007-980193. http://www.ncbi.nlm.nih.gov/pubmed/17578758. [DOI] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85(2–5):457–67. doi: 10.1016/s0960-0760(03)00218-8. http://www.ncbi.nlm.nih.gov/pubmed/12943736. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31(1):12–35. doi: 10.1038/sj.npp.1300783. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15920497. [DOI] [PubMed] [Google Scholar]
- Kurosawa A, Kageyama H, John TM, Hirota R, Itoh S. Effect of neonatal hydrocortisone treatment on brain monoamines in developing rats. Japanese Journal of Pharmacology. 1980;30(2):213–20. doi: 10.1254/jjp.30.213. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65(1):97–105. doi: 10.1079/pns2005478. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16441949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2252317. [DOI] [PubMed] [Google Scholar]
- Leret ML, Peinado V, Suarez LM, Tecedor L, Gamallo A, Gonzalez JC. Role of maternal adrenal glands on the developing serotoninergic and aminoacidergic systems of the postnatal rat brain. International Journal of Developmental Neuroscience. 2004;22(2):87–93. doi: 10.1016/j.ijdevneu.2003.12.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15036383. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci. 1994;746:275–88. doi: 10.1111/j.1749-6632.1994.tb39245.x. discussion 289–93. http://www.ncbi.nlm.nih.gov/pubmed/7825883. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116(1):1–7. doi: 10.1542/peds.2004-1275. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15995023. [DOI] [PubMed] [Google Scholar]
- Lorenz JM. The outcome of extreme prematurity. Semin Perinatol. 2001;25(5):348–59. doi: 10.1053/sper.2001.27164. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11707021. [DOI] [PubMed] [Google Scholar]
- Lynch A. Postnatal undernutrition: an alternative method. Dev Psychobiol. 1976;9(1):39–48. doi: 10.1002/dev.420090107. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=943351. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Johansson IM, Soderstrom I, Lai M, Gido G, Wieloch T, Seckl JR, Olsson T. Mineralocorticoid receptor expression and increased survival following neuronal injury. Eur J Neurosci. 2003;17(8):1549–55. doi: 10.1046/j.1460-9568.2003.02587.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12752372. [DOI] [PubMed] [Google Scholar]
- Macri S, Granstrem O, Shumilina M, Antunes Gomes dos Santos FJ, Berry A, Saso L, Laviola G. Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Horm Behav. 2009;56(4):391–8. doi: 10.1016/j.yhbeh.2009.07.006. http://www.ncbi.nlm.nih.gov/pubmed/19632235. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57(8):729–38. doi: 10.1001/archpsyc.57.8.729. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10920459. [DOI] [PubMed] [Google Scholar]
- Martens SE, Rijken M, Stoelhorst GM, van Zwieten PH, Zwinderman AH, Wit JM, Hadders-Algra M, Veen S. Is hypotension a major risk factor for neurological morbidity at term age in very preterm infants? Early Hum Dev. 2003;75(1–2):79–89. doi: 10.1016/j.earlhumdev.2003.09.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14652161. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends in Endocrinology and Metabolism. 2002;13(9):373–380. doi: 10.1016/s1043-2760(02)00690-2. http://www.sciencedirect.com/science/article/B6T3K-46SP63K-2/2/cb1b01bc16d542a4935192da8ea17891. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Herman JP. Mineralocorticoid receptors regulate bcl-2 and p53 mRNA expression in hippocampus. Neuroreport. 1998;9(13):3085–9. doi: 10.1097/00001756-199809140-00031. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9804321. [DOI] [PubMed] [Google Scholar]
- McEwen B, Brinton R, Harrelson A, Rostene W. Modulatory interactions between steroid hormones, neurotransmitters and neuropeptides in hippocampus. Adv Biochem Psychopharmacol. 1987;43:87–102. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2884822. [PubMed] [Google Scholar]
- McEwen B, Chao H, Spencer R, Brinton R, Macisaac L, Harrelson A. Corticosteroid receptors in brain: relationship of receptors to effects in stress and aging. Ann N Y Acad Sci. 1987;512:394–401. doi: 10.1111/j.1749-6632.1987.tb24975.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3327425. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoid-biogenic amine interactions in relation to mood and behavior. Biochem Pharmacol. 1987;36(11):1755–63. doi: 10.1016/0006-2952(87)90234-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3555503. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Corticosteroids and hippocampal plasticity. Ann N Y Acad Sci. 1994;746:134–42. doi: 10.1111/j.1749-6632.1994.tb39223.x. discussion 142-4, 178-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7825871. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48(8):721–31. doi: 10.1016/s0006-3223(00)00964-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11063969. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Dev. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. http://www.ncbi.nlm.nih.gov/pubmed/20331654. [DOI] [PubMed] [Google Scholar]
- Neal CRJ, VanderBeek BL, Vázquez DM, Watson SJJIPA. Dexamethasone Exposure During the Neonatal Period Alters ORL1 mRNA Expression in the Hypothalamic Paraventricular Nucleus and Hippocampus of the Adult Rat. Developmental Brain Research. 2004;146:15–24. doi: 10.1016/j.devbrainres.2003.09.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14643007. [DOI] [PubMed] [Google Scholar]
- Neal CRJ, Weidemann G, Kabbaj M, Vázquez DM. Effect of Neonatal Dexamethasone Exposure on Growth and Neurological Development in the Adult Rat. Am J Physiol Regul Integr Comp Physiol. 2004;287:R375–85. doi: 10.1152/ajpregu.00012.2004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15117721. [DOI] [PubMed] [Google Scholar]
- Ng PC, Lam CW, Lee CH, Chan IH, Wong SP, Fok TF, Lam CWK, Chan IHS, Wong SPS. Suppression and recovery of the hypothalamic function after high-dose corticosteroid treatment in preterm infants. Neonatology. 2008;94(3):170–5. doi: 10.1159/000143396. [DOI] [PubMed] [Google Scholar]
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG, 3rd, Dillard RG. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics. 1999;104(1 Pt 1):15–21. doi: 10.1542/peds.104.1.15. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10390254. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34(6):853–66. doi: 10.1016/j.neubiorev.2009.07.006. http://www.ncbi.nlm.nih.gov/pubmed/19631685. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Orlando, FL: 1986. [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, Hallman M, G. American Academy of Pediatrics. Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, Hallman M. Trial of early neonatal hydrocortisone: two-year follow-up. Neonatology. 2009;95(3):240–7. doi: 10.1159/000164150. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Kalabis GM, Gibb W, Matthews SG. Functional changes of mouse placental multidrug resistance phosphoglycoprotein (ABCB1) with advancing gestation and regulation by progesterone. Reprod Sci. 2007;14(4):321–8. doi: 10.1177/1933719107303856. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17644804. [DOI] [PubMed] [Google Scholar]
- Pharoah PO, Stevenson CJ, Cooke RW, Stevenson RC. Prevalence of behaviour disorders in low birthweight infants. Arch Dis Child. 1994;70(4):271–4. doi: 10.1136/adc.70.4.271. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8185358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivonello R, De Leo M, Vitale P, Cozzolino A, Simeoli C, De Martino MC, Lombardi G, Colao A. Pathophysiology of diabetes mellitus in Cushing's syndrome. Neuroendocrinology. 2010;92(Suppl 1):77–81. doi: 10.1159/000314319. http://www.ncbi.nlm.nih.gov/pubmed/20829623. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29(10):493–8. doi: 10.1016/j.tips.2008.07.005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18755516. [DOI] [PubMed] [Google Scholar]
- Saigal S. Follow-up of very low birthweight bavies to adolescence. Semin Neonatol. 2000;5:107–18. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- Sajaniemi N, Hakamies-Blomqvist L, Makela J, Avellan A, Rita H, von Wendt L. Cognitive development, temperament and behavior at 2 years as indicative of language development at 4 years in pre-term infants. Child Psychiatry Hum Dev. 2001;31(4):329–46. doi: 10.1023/a:1010238523628. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11227991. [DOI] [PubMed] [Google Scholar]
- Schanberg SM, Evoniuk G, Kuhn CM. Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development. Proc Soc Exp Biol Med. 1984;175(2):135–46. doi: 10.3181/00379727-175-41779. http://www.ncbi.nlm.nih.gov/pubmed/6364149. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a PGlycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin and cyclosporin A. J Clin Invest. 1995;96(4):1698–705. doi: 10.1172/JCI118214. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7560060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV. Molecular mechanisms of early life stress--lessons from mouse models. Neurosci Biobehav Rev. 2010;34(6):845–52. doi: 10.1016/j.neubiorev.2009.05.002. http://www.ncbi.nlm.nih.gov/pubmed/19447133. [DOI] [PubMed] [Google Scholar]
- Schothorst PF, van Engeland H. Long-term behavioral sequelae of prematurity. J Am Acad Child Adolesc Psychiatry. 1996;35(2):175–83. doi: 10.1097/00004583-199602000-00011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8720627. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18037004. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental 'programming' and the risk of affective dysfunction. Progress in Brain Research. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Barnes G, Lau C, Seidler FJ, Trepanier P, Weigel SJ, Whitmore WL. Development of polyamine and biogenic amine systems in brains and hearts of neonatal rats given dexamethasone: role of biochemical alterations in cellular maturation for producing deficits in ontogeny of neurotransmitter levels, uptake, storage and turnover. J Pharmacol Exp Ther. 1982;221(3):686–93. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6123585. [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31(5):904–11. doi: 10.1038/sj.npp.1300892. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16160705. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol Biochem Behav. 1997;56(3):507–13. doi: 10.1016/s0091-3057(96)00244-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9077590. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139(6):2718–26. doi: 10.1210/endo.139.6.6029. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9607777. [DOI] [PubMed] [Google Scholar]
- Stern JA, Winokur G, Eisenstein A, Taylor R, Sly M. The effect of group vs. individual housing on behaviour and physiological responses to stress in the albino rat. J Psychosom Res. 1960;4:185–90. doi: 10.1016/0022-3999(60)90010-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=13834443. [DOI] [PubMed] [Google Scholar]
- Stern JM. Offspring-induced nurturance: animal-human parallels. Dev Psychobiol. 1997;31(1):19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9222114. [DOI] [PubMed] [Google Scholar]
- Stjernqvist K, Svenningsen NW. Extremely low-birth-weight infants less than 901 g: development and behaviour after 4 years of life. Acta Paediatr. 1995;84(5):500–6. doi: 10.1111/j.1651-2227.1995.tb13682.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7633143. [DOI] [PubMed] [Google Scholar]
- Stoelhorst GM, Martens SE, Rijken M, van Zwieten PH, Zwinderman AH, Wit JM, Veen S. Behaviour at 2 years of age in very preterm infants (gestational age < 32 weeks) Acta Paediatr. 2003;92(5):595–601. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12839291. [PubMed] [Google Scholar]
- Stoelhorst GM, Rijken M, Martens SE, van Zwieten PH, Feenstra J, Zwinderman AH, Wit JM, Veen S, Leiden P, Stoelhorst GMSJ, Rijken M, Martens SE, van Zwieten PHT, Feenstra J, Zwinderman AH, Wit JM, Veen S, Leiden P. Developmental outcome at 18 and 24 months of age in very preterm children: a cohort study from 1996 to 1997. Early Human Development. 2003;72(2):83–95. doi: 10.1016/s0378-3782(03)00011-2. Follow-Up Project on. Follow-Up Project on. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–44. doi: 10.1016/j.neubiorev.2009.11.010. http://www.ncbi.nlm.nih.gov/pubmed/19931556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. doi: 10.1073/pnas.84.21.7735. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2444983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, Egan GF, Inder TE. Neonate hippocampal volumes: prematurity, perinatal predictors and 2-year outcome. Ann Neurol. 2008;63(5):642–51. doi: 10.1002/ana.21367. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18384167. [DOI] [PubMed] [Google Scholar]
- Uhr M, Holsboer F, Muller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14(9):753–9. doi: 10.1046/j.1365-2826.2002.00836.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12213137. [DOI] [PubMed] [Google Scholar]
- van der Heide-Jalving M, Kamphuis PJ, van der Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, Veen S, van Bel F. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatr. 2003;92(7):827–35. doi: 10.1080/08035250310002425. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12892163. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23(7):663–700. doi: 10.1016/s0306-4530(98)00029-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9854741. [DOI] [PubMed] [Google Scholar]
- Vázquez DM, López JF, Morano MI, Akil H. Short-term adrenalectomy increases glucocorticoid and mineralocorticoid receptor mRNA in selective areas of the developing hippocampus. Molecular and Cellular Neuroscience. 1993;4:455–471. doi: 10.1006/mcne.1993.1057. [DOI] [PubMed] [Google Scholar]
- Walker CD, Anand KJS, Plotsky PM. In: Development of the hypothalamic-pituitary-adrenal axis and the stress response. Coping with the Environment. McEwen BS, editor. Oxford University Press; New York: 2001. [Google Scholar]
- Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res. 2001;50(2):190–5. doi: 10.1203/00006450-200108000-00005. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11477202. [DOI] [PubMed] [Google Scholar]
- Weiler HA, Wang Z, Atkinson SA. Whole body lean mass is altered by dexamethasone treatment through reductions in protein and energy utilization in piglets. Biol Neonate. 1997;71(1):53–9. doi: 10.1159/000244397. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8996658. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595(2):195–200. doi: 10.1016/0006-8993(92)91049-k. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1467966. [DOI] [PubMed] [Google Scholar]
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–7. doi: 10.1136/fn.83.2.F154. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10952714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SG, Fitzpatrick KM, Levin R, Smotherman WP, Levine S. Alterations in the maternal behavior of rats rearing malnourished offspring. Dev Psychobiol. 1977;10(3):243–54. doi: 10.1002/dev.420100308. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=863120. [DOI] [PubMed] [Google Scholar]
- Wiener SG, Levine S. Perinatal malnutrition and early handling: interactive effects on the development of the pituitary-adrenal system. Dev Psychobiol. 1978;11(4):335–52. doi: 10.1002/dev.420110407. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=566686. [DOI] [PubMed] [Google Scholar]
- Wittekind CA, Arnold JD, Leslie GI, Luttrell B, Jones MP. Longitudinal study of plasma ACTH and cortisol in very low birth weight infants in the first 8 weeks of life. Early Hum Dev. 1993;33(3):191–200. doi: 10.1016/0378-3782(93)90145-k. http://www.ncbi.nlm.nih.gov/pubmed/8223315. [DOI] [PubMed] [Google Scholar]
- Zennaro MC, Caprio M, Feve B. Mineralocorticoid receptors in the metabolic syndrome. Trends Endocrinol Metab. 2009;20(9):444–51. doi: 10.1016/j.tem.2009.05.006. http://www.ncbi.nlm.nih.gov/pubmed/19800255. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Wang WP, Yang Y, Zhang YL, Pi YL, Tian XY, Jiang XF. Dexamethasone up-regulates the expression of glucocorticoid receptor in growth plate and inhibits the longitudinal growth of bone: experiment with rats. Zhonghua Yi Xue Za Zhi. 2007;87(36):2575–7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18067838. [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21(2 Suppl):52S–60S. doi: 10.1016/S0893-133X(99)00047-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10432489. [DOI] [PubMed] [Google Scholar]