Abstract
Most behaviors have numerous components based on reflexes, but the neural circuits driving most reflexes rarely are documented. The nasotrigeminal reflex induced by stimulating the nasal mucosa causes an apnea, a bradycardia, and variable changes in mean arterial blood pressure (MABP). In this study we tested the nasotrigeminal reflex after transecting the brainstem at the pontomedullary junction. The nasal mucosae of anesthetized rats were stimulated with ammonia vapors and their brainstems then were transected. Complete transections alone induced an increase in resting heart rate (HR; p < 0.001) and MABP (p < 0.001), but no significant change in ventilation. However, the responses to nasal stimulation after transection were similar to those seen prior to transection. HR still dropped significantly (p < 0.001), duration of apnea remained the same, as did changes in MABP. Results from rats whose transection were incomplete are discussed. These data implicate that the neuronal circuitry driving the nasotrigeminal reflex, and indirectly the diving response, is intrinsic to the medulla and spinal cord.
Keywords: diving response, cardiovascular, respiration, heart rate, medulla, SIDS
1. Introduction
The complexity of an animal’s behavior increases according to its place in phylogeny and is paralleled by the complexity of the neural systems driving behavior. Moreover, those behaviors that serve basic vegetative functions are usually less complex and more uniform across species. It therefore seems worthwhile to study those circuits which are the simplest, the most organized, and the most automatic and thus are applicable to a variety of species. These words paraphrase those of neurologist John Huglings Jackson (Jackson, 1884a; Jackson, 1884b), a man who believed in an evolutionary hierarchy within nervous systems. A behavior validating such a statement is the mammalian diving response, a system of at least three independent reflexes that appear to be simply organized and automatic and found in all mammals studied. The somatoautonomic diving response is very powerful and inhibits intrinsic rhythms like respiration and heart rate, as well as basic homeostatic reflex mechanisms such as the chemoreceptor (Panneton et al., 2010a) and baroreceptor (McCulloch et al., 1999; Kobayashi et al., 1999) reflexes.
By definition a reflex is an involuntary and nearly instantaneous movement in response to a stimulus. This then implies there must be a primary afferent neuron initiating the response, motor neurons to realize the response, and if the reflex arc is not monosynaptic, interneurons that lie between these poles. The substrate for “simple” reflex behaviors are thought to be neural circuits located within the brainstem and spinal cord; it is probable that some of these same circuits are influenced by more rostral parts of the brain and are utilized in more complex behaviors. Indeed, these Jacksonian beliefs have promoted numerous studies on pattern generators and muscle synergies in the brainstem and spinal cord for motor behaviors [e.g. see (Grillner et al., 1998; Grillner et al., 2000; Roh et al., 2011)] including those involving respiration (Onimaru and Homma, 1992; Dubayle and Viala, 1996; Onimaru and Homma, 2003; Potts et al., 2005). Although numerous areas within the brain modulate autonomic activity, an orderly functional organization must exist because specific autonomic responses result from a specific stimulus, and these adjustments are appropriate to physiological needs.
The mammalian diving response is called such because it is most prominent in aquatic mammals, such as seals and dolphins, but nevertheless appears in all mammals (Elsner et al., 1966; Elsner and Gooden, 1983), including humans (Goksör et al., 2002). It consists of three independent reflexes inducing: a cessation of breathing (apnea), a dramatic slowing of the heart (bradycardia), and an increase in peripheral vasoconstriction. The purpose of the diving response is to conserve vital oxygen stores in the blood of the organism during submersion, and limits perfusion to the two organs most essential for life, the heart and the brain. Although the peripheral manifestations of the diving response are well-documented (Elsner and Gooden, 1983; Blix and Folkow, 1983; Butler and Jones, 1997), much has yet to be learned of its central neural control. However, neural organization is difficult to study in awake behaving animals and laboratory preparations are commonly used to circumvent many of these problems (Panneton et al., 2010b). Indeed, similar responses of apnea, bradycardia and peripheral vasoconstriction are seen in the laboratory after stimulating the nasal mucosa with irritant vapors (Angell James and de Burgh Daly, 1972; White et al., 1974; McRitchie and White, 1974; White et al., 1975; Gandevia et al., 1978; Peterson et al., 1983; Panneton, 1990; Wallois et al., 1991; Nakamura and Hayashida, 1992; Panneton and Yavari, 1995; Houdi et al., 1995; Gieroba et al., 1995; Yavari et al., 1996; McCulloch and Panneton, 1997; McCulloch et al., 1999; Ho and Kou, 2000; Kratschmer, 2001; Nalivaiko et al., 2003; Mousa et al., 2005; Rybka and McCulloch, 2006; Panneton et al., 2008; Panneton et al., 2010b). If the neuronal circuitry for this nasotrigeminal reflex mimics that of diving, profound advances may be made in deciphering the organization of pathways driving the diving response, the most powerful autonomic response known. Moreover, if these reflex responses are simple, very organized and automatic, they should have neurons residing in the medulla and spinal cord that direct the responses.
The neuronal circuit for the nasotrigeminal reflex, as well as that of the diving response, is contained within the brainstem, since the responses are maintained in decerebrate preparations (White et al., 1975; Martner et al., 1977; Butler and Jones, 1982; Blix and Folkow, 1983; Elsner and Gooden, 1983; Butler and Jones, 1997; Panneton et al., 2010b). However transection at the level of the colliculi as done in these studies spares several pontine structures modulating respiration, such as the pneumotaxic area in the dorsolateral pons, the intertrigeminal area lateral to the trigeminal motor nucleus, and the apneustic area more caudally and ventrally. The brainstem was transected at more caudal levels in a few early studies (Lumsden, 1923a; Lumsden, 1923b; Ondina et al., 1960; Radulovacki et al., 2003) to determine loci where neurons drive respiration, but the maintenance of reflex circuitry driving behaviors such as the nasotrigeminal reflex was not investigated. Since the responses to stimulation of the nasal mucosa are similar to those of underwater submersion (Panneton et al., 2010b), we elected to stimulate the nasal mucosa of an anesthetized rat with ammonia vapors to induce an apnea, bradycardia and peripheral vasoconstriction in rats transected at the pontomedullary junction. Here we show that the cardiorespiratory changes to nasal stimulation are maintained despite such transection, suggesting the neuronal circuits driving the nasotrigeminal reflex lie within the medulla and spinal cord. It also implies that circuits defining the diving response are maintained at similar levels. Defining the neural circuit for the diving response may open broad avenues of understanding the mechanisms of suprabulbar control of autonomic function in general. This data has been published previously in abstract form (Panneton and Sun, 2002).
2. Materials and Methods
Thirteen adult (~275- 325 g) Sprague-Dawley male rats were obtained commercially (Harlan, Indianapolis, IN) and used in this study. All protocols were approved by the Animal Care Committee of Saint Louis University and followed the guidelines of the National Institutes of Health Guide for Care and Handling of Laboratory Animals.
The rats initially were anesthetized with injections (0.1ml/kg) of urethane (1000mg/ml) IP and prepared for surgery. Cannulae were inserted into the femoral artery and vein for recording blood pressure and the administration of drugs, respectively. Anesthesia was maintained prior to brainstem transection with urethane (IV; 100 mg/kg/hr) until responses to tail pinch and the blink reflex were absent. The trachea was transected and a polyethylene tube inserted toward the lungs for respiration while another passed through the nasopharynx to the choanae. A gentle suction was applied to the tracheal end of the nasopharyngeal tube for pulling ammonia vapors over the nasal mucosa for stimulation. Arterial blood pressure was recorded with a Gould P23 strain gauge transducer and amplified (Grass 7P122). Heart rate (HR) was determined by counting systolic peaks on the arterial pressure trace. Respiration was monitored with a low-pressure volumetric transducer (Grass PT5) and amplified (Grass 7P122) via a Y-shaped connector. Signals for respirations, heart rate and arterial blood pressure were passed through an A/D interface (1401 plus; Cambridge Electronic Design, Cambridge, UK) and stored in the computer for later analysis (Spike 2; Cambridge Electronic Design). The animals then were placed in a stereotaxic device (Kopf Instruments); interaural zero served as our zero point reference.
The brainstem was exposed via a dorsal incision and the posterior calvarium removed. After baseline parameters were determined, the vapors emanating from a cotton ball soaked in a 50% solution of ammonia hydroxide induced the nasotrigeminal reflex by gently pulling the vapors over the nasal mucosa for 5 sec. Three trials were done with approximately 5 min separating each trial. After each trial, residual ammonia vapors were removed immediately from the nasal cavity by continued suction through the choanae. The medial two-thirds of the brainstems then were transected stereotaxically with a blunted brass knife (3mm wide) at the rostral pole of the facial nucleus. The ventral third of the spinal trigeminal tract was spared bilaterally by raising the knife approximately 1.5mm above horizontal zero before completing the transection laterally. The cutting edge of the knife was polished but relatively blunt, so that major vessels (e.g., the basilar artery) were only compressed rather than severed which circumvented massive bleeding. New measurements of baseline respirations, heart rate and arterial blood pressure then were made followed by three more 5 sec stimulations every 5 min using ammonia vapors.
The rats then were perfused through the heart using first with 200ml of saline, followed immediately by fixative of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were extirpated and stored in the fixative solution with 20% sucrose overnight at 4°C. Frozen horizontal sections were cut (40µm) through the brainstem on a freezing microtome, serially collected in PB, mounted onto gelled slides, air dried, stained for Nissl with Neutral Red and coverslipped. Photomicrographs of sections were taken through a microscope (Nikon E800) equipped with a digital camera (MicroImager II), processed and saved with Northern Eclipse software (Empix), standardized in Adobe Photoshop (v. 9.0) using levels, brightness and contrast, and formatted using Adobe Illustrator CS2 software (v. 12.0).
Brains were analyzed qualitatively to determine the completeness of the transections. Transections were considered complete (n=6) if the ventral surface of the brain was cut over its middle two-thirds while incomplete transections (n=7) had parts of their ventral surfaces spared. Mean arterial blood pressure (MABP), heart rate (HR) and respirations were calculated in each animal for three trials for 5 sec periods either immediately before (Control data) or during (Experimental data) the nasal stimulus and averaged. A grand mean was then determined on the peak changes of MABP and HR induced by nasal stimulation in each group of animals. The duration of apnea induced by nasal stimulation was calculated from stimulus onset. Data are presented as M±S.E. and experimental data compared statistically (SPSS v.13.0 software) to control data using the Independent-Samples T-test. All analyses considered p < 0.05 as significant. Graphs were drawn with GraphPad Prism software and presented as box plots. Box plots present a vertical view of the data and show the shape of its distribution, its central value, and its spread. The box itself represents 50% of the data, 75th percentile marks the top of the box, the 25th percentile marks the bottom, while the median (50th percentile) is shown as a line through the box. Whiskers show the most extreme (maximum and minimum) values in the data set and extend a maximum of 1.5 times the range in the box.
3. Results
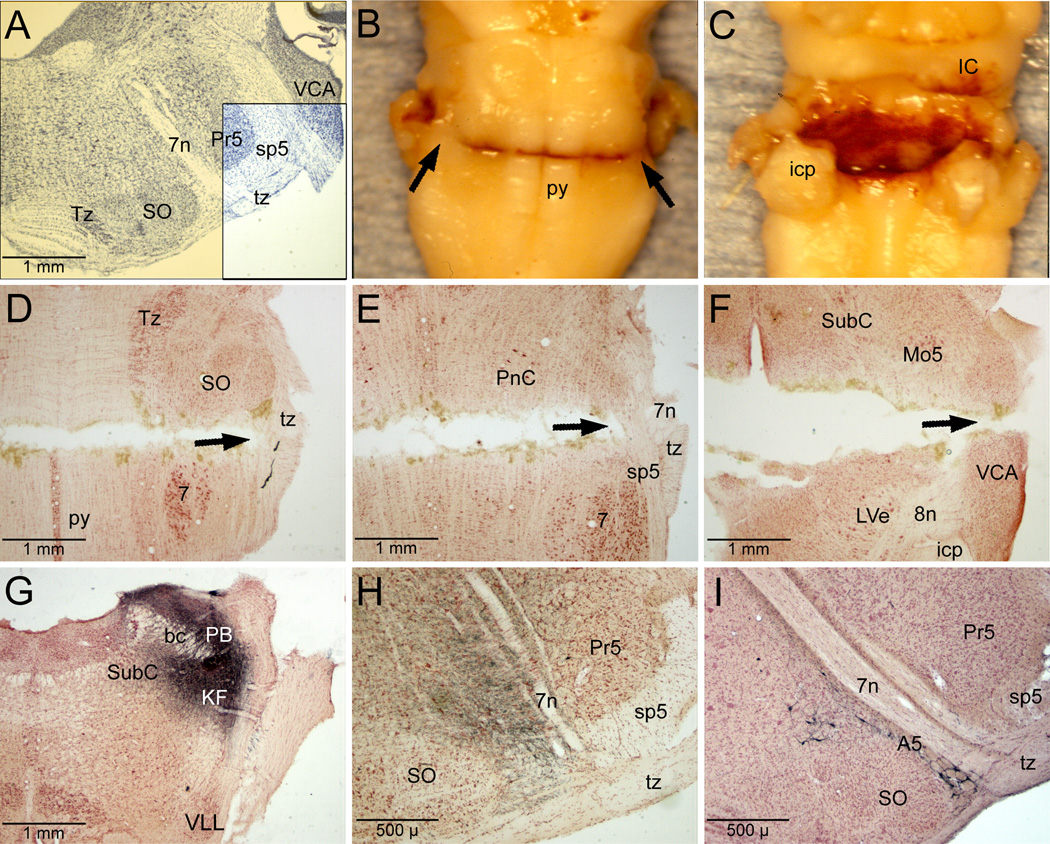
Six brains showed complete transection of the brainstem (shaded area; Fig. 1A) at the level of the trapezoid body (analogous to the acoustic striae) but sparing the ventral third of the spinal trigeminal tract (Fig. 1A, box; 1B, arrows). We considered seven brains to be incompletely transected, usually because the ventral surface was partially unscarred. Bleeding was minimized (Fig. 1C) by the design of the blade while physiologically all animals tolerated the transection apparently well since HR remained brisk (before: 295±11bpm to after: 378±18 bpm) and MABP was maintained (before: 73±4 mmHg to after: 95±7 mmHg), albeit at higher levels. Observations of the horizontal histological sections showed complete transection to the lateral edge of the superior olivary nucleus most ventrally (Fig. 1D, arrow), to the medial edge of the ventral spinal trigeminal tract through mid levels of the facial nucleus (Fig. 1E, arrow), and complete transection at the ventral part of the trigeminal motor nucleus, approximately 1.5mm dorsal to the horizontal zero (Fig. 1F, arrow). Such cuts also transected fibers descending from the peribrachial area (Fig. 1H) mediating cardiorespiratory behavior, especially those in the external lateral and external medial subnuclei of the parabrachial nucleus as well as the Kölliker-Füse nucleus (Fig. 1G). Although descending projections from rostral parts of the A5 catecholaminergic group (Fig. 1J) were transected, more caudal A5 neurons were spared.
Figure 1.
Photomicrographs of the rat brainstem illustrating the pontomedullary transections and some presumptive pathways destroyed. The brainstem was cut near the pontomedullary junction (A, shaded area) through the trapezoid body but spared the ventral third of the spinal trigeminal tract (A, unshaded boxed area), allowing primary afferent fibers innervating paranasal areas to proceed caudally. Only brains with a complete transection of their ventral surface were considered transected (B); note the spared ventrolateral corners with their spinal trigeminal tracts beneath (B, arrows). A dorsal view of a transected brainstem (C) shows the absence of major blood clots, since the tool used spared major blood vessels. Horizontal sections through a transected brainstem shows complete transection ventrally between the caudal superior olivary nucleus and the rostral the facial nucleus most ventrally (D), approximately 800µm dorsal to horizontal zero (E), and 1.5mm dorsal to horizontal zero (F). Arrow in D shows the spared trapezoid body, the arrow in E shows the spared spinal trigeminal tract, while that in F shows the complete transection. Such transections destroyed descending fibers originating in the dorsolateral pons (an injection of biotinylated dextran amine into cardiorespiratory areas of the peribrachial complex is shown in G). Labeled fibers form this injection descended medial to the exiting fibers of the seventh nerve (H); these fibers were transected. Immunostained sections with antibodies to tyrosine hydroxylase suggest that rostral neurons of the catecholamine A5 cell group were also destroyed (I), but more caudally placed A5 neurons probably survived. Abbreviations: A5, A5 catecholamine neuron group; KF, Kölliker-Füse nucleus; LVe, lateral vestibular nucleus; Mo5, motor trigeminal nucleus; PB, parabrachial complex; PnC, pontine reticular nucleus, pars caudalis; Pr5, principal sensory trigeminal nucleus; SO, superior olivary nucleus; SubC, subcoeruleus nucleus; Tz, nucleus of the trapezoid body; VCA, ventral cochlear nucleus, pars anterior; VLL, ventral nucleus of the lateral lemniscus; bc, brachium conjunctivum; icp, inferior cerebellar peduncle; py, pyramidal tract; sp5, spinal trigeminal tract; tz, trapezoid body; 7n, facial nerve; 8n, vestibular nerve.
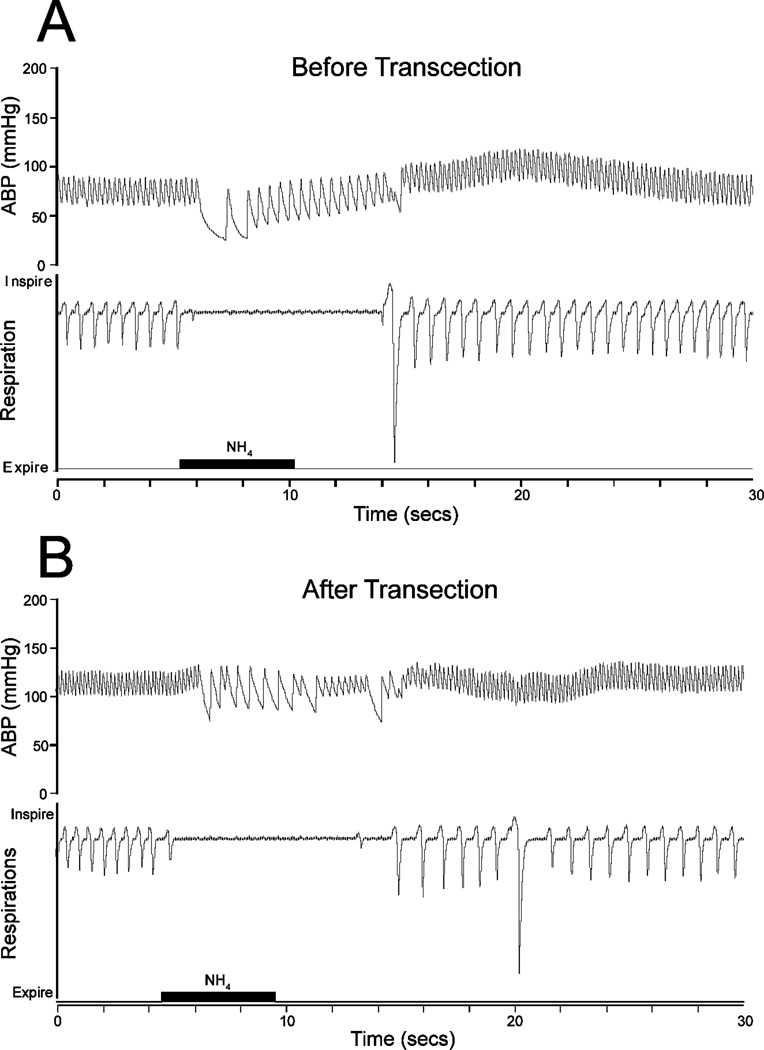
All animals tested showed an abrupt apnea and bradycardia but no change in MABP to nasal stimulation with ammonia vapors prior to transection (Fig. 2A). HR of all rats (n=13) prior to transection was 295±11bpm and MABP was 73±4 mmHg while responses to nasal stimulation induced a significant bradycardia bringing HR to 135±10 bpm and MABP to 73±7 mmHg. Normal respirations of rats prior to transection and categorized in the complete transection group were (108±2 breaths/min).
Figure 2.
Tracings illustrating the cardiorespiratory responses to nasal stimulation with ammonia vapors (black bar) for 5 sec both before (A) and after (B) pontomedullary transection. Note that normal respirations were not altered by such transections, that the apnea and bradycardia induced were similar before and after transection, but resting arterial blood pressure (ABP) was elevated by the cut.
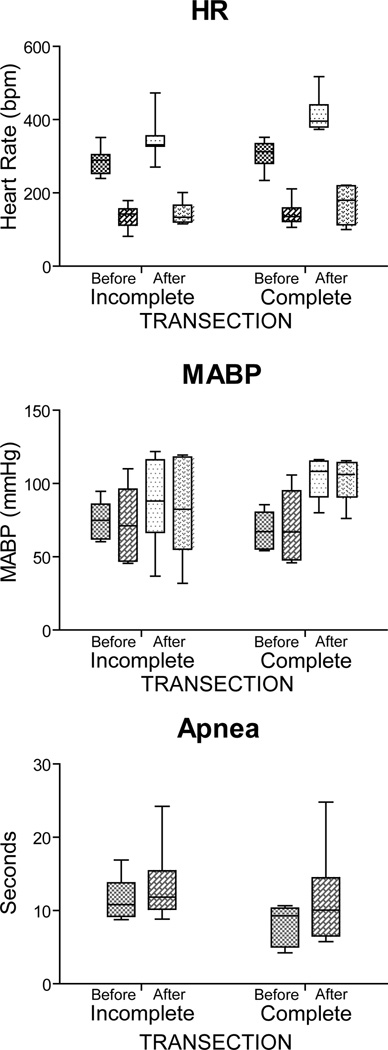
There were no differences in control data of HR or MABP when that from the incomplete or complete groups were compared (Before; Figure 3A, 3B). Prior to transection, rats in the incomplete transection category showed mean HR dropping 55% (p < 0.001) from 286±14 bpm to 129±15 bpm with nasal stimulation (Before, Incomplete; Figure 3A), MABP fell insignificantly (76±5 mmHg to 73±10mm Hg; Before, Incomplete; Figure 3B), and the duration of apnea was 12±1 sec (Fig. 3C). Prior to transection, rats in the complete transection category had HR fall 54% (p < 0.001) from 305±17 bpm to 142±15 bpm with nasal stimulation (Before, Complete; Figure 3A), MABP rose insignificantly from 69±5 mmHg to 72±10 mm Hg and the duration of apnea was 8±1 sec.
Figure 3.
Box plots illustrating the changes induced in heart rate, mean arterial blood pressure, and apnea after stimulating the nasal mucosa of rats with ammonia vapors both before and after transecting the brainstem at the pontomedullary junction. Transections judged Incomplete (n=7) are shown on the left while those judged Complete (n=6) are on the right. Mean bradycardia induced by nasal stimulation was similar despite degree of transection, but resting heart rate increased after transection, especially complete transections. Mean arterial blood pressure stayed relatively the same after ammonia stimulation, but resting pressures were increased after complete transections. The mean time for apnea induced by nasal stimulation was unchanged with transections, but became more variable. Lighter shades of boxes represent control data prior to nasal stimulation while darker shades represent cardiorespiratory consequences during nasal stimulation with ammonia vapors. See text for levels of significance.
The brainstems of the rats were then transected and three trials of nasal stimulation done again (Fig. 2B). Comparing control HR data before and after transection in the incompletely transected rats now showed that resting HR was significantly different (p < 0.001) from that prior to transection but MABP was not (p=0.064). Moreover, comparison of control HR before and after transection in the complete transection group also was significantly different (p < 0.001), as was MABP (p < 0.001), but resting respiration after transection remained at 105±2 breaths/min, no different from that prior to transection. Thus transections alone induced significant differences in resting HR in both transection categories, and MABP in the complete transection category, and no changes in resting respiration.
Comparing experimental data after nasal stimulation showed that HR dropped significantly (p < 0.001) to a level similar in both the complete and incomplete transection groups. Although the level of resting MABP after complete transection was significantly different than that seen prior to transection, differences after nasal stimulation were insignificant compared to resting levels of the transected rats. There were no significant changes in the length of apnea induced by either complete or incomplete transection but the apnea after transection was more variable, with apnea especially becoming longer.
4. Discussion
This studied showed that transections judged complete through the brainstem at the pontomedullary junction induced resting HR and MABP to rise significantly, but did not disrupt the rhythm of normal respiration similar to that seen by others (Ondina et al., 1960; Radulovacki et al., 2003). Moreover, the bradycardia and apnea induced by nasal stimulation with ammonia vapors persisted after transections through the pontomedullary junction, suggesting that the neural circuitry mediating these responses of the nasotrigeminal reflex is intrinsic to the medulla and spinal cord. This data supports that of others (Onimaru and Homma, 1992; Dubayle and Viala, 1996; Grillner et al., 1998; Grillner et al., 2000; Onimaru and Homma, 2003; Potts et al., 2005; Roh et al., 2011) showing that reflexes, muscle synergies or pattern generators are maintained in nervous systems comprised of only a medulla and spinal cord. It also supports the idea that behaviors that serve less complex basic vegetative functions are the simplest, the most organized, and the most automatic and are maintained in lower levels of the neuraxis.
4.1 Technical Considerations
Our qualitative assessment of the completeness of the transections could be questioned, but our assessment was verified quantitatively when data on the cardiorespiratory responses of those transections deemed incomplete versus complete were compared. For example, comparisons of HR in brains deemed incomplete transections versus complete transections were significantly different (p= 0.002) while comparison of MABP between these groups was also significant (p= 0.02). This may have been due to incomplete transection of fibers descending from the rostral part of the A5 group. Although both the cardiovascular (see discussion in Maiorov et al., 1999) and respiratory (Guyenet et al., 1993; Viemari et al., 2004; Hilaire et al., 2004; Li et al., 2008) function of the A5 group of catecholamine neurons has been debated for many years, the experiments presented herein however offer little new information towards solving this problem. A role of the peribrachial region in cardiorespiratory regulation is well-documented (Chamberlin, 2004; Alheid et al., 2004) and that of the intertrigeminal region in apneic reflexes noted (Chamberlin and Saper, 1998; Radulovacki et al., 2003; Radulovacki et al., 2004; Topchiy et al., 2009), but the influence of these areas over more caudal respiratory networks are negated since no fibers from neurons in these areas project through the ventral trigeminal complex, the only intact neuropil remaining after our transections (see Fig. 1H). Nonetheless, our designation of transection categories was supported with significant differences between these transections in resting HR and in resting MABP after complete transections, while resting respiration was maintained similar to other studies (Ondina et al., 1960; Radulovacki et al., 2003).
These rats were both anesthetized and decerebrated making comparisons to voluntary underwater submersion troublesome (Panneton et al., 2010b). Indeed either anesthesia in general (Elsner et al., 1966; Tchobroutsky et al., 1969; Whyane et al., 1971; Panneton et al., 2010b) or the anesthetic used (Doyle et al., 1988; Nakamura and Hayashida, 1992) induces variable degrees of attenuation of the diving response. Nevertheless, all rats showed significant bradycardia and apnea to stimulation of their nasal mucosa, but the responses were more variable than in voluntary submersion, supporting our previous study (Panneton et al., 2010b).
4.2 Rationale of Transection
We spared the ventral third of the spinal trigeminal tract where primary afferent fibers of the infraorbital (Panneton et al., 2010) and anterior ethmoidal (Panneton et al., 2006) nerves travel. Branches of the infraorbital nerve innervate the ala of the nose and upper lip while the anterior ethmoidal nerve (AEN) innervates the nasal vestibule and anterior-superior parts of the nasal mucosa (Panneton et al., 2006). For example stimulating these nerves electrically (Wallois et al., 1992; Dutschmann and Herbert, 1997; Dutschmann and Herbert, 1998; McCulloch et al., 1999; Dutschmann and Paton, 2002) induce cardiorespiratory behaviors similar to underwater submersion, while either cutting the AEN or anesthetizing paranasal areas/nasal mucosa eliminates the bradycardia (Angell James and de Burgh Daly, 1972; Dykes, 1974; Drummond and Jones, 1979; Yavari et al., 1996; Ho and Kou, 2000; Rybka and McCulloch, 2006). Nasal primary afferent fibers are important not only for the nasotrigeminal reflex, but also for initiating the diving response since snout immersion induces similar responses (Koppányi and Dooley, 1929; Whishaw and Schallert, 1977; Drummond and Jones, 1979; Schagatay and Van Kampen, 1995; Panneton et al., 2010b) The infraorbital nerve and the AEN project centrally to the whole trigeminal sensory complex (Panneton, 1991a; Panneton et al., 2006; Panneton et al., 2010) and most of their fibers descend in the ventral third of the spinal trigeminal tract towards caudal targets. We retained these fibers to prove that the nasotrigeminal reflex utilizes caudal medullary and spinal circuits. The present study suggests the more rostral projections of the AEN to the parabrachial complex (Panneton, 1991a; Panneton et al., 2006) and projections to the principal trigeminal nucleus from either nerve only modulate the responses since the bradycardia and apnea induced by nasal stimulation are blocked with small injections of either lidocaine or kyurenate into ventral parts of the medullary dorsal horn (Panneton and Yavari, 1995).
4.3 Responses to nasal stimulation
Transections at the pontomedullary junction induced both resting HR and MABP to rise significantly (p < 0.001) after complete transections. Nonetheless, the bradycardia induced always reached relatively similar nadirs, whether it was in anesthetized non-transected, partially-transected, or completely-transected rats. We noted similar responses in awake behaving rats during either voluntary or involuntary submersion (Panneton et al., 2010a; Panneton et al., 2010b) where heart rate dropped approximately 80% during submersion in 100% of the rats, 100% of the time. This suggests that the cardiac motor neurons modulating the bradycardia during nasal stimulation are influenced by lower brainstem circuits with little integration, possibly directly by neurons in the medullary dorsal horn (Panneton, 1991b; Panneton and Yavari, 1995; Panneton et al., 1996; Panneton et al., 2007).
All rats showed consistent bradycardia and apnea proving that the circuitry for this nasotrigeminal reflex is intrinsic to the medulla and spinal cord. If these autonomic adjustments use the same circuits as the cardiorespiratory changes to underwater submersion, e.g., the diving response, it implies that circuits driving diving behavior are also at medullary levels and caudal. However, the rise in MABP after complete transection conforms to the ideas of Jackson that more rostral (e.g., suprabulbar) parts of the brain generally modulate lower circuits and generally are inhibitory. Moreover, while the mean times for apnea were similar both before and after transection, apnea was more variable after transection. This supports the influence of suprabulbar structures, perhaps in the peribrachial complex, in modulating respiration.
4.4 Perspectives
Motor programs serving basic vegetative functions such as heart rate and respiration are hardwired genetically and reflexly initiated. Their output follows the synaptic organization of the medulla and spinal cord and has been molded through evolution. Indeed, some reflexive and rhythmic activities are demonstrated in anencephalic infants who possess only a brainstem and spinal cord. Many fetal and neonatal reflexes such as the rooting, Moro (startle) or Babinski reflexes appear near birth but are transitory, disappearing within 12–18 months as the child matures. Others such as the knee-jerk reflex, pharyngeal (gag) reflex, or blink reflex persist through adulthood. The maturing forebrain influences brainstem reflexes however; a child who harnesses his blink reflex and ‘winks’ volitionally usually is a celebratory moment for parents.
Perhaps of great conceptual interest to some is that many naturally-diving mammals can induce the diving response by a conscious act of will (Elsner and Gooden, 1983), apparently adjusting their autonomic nervous system volitionally with higher brain areas. Jacksonian adherents believe that higher levels of the brain (suprabulbar, cortical) usually control function through brainstem and spinal circuits. The data presented herein show that neural circuits driving the nasotrigeminal reflex, and perhaps the diving response, are contained within the medulla and spinal cord. It is of interest that Leiter and Böhm (2007) compiled data showing that ‘rebreathing asphyxial gases and reduced heat loss’ and modulation of ‘adult and fetal cardiac and respiratory reflexes’ are common risk factors for Sudden Infant Death Syndrome (SIDS). Moreover, Leiter and Böhm (Leiter and Böhm, 2007) proposed that the etiology of SIDS may be the activation of a persistent fetal reflex similar to the diving response. Defining the brainstem circuit for the nasotrigeminal reflex, and indirectly the powerful mammalian diving response, may open broad avenues of understanding for the mechanisms of suprabulbar control of autonomic function in general.
Highlights.
Nasotrigeminal reflex is maintained in medulla and spinal cord
Nasotrigeminal reflex is similar to the diving response
Nasotrigeminal reflex and diving response relationship to SIDS
Acknowledgements
This work was supported by NIH grant HL64772 to WMP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Resp. Physiol. Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Angell James JE, de Burgh Daly M. Reflex respiratory and cardiovascular effects of stimulation of receptors in the nose of the dog. J. Physiol. (Lond.) 1972;220:673–696. doi: 10.1113/jphysiol.1972.sp009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix AS, Folkow B. Cardiovascular adjustments to diving in mammals and birds. In: Sheperd JT, Abboud FM, editors. Handbook of Physiology - The Cardiovascular System. Bethesda, MD: American Physiological Society; 1983. pp. 917–945. [Google Scholar]
- Butler PJ, Jones DR. The comparative physiology of diving in vertebrates. Adv. Comp. Physiol. Biochem. 1982;8:179–364. doi: 10.1016/b978-0-12-011508-2.50012-5. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir. Physiol. Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. A brainstem network mediating apneic reflexes in the rat. J. Neurosci. 1998;18:6048–6056. doi: 10.1523/JNEUROSCI.18-15-06048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle RE, Panneton WM, Vogler GA, Romeo JP, Watson BJ, Higgins B. The muskrat in biomedical research. J. Lab. Anim. Sci. 1988;38:667–674. [PubMed] [Google Scholar]
- Drummond PC, Jones DR. The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J. Physiol. (Lond.) 1979;290:253–271. doi: 10.1113/jphysiol.1979.sp012770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubayle D, Viala D. Interactions between medullary and spinal respiratory rhythm generators in the in vitro brainstem spinal cord preparation from newborn rats. Exp. Brain Res. 1996;109:1–8. doi: 10.1007/BF00228620. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. Fos expression in the rat parabrachial and Kölliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Exp. Brain Res. 1997;117:97–110. doi: 10.1007/s002210050203. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The medial nucleus of the solitary tract mediates the trigeminally evoked pressor response. Neuroreport. 1998;9:1053–1057. [PubMed] [Google Scholar]
- Dutschmann M, Paton JFR. Influence of nasotrigeminal afferents on medullary respiratory neurones and upper airway patency in the rat. Pflugers Arch. 2002;444:227–235. doi: 10.1007/s00424-002-0797-x. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can. J. Physiol. Pharmacol. 1974;52:259–265. doi: 10.1139/y74-035. [DOI] [PubMed] [Google Scholar]
- Elsner R, Franklin DL, Van Citters RL, Kenney DW. Cardiovascular defense against asphyxia. Science. 1966;153:941–949. doi: 10.1126/science.153.3739.941. [DOI] [PubMed] [Google Scholar]
- Elsner R, Gooden B. Diving and asphyxia: A comparative study of animals and man. New York: Cambridge University Press; 1983. pp. 1–168. [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J. Physiol. (Lond.) 1978;276:383–394. doi: 10.1113/jphysiol.1978.sp012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieroba ZJ, MacKenzie L, Willoughby JO, Blessing WW. Fos-determined distribution of neurons activated during the Bezold-Jarisch reflex in the medulla oblongata in conscious rabbits and rats. Brain Res. 1995;683:43–50. doi: 10.1016/0006-8993(95)00320-p. [DOI] [PubMed] [Google Scholar]
- Goksör E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr. 2002;91:307–312. doi: 10.1080/08035250252833978. [DOI] [PubMed] [Google Scholar]
- Grillner S, Cangiano L, Hu GY, Thompson R, Hill R, Wallen P. The intrinsic function of a motor system - from ion channels to networks and behavior. Brain Res. 2000;886:224–236. doi: 10.1016/s0006-8993(00)03088-2. [DOI] [PubMed] [Google Scholar]
- Grillner S, Ekeberg OE, El Manira A, Lansner A, Parker D, Tegner J, Wallen P. Intrinsic function of a neuronal network - a vertebrate central pattern generator. Brain Res. Rev. 1998;26:184–197. doi: 10.1016/s0165-0173(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJM, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am. J. Physiol. 1993;264:R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari J-C, Coulon P, Simmonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Resp. Physiol. Neurobiol. 2004;279:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Ho CY, Kou YR. Protective and defensive airway reflexes evoked by nasal exposure to wood smoke in anesthetized rats. J. Appl. Physiol. 2000;88:863–870. doi: 10.1152/jappl.2000.88.3.863. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Dowell RT, Diana JN. Cardiovascular responses to cigarette smoke expsed to restrained conscious rats. J. Pharmacol. Exp. Ther. 1995;275:646–653. [PubMed] [Google Scholar]
- Jackson J. Evolution and dissolution of the nervous system. Lecture 1. Br. Med. J. 1884a;1:591–593. doi: 10.1136/bmj.1.1213.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. Evolution and dissolution of the nervous system. Lecture 3. Br. Med. J. 1884b;1:703–707. doi: 10.1136/bmj.1.1215.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Cheng ZB, Nosaka S. Inhibition of baroreflex vagal bradycardia by nasal stimulation in rats. Am. J. Physiol. 1999;276:H176–H184. doi: 10.1152/ajpheart.1999.276.1.H176. [DOI] [PubMed] [Google Scholar]
- Koppányi T, Dooley MS. Submergence and postural apnea in the muskrat. Am. J. Physiol. 1929;88:592–595. [Google Scholar]
- Kratschmer F. On reflexes from the nasal mucous membrane on respiration and circulation. Respir. Physiol. 2001;127:93–104. doi: 10.1016/s0034-5687(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. In: Poulin MJ, Wilson RJA, editors. Integration in Respiratory Control: From Genes to Systems. Springer Press; 2008. pp. 371–376. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J. Physiol. (Lond.) 1923a;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden T. The regulation of respiration. J. Physiol. (Lond.) 1923b;58:81–91. doi: 10.1113/jphysiol.1923.sp002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorov DN, Wilton ER, Badoer E, Petrie D, Head GA, Malpas SC. Sympathetic response to stimulation of the pontine A5 region in conscious rabbits. Brain Res. 1999;815:227–236. doi: 10.1016/s0006-8993(98)01150-0. [DOI] [PubMed] [Google Scholar]
- Martner J, Wadenvik H, Lisander B. Apnoea and bradycardia from submersion in "chronically" decerebrated cats. Acta Physiol. Scand. 1977;101:476–480. doi: 10.1111/j.1748-1716.1977.tb06031.x. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Faber KM, Panneton WM. Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res. 1999;830:24–31. doi: 10.1016/s0006-8993(99)01374-8. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM. Fos immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience. 1997;78:913–925. doi: 10.1016/s0306-4522(96)00633-1. [DOI] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM, Guyenet PG. The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the nasal mucosa. J. Physiol. (Lond.) 1999;516:471–484. doi: 10.1111/j.1469-7793.1999.0471v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie RJ, White SW. Role of trigeminal olfactory, carotid sinus and aortic nerves in the respiratory and circulatory response to nasal inhalation of cigarette smoke and other irritants in the rabbit. Aust. J. Exp. Biol. Med. Sci. 1974;52:127–140. doi: 10.1038/icb.1974.10. [DOI] [PubMed] [Google Scholar]
- Mousa TM, Gao L, Cornish KG, Zucker IH. Effects of angiotensin II on autonomic components of nasopharyngeal stimulation in male conscious rabbits. J. Appl. Physiol. 2005;98:1607–1611. doi: 10.1152/japplphysiol.01322.2004. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hayashida Y. Autonomic cardiovascular responses to smoke exposure in conscious rats. Am. J. Physiol. 1992;262:R738–R745. doi: 10.1152/ajpregu.1992.262.5.R738. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, De Pasquale CG, Blessing WW. Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: vago-sympathetic co-activation. Auton. Neurosci. 2003;105:101–104. doi: 10.1016/S1566-0702(03)00048-1. [DOI] [PubMed] [Google Scholar]
- Ondina DM, Yamamoto WS, Masland WS. Respiratory centers in the albino rat. Am J. Physiol. 1960;198:389–392. doi: 10.1152/ajplegacy.1960.198.2.389. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Arch. 1992;420:399–406. doi: 10.1007/BF00374476. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM. Controlled bradycardia induced by nasal stimulation in the muskrat, Ondatra zibethicus. J. Auton. Nerv. Syst. 1990;30:253–264. doi: 10.1016/0165-1838(90)90257-j. [DOI] [PubMed] [Google Scholar]
- Panneton WM. Primary afferent projections from the upper respiratory tract in the muskrat. J. Comp. Neurol. 1991a;308:51–65. doi: 10.1002/cne.903080106. [DOI] [PubMed] [Google Scholar]
- Panneton WM. Trigeminal mediation of the diving response in the muskrat. Brain Res. 1991b;560:321–325. doi: 10.1016/0006-8993(91)91251-u. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Anch MA, Gan Q. Topography of preganglionic parasympathetic cardiac motor neurons labeled after underwater submersion. FASEB J. 2007:21. [Google Scholar]
- Panneton WM, Gan Q, Juric R. Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience. 2006;141:889–906. doi: 10.1016/j.neuroscience.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. Cardiorespiratory and neural consequences of rats brought past their aerobic dive limit. J. Appl. Physiol. 2010a;109:1256–1269. doi: 10.1152/japplphysiol.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Juric R. The rat: a laboratory model for studies of the diving response. J. Appl. Physiol. 2010b;108:811–820. doi: 10.1152/japplphysiol.00600.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Sun W. Pressor responses to nasal stimulation are unaltered after disrupting the caudalmost ventrolateral medulla. Auto. Neurosci. 2008;144:13–21. doi: 10.1016/j.autneu.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp. Eye Res. 2010;90:388–396. doi: 10.1016/j.exer.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, McCulloch PF, Tan Y, Tan YX, Yavari P. Brainstem origin of preganglionic cardiac motoneurons in the muskrat. Brain Res. 1996;738:342–346. doi: 10.1016/s0006-8993(96)01048-7. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Sun W. Cardiorespiratory changes after nasal stimulation persist after partial pontine transection. Neurosci. 2002 Abstr. 27. [Google Scholar]
- Panneton WM, Yavari P. A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus: Evidence for excitatory amino acid transmission. Brain Res. 1995;691:37–45. doi: 10.1016/0006-8993(95)00597-j. [DOI] [PubMed] [Google Scholar]
- Peterson DF, Coote JH, Gilbey MP, Futuro-Neto HA. Differential pattern of sympathetic outflow during upper airway stimulation with smoke. Am. J. Physiol. 1983;245:R433–R437. doi: 10.1152/ajpregu.1983.245.3.R433. [DOI] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J. Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki M, Pavlovic S, Carley DW. Pontine intertrigeminal region attenuates sleep apneas in rats. Sleep. 2004;27:383–387. doi: 10.1093/sleep/27.3.383. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Pavlovic S, Saponjic J, Carley DW. Intertrigeminal region attenuates reflex apnea and stabilizes respiratory pattern in rats. Brain Res. 2003;975:66–72. doi: 10.1016/s0006-8993(03)02587-3. [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VCK, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J. Neurophysiol. 2011;106:1363–1378. doi: 10.1152/jn.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res. 2006;1075:122–132. doi: 10.1016/j.brainres.2005.12.112. [DOI] [PubMed] [Google Scholar]
- Schagatay E, Van Kampen M. Apneic snout immersion in trained pigs elicits a "diving response". Adv. Exp. Med. Biol. 1995;393:73–76. [PubMed] [Google Scholar]
- Tchobroutsky C, Merlet C, Rey P. The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Resp. Physiol. 1969;8:108–117. doi: 10.1016/0034-5687(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Topchiy I, Radulovacki M, Waxman J, Carley DW. Cardiorespiratory effects of intertrigeminal area stimulation in vagotomized rats. Brain Res. 2009;1250:120–129. doi: 10.1016/j.brainres.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Bevengut M, Coulon P, Hilaire G. Nasal trigeminal inputs release A5 inhibition received by the respiratory rhythm generator of the mouse neonate. J. Neurophysiol. 2004;91:746–758. doi: 10.1152/jn.01153.2002. [DOI] [PubMed] [Google Scholar]
- Wallois F, Macron JM, Jounieaux V, Duron B. Trigeminal nasal receptors related to respiration and to various stimuli in cats. Resp. Physiol. 1991;85:111–125. doi: 10.1016/0034-5687(91)90010-g. [DOI] [PubMed] [Google Scholar]
- Wallois F, Macron JM, Jounieaux V, Duron B. Influence of trigeminal nasal afferents on bulbar respiratory neuronal activity. Brain Res. 1992;599:105–116. doi: 10.1016/0006-8993(92)90857-6. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Schallert T. Hippocampal RSA (theta), apnea, bradycardia, and effects of atropine during underwater swimming in the rat. Electroenceph. Clin. Neurophysiol. 1977;42:389–396. doi: 10.1016/0013-4694(77)90175-4. [DOI] [PubMed] [Google Scholar]
- White S, McRitchie RJ, Korner PI. Central nervous system control of cardiorespiratory nasopharyngeal reflexes in the rabbit. Am J. Physiol. 1975;228:404–409. doi: 10.1152/ajplegacy.1975.228.2.404. [DOI] [PubMed] [Google Scholar]
- White SW, McRitchie RJ, Franklin DL. Autonomic cardiovascular effects of nasal inhalation of cigarette smoke in the rabbit. Aust. J. Exp. Biol. Med. Sci. 1974;52:111–126. doi: 10.1038/icb.1974.9. [DOI] [PubMed] [Google Scholar]
- Whyane TF, Smith NT, Eger EI, Stoelting RK, Whitcher CE. The effects of halothane anesthesia on reflex cardiovascular responses to simulated diving and the Valsalva maneuver. Anesthesiology. 1971;34:262–270. doi: 10.1097/00000542-197103000-00014. [DOI] [PubMed] [Google Scholar]
- Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J. Auton. Nerv. Syst. 1996;61:195–200. doi: 10.1016/s0165-1838(96)00072-0. [DOI] [PubMed] [Google Scholar]