Abstract
Tissue-based microsatellite instability analysis and immunohistochemistry for DNA mismatch repair proteins are accepted screening tools to evaluate cancer patients for Lynch Syndrome. These laboratory analyses are thus important tools in cancer prevention. Quality assurance review was performed to identify test discordances and problems. These results were then analyzed in conjunction with genetic testing outcomes. 646 consecutive tumors from 2002 to 2010 were examined. Microsatellite instability-low tumors were excluded so that 591 tumors comprised the final analyses. Discordance was defined as a discrepancy between immunohistochemistry and microsatellite instability analysis. Problem was defined as indeterminate or questionable immunohistochemistry or microsatellite instability results. All results and clinical and family histories were centrally reviewed by 2 pathologists and 1 genetics counselor. Discordances and problems were identified in 23/591 (3.9%) of the tumors. Twelve of 102 microsatellite instability-high carcinomas (11.8%) and one of 489 microsatellite stable tumors had discordant immunohistochemistry. Of these 13 tumors, 11 were from patients who had personal and/or family cancer histories concerning for a germline mismatch repair gene mutation. In addition to discordances, ten tumors with problematic immunohistochemistry profiles were identified. Accurate evaluation of microsatellite instability was possible in all tumors. In summary, concordance between immunohistochemistry and microsatellite instability was high, particularly for tumors that are microsatellite stable. Greater frequency of test discordance was identified in the tumors that were microsatellite instability-high. Thus, a major consequence of the use of immunohistochemistry by itself as a screen is the failure to identify colorectal and endometrial cancer patients who likely have Lynch Syndrome.
Keywords: Lynch Syndrome, molecular testing, microsatellite instability, immunohistochemistry
Introduction
Defects in the DNA mismatch repair (MMR) system cause replication errors or instability in DNA microsatellites. Germline mutations in MMR genes MLH1, MSH2, MSH6, and PMS2 cause the autosomal dominant hereditary disease Lynch syndrome. Over 90% of tumors from patients with this syndrome show high levels of microsatellite instability (MSI-High) (1). MSI also occurs in 10–15% of sporadic tumors, especially colorectal and endometrial carcinomas, due to hypermethylation of the MLH1 promoter (2). Screening for defects in the MMR genes has become important for the identification of patients with Lynch Syndrome and has treatment and prognostic significance for patients with sporadic colorectal cancer. Screening for Lynch Syndrome can not only effectively prevent subsequent cancers in the screened individual, but it also can prevent cancer development in relatives who also harbor a germline mutation of a MMR gene.
Traditionally, clinical screening methods to help identify families at risk for Lynch Syndrome included the use of the Amsterdam and Amsterdam II Criteria. Because these criteria have been thought to lack sensitivity, particularly when an extensive family history is unavailable, the revised Bethesda Criteria were developed. The Bethesda Criteria are more sensitive, focus on the patient medical history and introduced the category of microsatellite instability and tumor morphology into clinical guidelines. However, these criteria do not address patients with endometrial cancer and may fail to identify a large number of mutation carriers that do not meet the clinical criteria (3). To further help identify individuals at-risk for Lynch Syndrome, several clinical prediction models have been developed to quantify the risk for germline MMR mutations (4–6). These have comparable sensitivities to the Bethesda Criteria and can provide quantitative risk assessment (7). However, such prediction models require subsequent genetic testing and are not helpful in identifying patients with sporadic MSI-High colorectal cancers requiring alternative treatment regimens.
Two tissue-based screening methods used to assess tumors for MMR defects include PCR-based MSI testing and immunohistochemistry. In general, a number of large studies have concluded that MSI and immunohistochemistry have comparable sensitivity and specificity in detecting MMR defects (8–12). Because it is generally associated with lower cost, faster turnaround time, and wider availability in smaller pathology laboratories, some advocate that immunohistochemistry could be used as the sole method of screening in certain cancers (9, 13–16).
At our institution we perform both PCR-based MSI and immunohistochemistry concurrently. Immunohistochemistry-MSI analysis discordances have been acknowledged in the research setting, but the frequency and nature of these problems have not been formally examined in a quality assurance fashion in the clinical laboratory. In surgical pathology practice, correlation reviews (cytology-histology and frozen section-final pathology correlations) are commonly used tools for quality assurance (17). This type of quality assurance review has not been previously applied to the tissue-based approach to MMR testing. Therefore, we examined sources of discordances and problems in tissue based testing to help optimize this approach in our patient population.
Materials and Methods
Patient Population and Study Design
After obtaining institutional board approval we obtained the results and clinical records for all cancer patients who had MSI and/or immunohistochemistry testing between August 2002 and August 2010 at the University of Texas M.D. Anderson Cancer Center (n=736 patients). For 107 of these patients, only immunohistochemistry or MSI analysis had been previously performed, so these patients are excluded from further consideration. Only those patients with both MSI testing and immunohistochemistry results for the same tumor were included (n=629). Demographic, clinical and pathologic data were collected. Initial immunohistochemistry slides were examined by a group of 14 different gastrointestinal and gynecologic pathologists, while initial MSI analyses were evaluated by 7 GI pathologists and 5 Molecular Pathologists. Discordance was defined as a discrepancy between the result of MSI and immunohistochemistry. Problem was defined as a case with indeterminate or questionable immunohistochemistry or microsatellite instability results. The immunohistochemistry slides and the MSI chromatograms for all discordant/problematic cases were re-reviewed by two pathologists (RRB and ANB) and results and patient histories discussed with a genetics counselor (DS). Any additional testing performed, including MLH1 methylation, BRAF mutational analysis, and subsequent genetic sequencing and large rearrangement testing for the four most common MMR genes, was also documented.
Molecular Analyses
All immunohistochemistry and PCR-based molecular analyses were performed in CLIA- and College of American Pathology-approved laboratories. Immunohistochemistry was performed for DNA mismatch repair gene products MLH1 (G168-15, 1:25; BD Biosciences Pharmingen, San Diego, CA.), MSH2 (FE11, 1:100; Calbiochem, La Jolla, CA.), MSH6 (44, 1:300; BD Biosciences Pharmingen), and PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen) as previously described (18). Tumors showing loss of nuclear MLH1, MSH2, PMS2 or MSH6 were classified as negative for the protein expression of each respective marker. Stromal and normal tissues within the tumor served as internal positive controls.
MSI analysis was performed using six National Cancer Institute recommended microsatellites (19) with the addition of analysis for the mutational inactivation of transforming growth factor beta receptor type II (TGFBR2). Therefore, 7 microsatellites comprise the MSI panel. A tumor was designated as MSI-High if it demonstrated allelic shift at three or more markers and as MSI-Low with allelic shift in one or two markers. Those without allelic shifts were designated MS-Stable. A methylation-specific polymerase chain reaction MLH1 promoter methylation assay and BRAF V600E mutational testing by pyrosequencing were performed on a subset of cases (20–21).
Statistical Analysis
It is controversial whether MSI analysis or immunohisochemistry represents the “gold standard” when considering the tissue testing approach. Therefore, traditional measures of testing efficacy, such as sensitivity and specificity, are likely not appropriate here. Instead, the measures calculated were concordance (percent of pairs in agreement) and discordance (percent of pairs in disagreement). Specifically, we calculated concordance and discordance and their exact 95% confidence intervals for MSI-high tumors, MS-stable tumors, tumors with loss of a MMR protein by immunohistochemistry, and tumors with retained expression of all four MMR proteins.
Results
Patient tumor population
From August 2002 to August 2010, 629 patients had tumors analyzed by both immunohistochemistry and microsatellite instability analysis. Twenty-six of these patients had more than one tumor analyzed. Therefore, there are a total of 646 tumor analyses included in this study. The majority of tumors were colorectal adenocarcinomas (88%), followed by endometrial carcinomas (7%) (Table 1). Table 2 summarizes the distribution of tumors by microsatellite instability status. There were 102 (15.8% of the total) MSI-High carcinomas (78/102 colorectal [76%]; 17/102 endometrial [17%]; 7/102 other [7%]), 55 patients had MSI-Low carcinomas, (49/55 colorectal [89%]; 4/55 endometrial [7%]; 2/55 other [4%]). The remaining 489 tumors were MS-Stable.
Table 1.
Tumor analyses (total number of tumors analyzed = 646)
| Tumor Type | % Total (number of tumors) |
Median Patient Age (yrs) (range) |
|---|---|---|
| Colorectal adenocarcinoma | 88 (568) | 59 (18–88) |
| Endometrial carcinoma | 7 (45) | 55 (19–85) |
| Small Bowel adenocarcinoma | 2 (13) | 44 (29–59) |
| Colorectal adenoma | 1 (7) | 45 (42–56) |
| Ovarian carcinoma | 0.5 (3) | 57 (45–72) |
| Pancreatic adenocarcinoma | 0.5 (3) | 64 (61–69) |
| Other | 1 (7) | 40 (38–55) |
Table 2.
Distribution of tumor analyses by MSI status (total number of tumors analyzed = 646)
| MSI Status | % Total (number of tumors) |
|---|---|
| MSI-High | 15.8 (102) |
| MS-Stable | 75.7 (489) |
| MSI-Low | 8.5 (55) |
Immunohistochemistry-Microsatellite Instability Agreement
Table 3 summarizes the concordances and discordances between immunohistochemistry and microsatellite instability analysis for the MS-Stable and MSI-High tumors (n=591). MSI-Low tumors (n=55) were specifically excluded from these analyses. The nature of MSI-Low tumors is controversial and not well-understood, so it is not possible to determine if immunohistochemistry and microsatellite instability analyses for individual patients are concordant. Overall, for the MS-Stable and MSI-High tumors, the agreement between immunohistochemistry and MSI analysis was quite good (97.80% concordance, exact 95% CI 96.27 – 98.82). The highest level of agreement occurred in MS-Stable tumors with intact expression of all 4 MMR proteins. The lowest level of concordance occurred in MSI-High tumors with loss of at least one MMR protein by immunohistochemistry.
Table 3.
Summary of concordances and discordances between immunohistochemistry and microsatellite instability analysis1
| MSI-High (N = 102) | N | % | 95% CI |
|---|---|---|---|
| IHC shows loss of expression of at least one MMR protein | 90 | 88.24 | 80.35 – 93.77 |
| IHC shows intact expression of all 4 proteins | 12 | 11.76 | 6.23 – 19.65 |
| MS-Stable (N = 489) | |||
| IHC shows loss of expression of at least one MMR protein | 1 | 0.20 | 0.01 – 1.13 |
| IHC shows intact expression of all 4 proteins | 488 | 99.80 | 98.87 – 99.99 |
| IHC - Loss of at least one MMR protein (N = 91) | |||
| MSI-High | 90 | 98.90 | 94.03 – 99.97 |
| MS-Stable | 1 | 1.10 | 0.03 – 5.97 |
| IHC - Intact MMR protein expression (N = 500) | |||
| MSI-High | 12 | 2.40 | 1.25 – 4.15 |
| MS-Stable | 488 | 97.60 | 95.85 – 98.75 |
| Overall Agreement | |||
| IHC shows loss of expression of at least one MMR protein & MSI-High OR IHC shows intact expression of all 4 proteins & MSI-Stable | 578 | 97.80 | 96.27 – 98.82 |
| IHC shows loss of expression of at least one MMR protein & MSI-Stable OR IHC shows intact expression of all 4 proteins & MSI-High | 13 | 2.20 | 1.18 – 3.73 |
This table summarizes the results for n=591 tumor analyses, including MSI-High tumors (n=102) and MS-Stable tumors (n=489). Tumors that were MSI-Low were specifically excluded from this analysis, because of the uncertainty of what represents immunohistochemistry-MSI concordance/discordance in this group.
IHC – immunohistochemistry; MMR – DNA mismatch repair
Discordances were identified in 13 of the 591 (2.2%) tumors with both MSI and immunohistochemistry testing. A detailed summary of these discordances is presented in Table 4. Twelve of 102 (11.8%) MSI-High tumors had discordant immunohistochemistry tumor profiles, showing intact immunohistochemical expression of MLH1, MSH2, MSH6, and PMS2 proteins. 7/12 tumors were from patients were younger than 50 years at age of diagnosis, and 8/12 tumors were from patients who had a first degree relative with a Lynch Syndrome-associated cancer. Following genetic counseling, 9 patients (8 patients with MSI-High tumors but retained immunohistochemical expression of MLH1, MSH2, MSH6, and PMS2 and the one patient with MS-Stable endometrial carcinoma with immunohistochemical loss of MLH1 and PMS2) underwent germline testing; 4/9 (44.4%) were identified to have MMR gene alterations. One of these alteration positive patients (Patient 3) has an MLH1 mutation that is known to be pathogenic. The remaining identified gene alterations are variants of undetermined significance. Patient 7 had an inframe duplication of exons 3–5 of MLH1. This duplication is not predicted to result in protein truncation, but it is possible that this would affect protein function. Of the 489 MS-Stable tumors, only 1 had loss of a MMR protein by immunohistochemistry. This was an endometrial carcinoma with loss of MLH1 and PMS2 by immunohistochemistry and no evidence of MLH1 methylation. No mutations of MLH1 were detected on subsequent sequencing or large rearrangement analysis. The patient declined genetic testing for PMS2 and is now deceased.
Table 4.
Summary of tumors with discordant MSI and immunohistochemistry results
| IHC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | PMS2 | MSI Status |
MLH1 Methylation |
Germline1 Alteration |
BRAF mutation |
Tumor Location |
Age | AC/FH | |
| 1 | + | + | + | + | MSI-H | Negative | MSH2 | Neg | Rectum | 48 | Neg/No |
| 2 | + | + | + | + | MSI-H | NP | NP | NP | Colon | 47 | Neg/No |
| 3 | + | + | + | + | MSI-H | Negative | MLH1 | NP | Colon | 62 | Pos/Yes |
| 4 | + | + | + | + | MSI-H | NP | MLH1, PMS2 | NP | Colon | 57 | Neg/No |
| 5 | + | + | + | + | MSI-H | Present | NP | NP | Small Bowel | 59 | Neg/No |
| 6 | + | + | + | + | MSI-H | NP | Negative | NP | Colon | 47 | Neg/Yes |
| 7 | + | + | + | + | MSI-H | Negative | MLH1 | Neg | Colon | 29 | Pos/Yes |
| 8 | + | + | + | + | MSI-H | NP | Negative | NP | Colon | 43 | Neg/Yes |
| 9 | + | + | + | + | MSI-H | Negative | Negative | Neg | Uterus | 51 | Neg/Yes |
| 10 | + | + | + | + | MSI-H | Negative | Negative | Neg | Colon | 63 | Neg/Yes |
| 11 | + | + | + | + | MSI-H | Negative | NP | Neg | Uterus | 32 | Pos/Yes |
| 12 | + | + | + | + | MSI-H | Negative | NP | Neg | Colon | 47 | Neg/Yes |
| 13 | − | + | + | − | MSS | Negative | Negative | Neg | Uterus | 85 | Neg/Yes |
Abbreviations: MSI, microsatellite instability; MSI-H, MSI-High; MSS, microsatellite stable; NP, not performed; Neg, negative; Pos, positive; AC, Amsterdam II Criteria; FH, family history of a Lynch Syndrome-associated cancer
The MLH1 mutation (618del [1852del3]) in Patient 3 is known to be pathogenic. This is an in-frame deletion of 3 nucleotides that results in the omission of lysine from amino acid position 618, but does not lead to premature protein termination. The germline alterations in Patients 1, 4, and 7 are currently classified as variants of undetermined significance. For Patient 1, there is a point mutation (T677R) that substitutes arginine for threonine at 677. For Patient 4, three different mutations were identified, one in PMS2 (PMS2 c. 1437C>G [p.His479Gln]) and two in MLH1 (MLH1 c.290A>G [p.Tyr97Cys] and MLH1 c.299G>A [R100Q, Arg100Gln]); none of these are currently predicted to result in protein truncation. Patient 7 had an inframe duplication of exons 3–5 of MLH1. This is not predicted to cause protein truncation, but it possible that the duplication would affect protein function. Patients 6 and 10 had negative MLH1, MSH2, and MSH6 germline testing. Patient 8 had negative MLH1, MSH2, MSH6, and PMS2 germline testing. Patient 9 had negative MSH6 germline testing. Patient 13 had negative and MLH1 and MSH2 germline testing and declined further testing.
53/55 MSI-Low tumors had positive immunohistochemical expression of MLH1, MSH2, MSH6, and PMS2. None of these patients had further genetic testing. One 53 year old patient with an MSI-Low rectal adenocarcinoma had loss of MSH6 by immunohistochemistry; subsequently, a deleterious MSH6 mutation was discovered. A second 68 year old patient with an MSI-Low rectal adenocarcinoma had immunohistochemical loss of MSH2 and MSH6, suggestive of MSH2 mutation. This patient declined genetic testing to confirm this, however.
Problems – Immunohistochemistry
Problems were identified in ten patients. These problems do not overlap with the discordances presented in Table 4. The problems were grouped as selection error (n=2), pathologist error in immunohistochemistry interpretation (n=6), or unusual pattern of immunohistochemistry expression (n=2). Selection error involved two different patients, each with two separate primary colorectal adenocarcinomas. The proximal tumor in both was MS-Stable with intact nuclear expression for all 4 MMR proteins by immunohistochemistry. The distal tumor in both patients was subsequently tested and found to be MSI-High with immunohistochemical loss of MSH2 and MSH6. One of these patients agreed to further genetic testing and was found to have an MSH2 mutation.
Six colorectal adenocarcinomas had pathologist difficulty/error in immunohistochemistry interpretation. For two of these, our central review changed the immunohistochemistry result. The first tumor with pathologist error was from a 32 year old with an MSI-High rectal adenocarcinoma that was initially interpreted as having positive expression for all 4 MMR proteins. Re-review showed that the tumor had diffuse, strong expression for MLH1, MSH2, and PMS2. Most of the tumor cells, however, were entirely negative for MSH6. A few tumor cells had faint nuclear expression for MSH6 that was much weaker than that of adjacent stromal cells and normal mucosa (Figure 1). This patient was subsequently identified to have an MSH6 mutation. The second tumor with pathologist error was a rectal adenocarcinoma that was MS-Stable and initially interpreted as negative for MLH1 by immunohistochemistry. Re-review revealed that the background stromal cells were also negative for MLH1 and that the immunostaining had therefore not worked properly. Immunohistochemistry was repeated using a different tumor block and showed convincing MLH1 protein expression in the tumor (Figure 2). The remaining 4 tumor analyses in this category involved difficulties with MSH6 immunohistochemistry. Two of these were MSI-High carcinomas (one colorectal, one endometrial) in which the tumors were positive for MLH1, MSH2, and PMS2, but the MSH6 immunohistochemistry did not work (internal control benign tissue also did not stain). Both of these were from patients who had a family history of a Lynch Syndrome-associated cancer, but neither fulfilled Amsterdam Criteria. Neither of these patients consented to further genetic testing. The last two tumors in this category were MS-Stable colorectal adenocarcinomas which had strong, diffuse nuclear expression for MLH1, MSH2, and PMS2, but the MSH6 expression in the tumor was initially interpreted as only focal or weak. However, upon our re-review, the MSH6 immunohistochemistry was interpreted as positive (retained expression), so these patients did not undergo genetic testing.
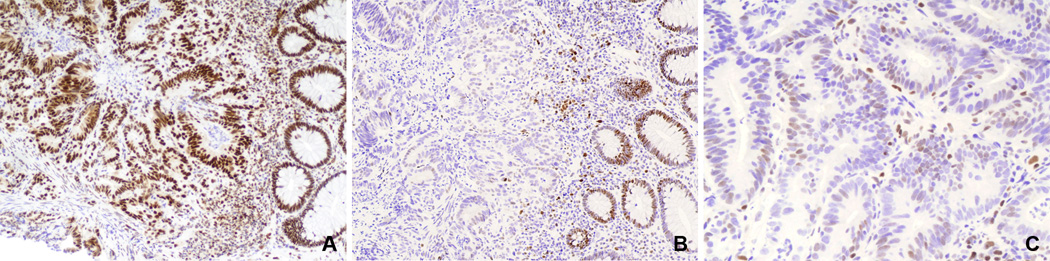
Figure 1.
Immunohistochemistry in an MSI-High colorectal adenocarcinoma initially interpreted as weakly positive for MSH6 by immunohistochemistry. A) The tumor and adjacent normal crypts have strong MLH1 nuclear expression (100X). B) MSH6 is negative in the tumor cells, but strongly positive in adjacent benign crypts and stromal cells (100X). C) Higher magnification of MSH6 immunohistochemistry with slight nuclear blush of staining in neoplastic cells. This staining is far lighter than that of stromal internal controls or adjacent non-neoplastic crypts (200X).
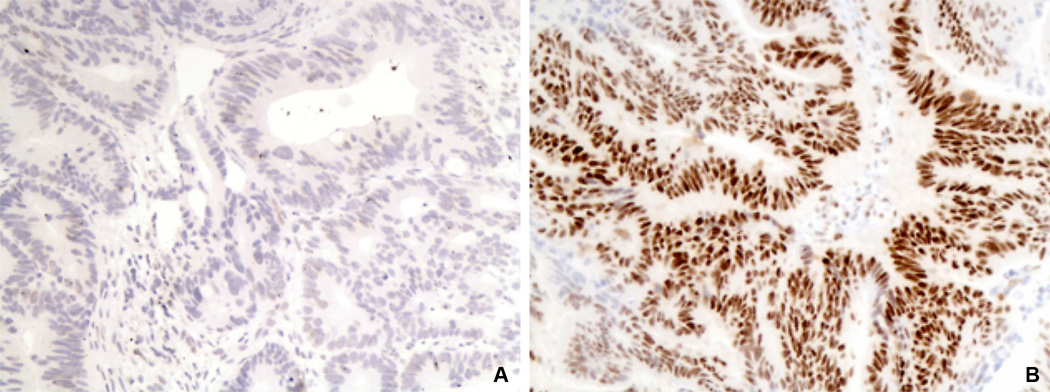
Figure 2.
Immunohistochemistry for a microsatellite-stable colorectal adenocarcinoma initially interpreted as negative for MLH1 (A and B, 200X). A) Original MLH1 slide shows that both neoplastic glands and adjacent stromal cells are negative for MLH1. This is a sub-optimal IHC test that cannot be adequately interpreted. B) Repeat MLH1 immunohistochemistry using a different paraffin block of the same tumor shows strong MLH1expression in both the tumor and adjacent stromal cells.
Two tumors with unusual immunohistochemical staining patterns were identified. One unusual staining pattern in a MS-Stable endometrial adenocarcinoma showed strong nuclear staining for MSH2 and MSH6, but the tumor had definite positive foci and definite negative foci for both MLH1 and PMS2. In the negative foci, internal positive control stromal cells retained expression of both MLH1 and PMS2. Methylation of MLH1 was detected in this tumor, so this was interpreted as a sporadic endometrial carcinoma. The second unusual pattern was a MSI-High colorectal adenocarcinoma with strong expression of MLH1 and MSH2 and complete loss of MSH6 and PMS2 (Figure 3). These tests were subsequently repeated twice with re-review of each set with the same result. This patient did not assent to genetic testing.
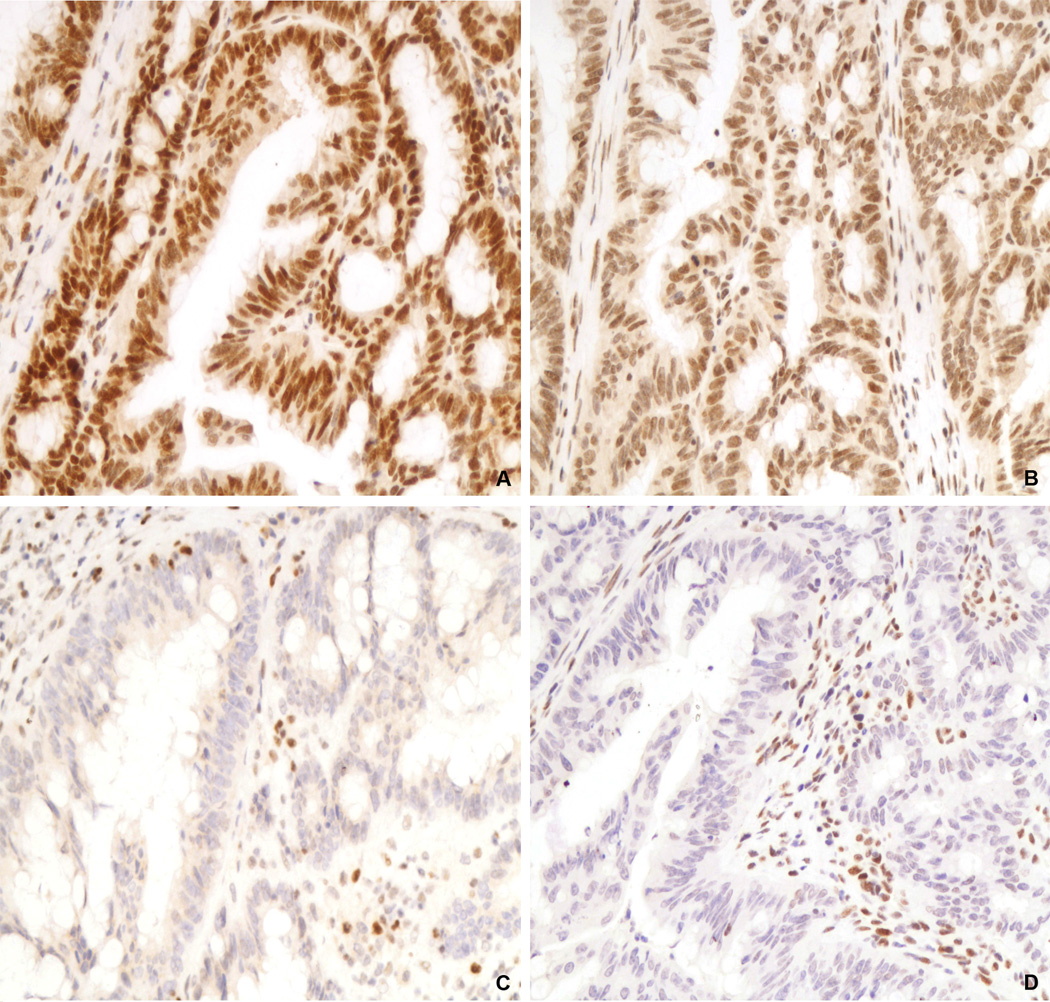
Figure 3.
Unusual immunohistochemical staining pattern in an MSI-High colorectal adenocarcinoma positive for MLH1 (A) and MSH2 (B) and negative for PMS2 (C) and MSH6 (D). For PMS2 and MSH6, note the presence of nuclear staining in the adjacent benign stromal cells, indicating that the immunohistochemistry tests are working appropriately. A-D, 200X.
Problems – Microsatellite Instability
Given the intense scrutiny of the immunohistochemistry tests summarized above, we next reviewed the results of all 646 tumor analyses for problems in DNA amplification in the MSI analysis to provide better balance to the quality assurance review. Overall, only 0.6% of the microsatellites amplified suboptimally. No tumor had more than one failed microsatellite amplification. Thus, accurate determination of microsatellite instability status was possible for all 646 tumor analyses. Only 5 microsatellites, or 1% of the total tested, for the tumors in the MSI-High category failed to amplify. This included one each in five different tumors. This did not pose a diagnostic problem since the remaining 6 microsatellites did amplify. For the 55 MSI-Low tumors, only 6 of 385 microsatellites (2%) tested failed to amplify (one each in six different tumors). Even if this failed microsatellite was actually unstable, this would not be sufficient to re-designate the tumor as MSI-High, as instability in at least 3/7 microsatellites is required for a tumor to be MSI-High. For the 489 MS-Stable tumors, only 13 of 3423 microsatellites (0.4%) failed to amplify, one each from 13 different tumors.
Discussion
Because immunohistochemistry is less expensive, more widely available, relatively simple to perform, and thought to be comparable to MSI analysis, a number of studies have advocated the use of immunohistochemistry alone in the initial evaluation for MMR defects (14–16, 22). Indeed, we confirm that, in general, concordance between immunohistochemistry and MSI analysis is quite high. However, 11.8% of the MSI-High carcinomas had intact immunohistochemical expression of MLH1, MSH2, MSH6, and PMS2. A previous population-based examination of 500 unselected colon cancer patients demonstrated a comparable percentage (9.7%) of MSI-High cancers with intact immunohistochemical expression of MMR proteins (23). Importantly, our study suggests that a significant number of possible Lynch Syndrome patients would not be detected if only immunohistochemistry was used as a screening technique. When family history and the molecular diagnostics tests are considered together, only one (#5) of the 12 MSI-High carcinomas with intact MMR immunohistochemistry summarized in Table 4 is clinically thought to be sporadic. This percentage of likely Lynch Syndrome cases is relatively high, especially compared to that of other population-based screening studies (23). This higher percentage may be due to the fact that the vast majority of the patients in our study were initially clinically suspected as having Lynch Syndrome due to young age of cancer onset or family history of a Lynch Syndrome-related cancer. Reflex testing of all colorectal cancer patients did not begin at our institution until September, 2009. Previously, Thibodeau et al. showed that, in a large series of approximately 1400 colorectal cancer patients, there are more immunohistochemistry-MSI discrepancies when the patient population screened had moderate to high-risk for Lynch Syndrome (24). Interestingly, in our current series, we found that 7/13 of our immunohistochemistry/MSI discordances were prior to September 2009, and 6/13 were after this date. So, approximately half of the discordances occurred after commencement of universal screening for colorectal cancer.
In the present study, 12 cancer patients had MSI-High tumors, but intact MMR protein expression by immunohistochemistry. Of these 12, 8 agreed to genetic testing. Genetic testing in these patients is problematic, as the immunohistochemistry results cannot help to direct the testing. Seven of these 8 were suspicious for Lynch Syndrome based on lack of tumor MLH1 methylation, lack of BRAF mutation, young age of cancer onset, and/or presence of Amsterdam Criteria/family history of a Lynch Syndrome cancer. Missense mutations could potentially lead to full-length, but non-functional, mismatch repair proteins, which would help to explain the immunohistochemistry-MSI discordances. However, only 1 of these 8 (12.5%) patients had a pathologic MMR mutation detected (Table 4, #3, MLH1 mutation). The remaining 7 patients either had variants of undetermined significance or no germline variation detected. Tumor 7 was from a patient with an MLH1 inframe duplication of exons 3–5; while this duplication is not thought to cause protein truncation, it could possibly affect protein function. In our previous studies examining endometrial cancer patients younger than 50 years of age (20), 11 patients had MSI-High endometrial cancers with loss of a MMR protein detected by immunohistochemistry. From these 11 patients, we detected 9 pathologic mutations (81.8%). Three more endometrial cancer patients had MSI-High tumors but intact MMR protein expression by immunohistochemistry; for these 3 patients, no mutations were identified by genetic testing. Collectively, these results suggest that when immunohistochemistry detects loss of expression of a MMR protein, there is a higher likelihood of ultimately detecting a pathologic MMR gene mutation. Conversely, for tumors that are MSI-High but have retained positive expression of MMR proteins by immunohistochemistry, there could be a lower chance of ultimately discovering a pathologic Lynch Syndrome mutation. The reasons for this at this time are not clear. Given time and more experience, it is possible that some of these identified variants of undetermined significance become reclassified as pathologic mutations. Or, mutations in less common genes regulating DNA mismatch repair may be responsible for the high levels of microsatellite instability. It is also possible that alternative, not yet identified, genetic mechanisms other than mismatch repair are generating tumor microsatellite instability.
In addition to the discordances, our quality assurance review identified several different problems in the tissue-based approach, particularly with immunohistochemistry. Several of these problems are readily correctable, particularly the recognition of lack of internal positive control staining and the testing of all tumors in patients with synchronous primaries. Other problems, such as interpretation of weak or focal immunohistochemical staining (Figure 1), may require considerable experience to accurately interpret (25). Problems in the MSI analysis, chiefly failure of microsatellite DNA to amplify for adequate testing, were also identified in our study. Of the 646 tumors reviewed, no tumor had more than one microsatellite fail PCR amplification. Given that a panel of 7 microsatellites is employed, rather than just one or two, the failure of one microsatellite to amplify still allows for distinguishing MSI-High from MS-Stable.
Although our quality assurance review identified more short-comings in immunohistochemistry-based testing, it should be emphasized that immunohistochemistry plays an important role in the evaluation of patients with MSI-High carcinomas and can help direct future germline testing. The identification of immunohistochemical loss of MSH2, MSH6, or PMS2 in an MSI-High tumor is virtually diagnostic for Lynch Syndrome, even when germline testing fails to reveal a mutation. These patients and their relatives should still be offered cancer prevention services at an earlier age.
Considering the number of possible Lynch Syndrome patients who would be missed by screening by immunohistochemistry alone, we cannot recommend immunohistochemistry as the sole tissue-based screening technique. Rather, for population-based screening of colorectal cancer patients, it might be preferred to use MSI analysis as an initial screen and then perform immunohistochemistry in MSI-High cases, as recommended by others (12, 25–27). Alternatively, MSI and immunohistochemistry can be initiated concurrently in patients with moderate or high-risk of Lynch Syndrome (24). Of course, it is imperative that patients have access to genetic counseling and subsequent germline mutation analysis if these tissue-based tests are informative.
The tissue-based testing of endometrial cancer patients is more problematic. Endometrial cancers and colon cancers from the same patient can exhibit different levels of MSI, with the endometrial cancers often being MSI-Low or MS-Stable (28). MSH6 mutation carriers are especially prone to developing endometrial cancer, and these tumors are not typically MSI-High (29,30). Experience with MSI analysis is even more limited with other cancer types associated with Lynch Syndrome, such as cancers of the ureter, ovary, and small intestine. Thus, concurrent MSI analysis and immunohistochemistry should be performed in patients with these extra-colonic malignancies.
Acknowledgements
NIH 2P50 CA098258-06 SPORE in Uterine Cancer (RRB) and NIH CA016672 Cancer Center Support Grant.
Footnotes
This original research was presented in part at the 100th United States and Canadian Academy of Pathology Annual Meeting, February 27-March 4, 2011, San Antonio, Texas
Disclosure: The authors have no conflicts of interest to disclose. Devki Saraiya is currently employed by Myriad Genetic Laboratories, Inc., as a Regional Medical Specialist (Certified Genetic Counselor). However, all content and writing for this manuscript were completed while she was an employee of M.D. Anderson Cancer Center.
References
- 1.Lynch HT, de la Chapelle A. Hereditary Colon Cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham JM, Kim CY, Christensen ER, Tester DJ, Parc Y, Burgart LJ, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet. 2001;69:780–790. doi: 10.1086/323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296:1479–1487. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmana J, Stockwell DH, Steyerberg EW, Stoffel EM, Deffenbaugh AM, Reid JE, et al. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. JAMA. 2006;296:1469–1478. doi: 10.1001/jama.296.12.1469. [DOI] [PubMed] [Google Scholar]
- 6.Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 7.Grovel S, Syngal S. Risk assesment, genetic testing and management of Lynch syndrome. J Natl Compr Canc Netw. 2010;8:98–105. doi: 10.6004/jnccn.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanza G, Gafa R, Santini A, Maestri I, Guerzoni L, Cavazzini L. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24:2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 10.Ruszkiewicz A, Bennett G, Moore J, Manavis J, Rudzki B, Shen L, et al. Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumours. Pathology. 2002;34:541–547. doi: 10.1080/0031302021000035965-2. [DOI] [PubMed] [Google Scholar]
- 11.Sheng JQ, Zhang H, Ji M, Fu L, Mu H, Zhang MZ, et al. Genetic diagnosis strategy of hereditary non-polyposis colorectal cancer. World J Gastroenterol. 2009;15:983–989. doi: 10.3748/wjg.15.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedroni M, Roncari B, Maffei S, Losi L, Scarselli A, Di Gregorio C, et al. A mononucleotide markers panel to identify hMLH1/hMSH2 germline mutations. Dis Markers. 2007;23:179–187. doi: 10.1155/2007/703129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigau V, Sebbagh N, Olschwang S, Paraf F, Mourra N, Parc Y, et al. Microsatellite instability in colorectal carcinoma. The comparison of immunohistochemistry and molecular biology suggests a role for hMSH6 [correction of hMLH6] immunostaining. Arch Pathol Lab Med. 2003;127:694–700. doi: 10.5858/2003-127-694-MIICC. [DOI] [PubMed] [Google Scholar]
- 14.Rosen DG, Cai KQ, Luthra R, Liu J. Immunohistochemical staining of hMLH1 and hMSH2 reflects microsatellite instability status in ovarian carcinoma. Mod Pathol. 2006;19:1414–1420. doi: 10.1038/modpathol.3800672. [DOI] [PubMed] [Google Scholar]
- 15.Marcus VA, Madlensky L, Gryfe R, Kim H, So K, Millar A, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol. 1999;23:1248–1255. doi: 10.1097/00000478-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Hampel H. Point: justification for lynch syndrome screening among all patients with newly diagnosed colorectal cancer. J Natl Compr Canc Netw. 2010;8:597–601. doi: 10.6004/jnccn.2010.0044. [DOI] [PubMed] [Google Scholar]
- 17.Roy JE, Hunt JL. Detection and classification of diagnostic discrepancies (errors) in surgical pathology. Adv Anat Pathol. 2010;17:359–365. doi: 10.1097/PAP.0b013e3181ece0db. [DOI] [PubMed] [Google Scholar]
- 18.Cai KQ, Albarracin C, Rosen D, Zhong R, Zheng W, Luthra R, et al. Microsatellite instability and alteration of the expression of hMLH1 and hMSH2 in ovarian clear cell carcinoma. Hum Pathol. 2004;35:552–559. doi: 10.1016/j.humpath.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 20.Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 21.Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 22.Evans GD, Lalloo F, Mak T, Mak T, Speake D, Hill J. Is it time to abandon microsatellite instability as a pre-screen for selecting families for mutation testing for mismatch repair genes? J Clin Oncol. 2006;20:1960–1962. doi: 10.1200/JCO.2005.05.3207. [DOI] [PubMed] [Google Scholar]
- 23.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;10:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baudhuin LN, Burgart LJ, Leontovich O, Thibodeau SN. Use of microsatellite instability and immunohistochemistry testing for the identification of individuals at risk for Lynch Syndrome. Fam Cancer. 2005;4:255–265. doi: 10.1007/s10689-004-1447-6. [DOI] [PubMed] [Google Scholar]
- 25.Chapusot C, Martin L, Puig PL, Ponnelle T, Cheynel N, Bouvier AM, et al. What is the best way to assess microsatellite instability status in colorectal cancer? Study on a population base of 462 colorectal cancers. Am J Surg Pathol. 2004;28:1553–1559. doi: 10.1097/00000478-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Barrow E, McMahon R, Evans DG, Levine E, Hill J. Cost analysis of biomarker testing for mismatch repair deficiency in node-positive colorectal cancer. Br J Surg. 2008;95:868–875. doi: 10.1002/bjs.6172. [DOI] [PubMed] [Google Scholar]
- 27.Arnold CN, Goel A, Compton C, Marcus V, Niedzwiecki D, Dowell JM, et al. Evaluation of microsatellite instability, hMLH1 expression and hMLH1 promoter hypermethylation in defining the MSI phenotype of colorectal cancer. Cancer Biol Ther. 2004;3:73–78. doi: 10.4161/cbt.3.1.590. [DOI] [PubMed] [Google Scholar]
- 28.Kuismanen SA, Moisio AL, Schweizer P, Truninger K, Salovaara R, Arola J, et al. Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am J Path. 2000;160:1953–1958. doi: 10.1016/S0002-9440(10)61144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berends M, Wu Y, Sijmons R, Mensink RG, van der Sluis T, Hordijk-Hos JM, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Gen. 2002;70:26–37. doi: 10.1086/337944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda M, Gonzalez S, Lazaro C, Blanco I, Capella G. Detection of genetic alterations in hereditary colorectal cancer screening. Mutat Res. 2010;693:19–31. doi: 10.1016/j.mrfmmm.2009.11.002. [DOI] [PubMed] [Google Scholar]