Abstract
Insufficient dose of dietary methyl groups are associated with a host of conditions ranging from neural tube defects to cancer. On the other hand, it is not certain what effect excess dietary methyl groups could have on cancer. This is especially true for prostate cancer (PCa), a disease that is characterized by increasing DNA methylation changes with increasing grade of the cancer. In this three-part study in animals, we look at (i) the effect of excess methyl donors on the growth rate of PCa in vivo, (ii) the ability of 5-aza-2'-deoxycytidine, a demethylating agent, to demethylate in the presence of excess dietary methyl donors and (iii) the effect of in utero feeding of excess methyl donors to the later onset of PCa. The results show that when mice are fed a dietary excess of methyl donors, we do not see (i) an increase in the growth rate of DU-145 and PC-3 xenografts in vivo, or (ii) interference in the ability of 5-aza-2'-deoxycytidine to demethylate the promoters of Androgen Receptor or Reprimo of PCa xenografts but (iii) a protective effect on the development of higher grades of PCa in the “Hi-myc” mouse model of PCa which were fed the increased methyl donors in utero. We conclude that the impact of dietary methyl donors on PCa progression depends upon the timing of exposure to the dietary agents. When fed before the onset of cancer, i.e. in utero, excess methyl donors can have a protective effect on the progression of cancer.
INTRODUCTION
The dynamics of DNA methylation are controlled by the availability of methyl groups (methyl donors), the utilization of these groups by DNA methyl transferase (DNMT), DNA demethylation processes – whether active or passive, cell responsiveness to DNA methylation defects, as well as abundance of methyl group acceptors and enzymes in the cell (1). Dietary sources of methyl donors include folate, methionine, vitamin B12, betaine, and choline (2). The “ultimate” methyl donor, S-adenosylmethionine (SAM) is eventually derived from these dietary sources and also from cellular recycling of S-adenosylhomocysteine (SAH). In some studies, insufficient dietary methyl donors resulted in a decreased availability of SAM in vivo (3), (4); while a diet proficient in the same compounds consequently resulted in an increase in SAM. Another example of this dependence was seen in rats where dietary betaine caused increased synthesis of betaine-homocysteine methyltransferase – the enzyme that transfers methyl groups to SAH to produce SAM (5). The effect of methyl donor enriched diets on one of the end products, methylation of DNA or histones is not so straight-forward. In one study in rats, disruption of folate metabolism resulted in global hypomethylation of hepatic DNA (6), and in another study in mice, an excess of methionine intake resulted in methylation of genes in the mouse frontal cortex (7). On the other hand, Kovacheva et al. have demonstrated that when choline is in short supply in the diet, a reverse adaptive epigenetic response occurs that causes an upregulation of DNMT-1 and increase in global DNA methylation in rat fetal liver and brain (8).
In the absence of knowledge of the kinetics of DNMT-1 from existing studies examining DNMT-1 and SAM, it is difficult to infer what the influence of methyl donors on the activity of DNMT-1 might be. However, a few studies have provided some insight into the relationship. Psychotic patients exhibit elevated levels of SAM, and in their telencephalic GABAergic neurons, DNMT-1 mRNA expression is increased along with the methylation of promoters like reelin, in conjunction with psychosis (9). Thus, an association between levels of SAM, from diet or otherwise, and DNMT-1, and methylation of genes is apparent. Consequently, for oncology such an association has been most convincingly demonstrated in experimental studies of colorectal cancer where Young-In Kim has brought to light the importance of optimal timing and dose of folate in determining predisposition and development of tumors (10).
While observational studies of human populations suggest that lower folate status is associated with an increased risk of colorectal neoplasia, a combined analysis of three large randomized trials of folic acid supplementation for the prevention of advancing to higher grades of adenomas in patients with an adenoma history, revealed no association (11). On the other hand Mason et al. show that folic acid fortification may have reversed the downward trend in colorectal cancer in the US and Canada resulting in increased incidence of colorectal cancer by as much as 10% (12). Thus, results from human dietary intervention studies with methyl donors show a varied, and at times, contradictory response of dietary methyl donor content to cancer chemoprevention (13). What is even more ambiguous in the case of human populations is the effect of dietary methyl donors on DNA methylation levels and how these changes may be implicated in the carcinogenesis process.
Epigenetic dysregulation is well characterized in prostate cancer (PCa). Worse prognosis of PCa is characterized by increased DNMT-1 expression (14). In PCa, at least 30 genes including the Androgen Receptor (AR) are hypermethylated (15). The publication of a study showing increased prostate cancer in patients with higher levels of plasma folate (16) only adds to the enigma of an association between increased methyl donors and PCa. We hypothesized that not only would the growth of PCa be affected by the presence of methyl donors present in a fortified diet or as supplements, but efficacy of pharmacologically useful demethylating agents be influenced by such fortification or supplementation. The PCa cells DU-145 and PC-3 are well studied PCa cell lines which have served as models for methylation studies. While promoters of many genes are methylated in these two cell lines, the DU-145 cell line is exceptionally suitable as the AR promoter is methylated in it. The AR is crucial for the growth and treatment of PCa. Therefore in this study, first the effect of a methyl proficient diet on the growth of established DU-145 and PC-3 PCa xenografts was evaluated. In the second part of the study, the ability of the drug 5-aza-2'-deoxycytidine (AdC) to demethylate in the presence of excess methyl donors in the diet was examined. Lastly, in the third part of the study, in order to examine the significance of timing of exposure to excess dietary methyl donors, the effects of a methyl-proficient diet on the initiation and progression of prostate tumors in a developmental mouse model of PCa fed increased methyl donors in utero was examined. By these studies, we demonstrate that in animals prostate carcinogenesis or demethylation by AdC is not adversely affected by excess dietary methyl donors but, depending upon timing of exposure the latter may be protective towards the progression of PCa. The finding is significant in view of the ambiguity in epidemiological studies linking dietary methyl donors to cancer occurrence/progression.
MATERIALS AND METHODS
Cell Culture and Reagents
The DU-145 and PC-3 PCa cell lines, were obtained from American Type Culture Collection (Manassas, Virginia) and low passage numbers aliquoted and frozen at -80°C until use. They were cultured and maintained in 5% CO2 at 37°C in RPMI 1640 (Invitrogen, Grand Island, NY), supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY). The DU-145 and PC-3 PCa cell lines were tested before animal experimentation and found to be mycoplasma free by a PCR based MycoDtect kit from Greiner Bio-One performed at the Johns Hopkins Core facility. The Myc-PCa cell line was a generous gift from Dr. Charles Sawyers and was cultured in DMEM medium (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C, 5% CO2. The Myc-PCa cell line was not tested after arrival in our laboratory. The AdC was obtained from Sigma-Aldrich (St.Louis, MO). A stock solution of 1mg/mL was prepared in water and stored at -80°C and diluted before use. Antibody to Ki-67 was obtained prediluted from Ventana Medical Systems (Tucson, AZ) and secondary rabbit antibody was from DAKO Cytomaton (Denmark).
Animals
Athymic nu/nu male mice were purchased from Cancer Research Facility, Frederick, MD. “Hi-myc” mice were obtained from the Mutant Mouse Regional Resource Center (MMRCC), Frederick, MD.
Diets
Two groups of mice were fed either regular rodent diet (Teklad Global 18% Protein Rodent Diet) called Reg diet or the same regular diet supplemented with the methyl donors: L-methionine, choline chloride, betaine, folic acid, vitamin B12 and zinc sulphate, called Me diet (Table 1).
Table 1.
Composition of Methyl Diet
| Formula | g/kg |
|---|---|
| 2018, Teklad Global 18% Protein Rodent Diet | 969.67 |
| L-methionine | 7.5 |
| Choline chloride | 5.76 |
| Betaine, anhydrous | 15 |
| Folic acid | 0.015 |
| Vitamin B12 (0.1% in mannitol) | 1.5 |
| Zinc sulphate | 0.555 |
Tumor Growth in vivo
Evaluation of effect of Me diet on growth of PCa and interference of AdC effects in vivo was done by growing xenografts of DU-145 and PC-3 cells in athymic nu/nu male mice with a median weight of 30g. All animal experiments were carried out with the approval of the ethical committee (University Animal Care and Use Committee, Johns Hopkins University). DU-145 and PC-3 cells were cultured as described. One million DU-145 cells and 50,000 PC-3 cells suspended in 50% matrigel were transplanted subcutaneously into the hind flank of each animal through 25G5/8 needles. Mice were randomized into 4 groups with 8-10 mice per group. Two groups were fed Reg diet and the other two Me diet. One group from each dietary group was treated 5 times a week with 0.25mg/kg body weight AdC for 4 weeks – a schedule determined after previous optimizations involving doses up to 1mg/kg body weight (17). The two remaining groups were injected equivalent amounts of saline. Drug / saline treatment was started 2 weeks after implantation, at which point the tumors were palpable. Tumor size was measured once in 4-5 days and tumor volume was calculated according to the formula 0.5326*L*W*H (18). The weights of all mice were measured weekly. Mice were sacrificed after 4 weeks of treatment with AdC or saline by CO2 overdose followed by cervical dislocation.Tumors were excised and portioned. Portions were either immediately frozen in liquid nitrogen, or fixed in 10% neutral buffered formalin for 24hrs, washed and subsequently embedded in paraffin.
Immunohistochemistry
Paraffin embedded tissues were sectioned to 4μm thickness and fixed on poly-L-lysine-coated slides. Slides were deparaffinised, rehydrated, and washed twice in PBS just prior to staining. Ki-67 staining was performed as described earlier (19). Stained slides were scanned using the Bacus Laboratories Image Scanning System (BLISS). The images were uploaded onto a computer and quantitated using Frida, software for quantitation developed by the Johns Hopkins Medical Institutions’ Pathology Division.
Bisulphite Sequencing of AR and Reprimo
Genomic DNA was isolated from frozen portions of DU-145 xenografts using a SDS based lysis buffer followed by phenol-chloroform isolation. Presence of methylation in promoter region of AR and Reprimo was determined by the methods of Frommer and Clark (20). In brief: DNA (1μg) was converted with sodium bisulfite using the EZ DNA methylation kit (Zymo Research, Orange, CA) according to the manufacturer's instructions. A 377 bp AR and a 305 bp Reprimo promoter region was amplified from the bisulfite treated DNA by a PCR reaction using AR and Reprimo-promoter specific primers designed using mspprimer.org, set at the default parameters. The primer sequences are AR forward: AAAAGGAGGTGGGAAGGTAA and AR reverse: AATCCTACCAAACACTTTCCTTACTTC, and Reprimo forward: AGAAGAGTATAGTGATTTTTGTTTG and Reprimo reverse: ACAACTAAACTCTTCTAAAACC. PCR was performed with High-fidelity Taq polymerase (Invitrogen) following the protocol: an initial incubation at 95°C for 2mins, followed by 35 cycles of 95°C for 30s, 55°C for AR and 60°C for Reprimo for 30s and 72°C for 40s, followed by one cycle of 72°C for 10mins. PCR products were separated on 2% agarose gels and visualized by SYBR Gold staining. The correct band size was the only product on the gel and was excised and the DNA extracted using a DNA-gel extraction kit (Qiagen). The purified DNA was cloned into TOPO vector using the TOPO TA cloning kit (Invitrogen). Up to 10 clones from each sample were amplified with the TempliPhi™ (Amersham Biosciences, Piscataway, NJ) reaction, and sequenced using the single strand sequencing method by Agencourt Biosciences. Methylation status at each CpG position was analyzed on the sequencing results using software developed by Johns Hopkins Oncology Division. The methylated sites in the promoter region of 4-5 clones were quantitated only after selecting those sequences that passed the quality control test of greater than 89% conversion of all cytosines to uracil.
Development of Prostate Tumors in “Hi-myc” Mice on Me Diet
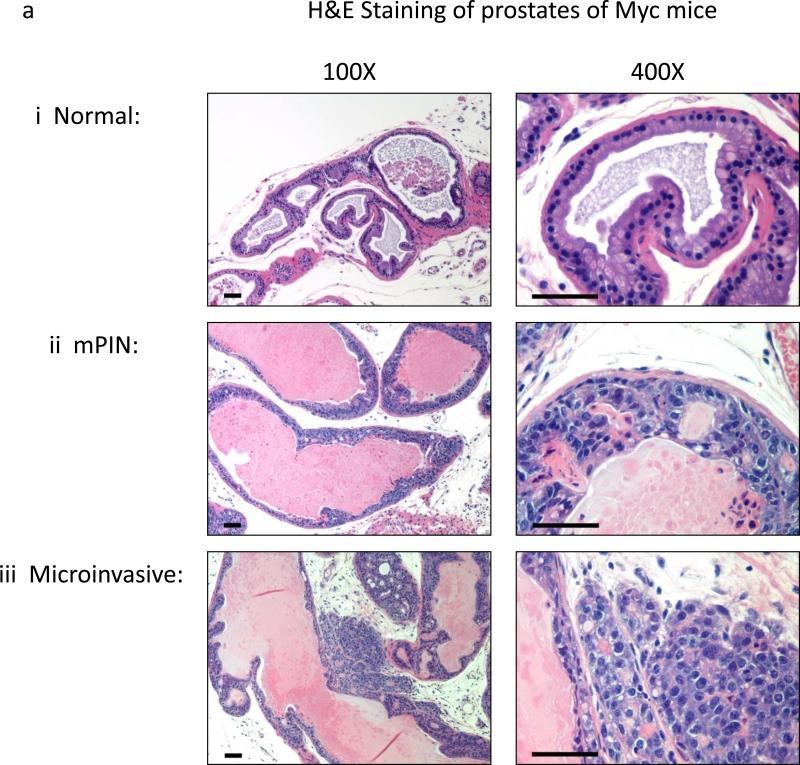
The “Hi-myc” mouse was used to study the effect of timing of exposure to methyl donors. This model has an advantage over existing mouse models to study PCa because the development of adenocarcinoma type of PCa in this model closely recapitulates the same pathological occurrence in human PCa patients (21). “Hi-myc” mice were fed either Reg or Me diet during gestation, birth of pups and until pups were one month of age. At this time, pups were weaned off the diet and maintained on Reg diet until sacrificed. To see the effects the diets had on the pathology of prostate tumors, the mice were sacrificed at different time points from 3 months until 7 months of age. Prostates were excised into different lobes. The lobes were either immediately frozen in liquid nitrogen, or fixed in 10% neutral buffered formalin for 18hrs and subsequently embedded in paraffin. Paraffin embedded tissues were sectioned just prior to staining to make 4μm thick sections fixed on poly-L-lysine-coated slides. These were then stained with Haematoxylin and Eosin (H&E) by the Pathology Core Facility at Johns Hopkins University, Baltimore. Slides were viewed on a Nikon Eclipse E400 microscope fitted with Nikon Plan Apochromat objectives . Images were then captured with Nikon DS-5M-L1 Digital Sight Camera System. After capture, images were white balanced with Adobe Photoshop CS2 and a scale bar was added. For histopathological analysis, the pathologist was blinded and the images of H&E stained prostate tissues were scored into four grades from 1-4, where 1=Benign, 2=mPIN, 3=Microinvasive, and 4=Invasive adenocarcinoma as defined by the Mouse Models of Human Cancer Consortium prostate pathology committee (22). Measurements of significant differences between groups were carried out by One-way ANOVA followed by Bonferroni's multiple comparison test. There were 7-14 mice in each group used for the comparison.
Global DNA Methylation
Global methylation levels were determined in 5 months old “Hi-myc” mouse fed either Reg or Me diet. DNA was isolated from frozen portions of the prostatic tissue. The ratio of methylated Cytosine to Cytosine was measured by mass spectrometry following previously published protocols by the Analytical Pharmacology Core at Johns Hopkins Kimmel Cancer Center (M. Rudek-Renaut, Director).
Quantitating Methyl Donors: Folate, Methionine and Homocysteine
To quantitate the levels of circulating and tissue methyl donors during the exposure to Me diet, five month old “Hi-myc” mice were fed either Reg or Me diet for 1 week. On the last day of feeding, blood was collected by cardiac puncture. Mice were sacrificed after blood was collected and prostates isolated. Folate levels were measured in unhemolysed plasma by the Chemical Core Facility at Johns Hopkins Hospital. Methionine, and homocysteine levels in plasma and prostate homogenates were determined by standard biochemical assays as routinely performed in the Analytical Pharmacology Core at Johns Hopkins Kimmel Cancer Center (M. Rudek-Renaut, Director). A standard curve prepared in charcoal dextran stripped serum was used to determine concentrations of unknowns.
Immunoblotting for c-Myc and Ki-67 expression affected by folate supplementation in vitro
Myc-PCa cells were cultured in T25 flasks and incubated: (i) either for 1 week in DMEM media or (ii) 1 week in DMEM media supplemented with folate (100μg/mL) and (iii) 1 week in DMEM media supplemented with folate (100μg/mL) followed by growth in unsupplemented DMEM media. Immunoblotting: Cells were incubated for 30mins at 4°C in NETN lysis buffer supplemented with a cocktail of protease inhibitors (Complete Mini®, Roche, Mannheim, Germany) for 15mins. The solution was centrifuged at 10,000g at 4°C for 15mins and supernatants collected. The protein concentration of the lysates was determined using the BCA assay (Pierce, Rockford, IL). Samples were mixed in a ratio of 1:2 in Laemmli buffer and denatured by heating at 98°C for 5min. Fifty μg of protein was separated on 10% or 6% Tris-SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA) for myc protein, at 100V for 1h. The separated proteins were electrophoretically transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) at 380mA for 1h. The membranes were blocked with TBS plus 5% nonfat milk (20mM Tris-HCl, pH 7.6, 137mM NaCl) followed by incubation overnight with primary antibodies diluted in blocking solution, 200-fold for c-Myc (SantaCruz, USA). This was followed by incubation for 1h in the appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham, Buckinghamshire, UK). For detection, an ECL kit was used according to the manufacturer's instructions (Amersham, Buckinghamshire, UK).
Statistical Analysis
All figures and statistical analysis were done using GraphPad Prism 5 software.
RESULTS
In vivo Effect of Methyl Proficient Diet on PCa xenografts
i. On Growth of Xenografts
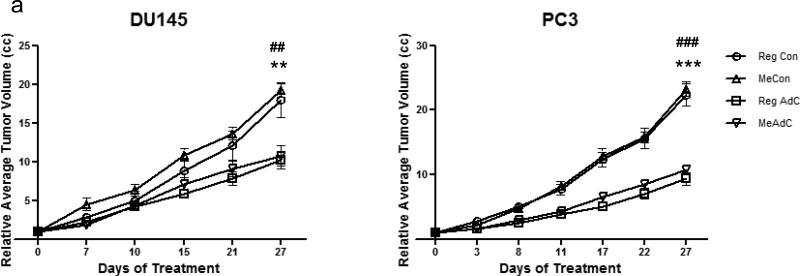
Xenografted DU-145 cells and PC-3 cells developed into palpable tumors 2 weeks after inoculation into immunodeficient mice at which point measurements were begun. In 4 weeks, the DU-145 tumor volume increased 18 fold in mice on Reg diet while it increased 19 fold in mice on Me diet. In the case of PC-3 cells, in 4 weeks, the tumor volume increased 22 fold in Reg diet-fed mice and 23 fold in the Me diet-fed mice. Thus, there was no difference in the rate at which these xenografts grew in mice whether fed standard Reg diet or one fortified with methyl donors – i.e. Me diet (Figure 1a).
Fig.1. Growth of DU-145 and PC-3 xenografts in mice fed Reg or Me Diet.
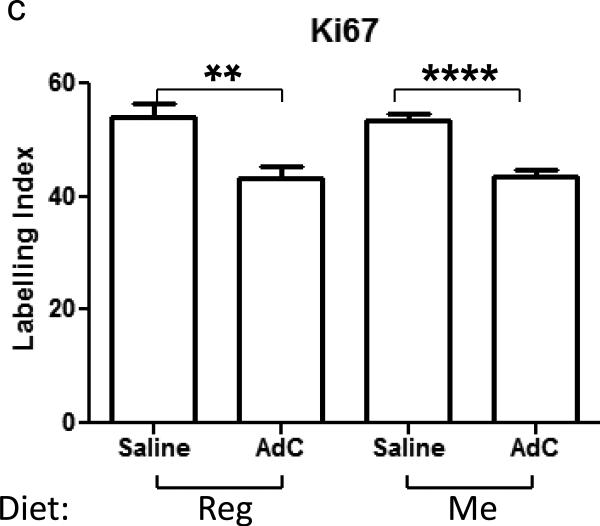
(a) Xenograft volume measurements during a four week treatment period,* = P < 0.05 by ANOVA shows significant difference between saline and AdC treatment in Reg diet-fed mice and # = P < 0.05 by ANOVA shows significant difference between saline and AdC treatment in Me diet-fed mice, (b) Representative immunohistochemistry of Ki-67 staining of DU-145 xenografts, (c) quantitation of Ki-67 staining *, P < 0.05 by Student's t test shows significant difference in proliferation index between saline and AdC treatment in both Me and Reg diet-fed group.
ii. On Effect of AdC
At the end of the 4 week AdC/saline treatment, the relative volume of DU-145 xenografts had only increased 10 fold from the time of initiation of the experiment in mice on the AdC + Reg diet and similarly, 11 fold in the AdC + Me diet; for PC-3 xenografts the volume of tumors increased 9 fold from the time of initiation of the experiment in mice on the AdC + Reg diet and 11 fold in the AdC + Me diet. Thus, treatment with AdC significantly slowed the growth of the xenografts irrespective of which diet the mice were fed (Figure 1a).
No signs of overt toxicity measured as significant differences in either the mean weights, histology of internal organs, mean blood chemistries, and hematopoietic values, were found between saline-treated mice and those that received 4 weeks of AdC (data not shown), either on Reg/Me diet. Histological analysis of DU-145 tumors from saline-treated mice revealed a small central core of necrosis, which constituted ~20% of the area of the tumor section. None of the tumor sections from mice treated with AdC were necrotic indicating that the metronomic mode of administering AdC in this study resulted in normalized and thus well-perfused xenografts, irrespective of the dietary group the mice belonged to.
iii. On Proliferation
To further define the effects of AdC and Me diet on markers of cell proliferation in human PCa cells growing as xenografts, sections of the xenografts were then analyzed for cell proliferation by immunohistochemical staining with antibodies for Ki-67, and counter staining of all nuclei by Mayer's hematoxylin. Figure 1b shows representative slides of tissues from untreated mice either on Reg diet or Me diet and 4-week AdC/saline-treatment. Brown nuclear staining are areas positive for cells undergoing proliferation. The purple areas of nuclear staining are areas of live cells that are not in a proliferative state while the areas in which no staining is seen are the areas of necrosis. The proliferative areas were quantitated for Ki-67 expression by determining the percentage of brown nuclei/field over a total of brown and blue nuclei in that field. This revealed no difference in the percentage of Ki-67 positive staining nuclei between xenografts of mice on Reg and on Me diet. However, the xenografts from mice treated with AdC, showed a 19% to 20% decrease in proliferation index compared to saline treated mice in the Reg and Me diet-fed groups, respectively (P < 0.05 between AdC and saline treated mice on Reg/Me diet by ANOVA followed by Bonferroni's test) (Figure 1c).
Effect of Methyl Proficient Diet on Demethylation of AR and Reprimo by 5-aza-2'-deoxycytidine in vivo
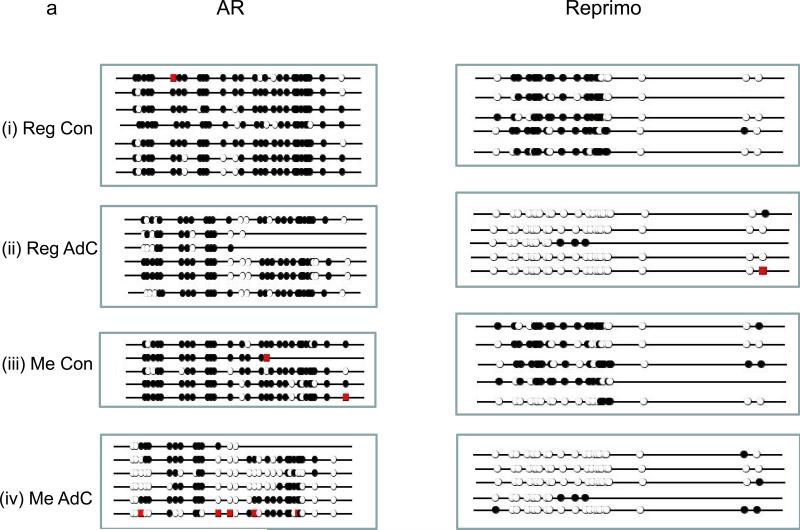
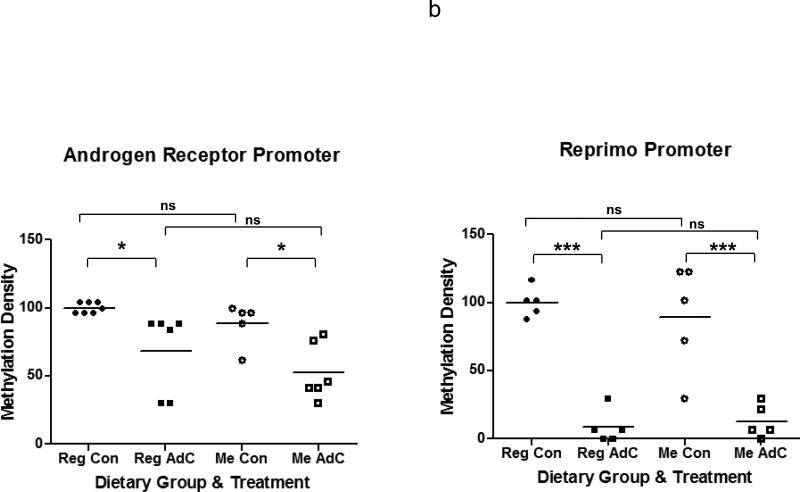
Since demethylation of hypermethylated genes in tumor clones is a target effect of AdC (23), we looked at (de)methylation of AR and Reprimo (Figure 2) in DU-145 cells treated with AdC / saline while being exposed to Reg or Me diet. The dose and schedule of AdC was previously demonstrated to be growth inhibitory and resulting in demethylation while being similar to the dose and schedule used in humans (17, 23). The examined regions of the AR and Reprimo promoters are methylated by 90% and 66%, respectively, in untreated DU-145 cells growing as xenografts on mice fed Reg diet. Treatment with AdC for 4 weeks resulted in a drop of the methylation, irrespective of the dietary group the mice belonged to. After normalizing the baseline to the methylation levels of the Reg diet group, we observed a 32% decrease in methylation density of AR promoter and 92% decrease of Reprimo promoter in AdC treated Reg diet-fed mice. The AR and Reprimo promoters were similarly methylated by 79% and 58%, respectively, in untreated DU-145 cells growing as xenografts on mice fed Me diet. Again, after normalizing the baseline to the methylation density of Reg diet-fed mice, we observed a decrease in methylation density of Me diet-fed mice that were treated with AdC for 4 weeks of 47% for AR and 87% for Reprimo. To summarize, with AdC treatment, there is a 32% and 47% decrease in methylation of AR promoter in Reg and Me diet-fed mice, respectively, while there is a greater decrease in the methylation of the Reprimo promoter of 92% and 87% in Reg and Me diet-fed mice, respectively. In saline treated mice, while there is a trend towards a decrease in methylation of promoters in Me diet-fed mice compared to the promoters of the same genes in mice fed Reg diet, this decrease was not significant.
Fig.2. Methylation in AR and Reprimo promoter regions from DU-145 xenografts in mice fed Reg or Me Diet.
(a) Bisulphite treated sequences from a 378 bp region of the AR (left panel) and 305 bp region of the Reprimo promoter (right panel) from xenografts of DU-145 tumors. (i) Reg diet control, (ii) Reg diet + AdC (iii) Me diet control (iv) Me diet + AdC. Filled circle – methylated CpG island, Open circle – unmethylated CpG island, Red square – incomplete bisulphite modification of C. (b) Scatter plot showing quantitation and significance of methylation density between groups. Significant at P ≤ 0.05 by ANOVA followed by Bonferroni's multiple comparison test, are marked (*); ns= not significant.
Effect of Methyl Proficient Diet on Development of Prostate Tumors in “Hi-myc” Mice
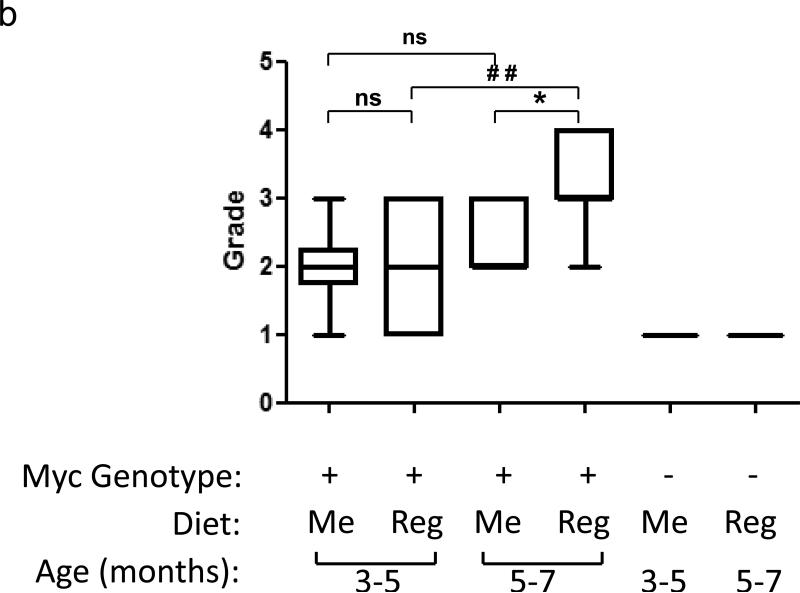
No difference was noted between the total body weights or the weights of prostates between age matched Me diet-fed and Reg diet-fed “Hi-myc” mice. H&E staining and grading by a blinded pathologist revealed different stages of mouse prostate cancer progression (Figure 3a). Figure 3b is a graphical representation of the different grades seen in prostates from mice of different genotypes (myc positive or negative), ages (3-7 months) and dietary groups (Me diet or Reg diet). The mice not expressing the myc transgene (myc-negative) showed no evidence of any cancerous changes, irrespective of their age or dietary regimen. Thus, all prostates from 3-7 month old myc negative mice were of grade 1. In the myc-expressing mice, the grades ranged from 2 to 3 in the 3 - 5 month old age range and 2 to 4 in the higher age range of 5 - 7 months. Age-dependent increases in grade were significant only in the Reg diet-fed mice (p <0.05, ANOVA, followed by Bonferroni's multiple comparison test) as no age dependent increase in grade between Me diet-fed 3 – 5 month old age group vs. Me diet-fed 5 – 7 month old age group was seen. However, as the Me diet-fed mice grew older than 6 months, an age dependent increase in grades now began to appear. Me diet-fed 6 – 7 month old mice had higher grades than Me diet-fed 5 – 6 month old mice. There was a noticeable absence of a higher grade (grade 4) tumor in Me diet-fed mice vs. Reg diet-fed mice.
Fig.3. Development of Prostate Tumors in “Hi-myc” Mice fed Reg or Me Diet.
(a) Left column: 100 X Magnification; Right column: 400 X Magnification of H&E Staining of Different Grades of tumors – i) 6 month old Myc negative mice showing normal histology, ii) 5 month old Me diet-fed mice with mPIN lesions and iii) 6 month old Reg diet-fed mice showing microinvasive cancer, (b) Box and whiskers plot comparing histological grades of PCa in groups of mice grouped on age, dietary exposure and myc status (Myc = myc positive, Neg = myc negative) where grades 1=Benign, 2=mPIN, 3=Microinvasive, and 4=Invasive adenocarcinoma. Significant difference between the grades due to age (#) or dietary exposure (*) at P ≤ 0.05 by ANOVA followed by Bonferroni's multiple comparison test, are marked; ns= not significant.
Effect of Methyl Proficient Diet on Levels of Circulating and Tissue Me donors - Folate, Methionine and Homocysteine
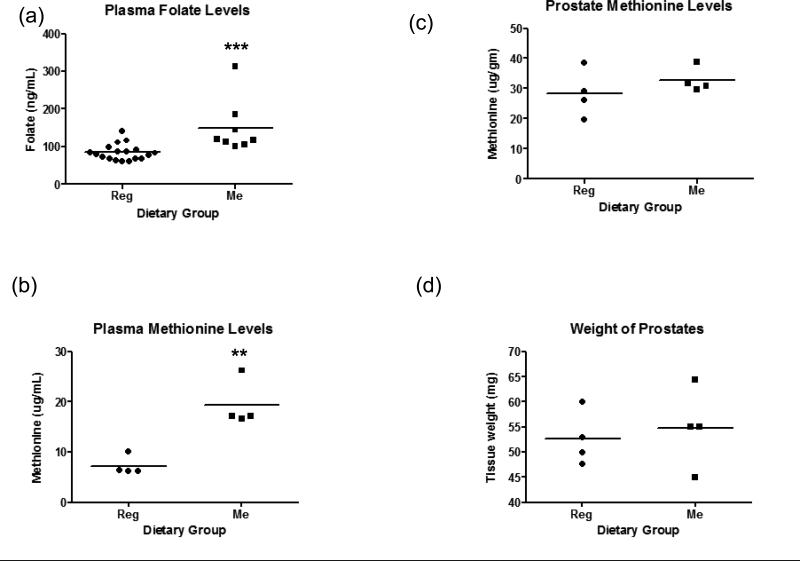
Folate levels were 84.59 ± 5.127ng/mL, n=18 and 150.2 ± 25.19ng/mL, n=8 in Reg diet and Me diet-fed mice, respectively (Figure 4a). Thus, mice on the Me diet have ~2 fold higher mean levels of circulating folate in their plasma than mice on Reg diet (P=0.0014). Differences in sample numbers analysed are due to the fact that blood samples that showed traces of hemolysis were excluded from the analysis to avoid confounding of results by folate from RBCs. Methionine levels in mice were 7.24 ± 0.9872 μg/mL in Reg diet-fed group and 19.32 ± 2.328 μg/mL in Me diet-fed group; n=4 for each group (Figure 4b). Thus, mice fed the Me diet had ~3 fold higher mean levels of circulating methionine in their plasma than mice fed Reg diet (P=0.031). The prostate methionine levels were in the range of 20 – 40μg/gm of prostate organ wet weight in the case of Reg diet-fed mice and 30-40μg/gm of prostate organ wet weight in the case of Me diet-fed mice (Figure 4c). The mean levels of methionine in the prostate of Me diet-fed mice (32.88 ± 2.024ug/gm) though not significant, tended to be higher than the corresponding mean levels in the prostates of Reg diet-fed mice (28.44 ± 3.930 μg/gm); n=4 for each group. Homocysteine levels in plasma in both dietary groups were below levels of quantitation of the assay chosen for this analysis. The gross anatomy of isolated prostates was the same for prostates isolated from each of the dietary groups (data not shown). The mean wet weights of prostates between dietary groups was similar too (Figure 4d).
Fig.4. Methyl Donor Levels and Weight of Prostates in Mice fed Reg or Me Diet.
Dot plots showing (a) Plasma folate levels in Reg diet and Me diet-fed “Hi-myc” mice. ***, P < 0.005 by Student's t test, n=18 in Reg diet-fed group and n=8 in Me diet-fed group. (b) Plasma methionine levels in Reg diet and Me diet-fed mice. **, P < 0.05 by Student's t test, n=4 in each group. (c) Prostate methionine levels in Reg diet and Me diet-fed “Hi-myc” mice, n=4 in each group. (d) Weights of prostate in Reg diet and Me diet-fed “Hi-myc” mice, n=4 in each group; Bar drawn at mean.
Effect of Methyl Proficient Diet on Global Methylation Levels
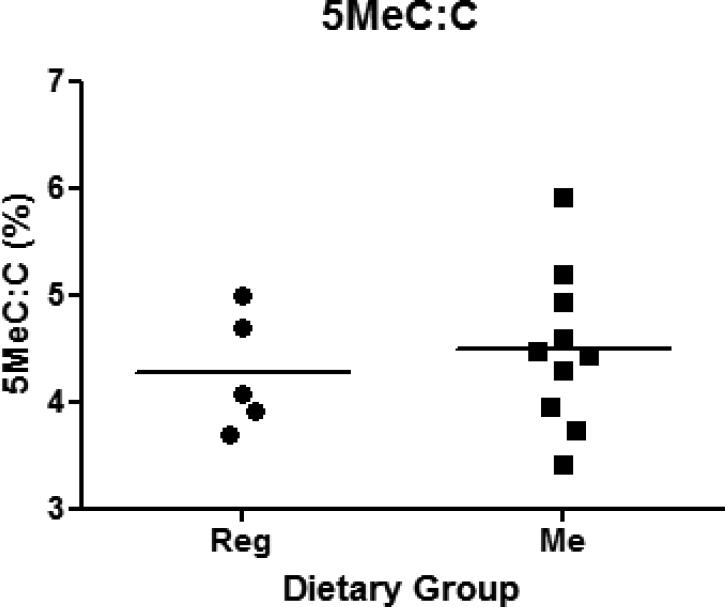
5 “Hi-myc” mice fed Reg diet and 10 “Hi-myc” mice fed Me diet following the same regimen as described in the histopathological section, were sacrificed at 5 months of age. The mean ratio of methyl-Cytosine to Cytosine in DNA was determined by mass spectrometry to be 4.3% and 4.5% in the Reg diet and Me diet-fed groups, respectively (Figure 5). Thus, no significant difference in global methylation levels in DNA from prostates between the two dietary groups was observed. Similarly, no significant difference in methylation of promoter of AR in the two groups was seen (results not shown).
Fig.5. Global Methylation Levels in Mice fed Reg or Me Diet.
Dot plot showing ratio of 5-methyl-C : C in prostates of 5 month old Reg and Me diet-fed “Himyc” mice. Bar drawn at mean. n=5 in Reg group and n=10 in Me diet group.
Effect of Methyl Donors on Expression of the c-Myc Oncogene
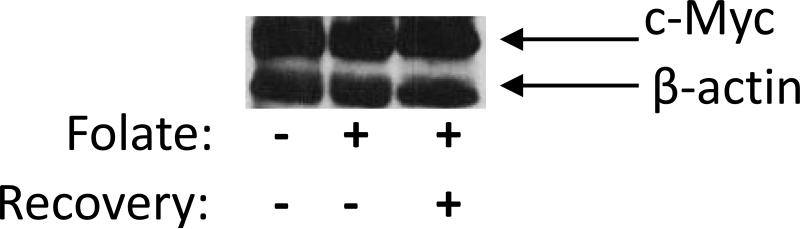
In order to rule out the possibility that dietary methyl donors were affecting c-Myc transgene expression even before the surge in androgen levels in the Hi-myc mice, we examined c-Myc protein levels in Myc PCa cells by Western blots (Figure 6) and found similar amounts of c-Myc protein for regular media, one fortified with folate, and one in cells that were first grown in fortified media for 7 days followed by regular media for another 7 days.
Fig.6. Immunoblotting Myc Levels in Myc-PCa cells on regular and folate fortified media.
Immunoblotting of protein extracts of Myc-PCa cells for c-Myc with actin as the loading control. Myc-PCa cells grown in regular media (lane1), one fortified with folate (lane2), and one in cells that were first grown in fortified media for 7 days followed by regular media for another 7 days (lane3).
DISCUSSION
This study was undertaken to determine the effects of excess amounts of methyl donors in the diet on methylation of DNA, growth rate of tumors, efficacy of the demethylating drug 5-aza-2'-deoxycytidine (AdC) to demethylate DNA and finally on the initiation and development of prostate tumors. The diet used in this study is similar to the 3SZM diet used by Cooney et al. and Cropley et al. except for the amounts of zinc sulphate (4 fold higher) and choline chloride (3 fold lower)(24, 25). The changes were made based on the premise that the levels of choline in diets used in the published studies are responsible for the increased toxicity seen in the latter, as well as some other studies where a similar diet was used.
Exposure to excess methyl donors did not influence the growth rate of athymic nude mice on the diet or the xenografted PCa cells. We hypothesized that Me diet would affect the efficacy of demethylating drugs merely by releasing into circulation excess methyl donors that could potentially methylate newly synthesized DNA before AdC binds to that DNA strand. However, the heterogeneous loss of CpG methylation within each single allele examined (by bisulphite sequencing) strongly supports demethylation within the xenografts despite the presence of excess circulating methyl donors. These results could be a function of having stoichiometrically more AdC than Me donors in circulation. Unlike methotrexate – an antifolate drug used for the treatment of rheumatoid arthritis, psoriasis, ectopic pregnancies and cancer – whose efficacy is affected by dietary folate (13), the current data suggest that AdC used at clinically relevant dose is not similarly affected by the presence of excess methyl donors in the diet.
In chemoprevention studies, agents are administered at a high dose that approximates the maximum tolerated dose for that agent. Because the excess methyl donors did not influence growth of preformed tumors or the ability of AdC to demethylate, it does not mean that the diet was insufficiently loaded with methyl donors or a lack of oral bioavailability. Measurements of circulating methyl donors - folate and methonine, showed that these donors were present in at least 2-fold and 3-fold greater amounts, respectively, than the levels circulating in mice on Reg diet. Thus, the levels of methyl donors were actually much higher than the recommended daily requirement of rodents. A similar methyl-proficient diet was shown to have epigenetic effects in the agouti mouse model (26). The agouti locus may be more sensitive to methylation changes than either the AR or Reprimo loci examined here. The time frame of exposure to dietary methyl donors and the time elapsed since the exposure and analysis may be differentiating factors in measuring methyl dependent effects too. The body weights of age matched athymic nude or “Hi-myc” mice on Reg or Me diet were not different indicating that the total caloric intake of mice from either dietary group was not different. Thus, a lowered caloric intake could not have occurred, eliminating the reasoning that the protective effects of the Me diet on the growth of the cancer is due to the protective effects of caloric restriction on cancer- as has been observed in other experiments (27).
The results showing lack of effect of excess methyl groups on growth of preformed cancer cells in vivo also add to the evidence supporting the role of the methylating enzyme rather than excess dietary methyl donors in determining the methylation state. Eads et al. have shown that DNMT-1 hypomorphic alleles reduce the frequency of CpG island methylation in the normal mucosa and intestinal polyps (28). An increased level of DNMT-1 gene expression in some types of cancer (29) changes in the set-and-site specificity of DNA-methyltransferases, and an appearance of new proteins with DNA-methyltransferase activities in tumor (hepatoma) cells of rats fed a methyl-deficient diet, have been observed (30). Other demonstrated mechanisms of feedback regulation of DNMT-1 are: presence of methylation sensors in certain genetic elements (presence of unmethylated CpGs) (31) and RNA-mediated feedback of methylation (32). Are these mechanisms affected by excess dietary methyl donors, and if so how do they modulate DNMT-1 to cause unwarranted epigenetic changes that result in tumor suppressor gene silencing (33)- should be the focus of future studies.
No change in methylation of AR or Reprimo promoters was noted in DU-145 tumors from mice fed the methyl proficient-diet compared to the same regions from mice fed their regular diet, may imply that a threshold of circulating methyl donors is already present in Reg diet. An increase in methyl donors beyond the levels already present does not have a noticeable effect on the promoters assayed here. The lack of noticeable differences may be a result of the fact that some of the methyl donors - L-methionine, betaine, zinc sulphate, Vitamin B12, and folic acid are water soluble. When tissue storage capacity of water soluble vitamins is saturated, the rate of excretion of these increases sharply.
The markers of methylation change used in this study (AR and Reprimo) are significant because they are known to be methylated in PCa. Since the results also show that the extent of DNA demethylation by demethylating agents is gene-specific - AR undergoes less demethylation by AdC compared to Reprimo, it lends credence to the fact that different regions of the genome are differently susceptible to (de)methylation. While it has not been explored here, it may be that the structure of the chromatin around the gene may influence the methylation state of that gene / promoter. Other X-chromosome genes have also been shown to be differentially demethylated when exposed to AdC when compared to genes on other chromosomes (34). Therefore, overall changes in methylation state, global methylation, may not provide a comprehensive picture of the dynamics involved in achieving the final methylation state. Indeed, global methylation differences were not apparent in the third part of this study where the ratio of Methyl-Cytosine to Cytosine was measured for DNA from 5 month old “Hi-myc” mice fed Me diet when in utero and weaned off the diet when they were 1 month old compared to DNA from Reg diet-fed “Hi-myc” mice of the same age. Further, it may be that a threshold of methylation density exists in promoters and once this threshold methylation density is reached, no further increase in methyl donors changes the methylation on these sites.
As reported in the original paper of the “Hi-myc” mice (21), in this study too, there was an age-dependent increase in the grade of tumors in myc positive genotypes on Reg diet. In the present paper, “Hi-myc” mice were fed either Reg or Me diet while in utero, at birth and until one month of age. At this time, Me diet-fed mice were weaned off the diet and fed Reg diet until they were sacrificed at different time points between 3 months and 7 months of age to see the effects the diets had on the pathology of prostate tumors. 5-7 month old Myc mice fed Reg diet developed higher grades of tumors as compared to age-matched mice fed Me diet. This result was contrary to our hypothesis as well as the observation in xenografts where the phenotype / proliferation / methylation of promoters of xenografts from Reg diet-fed mice was not different from xenografts from Me diet-fed mice. In the case of the “Hi-myc” mice that were fed Me diet in utero and after birth only for an additional month, the significant differences in grades between groups of mice on different dietary regimens were only seen in the 5-7 month age group. If the different diets contributed to these differences in grade then it must be a residual effect of the exposure that is retained in some form of a “memory molecule” that manifests itself at the conducive time. In the present study, the conducive time was when the mice were of the ages of 5 months or higher. This is the period when the transition from mPIN to cancer takes place. According to the original paper describing the development of tumors in “Hi-myc” mice, at 6 months or higher, all “Hi-myc” mice developed invasive cancer. A previous exposure to Me diet may be influencing the mechanisms at play during the transitionary period from mPIN to invasive cancer. Our in vitro studies show that exposure to methyl donors does not affect the expression of the c-myc-transgene. Further studies are warranted, including maintaining Hi-myc mice on Reg and Me diet since weaning and not in utero and observing cancerous changes that may be occurring.
Such a delayed effect of maternal Me donor supplementation was also described by Cropley et al.,(25). Cropley et al.,report that maternal supplementation only during mid-gestation substantially affects offspring coat color. Importantly, they also show that this effect is inherited by the next generation, presumably through germ-line modifications during grandmaternal dietary supplementation of increased methyl donors. The genetic element in their case is the agouti allele Avy or Aiapy. What genetic element(s) are at play in the present study, that confer protection by delaying the onset of higher grades of cancer is under investigation. An in vitro study aimed at deciphering the role of remethylation of the genome in glioma cells by exposure to increased levels of folate shows that Sp1/Sp3-mediated transcriptional upregulation of DNMT3a and 3b proteins may be responsible for limiting the aggressiveness of glioma when exposed to increased folate (35). However, this upregulation was reported in an in vitro study where high levels of folate can be achieved and maintained, unlike in vivo studies where an increase in the circulating levels of methyl donors does not translate into proportionately higher levels in tissues. What we have not examined in the present study is the effect of continued exposure of Me diet beyond 1 month of age. We cannot extrapolate the present data to hypothesize on what protective effects or otherwise would be seen then.
The protective effect in the developmental model of prostate cancer gives credence to the epidemiological observations stating the cancer-protective effects of increased folate consumption before the initiation of the cancer. The current study allays fears of a “methylising” effect of diets rich in methyl-donors. Future epidemiological studies may benefit from also examining total methyl-donor levels instead of focusing on folate levels alone, along with polymorphisms that may affect tissue levels of the methyl-donors, rather than the simplistic models many epidemiological studies are based on.
CONCLUSION
In the present study, neither the growth rate of DU-145 and PC-3 xenografts nor the in vivo demethylation capacity of 5-aza-2'-deoxycytidine is affected by presence of excess dietary methyl donors. The methyl-proficient diet is protective against formation of higher grade of tumors in a developmental model of PCa. The protective effect lies in the timing of exposure to excess methyl donors. When Me diet is fed before the formation of tumors, a protective effect of the progression of PCa is seen. This protection is seen despite the lag between exposure to the diet and determination of the effect. On the other hand, excess dietary methyl donors do not influence the growth of preformed tumors.
ACKNOWLEDGEMENTS
This research was supported in part by NIH grants P30 CA006973, UL1 RR025005, and U01 CA070095
We thank Muwen Wang for help with sacrificing “myc” mice and isolating prostates.
REFERENCES
- 1.Ross SA. Diet and DNA methylation interactions in cancer prevention. Ann N Y Acad Sci. 2003;983:197–207. doi: 10.1111/j.1749-6632.2003.tb05974.x. [DOI] [PubMed] [Google Scholar]
- 2.Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–5. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- 3.Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–7. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman DR, Haning JA, Cornatzer WE. Effects of a methyl-deficient diet on rat liver phosphatidylcholine biosynthesis. Can J Biochem. 1981;59:543–50. doi: 10.1139/o81-075. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JD, Martin JJ, Harris BJ, Kyle WE. Regulation of hepatic betaine-homocysteine methyltransferase by dietary betaine. J Nutr. 1983;113:519–21. doi: 10.1093/jn/113.3.519. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Aperte E, Ubeda N, Achon M, Perez-Miguelsanz J, Varela-Moreiras G. Impaired methionine synthesis and hypomethylation in rats exposed to valproate during gestation. Neurology. 1999;52:750–6. doi: 10.1212/wnl.52.4.750. [DOI] [PubMed] [Google Scholar]
- 7.Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–9. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, et al. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem. 2007;282:31777–88. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- 9.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–6. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, et al. Folic acid and prevention of colorectal adenomas: A combined analysis of randomized clinical trials. Int J Cancer. doi: 10.1002/ijc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–9. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 13.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann MJ, Engers R, Florl AR, Otte AP, Muller M, Schulz WA. Expression changes in EZH2, but not in BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation changes in prostate cancer. Cancer Biol Ther. 2007;6:1403–12. doi: 10.4161/cbt.6.9.4542. [DOI] [PubMed] [Google Scholar]
- 15.Schulz WA, Hatina J. Epigenetics of prostate cancer: beyond DNA methylation. J Cell Mol Med. 2006;10:100–25. doi: 10.1111/j.1582-4934.2006.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomaszewski JJ, Cummings JL, Parwani AV, Dhir R, Mason JB, Nelson JB, et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011 doi: 10.1002/pros.21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabbeer S, Washington E, Kachhap S, Kortenhorst M, Carducci MA. Studies on 5-aza-2'-deoxycytidine in prostate cancer cells in vitro and in vivo. AACR Meeting Abstracts. 2005;2005:771–a. [Google Scholar]
- 18.Miayake H, Tolcher A, Gleave ME. Chemosensitization and delayed androgen-independent recurrence of prostate cancer with the use of antisense Bcl-2 oligodeoxynucleotides. J Natl Cancer Inst. 2000;92:34–41. doi: 10.1093/jnci/92.1.34. [DOI] [PubMed] [Google Scholar]
- 19.Shabbeer S, Kortenhorst MS, Kachhap S, Galloway N, Rodriguez R, Carducci MA. Multiple Molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo. Prostate. 2007;67:1099–110. doi: 10.1002/pros.20587. [DOI] [PubMed] [Google Scholar]
- 20.Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–7. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 22.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 23.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci MA, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Research. 2006;66:6361–9. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 24.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 25.Cropley JE, Suter CM, Beckman KB, Martin DI. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A. 2006;103:17308–12. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 28.Eads CA, Nickel AE, Laird PW. Complete genetic suppression of polyp formation and reduction of CpG-island hypermethylation in Apc(Min/+) Dnmt1-hypomorphic Mice. Cancer Res. 2002;62:1296–9. [PubMed] [Google Scholar]
- 29.Casillas MA, Jr., Lopatina N, Andrews LG, Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Mol Cell Biochem. 2003;252:33–43. doi: 10.1023/a:1025548623524. [DOI] [PubMed] [Google Scholar]
- 30.Vanyushin BF. Enzymatic DNA methylation is an epigenetic control for genetic functions of the cell. Biochemistry (Mosc) 2005;70:488–99. doi: 10.1007/s10541-005-0143-y. [DOI] [PubMed] [Google Scholar]
- 31.Slack A, Cervoni N, Pinard M, Szyf M. Feedback regulation of DNA methyltransferase gene expression by methylation. Eur J Biochem. 1999;264:191–9. doi: 10.1046/j.1432-1327.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 32.Vanyushin BF. DNA methylation in plants. Curr Top Microbiol Immunol. 2006;301:67–122. doi: 10.1007/3-540-31390-7_4. [DOI] [PubMed] [Google Scholar]
- 33.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 34.El Kharroubi A, Piras G, Stewart CL. DNA demethylation reactivates a subset of imprinted genes in uniparental mouse embryonic fibroblasts. The Journal of Biological Chemistry. 2001;276:8674–80. doi: 10.1074/jbc.M009392200. [DOI] [PubMed] [Google Scholar]
- 35.Hervouet E, Debien E, Campion L, Charbord J, Menanteau J, Vallette FM, et al. Folate supplementation limits the aggressiveness of glioma via the remethylation of DNA repeats element and genes governing apoptosis and proliferation. Clin Cancer Res. 2009;15:3519–29. doi: 10.1158/1078-0432.CCR-08-2062. [DOI] [PubMed] [Google Scholar]