Abstract
Contact hypersensitivity (CHS) is a CD8 T cell-mediated response to hapten skin sensitization and challenge. The points at which IL-1 receptor (IL-1R) signaling is required during this complex, multistep immune response have not been clearly delineated. The role of IL-1R signaling during 2, 4 dinitrofluorobenezene (DNFB) sensitization to induce hapten-specific CD8 effector T cells and in the trafficking of the effector T cells to the DNFB challenge site to elicit the response were investigated using IL-1R deficient mice. DNFB-sensitized IL-1R−/− mice had low CHS responses to hapten challenge that were caused in part by marked decreases in hapten-specific CD8 T cell development to IL-17 and IFN-γ producing cells during sensitization. Hapten-primed wild-type CD8 T cell transfer to naïve IL-1R−/− mice did not result in T cell activation in response to hapten challenge indicating a need for IL-1R signaling for the localization and/or activation of the CD8 T cells at the challenge site. Decreased CD8 T cell priming in sensitized IL-1R−/− mice was associated with marked decreases in hapten-presenting dendritic cell migration from the sensitized skin to draining lymph nodes. Transfer of hapten-presenting dendritic cells from wild-type donors to naïve IL-1R−/− mice resulted in decreased numbers of the dendritic cells in the draining lymph nodes and decreased priming of hapten-specific CD8 T cells when compared to dendritic cell transfer to naïve wild-type recipients. These results indicate that IL-1R signaling is required at multiple steps during the course of sensitization and challenge to elicit CHS.
Keywords: Skin, IL-1 receptor, CD8 T cells, neutrophils, dendritic cells
Introduction
Allergic contact hypersensitivity (CHS) is a T cell mediated immune response to epidermal sensitization and subsequent challenge with a hapten. Both sensitization to induce the hapten-specific effector T cells and elicitation to activate expression of their effector function require interactions between the innate and adaptive immune compartments that occur in distinct stages. Application of hapten to the skin induces an inflammatory response that provokes cutaneous dendritic cell activation and CCR7-mediated migration through the efferent lymphatics to the skin draining lymph nodes where the dendritic cells prime hapten-specific populations of effector T cells (1–5). The skin-derived dendritic cells program antigen-reactive T cells to express receptors such as E-selectin ligand and CCR4 that direct trafficking to the skin (6–9). Hapten challenge of sensitized individuals provokes the trafficking of the primed effector T cells to the skin challenge site and activation to mediate the characteristic edema of the response. Hapten-primed CD8 T cells are the primary effector T cells mediating responses to dinitrofluorobenzene (DNFB), oxazolone and urushiol, the reactive hapten in poison ivy and poison oak (10–13). More recent studies have indicated that separate populations of hapten-specific IFN-γ- and IL-17-producing CD8 T cells are primed in response to DNFB and oxazolone sensitization and that both are required for the elicitation of the response to hapten challenge (14–17).
Hapten challenge of sensitized animals induces the rapid recruitment of the primed CD8 T cells to the challenge site. Within 3 hrs. after challenge the expression of IL-17 and IFN-γ is detected in the challenge site through localization of the primed CD8 T cells to the vasculature of the skin challenge site and activation by hapten-presenting endothelial cells (16, 17). While the CD8 T cells are activated within the vasculature of the challenge site to produce IFN-γ and IL-17 within 3 hrs. after challenge, it is important to note that the CD8 T cells do not penetrate the vascular barrier and infiltrate the skin parenchymal tissue at this early time. Both the IL-17 and IFN-γ are required to stimulate endothelial cells and possibly other cells in the challenge site to produce the neutrophil chemoattractants CXCL1 and CXCL2, that direct neutrophils into the parenchymal tissue of the skin challenge site. Neutrophil activation during this early infiltration is required for the subsequent infiltration of the effector CD8 T cells through the endothelial barrier in the challenge site to mediate the CHS response (16, 18). Furthermore, the intensity of this neutrophil activation regulates the numbers of hapten-primed CD8 T cells infiltrating the parenchymal tissue of the challenge site and the magnitude of the edematous response elicited (19).
The inflammatory mediators that are required for localization of the effector CD8 T cells to initiate the innate immune response component of CHS remain poorly defined. IL-1 is rapidly produced by cells in the skin in response to hapten application for sensitization and challenge (20–23). Studies from several investigators using a variety of tools have indicated a requirement for IL-1 in CHS responses. Sensitization of IL-1-deficient mice with trinitrochlorobenzene results in decreased T cell proliferative responses to hapten-labeled stimulator cells and decreased CHS responses to hapten challenge (24, 25). Anti-IL-1 antibodies or IL-1 receptor antagonists administered during sensitization also inhibit T cell priming for CHS responses and inhibit elicitation of the response when given during challenge of sensitized animals (20, 26, 27). Low CHS responses are also elicited following sensitization and challenge of mice with deficiencies in IL-1 receptor expression or in components of the inflammasome required for IL-1β processing (28–35). Although these studies indicate a requirement for IL-1 receptor signaling at some point during sensitization of hapten primed T cells and at some point during the recruitment and/or activation of these T cells to mediate CHS, the points where IL-1 signaling mediates effects that are required for the progression of sensitization and elicitation remain unclear. In this study we utilized IL-1 receptor-deficient mice to directly examine the requirement for IL-1 receptor signaling for hapten-presenting dendritic cell migration from the sensitized skin to the draining lymph nodes, for the priming of the two effector CD8 T cell populations, and for the recruitment of the hapten-primed effector CD8 T cells to the challenge to produce the IFN-γ and IL-17 that initiates the elicitation of the response.
Materials and Methods
Mice
C57/BL6 (H-2b) mice were obtained through Dr. Clarence Reeder (National Cancer Institute, Frederick, MD). IL1R−/− mice on the C57BL/6 background (B6.IL1R−/−) were obtained from Jackson Labs (Bar Harbor, ME). Female mice, 8–10 weeks of age, were used throughout these studies.
Hapten sensitization and elicitation of CHS
Mice were sensitized to 2,4-dinitrofluorobenzene (DNFB) by painting the shaved abdomen with 25 μl 0.25% DNFB (Sigma Aldrich, St. Louis, MO) in acetone/olive oil (4:1) and 10 μl to each paw on days 0 and +1 (13, 36). On day +5, the ear thickness of hapten sensitized and control, non-sensitized wild-type C57BL/6 mice were measured using an engineer's micrometer (Mitutoyu, Tokyo, Japan) and the mice were challenged on each side of each ear with 10 μl DNFB. The increase in ear thickness was measured 24 hrs. later and expressed in μm. The ear swelling response is given as the mean increase of each group of 4 individual animals ± SEM. Mice were sensitized to TRITC by painting the shaved abdomen with 25 μl 6.67 mg/ml TRITC (Sigma Aldrich, St. Louis, MO) in acetone/dibutylphthalate (1:1) and 10 μl to each paw and to FITC (Sigma Aldrich) by painting the shaved abdomen with 100 μl of a 1% solution in acetone/olive oil (4:1) and 10 μl on each paw.
Antibodies and cytokines
For flow cytometry the following antibodies were used: anti-CD3 mAb, anti-mouse CD11c mAb, anti-CD45 mAb, anti-CD11b mAb, anti-CCR7 mAb, (BD Pharmingen, San Diego, CA), F4/80, anti-CD207 mAb, and rat anti-mouse Gr-1 mAb (eBioScience, San Diego, CA). Antibodies used for in vivo depletion of CD8 or CD4 T cells or Gr-1+ cells were purchased from BioXCell, West Lebanon, NH). CD8 T cells were depleted by injecting mice with 100 μg of each anti-CD8 mAb, YTS 169 and TIB-150, on three consecutive days before sensitization. CD4 T cells were depleted before sensitization using 100 μg of each anti-CD4 mAb, GK1.5 and YTS 191. Gr-1+ cells that include neutrophils and some monocytes were depleted at the time of hapten challenge of sensitized mice by injecting 150 μg anti-Gr-1 mAb RB6.8C5 i.p. In each experiment utilizing mAb depletion of T cells or Gr-1+ cells, treated sentinel mice were used to evaluate the efficiency of cell depletion by antibody staining and flow cytometry analysis of spleen and lymph node cells (LNC) or thioglycollate-induced peritoneal exudate cells and was always >95% for CD4 and CD8 T cells and always > 85% for Gr-1+ cellss when compared to cells from control, rat IgG treated mice.
Histological analyses of CHS
Hapten challenged ears were excised from DNFB-sensitized and nonsenstized wild-type C57BL/6 and B6.IL1R−/− mice 24 hrs. after challenge with 0.2% DNFB. For staining to detect cellular infiltration into the challenge site, the skin tissue was fixed in 10% formalin, embedded in paraffin, and 8 μm sections were prepared, stained with hematoxylin and eosin, and viewed by light microscopy.
Enumeration of hapten-specific T cells producing IFN-γ and IL-17
Frequencies of hapten-specific T cells producing IFN-γ and IL-17 were determined using ELISPOT assays as previously described (16, 37). Briefly, ELISPOT plates (Unifilter 350, Polyfiltronics, Rockland, MA) were coated with 4 μg/ml anti-IFN-γ or 2 μg/ml anti-IL-17 mAb and incubated overnight at 4° C. The plates were blocked with 1% BSA in PBS and washed four times with PBS. LNC suspensions from non-sensitized or DNFB-sensitized mice were prepared on day +5 post-sensitization, CD8 T cells were enriched by removing the CD4 T cells with anti-CD4 mAb conjugated magnetic beads (Invitrogen, Carlsbad, CA) and magnetic removal of the cells, and the remaining cells were used as responder cells in the assay. Syngeneic spleen cells from naïve mice were treated with 50 μg/ml mitomycin C and then labeled with 100 μg/ml DNBS for use as stimulator cells. Stimulator cells were plated at 5 × 105cells/well with either 2 × 105, 5 × 105, or 1 × 106 responder cells/well in serum-free HL-1 medium (Bio-Whittaker, Walkersville, MD) supplemented with 1mM L-glutamine and 1mM antibiotic. Responder cells plated with unlabeled splenocytes were used as a negative (hapten-specificity) control. After 24 hrs. of culture at 37°C in 5% CO2, cells were removed from the plate by extensive washing with PBS/0.05% Tween-20 (PBS-T). Biotinylated anti-IFN-γ mAb (2 μg/ml) or biotinylated anti-IL17 mAb (1 μg/ml) was added and the plate was incubated overnight at 4°C. The following day, the plate was washed three times with PBS-T and conjugated streptavidin-alkaline phosphatase was added to each well. After 2 hrs. at room temperature, the plates were washed with PBS-T and nitroblue tetrazolium/5-bromo-4-cholor-30-indolyl substrate (Bio-Rad Laboratories, Hercules, CA) was added to each well. The resulting spots were counted with an ImmunoSpot Series I Analyzer (Cellular Technology Ltd., Cleveland, OH) that was designed to detect ELISA spots with predetermined criteria for spot size, shape, and colorimetric density.
Analysis of tissue infiltrating cells by flow cytometry
On day +5, sensitized and naïve C57BL/6 and B6.IL1R−/− mice were challenged with DNFB. After 6 or 18 hrs., the challenged skin was excised and incubated in 0.5% dispase (Invitrogen) for 18 hrs. at 4°C. The next day, the epidermis was separated from the dermis, incubated in 0.5% trypsin (Sigma Aldrich) for 60 min. at 37° C, 5% CO2, and pressed through dialysis tubing to isolate individual cells. The cells were washed twice in HBSS, incubated in 0.2% DNase (Roche, Indianapolis, IN) for 10 min at room temperature and washed again. Aliquots of 1 × 106 cells were washed in staining buffer (Dulbecco's PBS with 2% FCS/0.2% NaN3) and incubated in Fc block (BD Pharmingen) diluted 125:10,000 in the staining buffer for 20 min on ice. The cells were washed and stained with fluorochrome-labeled anti-mouse mAb to CD45 and Gr-1, CD11b, F4/80, or CD3 to identify infiltrating neutrophils, monocytes/macrophages and T cells and analyzed by two-color flow cytometry using a FACScan and CellQuest software (Becton-Dickinson, San Jose, CA). The cells were gated to exclude residual tissue debris and non-viable cells and sample data were collected on 20,000 cells.
Quantitation of CXCL1 production in the skin challenge site by immunoassay
Production of CXCL1 in the skin following hapten application was determined using a CXCL1-specific ELISA. Mice were sensitized with 0.25% DNFB by application of the hapten to the ears (10 μl/ side/ear) on days 0 and +1. The shaved trunk skin of both sensitized and nonsensitized mice was challenged with 25 μl DNFB and the challenged skin was removed at 6 or 18 h following challenge, weighed, and homogenized in 500 μl proteinase inhibitor cocktail (Sigma Aldrich) with gentle shaking for 30 min. Following centrifugation at 12000 × g for 10 min the supernatants were collected and the total protein concentration was quantified using a Coomasie Plus Protein Assay Reagent Kit (Pierce, Rockford, IL). All samples were diluted to an equivalent total protein concentration and tested for CXCL1 levels by sandwich ELISA using commercially available anti-CXCL1 mAb to coat and develop the plate and recombinant CXCL1 to generate a standard curve.
Analysis of gene expression by quantitative RT-PCR
RNA was obtained from skin homogenates excised following hapten sensitization or hapten challenge by dissolving the skin in TRIZOL reagent (Invitrogen) with subsequent chloroform extraction. cDNA was synthesized from 2 μg RNA using the TaqMan Reverse Transcription Reagent Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. PCR was performed using custom primers and FAM dye-labeled probes (Applied Biosystems, Foster City, CA) for mouse CXCL1, IFN-γ, IL-17, TNF-α ICAM-1, CCL19, CCL21, and Mrpl 32 (gene assay ID#: Mm00433859_m1, Mm00801778_m1, Mm00439619_m1, Mm00443258_m1, Mm00516023_m1 Mm00434165_m1, Mm03646971_gH, and, Mm00777741_sH respectively).
The comparative CT method for relative quantitation of cytokine gene expression was used where log measurements for each sample are made during amplification and the expression level of the Mrpl 32 housekeeping gene is subtracted from the expression level for each test cytokine gene. For each test cytokine, the expression level of a single RNA sample prepared from the unchallenged skin of non-sensitized wild-type mice was used as the calibrator and was arbitrarily set at 1.0 and the expression levels of all other samples were then normalized to the calibrator. Duplicate runs of each individual RNA sample prepared from a single mouse of 4 mice per group were tested and the data expressed as mean test cytokine expression level ± SEM.
Adoptive transfer of CD8 T cells
C57BL/6 and IL1R−/− mice were depleted of CD4+ T cells and then sensitized with DNFB. On day +4 following sensitization, LNC suspensions were prepared from the sensitized mice and aliquots of 4 × 106 cells were transferred i.v. into naïve C57BL/6 or IL1R−/− recipients that were immediately challenged with 10 μl of 0.2% DNFB to each side of each ear or 25 μl of 0.2% DNFB to the shaved abdomen.
Sensitization with TRITC and detection of hapten-expressing dendritic cells
Groups of C57BL/6 and B6.IL1R−/− mice were sensitized by application of 25 μl 6.67 mg/ml TRITC (Sigma Aldrich) to the shaved abdomen and 10 μl to each paw on day 0. On day +1 and on day +2, skin draining lymph nodes were removed, single cell suspensions were prepared, washed, and aliquots stained with combinations of FITC-conjugated anti-I-Ab mAb, and APC-conjugated anti-CD207 mAb, PerCP- or APC-conjugated anti-CD11c mAb, and PerCP-conjugated anti-CCR7 mAb. The cells were gated on the class II MHC expressing cells and the TRITC+/CD11c+, CD11c+/CD207+, and CD11c+/CCR7+ cells analyzed by flow cytometry with sample data collected on 50,000 cells.
Hapten-presenting dendritic cell transfer
C57BL/6 mice were sensitized to 0.25% DNFB on the shaved abdomen and paws, as previously described (36). On day +2, skin-draining lymph nodes were removed and single cell suspensions were prepared. Cells were incubated with anti-CD11c+ mAb coated magnetic beads (Miltenyi Biotec, Auburn CA) to isolate purified populations of CD11c+ cells. Aliquots of 3 × 105 CD11c+ cells were injected intradermally to naïve C57BL/6 or IL1R−/− mice. On day +4 following transfer, skin draining lymph nodes from recipient mice as well as sensitized or naive mice were removed and single cell suspensions were prepared for use as responders in IFN-γ ELISPOT assays.
Statistical analysis
Statistical analysis to assess differences between experimental groups was performed using Students' t test. Differences were considered significant when P < 0.05.
Results
Low magnitude CHS responses elicited in sensitized IL1R−/− mice
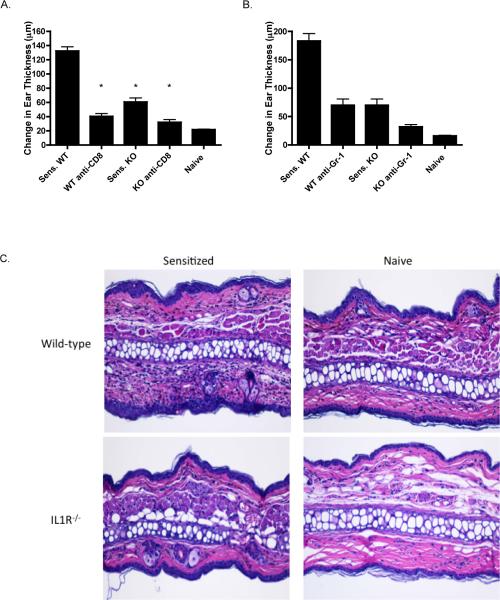
To initiate studies investigating the role of IL-1 receptor signaling in the induction and elicitation of CHS, the magnitude of CHS responses to DNFB were compared in wild-type and IL-1R−/− mice. Groups of wild-type C57B/6 and IL1R−/− mice were sensitized with DNFB and then challenged on the ear to elicit the response. When measured 24 h after challenge, the increase in ear thickness of the sensitized IL1R−/− mice was less than half that elicited in sensitized wild-type mice (Figure 1A and B). Consistent with previous results, CHS responses in sensitized mice depleted of CD8 T cells were nearly decreased to the swelling response observed in the hapten challenged ears of naïve mice (Figure 1A). In addition, depletion of Gr-1+ cells, which include neutrophils, at the time of hapten challenge of DNFB sensitized wild-type and IL-1R−/− mice also decreased the magnitude of the CHS response in both groups of mice (Figure 1B). DNFB challenged ears of sensitized and unsensitized wild-type C57BL/6 and B6.IL-1R−/− mice were excised 24 hrs. after hapten challenge and prepared sections were stained with hematoxylin and eosin. Challenged ears excised from sensitized wild-type mice exhibited the characteristic leukocytic infiltration accompanied by tissue edema and these characteristics were absent in challenged ears from sensitized B6.IL-1R−/− mice as well as in hapten challenged ears from naïve control wild-type C57BL/6 and B6.IL-1R−/− mice (Figure 1C).
Figure 1.
Decreased CHS responses elicited in hapten sensitized IL-1R −/− mice. A. Groups of wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with 0.25% DNFB on days 0 and +1. The indicated groups of mice were treated with anti-CD8 mAbs on three consecutive days prior to sensitization. On day +5 following sensitization, sensitized and nonsensitized (naïve) mice were challenged on the ear with 0.2% DNFB and the increase in ear thickness measured 24 hrs. later. *p < 0.02. B. Groups of IL-1R−/− and WT C57BL/6 mice were sensitized with 0.25% DNFB on days 0 and +1. On day +5 following sensitization, sensitized and nonsensitized (naïve) wild-type C57BL/6 mice were challenged on the ear with 0.2% DNFB and the increase in ear thickness measured 24 hrs. later. The indicated groups of sensitized mice were treated with a single dose of anti-Gr-1 mAb at the time of hapten challenge. The mean increase in ear thickness ± SEM for 4 individual mice per group is shown. *p < 0.045. Results are representative of two individual experiments. C. Ears from DNFB sensitized and unsensitized wild-type C57BL/6 and B6.IL-1R−/− mice were challenged with 0.2% DNFB and 24 hrs. later the ears were excised, fixed in formalin, and prepared sections were stained with hematoxylin and eosin and representative images were captured by light microscopy. Magnification, 200×.
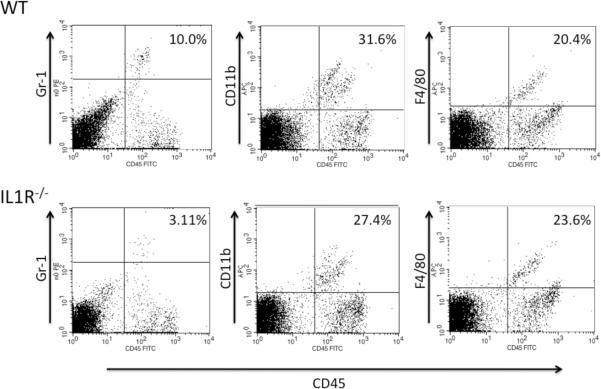
Previous studies have demonstrated that prior CXCL1-mediated neutrophil infiltration into the skin challenge site at 6 h after challenge is required to induce subsequent T cell infiltration (16, 18). To determine if the lower CHS response elicited in sensitized IL1R−/− mice was associated with decreased infiltration of neutrophils into the skin challenge site, challenged skin was excised at 6 post-challenge, digested, and cell suspensions were prepared and stained with antibodies to identify infiltrating neutrophils and macrophages. The infiltration of Gr-1+ cells that include neutrophils was markedly decreased into the challenge site of sensitized IL1R−/− mice when compared with the challenge site of sensitized wild-type mice (Figure 2). In contrast, little-no difference was observed with the presence of CD11b+ cells that include monocytes and F4/80+ monocytes and macrophages in the skin challenge site of sensitized wild-type C57BL/6 and B6.IL-1R−/− mice. As previously observed (16, 17), T (CD3+) cell infiltration into the challenge site was not observed at 6 hrs. after challenge of sensitized mice (data not shown).
Figure 2.
Decreased cellular infiltration in skin challenge site of DNFB sensitized and challenged IL-1R−/− mice at 6 hrs. post-challenge. Wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with DNFB on days 0 and +1 and challenged with 0.2% DNFB. Challenged skin was excised 6 hrs. later, digested to prepare single cell suspensions of the tissue, and cell aliquots were stained with fluorescent antibodies to identify infiltrating neutrophils, monocytes, and macrophages. Representative samples from 4–5 different animals analyzed are shown. Results are representative of two individual experiments.
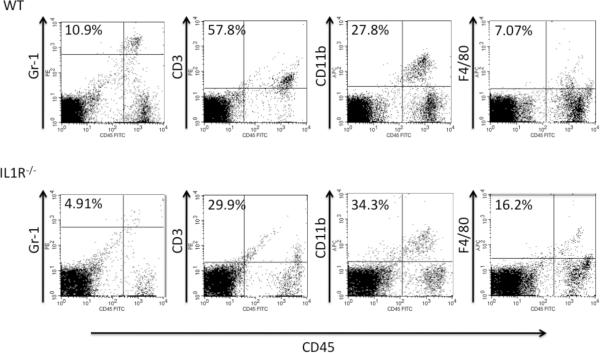
The infiltration of different leukocyte populations into the skin challenge site of sensitized wild-type C57BL/6 and B6.IL-1R−/− mice 18 hrs. after challenge was also assessed. In the hapten challenge site of sensitized wild-type animals, infiltrating Gr-1+ cells that include neutrophils and CD3+ cells (i.e. T cells) into the skin challenge site were clearly present whereas infiltration of these leukocyte populations into the challenge site of sensitized IL1R−/− mice was markedly decreased (Figure 3). Interestingly, the presence of F4/80+ cells, macrophages, was decreased in the challenge site of the wild-type and IL-1R−/− mice at this time when compared to 6 hrs. after challenge.
Figure 3.
Decreased cellular infiltration in skin challenge site of DNFB sensitized and challenged IL-1R−/− mice at 18 hrs. post-challenge. Wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with DNFB on days 0 and +1 and challenged with 0.2% DNFB. Challenged skin was excised 18 hrs. later, digested to prepare single cell suspensions of the tissue, and cell aliquots were stained with fluorescent anti-CD11b and anti-Gr-1 antibodies to identify infiltrating neutrophils and monocytes, with fluorescent anti-F4/80 antibody to identify macrophages, and with fluorescent anti-CD3 mAb to detect infiltrating T cells. Representative samples from 4–5 different animals analyzed are shown. Results are representative of two individual experiments.
Decreased production of CXCL1 induced in the challenge site of IL-1R−/− mice during the elicitation of CHS
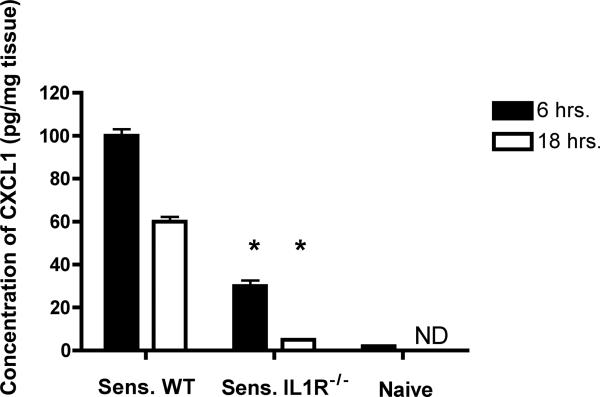
To investigate potential mechanisms underlying the low neutrophil infiltration into the skin challenge site of sensitized IL1R−/− mice, the production of the neutrophil chemoattractant CXCL1 in the site was tested. Hapten challenged skin was excised from groups of sensitized wild-type and IL-1R−/− mice 6 and 18 hrs. after challenge and prepared tissue protein was tested for quantity of CXCL1 by ELISA. CXCL1 production was decreased in the skin challenge site of sensitized IL1R−/− mice more than 70% to that induced in the challenge site of sensitized wild-type mice at both time points (Figure 4).
Figure 4.
Decreased early CXCL1 production in the skin challenge site of sensitized IL-1R−/−mice. Groups of wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized and challenged with DNFB. Challenged skin from each mouse was removed 6 or 18 hrs. after hapten challenge, weighed, and homogenized. Tissue-free supernatants were tested by ELISA to determine amounts of CXCL1. The mean concentration of CXCL1 ± SEM for 4 individual mice/group is shown. Results are representative of two individual experiments of 4 individual mice in each. *p ≤ 0.04.
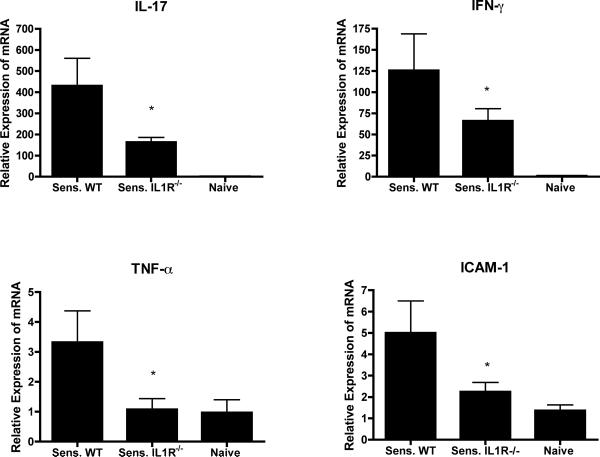
Since the production of CXCL1 within the site at 6 hrs. after challenge is dependent on the hapten-mediated activation of hapten-primed CD8 T cell populations producing IFN-γ and IL-17, the expression of these cytokines in the challenge site at this time point was tested. Whole cell RNA was prepared from challenge skin homogenates of sensitized wild-type and IL-1R−/− mice and unsensitized wild-type mice and tested for expression levels of IFN-γ and IL-17, as well as TNFα and ICAM-1, which are required for the localization of the CD8 T cells to the hapten challenge site (17). Expression levels of IFN-γ and IL-17 were significantly decreased in challenged skin of sensitized IL-1R−/− mice when compared to wild-type mice (Figure 5). Furthermore, expression levels of TNFα and ICAM-1 in the challenged skin of IL-1R−/− mice were near levels expressed in the skin following hapten challenge of naïve wild-type mice.
Figure 5.
Decreased expression of early inflammatory mediators in response to challenge of sensitized IL-1R−/− mice. Groups of wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized and challenged with DNFB. Challenged areas of skin and skin from naïve nonchallenged mice were excised 6 hrs. after challenge, snap-frozen, and homogenized. Whole cell RNA was prepared and was used to assess mRNA expression of TNF- α, IL-17, IFN- γ, and ICAM-1 in the skin samples by quantitative RT/PCR. The mean expression level for each of 4 samples per group ± SEM is shown. The results are representative of two individual experiments. *p ≤ 0.05.
IL1 signaling is required to induce hapten-primed CD8 T cells to activate endothelial cells in the skin challenge site to produce CXCL1
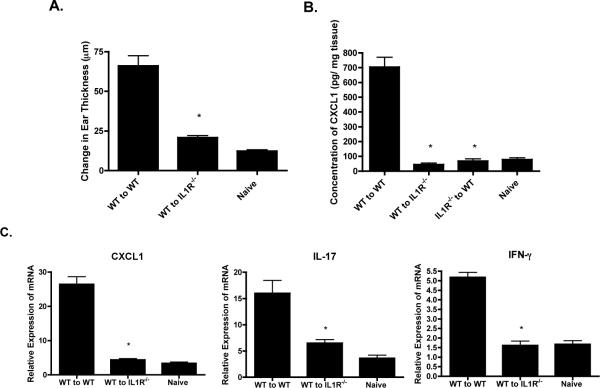
Since there was suboptimal expression of cytokines associated with the CD8 T cell mediated response following hapten challenge of DNFB sensitized IL-1R−/− mice, the ability of hapten-primed CD8 T cells to function in the IL-1R-deficient environment was investigated. First, aliquots of hapten-primed CD8 T cells from the lymph nodes of sensitized wild-type C57BL/6 mice were transferred to naïve wild-type or B6.IL-1R−/− mice and the recipients as well as a group of non-recipient wild-type mice were challenged on the ears with DNFB and the increase in ear thickness was determined 24 hrs. later. Transfer of the hapten-primed CD8 T cells induced CHS to hapten challenge in wild-type recipients whereas the response in IL-1R−/− recipients was significantly lower and similar to the increase in ear swelling observed following challenge of naïve wild-type mice (Figure 6A).
Figure 6.
Deficient activation of transferred immune WT T cells in IL1R−/− mice. Wild-type C57BL/6 and B6.IL-1R−/− mice were depleted of CD4 T cells and then sensitized with 0.25% DNFB on days 0 and +1. On day +4, cell suspensions were prepared from skin draining lymph nodes and 4 × 106 T cell aliquots were transferred i.v. to naïve wild-type or naïve IL-1R−/− mice that were then immediately challenged with 0.2% DNFB on the ears to determine passive transfer of the CHS response or on a shaved area of trunk skin to determine activation of the transferred T cells within the challenge site. A. The increase in ear thickness was measured 24 hrs. later in ear challenged groups of 4 recipient mice or challenged non-recipient wild-type C57BL/6 mice. The mean increase in ear thickness ± SEM for 4 individual mice per group is shown. *p ≤ 0.002. B. Groups of sensitized recipient mice and non-recipient wild-type C57BL/6 mice were challenged with 0.2% DNFB on a shaved area of trunk skin and the challenged skin was removed 6 hrs. later, weighed, and snap-frozen. Frozen skin was homogenized and tissue free supernatants were tested by ELISA to determine amounts of CXCL1. The mean concentration of CXCL1 ± SEM for 4 individual mice/group is shown. *p ≤ 0.02. C. Frozen skin was homogenized and whole cell RNA was prepared and used to assess mRNA expression of CXCL1, IL-17, and IFN- γ in the skin samples by quantitative RT/PCR. The mean expression level for each of 4 samples per group ± SEM is shown. *p ≤ 0.05.All results are representative of two individual experiments.
The early CXCL1 required for the progression of the CHS response is produced primarily by endothelial cells in the challenge site that are stimulated by effector CD8 T cell produced IFN-γ and IL-17 (17). Since early CXCL1 was not induced during challenge of sensitized IL-1R−/− mice, the ability of CD8 T cells from sensitized wild-type mice to restore early CXCL1 production in the skin challenge site of IL1R−/− mice was tested. Wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with DNFB and four days later purified CD8+ T cells were prepared from the skin draining lymph nodes and transferred to naïve wild-type or IL1R−/− recipients. The recipients were then challenged and the challenged skin was excised 6 hrs. later to prepare tissue free homogenates and test CXCL1 production by ELISA. Whereas hapten-primed CD8 T cells from sensitized wild-type donors induced high levels of CXCL1 production in the challenged skin of naïve wild-type recipients, these CD8 T cells failed to induce CXCL1 production in the challenged skin of naive IL1R−/− recipients (Figure 6B). CD8 T cells from the lymph nodes of sensitized IL1R−/− mice did not induce CXCL1 production upon transfer and challenge of naïve wild-type recipients.
The activation of the transferred hapten-primed CD8 T cells from sensitized wild-type donors within the skin challenge site of the naïve wild-type and IL-1R−/− recipients was then tested. The hapten challenged skin of the naïve recipients was excised 6 hrs. after challenge, whole cell RNA was isolated and tested by qRT/PCR for expression of IL-17 and IFN-γ in the skin challenge site. Again, transferred CD8 T cells from sensitized wild-type donors failed to induce the expression of CXCL1 in the skin challenge site of the naïve IL-1R−/− recipients and this was associated with induced expression levels of IL-17 and IFN-γ that were equivalent to those observed in the hapten challenged skin of naïve wild-type mice (Figure 6C). These results indicate that the transferred wild-type effector CD8 T cells are not being activated within the challenge site of the IL-1R−/− recipients and this accounts for the low production of CXCL1 induced in response to hapten challenge in the site and, in part, accounts for the inability to passively transfer CHS responses to the naïve IL-1R−/− mice.
T cell priming is deficient in sensitized IL1R−/− mice
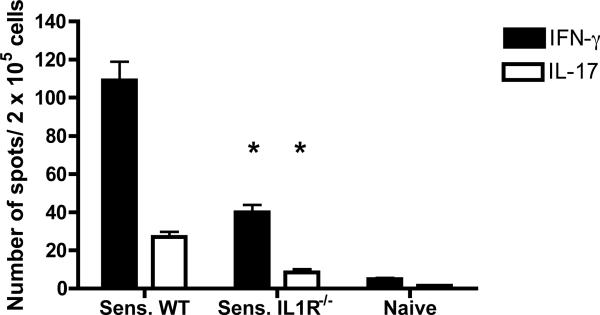
Since T cell activities inducing inflammatory events in the hapten challenge site of sensitized IL1R−/− mice were compromised, the magnitude of CD8 T cell priming in response to hapten sensitization was compared in IL1R−/− and wild-type mice. To test this, CD8 T cell enriched cell suspensions were prepared from skin draining lymph nodes of DNFB sensitized wild-type and IL1R−/− mice, aliquots were cultured with hapten labeled syngeneic splenocytes, and the numbers of hapten-specific CD8 T cells producing either IFN-γ or IL-17 were determined by ELISPOT analysis (Figure 7). As previously observed in hapten sensitized wild-type C57BL/6 mice (16), numbers of hapten-specific CD8+ T cells producing IFN-γ were approximately five fold higher than numbers of hapten-specific CD8+ T cells producing IL-17. The numbers of hapten-specific CD8+ T cells from sensitized IL1R−/− mice producing either IFN-γ or IL-17 were decreased by > 65% compared to those induced in the sensitized wild-type mice.
Figure 7.
Deficient priming of hapten-specific CD8 T cell priming in sensitized IL1R−/− mice. Wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with DNFB on days 0 and +1. On day +5, LNC suspensions were prepared from naïve and the DNFB sensitized mice and CD4 T cells were removed with anti-CD4 mAb conjugated to magnetic beads. Aliquots of 2 × 105 enriched CD8 T cells were cultured with 5 × 105 DNBS-labeled or unlabeled syngeneic splenocytes on anti-IFN-γ or anti-IL-17 mAb coated ELISPOT plates. After 24 hrs., cells were removed and the ELISPOT assay was developed to detect numbers of IFN-γ- or IL-17-producing cells. The mean number ± SEM of cytokine-producing T cells per 2 × 105 cells in triplicate cultures for 3 mice is shown after subtraction of spots from control wells containing the T cells with unlabeled stimulator cells (> 5 spots per well). Results are representative of two individual experiments. p < 0.02.
IL-1 receptor signaling is for required for migration of hapten-presenting dendritic cells into the skin-draining lymph nodes
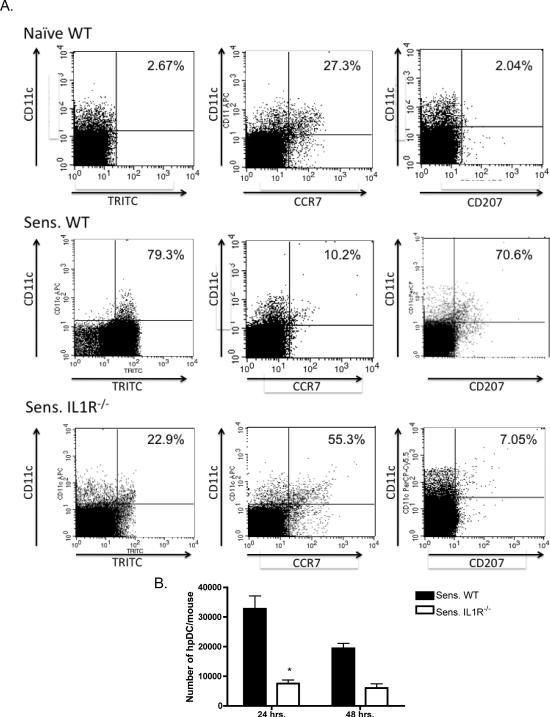
The migration of hapten-presenting dendritic cells from the sensitized skin into the skin-draining lymph nodes of IL-1R−/− mice was investigated as an underlying mechanism for the low hapten-specific CD8 T cell priming induced in response to DNFB sensitization. Groups of wild-type C57BL/6 and B6.IL1R−/− mice were sensitized with the fluorescent hapten TRITC and the skin-draining lymph nodes were removed 24 and 48 hrs. after hapten application and single cell suspensions were prepared and stained with anti-class II MHC, anti-CD11c mAb, anti-CD207, and anti-CCR7 mAb to determine the presence of hapten-labeled skin-derived and lymph node resident dendritic cells by flow cytometry. Small numbers of lymph node class II MHC+/CD11c+ cells from naive mice expressing TRITC were considered background and many of the class II MHC+/CD11c+ cells from naive mice expressed CCR7 and not CD207 indicating that they were resident dendritic cells (Figure 8A). The majority of the class II MHC+/CD11c+ cells in the skin-draining lymph nodes of sensitized wild-type mice expressed TRITC as well as CD207 indicating emigration from the skin. In contrast, there was a marked decrease in class II MHC+/CD11c+ cells in the skin-draining lymph nodes of sensitized IL1R−/− mice. However, many of the class II MHC+/CD11c+ cells in TRITC sensitized IL-1R−/− mice did express TRITC as well as CCR7 but not CD207 indicating the acquisition of hapten by lymph node resident dendritic cells following sensitization of IL-1R−/− mice. Quantitation of the flow cytometry results indicated significant decreases in the number of hapten-presenting dendritic cells in the skin draining lymph nodes of TRITC-sensitized IL1R−/− mice when compared with sensitized wild-type mice at both 24 and 48 hrs. after TRITC application to the skin (Figure 8B).
Figure 8.
Deficient migration of hapten-presenting dendritic cells from the sensitized skin to the draining lymph nodes in sensitized IL-1R−/− mice. A. Groups of wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with the hapten TRITC on day 0 and on each of the following two days skin-draining single cell suspensions were prepared from six skin draining lymph nodes. Cells were washed and aliquots stained with FITC-anti-I-Ab mAb, APC or PerCP-anti-CD11c mAb, APC-anti-CD207 mAb, and PerCP-anti-CCR7 mAb. The I-Ab expressing cells were gated and the presence of TRITC+/CD11c+, CD11c+/CCR7+, and CD11c+/CD207+ dendritic cells determined by flow cytometry analysis. Results shown are for cells analyzed at 24 hrs. after TRITC application to the skin and are representative of two different experiments. Percentages in the upper right hand quadrants indicate the percentage of class II MHC+/CD11c+ cells expressing TRITC, CCR7 or CD207. B. Total counts of the cells from six skin draining lymph node and the percentages of TRITC+CD11c+ cells from each mouse were used to determine the total number of TRITC+ (presenting) dendritic cells (hpDC) per 6 lymph nodes for each mouse at 24 and 48 hrs. after application of TRITC to the skin. The data shown are the means of total numbers of hapten-expressing dendritic cells per group ± SEM from 4−5 mice/group. p < 0.02. Results are representative of two different experiments.
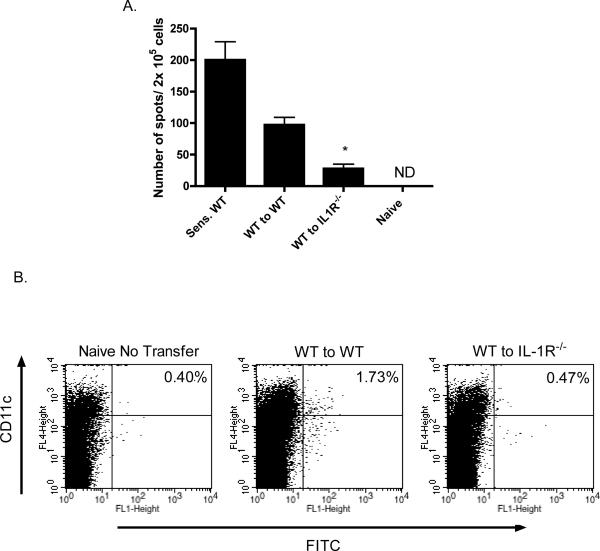
We then asked if the deficiency in hapten-presenting dendritic cell migration into the skin-draining lymph nodes in IL1R−/− mice could be overcome and T cell priming could be restored by transfer of hapten-expressing dendritic cells from sensitized wild-type mice to IL1R−/− mice. Wild-type mice were sensitized with DNFB on days 0 and +1 and on day +2 CD11c+ cells were purified from skin-draining lymph nodes and transferred intradermally into groups of wild-type or IL1R−/− naïve mice. Five days after the transfer, skin-draining lymph nodes were removed from the recipient mice and numbers of hapten-specific CD8 T cells producing IFN-γ were tested by ELISPOT assay. Transfer of the dendritic cells potently induced hapten-specific CD8 T cells producing IFN-γ in wild-type recipients though the numbers were lower than the numbers observed in lymph nodes of directly sensitized wild-type mice (Figure 9A). Hapten-specific T cells producing IFN-γ were decreased by more than 75% in IL1R−/− recipients of the dendritic cells from sensitized wild-type donors.
Figure 9.
Deficient migration of hapten-presenting wild-type dendritic cells to lymph nodes in IL-1R−/− mice. A. C57BL/6 mice were sensitized with 0.25% DNFB on days 0 and +1. On day +2, skin-draining lymph nodes were removed and single cell suspensions prepared. Purified populations of CD11c+ cells were prepared using anti-CD11c mAb-coated magnetic beads. Aliquots of 3 × 105 CD11c+ cells were injected intradermally to naïve wild-type C57BL/6 and B6.IL-1R−/− mice. On day +4 after the transfer, skin-draining lymph nodes were removed from recipient mice as well as from DNFB sensitized and naïve wild-type mice and tested for numbers of hapten-specific CD8 T cells producing IFN-γ by ELISPOT assay. *p < 0.01. B. Wild-type C57BL/6 mice were sensitized with 1% FITC on day 0. On day +2, skin-draining lymph nodes were removed, single cell suspensions were prepared, and purified populations of CD11c+ cells were prepared using anti-CD11c mAb-coated magnetic beads. Aliquots of 3 × 105 CD11c+ cells were injected intradermally into naïve wild type or IL-1R−/− mice. After 24 hrs., skin-draining lymph nodes were removed from recipient mice as well as from naïve wild-type mice and aliquots of prepared single cell suspensions were stained with PE-conjugated anti-CD11c mAb and the presence of the transferred FITC+/CD11c+ cells assessed by flow cytometry. Percentages in the upper right hand quadrants indicate the percentage of CD11c+ cells expressing FITC. Results are representative of two different experiments.
Since transfer of wild-type hapten-presenting dendritic cells to IL1R−/− mice resulted in poor priming of hapten-specific CD8 T cells, the trafficking of transferred wild-type hapten-presenting dendritic cells to the skin-draining lymph nodes in the absence of recipient IL1 receptor signaling was assessed. CD11c+ cells were prepared from lymph nodes of FITC sensitized wild-type mice and transferred intradermally to naive wild-type or IL1R−/− mice. Two days later, recipient skin-draining lymph nodes were taken and the presence of the transferred FITC+CD11c+ cells was assessed by flow cytometry (Figure 9B). Whereas the transferred FITC+CD11c+ cells were clearly present in the skin-draining lymph nodes of wild-type recipients, in the skin-draining lymph nodes of the IL1R−/− recipients these cells were near the background levels of the non-recipient wild-type mice suggesting defective trafficking of the transferred wild-type dendritic cells to the lymph nodes of the IL-1R-deficient mice.
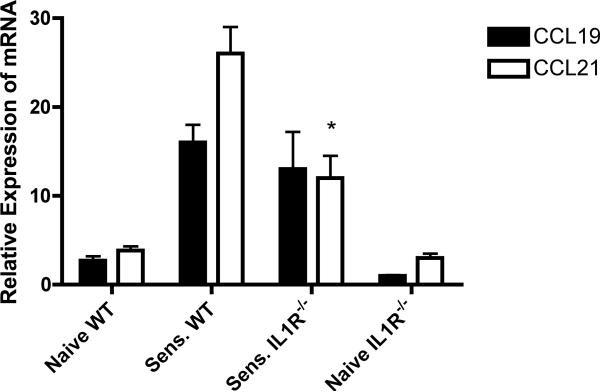
Previous studies from this laboratory have demonstrated the requirement for CCR7 binding chemokines for hapten-presenting dendritic cell trafficking from the sensitized skin to the skin draining lymph nodes (1). When the mRNA expression levels of CCL19 and CCL21 were compared in the unchallenged and hapten-challenged skin of wild-type and IL-1R−/− mice, the levels in unchallenged skin was similar in both groups of mice but there was a significant decrease in the expression of CCL21 in the skin of IL-1R−/− mice induced by hapten application (Figure 10).
Figure 10.
Decreased induction of CCL21 expression in the sensitized skin of IL-1R/− mice. Groups of wild-type C57BL/6 and B6.IL-1R−/− mice were sensitized with 0.25% DNFB on days 0 and +1. Skin was excised from sensitized mice and naïve C57BL/6 and IL1R−/− mice 3 hrs. after the second application of hapten. Skin was homogenized and whole cell RNA was prepared and used to assess mRNA expression of CCL19 and CCL21 in the skin samples by quantitative RT/PCR. The mean expression level for each of 4 samples per group ± SEM is shown. *p < 0.04.
Discussion
Hapten application to sensitize and elicit CHS responses initiates an intricate series of inflammatory events that result in hapten-specific effector CD8 T cell priming and culminate in the migration and activation of the T cells in the skin challenge site to mediate the characteristic edema of the response. Studies from this and other laboratories have shown that hapten application to the skin rapidly induces the production of the acute phase cytokines IL-1α, IL-1β and TNFα (17, 20–22). Early localization of hapten-primed CD8 T cells to the challenge site requires the TNFα induced expression of ICAM-1 resulting in their activation to produce IL-17 and IFN-γ that, in turn, stimulates endothelial cells within the challenge site to produce the neutrophil chemoattractants CXCL1 and CXCL2 as early as 3 hrs. after challenge (17). Consistent with the requirement for neutrophil infiltration and activation for the subsequent infiltration of the CD8 T cells into the challenge site, antagonism of this chemokine production inhibits neutrophil and subsequent CD8 T cell infiltration into the site as well as the CHS response. The mechanisms directing localization of the effector CD8 T cells to the challenge site and the infiltration of neutrophils into the skin challenge site remain unclear. The initial goal of this study was to test the role of IL-1 signaling in the early events that lead to hapten-primed CD8 T cell localization and activation to induce CXCL1 production in the challenged skin of sensitized mice.
Although both hapten sensitization and challenge to elicit CHS responses to various haptens are clearly inhibited by neutralization or the absence of IL-1 (20, 24–29, 32, 33), the points during the sensitization and elicitation phases of the response where IL-1R signaling is required have not been clearly identified. The initial results of this study confirm those of previous studies indicating absent or decreased CHS responses when mice are sensitized and challenged in the absence of IL-1R signaling (26, 30). In trinitrochlorobenzene sensitized mice with a targeted deletion in the IL-1α or both IL-1α and IL-1β gene, T cell proliferative responses during culture with hapten-labeled stimulator cells are markedly decreased suggesting decreased hapten-specific T cell priming in sensitized IL-1−/− mice (24, 25). In the current study we have shown the decreased priming of the hapten-specific CD8 T cell populations producing IL-17 and IFN-γ that are the effector T cells required for elicitation of the response. Recent studies from Chung and colleagues (38) have indicated that IL-1R signaling to antigen-specific CD4 T cell is required for their development to IL-17 producing cells. Hapten-specific CD8 T cell development to IL-17 producing cells was virtually absent following sensitization of IL-1R−/− mice whereas CD8 T cells producing IFN-γ were reduced more than 60% of that observed in sensitized wild-type mice but the presence of these T cells was observed at low numbers. This defective CD8 T cell priming could be due to decreases in hapten presentation in the lymph nodes or a requirement for IL-1-mediated signaling by CD8 T cells developing to IL-17 producing cells. Although both are likely to contribute to this deficiency, the presence of low level hapten-specific CD8 T cells producing IFN-γ suggests that the absence of IL-17 producing CD8 T cells is consistent with their need for IL-1R signaling during development.
This defective effector CD8 T cell priming led us to test whether hapten-presenting dendritic cell populations were trafficking from the skin sensitization site to the skin draining lymph nodes. Although several investigators had shown a decreased percentage of hapten-expressing Langerhans cells in the lymph nodes when the IL-1 signaling pathway was absent or antagonized during hapten sensitization (20, 24, 27), it has remained unclear whether this decrease was due to inhibition of dendritic cell activation within the sensitized skin or due to the inhibited migration of the dendritic cells from the sensitized skin to the draining lymph nodes. In wild-type mice the activation of dendritic cell populations in the epidermis and dermis during inflammatory events induced by hapten application result in dendritic cell acquisition of hapten but also in the down regulation of molecules tethering the cells within the skin and the upregulation of molecules (e.g. CCR7) that direct their migration into the efferent lymphatics and then into the T cell rich areas of the draining lymph nodes (39, 40). Following sensitization of IL-1R−/− mice, there was a marked decrease in the presence of hapten-presenting dendritic cells in the lymph nodes. There is no marked difference in the number of CD11c+/class II MHC+ cells in the epidermis or in the spleen (D. D. Kish, data not shown). This suggests that the defect may lie in the ability of the skin-derived dendritic cells to traffic to the lymph nodes. In support of this, when hapten-presenting dendritic cells isolated from the draining lymph nodes of hapten sensitized wild-type mice were injected intradermally into naïve wild-type vs. IL-1R−/− mice, the injected dendritic cells trafficked to the lymph nodes of wild-type recipients and primed hapten-specific CD8 T cells much more efficiently than the dendritic cells injected into IL-1R−/− recipients. Consistent with this result, the expression of the CCR7 ligand CCL21 was expressed at significantly lower levels in hapten sensitized skin of IL-1R−/− mice vs. wild-type mice. It is important to note that mRNA expression levels of the other CCR7 ligand, CCL19, were similar in the two groups of mice. These results clearly demonstrate a defect in this hapten-presenting dendritic cell migration following sensitization that is likely to contribute to the decreased effector CD8 T cell priming observed in the sensitized IL-1R−/− mice.
Our previous results have indicated that administration of anti-CCL19 mAb at the time of hapten sensitization inhibits hapten-presenting dendritic cell trafficking from the skin to the lymph nodes and CHS responses to subsequent hapten challenge (1). However, the level of hapten-specific CD8 T cell priming in this previous study was not altered by the anti-CCL19 antibody treatment. Since hapten also drains through the efferent lymphatics into the lymph node and is picked up by lymph node resident dendritic cells and other antigen presenting cells (41), we had postulated that the low numbers of hapten-reactive CD8 T cells primed were through this mechanism. The results of the current study indicate that all of the CD11c+ dendritic cells in the lymph nodes of sensitized IL-1R−/− expressed the hapten and it is likely that the majority of these cells have acquired the hapten through this process rather than through the trafficking of dendritic cells from the sensitized skin to the lymph nodes.
In addition to the decreases in hapten-specific CD8 T cell priming in IL-1R−/− mice, two points indicate that it is more than a decrease in the number of effector CD8 T cells primed underlying the absence of a CHS response. First is the low induction of CXCL1 within the challenge site 6 hrs. after challenge of sensitized IL-1R−/− mice as well as the nearly absent neutrophil infiltration into the site at this time. Second is the inability of transferred hapten-primed CD8 T cells from wild-type donors to correct these defects following challenge of naïve IL-1R−/− mice. Together these results suggest that IL-1R signaling is required for the endothelial cells to produce optimal levels of the neutrophil chemoattractant and for the infiltration of the neutrophils into the skin challenge site parenchymal tissue. Recent studies from Rao and colleagues (42) have indicated that human endothelial cells produce IL-1β and that antagonism of the IL-1R attenuates T cell infiltration into vascular allografts. In addition these and our results indicating the low expression of IL-17 and IFN-γ 6 hrs. after challenge of sensitized IL-1R−/− mice suggest that trafficking and/or activation of the CD8 T cells at the site may be impaired in the absence of ILI-1R signaling. There is a clear problem in the localization of the T cells within the challenge site of the challenged IL-1R−/− mice as transfered hapten-primed CD8 T cells from wild-type donors were unable to become activated within the challenge site of the IL-1R−/− mice. There is also a clear defect in the upregulation of TNFα and ICAM-1 in the challenge site of the challenged site of the IL-1R−/− mice and we have previously reported the need for these two inflammatory components for localization and activation of the CD8 T cells within the challenge site.
Collectively, the results of the current studies extend studies indicating the absence of CHS responses in the absence of IL-1R signaling. The current study has identified the need for IL-1R signaling for the directed migration of skin-derived dendritic cells to the draining lymph nodes to prime T cells and defects in the localization of hapten-primed effector T cell populations within the challenge site. Each of these events is essential for optimal elicitation of CHS responses and accounts for the minimal responses observed following hapten sensitization and challenge of the IL-1R−/− mice. In light of recent clinical reports that treatment with IL-1R antagonist is efficacious in reducing inflammation and improving islet beta-cell function in patients with type 2 diabetes mellitus and in reducing skin inflammation and arthritis in psoriatic patients (43–45), this strategy would be expected to have efficacy in decreasing inflammation during ongoing allergic contact dermatitis responses.
Acknowledgements
The authors thank Drs. Anna Valujskikh, Booki Min and Wink Baldwin for valuable discussions and advice during the course of this work and the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in the study. This work was supported by grant RO1 AI45888 from the National Institutes of Allergy and Infectious Diseases.
Abbreviations used in this article
- CHS
contact hypersensitivity
- DNFB
2,4-dinitrofluorobenzene
- hpDC
hapten-presenting dendritic cells
- IL-1R
interleukin-1 receptor
Footnotes
Disclosure of Conflicts of Interest The authors have no financial conflict of interest to declare with the work presented in this report.
References
- 1.Engeman TM, Gorbachev AV, Gladue RP, Heeger PS, Fairchild RL. Inhibition of functional T cell priming and contact hypersensitivity responses by treatment with anti-secondary lymphoid chemokine antibody during hapten sensitization. J. Immunol. 2000;164:5207–5214. doi: 10.4049/jimmunol.164.10.5207. [DOI] [PubMed] [Google Scholar]
- 2.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 3.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. J. Exp. Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeki H, Moore AM, Brown MJ, Hwang ST. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 5.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 6.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 7.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hammann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprograming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 8.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J. Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 9.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cels from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bour H, Peyron E, Gaucherand M, Garrigue J-L, Desvignes C, Kaiserlian D, Revillard J-P, Nicolas J-F. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur. J. Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 11.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J. Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 12.Kalish RS, Johnson KL. Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J. Immunol. 1990;145:3706–3713. [PubMed] [Google Scholar]
- 13.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon-γ-produing (Tc1) effector CD8+ T cells and Interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He D, Wu L, Kim HK, Li H, lmets CA, Xu H. IL-17 and IFN-γ mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 2009;183:1463–1470. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kish DD, Li X, Fairchild RL. CD8 T cells producing IL-17 and IFN-γ initiate the innate immune response required for responses to antigen skin challenge. J. Immunol. 2009;182:5949–5959. doi: 10.4049/jimmunol.0802830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kish DD, Volokh N, Baldwin WM, III, Fairchild RL. Hapten application to the skin induces an inflammatory program directing hapten-primed effector CD8 T cell interacction with hapten-presenting endothelial cells. J. Immunol. 2011;186:2117–2126. doi: 10.4049/jimmunol.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiIulio NA, Engeman TM, Armstrong D, Tannenbaum C, Hamilton TA, Fairchild RL. Groα-mediated recruitment of neutrophils is required for elicitation of contact hypersensitivity. Eur. J. Immunol. 1999;29:3485–3495. doi: 10.1002/(SICI)1521-4141(199911)29:11<3485::AID-IMMU3485>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Engeman TM, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J. Leukoc. Biol. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- 20.Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans cell-derived IL-1β in the initiation of primary immune responses in skin. J. Immunol. 1993;150:3698–1704. [PubMed] [Google Scholar]
- 21.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc. Natl. Acad. Sci. USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kermani F, Flint MS, Hotchkiss SAM. Induction and localization of cutaneous interleukin-1β mRNA during contact sensitization. Toxicol. Appl. Pharmacol. 2000;169:231–237. doi: 10.1006/taap.2000.9085. [DOI] [PubMed] [Google Scholar]
- 23.Kupper TS, Groves RW. The interleukin-1 axis and cutaneous inflammation. J. Invest. Dermatol. 1995;105:62S–66S. doi: 10.1111/1523-1747.ep12316087. [DOI] [PubMed] [Google Scholar]
- 24.Nakae S, Naruse-nakajima C, Sudo K, Horai R, Asano M, Iwakura Y. IL-1α, but not IL-1β, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. International Immunol. 2001;13:1471–1478. doi: 10.1093/intimm/13.12.1471. [DOI] [PubMed] [Google Scholar]
- 25.Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, Chaplin DD. Mice deficient in IL-1β manifest impaired contact hypersensitivity to trinitrochlorobenzene. J. Exp. Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo S, Pastore S, Fujisawa H, Shivji GM, McKenzie RC, Dinarello CA, Sauder DN. Interleukin-1 receptor antagonist suppresses contact hypersensitivity. J. Invest. Dermatol. 1995;105:334–338. doi: 10.1111/1523-1747.ep12320329. [DOI] [PubMed] [Google Scholar]
- 27.Cumberbatch M, Dearman RJ, Groves RW, Antonopoulos C, Kimber I. Differential regulation of epidermal Langerhans cell migration by interleukins (IL)-1α and IL-1β during irritant- and allergen-induced cutaneous immune responses. Toxiol. Appl. Pharmacol. 2002;182:126–135. doi: 10.1006/taap.2002.9442. [DOI] [PubMed] [Google Scholar]
- 28.Antonopoulos C, Cumberbatch M, Dearman RJ, Daniel RJ, Kimber I, Groves RW. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J. Immunol. 2001;166:3672–3677. doi: 10.4049/jimmunol.166.6.3672. [DOI] [PubMed] [Google Scholar]
- 29.Antonopoulos C, Cumberbatch M, Mee JB, Dearman RJ, Wei X-Q, Liew FY, Kimber I, Groves RW. IL-18 is a key proximal mediator of contact hypersensitivity and allergen-induced Langerhans cell migration in murine epidermis. J. Leukoc. Biol. 2008;83:361–367. doi: 10.1189/jlb.0604352. [DOI] [PubMed] [Google Scholar]
- 30.Klekotka PA, Yang L, Yokoyama WM. Contrasting roles of the IL-1 and IL-18 receptors in MyD88-depedent contact hypersensitivity. J. Invest. Dermatol. 2010;130:184–191. doi: 10.1038/jid.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the IL-1-β-processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H, Gehrke S, Contassot E, Roques S, Tschopp J, Freidmann PS, French LE, Gaide O. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J. Immunol. 2008;180:5826–5832. doi: 10.4049/jimmunol.180.9.5826. [DOI] [PubMed] [Google Scholar]
- 34.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 35.Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF. Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J. Exp. Med. 2010;207:2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorbachev AV, Fairchild RL. CD4+ T cells regulate CD8+ T cell-mediated cutaneous immune responses by restricting effector T cell development through a Fas ligand-dependent mechanism. J. Immunol. 2004;172:2286–2295. doi: 10.4049/jimmunol.172.4.2286. [DOI] [PubMed] [Google Scholar]
- 37.Gorbachev AV, Fairchild RL. CD4+CD25+ regulatory T cells utilize FasL as a mechanism to restrict DC priming functions in cutaneous immune responses. Eur. J. Immunol. 2010;40:2006–2015. doi: 10.1002/eji.200939387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 41.Pior J, Vogl T, Sorg C, Macher E. Free hapten molecules are dispersed by way of the blood stream during contact sensitization to fluorescein isothiocyanate. J. Invest. Dermatol. 1999;113:888–893. doi: 10.1046/j.1523-1747.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 42.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J. Exp. Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guigue P, Pages C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonis anakinra: lack of correlation with IL1RN mutations. Ann. Int. Med. 2010;153:66–67. doi: 10.7326/0003-4819-153-1-201007060-00030. [DOI] [PubMed] [Google Scholar]
- 44.Jung N, Hellmann M, Hoheisel R, Lehmann C, Haase I, Perniok A, Hallek M, Rubbert A. An open-label pilot study of the efficacy and safety of anakinra in patients with psoriatic arthritic refractory to or intolerant of methotrexate (MTX) Clin. Rheumatol. 2010;29:1169–1173. doi: 10.1007/s10067-010-1504-5. [DOI] [PubMed] [Google Scholar]
- 45.Larsen CM, Faulenbach MV, Volund A,A, Ehses J. a., Seifert B, Mandrip-Poulsn T, Donath MY. Interleukin-1-receptor antaonist in type 2 diabetes mellitus. N. Engl. J. Med. 2011;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]