Abstract
Stably expressed housekeeping genes (HKGs) are necessary for standardization of transcript measurement by quantitative real time PCR (qRT-PCR). Peripheral nerve injury disrupts expression of numerous genes in sensory neurons, but the stability of conventional HKGs has not been tested in this context. We examined the stability of candidate HKGs during nerve injury, including the commonly used 18s ribosomal RNA (18s rRNA), β tubulin I (Tubb5) and β tubulin III (Tubb3), actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hypoxanthine phosphoribosyl transferase 1 (HPRT1), and mitogen activated protein kinase 6 (MAPK6). Total RNA for cDNA synthesis was isolated from dorsal root ganglia of rats at 3, 7 and 21 days following either skin incision alone or spinal nerve ligation, after which the axotomized and adjacent ganglia were analyzed separately. Relative stability of HKGs was determined using statistical algorithms geNorm and NormFinder. Both analyses identified MAPK6 and GAPDH as the two most stable HKGs for normalizing gene expression for qRT-PCR analysis in the context of peripheral nerve injury. Our findings indicate that a priori analysis of HKG expression levels is important for accurate normalization of gene expression in models of nerve injury.
Keywords: Neuropathic pain, Gene expression, Nerve injury, Spinal nerve ligation, Quantitative Real time PCR, Housekeeping genes
Introduction
Painful peripheral nerve injury results in functional changes in primary sensory neurons that are accompanied by altered expression of numerous genes (Wang et al. 2002; Valder et al. 2003; Costigan et al. 2002; Hoffman and Cleveland 1988; Lund et al. 2002; Moskowitz et al. 1993). Various methods may be employed to quantify gene expression at the level of mRNA, including Northern blotting, RNase protection assay, and quantitative RT-PCR (qRT-PCR). Each of these methods has distinct advantages and disadvantages for determination of changes in gene expression, but a critical common component for all these methods is accurate normalization of target gene expression to a stable reference gene. An optimal reference gene or combinations of genes should control for non-biological variations in RNA preparation and the reverse transcription reaction. Since housekeeping genes (HKGs) serve basic functions that maintain normal homeostasis of the cell, they are generally considered to have minimal variability in their steady state level of expression. There is growing recognition, however, that expression of commonly used reference genes may, in fact, be unstable depending on the experimental condition (Takagi et al. 2008; Tatsumi et al. 2008), and that this may compromise conclusions regarding changes in the gene of interest. It has also been determined that use of more than one reference gene results in a more accurate normalization than that of a single gene (Vandesompele et al. 2002; Andersen et al. 2004).
In this study, we sought to identify the optimal reference genes in a relevant model of neuropathic pain following nerve injury. Specifically, we examined rat dorsal root ganglia (DRGs) 3, 7 and 21 days after spinal nerve ligation (SNL), which results in hyperalgesia identifiable by noxious mechanical stimulation of the plantar skin (Hogan et al. 2004; Wu et al. 2010). In this model, neurons of the L5 DRG are transected, whereas the adjacent L4 DRG neurons are subjected to minimal trauma combined with exposure to inflammatory mediators generated in response to the injury (Gold 2000). Since the contributions of these neuronal populations to neuropathic pain are distinct, we evaluated gene expression stability in the L4 and L5 DRGs separately. A set of seven genes commonly used for normalization in PCR was analyzed, including 18S rRNA, two different components of the cytoskeletal structures of the cell microtubular network, specifically β tubulin I (Tubb5) and β tubulin III (Tubb3), the microfilament protein actin (detected as a combination of the β and γ isoforms due to near identical homology), two different enzymes involved in metabolic pathways, specifically glyceraldehydes 3-phosphate dehydrogenase (GAPDH) and hypoxanthine phosphoribosyl transferase 1 (HPRT1), and the signaling molecule mitogen activated protein kinase 6 (MAPK6). Determination of the most stable reference genes was performed using two different statistical algorithms, geNorm and NormFinder (Vandesompele et al. 2002; Andersen et al. 2004). In addition to identifying the required number and identity of the most stable reference genes, we applied these findings to test the concept that the choice of reference genes may influence the apparent expression level of a sample gene of interest in the context of nerve injury, choosing for this purpose the stromal interaction molecule-1 (STIM1), as we have previously found it to be unaffected by injury in determinations controlled by a single untested reference gene, Tubb5 (Gemes et al. 2011).
Methods
All methods and use of animals were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Injury model
Male Sprague-Dawley rats (Taconic Farms Inc., Hudson, NY) weighing 160 to 180g were subjected to spinal nerve ligation (SNL) in a manner modified from the original technique (Kim and Chung 1992). Rats were anesthetized with 2% isoflurane in oxygen and the right paravertebral region was exposed. After removal of the L6 transverse process, the L5 and L6 spinal nerves were ligated with 6-0 silk suture and transected distal to the ligature. The fascia was closed with 4-0 resorbable polyglactin suture and the skin closed with staples. Control animals received anesthesia, skin incision and stapling only. After surgery, the rats were returned to their cages and kept under normal housing conditions with access to pellet food and water ad lib.
Sensory testing
Rats underwent sensory testing for hyperalgesic behavior as previously described (Hogan et al. 2004; Wu et al. 2010). Briefly, noxious mechanical stimulation was produced by touching the right plantar skin with a 22G spinal needle with adequate pressure to indent but not penetrate the skin. Whereas control animals respond with only a brief reflexive withdrawal, rats following SNL may display a complex hyperalgesia response that incorporates sustained elevation of the paw, licking, chewing, and grooming. The frequency of hyperalgesia responses out of 10 touches was tabulated for each test day. Sensory testing was carried out on day 3 after surgery for animals from which tissue was harvested that day, on days 6 and 7 for animals from which tissue was harvested on day 7, and days 20 and 21 for animals from which tissue was harvest 21 days after surgery. The animals were allowed to rest for 2–3 hours after sensory testing before the tissue was harvested.
Quantitative real-time PCR analysis
Total RNA from the homogenized DRGs was isolated from the aqueous phase following the manufacturer’s instructions using Trizol reagent (Invitrogen, Carlsbad, CA). Control L4 and L5 DRGs were processed together as a single sample from one control animal, whereas L4 and L5 DRGs of a single SNL animal were processed separately (n=3). Although L6 DRGs were ligated as part of the standardized SNL technique, they were not used for analysis. After DNase treatment, cDNA was synthesized from amounts of RNA that were standardized for each experiment (ranging from 265ng to 625ng for different experiments) using random hexamer primers (Superscript III first strand synthesis kit, Invitrogen). cDNA (2µl of 50µl cDNA reaction mix) was taken in each reaction for real time PCR carried out using IQ Syber Green supermix (Biorad Laboratories, Hercules, CA) on a Biorad CFX96 Real Time PCR Machine and specific primers to quantify the cDNA levels of various housekeeping genes; Tubb5, GAPDH, MAPK6, actin, Tubb3, HPRT1 and 18S rRNA (Table 1). The efficiency of the primers was 92–100% with r2>0.990. For each sample, two inter-run determinations were carried out and two replicates in each run were averaged. Expression of the two additional target genes STIM1 and galanin were also determined from the same set of samples. For the comparative CT method, day 3 control samples were used as the reference point for calculation of fold differences in expression of all the HKGs, galanin, and STIM1 in the various time and injury groups. Additional statistical analysis to determine the overall influence of injury and time was performed on the ΔCT values of the HKGs using 2-way ANOVA (factors were day and injury, and all candidate HKGs were combined into a single group for a given day and injury category), followed by Bonferroni post hoc comparisons. Galanin expression was analyzed by 2-way ANOVA, followed by post hoc comparisons of each group with the control from that day, using Bonferroni’s correction.
Table 1.
Names, sequences and amplicon lengths of primers for the 7 housekeeping and target genes
| Gene | Primer Sequences | Product Size |
|---|---|---|
| Tubb5 | FP:5` CATGGACGAGATGGAGTTCA 3` RP: 5` GAAACAAAGGGCAGTTGGAA 3` |
197 bp |
| GAPDH | FP:5` AGACAGCCGCATCTTCTTGT 3` RP: 5` TGATGGCAACAATGTCCACT 3` |
142 bp |
| MAPK6 | FP:5` TAAAGCCATTGACATGTGGG 3` RP: 5` TCGTGCACAACAGGGATAGA 3` |
129 bp |
| Actin | FP:5`AAGATCATTGCTCCTCCTGA 3` RP: 5TACTCCTGCTTGCTGATCCA 3` |
104 bp |
| Tubb3 | FP:5` TGAGGCCTCCTCTCACAAGT 3` RP: 5` TGCAGGCAGTCACAATTCTC 3` |
237 bp |
| HPRT1 | FP:5` AAGCTTGCTGGTGAAAAGGA 3` RP: 5`CCGCTGTCTTTTAGGCTTTG 3` |
185 bp |
| 18srRNA | FP:5` ACCGCGGTTCTATTTTGTTG 3` RP: 5` CTGATCGTCTTCGAACCTCC 3` |
185 bp |
| Galanin | FP:5`TACGCCCGGTTCCCACCACT 3` RP: 5`GCCAGCGCTGTTCAGGGTCC 3` |
147 bp |
| Stim 1 | FP:5`GTGCGCTCGTCTTGCCCTGT 3` RP: 5TGCGGACGGCCTCAAAGCTG 3` |
200 bp |
Analysis of ΔΔCT values used for the target gene STIM1, normalized with MAPK6/GAPDH, was performed using 2-way ANOVA. The influence of reference gene choice on apparent STIM1 expression in day 7 samples was analyzed by Mann-Whitney U test. Graphs were plotted using 2ΔCT values for HKGs data as well as for galanin and 2−ΔΔCT values for STIM1 gene expression.
geNorm & NormFinder programs
Real time analysis data for all the HKGs in different samples obtained from comparative CT determinations was employed according to the specified methods for geNorm [http://medgen.ugent.be/~jvdesomp/genorm/] and NormFinder [http://www.mdl.dk/publicationsnormfinder.htm]. geNorm calculates the gene expression stability measure (M) for a reference gene as the average pairwise variation for that gene with all other tested reference genes. Stepwise exclusion of the gene with the highest M and recalculation of M for the remaining genes allows ranking of the tested genes according to their expression stability until the two most stable genes are identified. To determine the required minimum number of HKGs, a normalization factor (NF, derived from the geometric mean of the included genes) is calculated for the two most stable genes, for the three most stable genes, and so forth. The pairwise variation of NF between groups containing sequentially larger numbers of normalization genes is calculated. According to empirical testing of this approach (Vandesompele et al. 2002), the point in this sequence at which this variation becomes less than 0.15 specifies the number of genes necessary for normalization, as additional genes add no further stability.
NormFinder calculates the intra-group and inter-group variations in the expression of all the HKGs across all the samples. From this analysis, a stability value is calculated for each HKG with a standard error. The lowest stability value is considered the single most stable expressing HKG, and low standard error is used as a secondary determining factor. Unlike geNorm, it does not determine the number of reference genes required for reliable normalization of target gene expression (Andersen et al. 2004).
Results
Animal behavior
Control animals (n=9) showed a 0±0% hyperalgesia response rate. All SNL animals (n=9) showed a hyperalgesia response rate greater than 20% and showed an average hyperalgesia rate of 54.4±5.5% (P<0.001 compared to control).
HKG expression
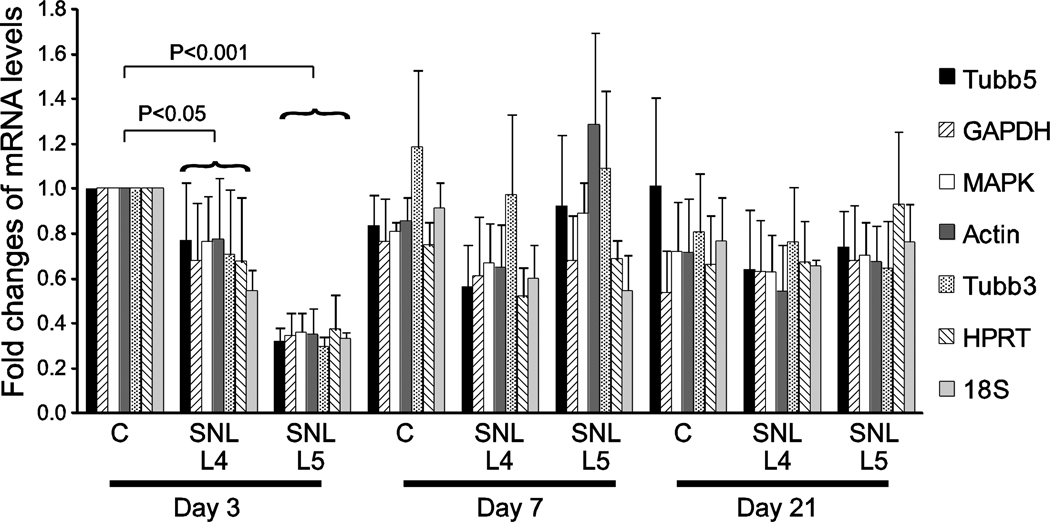
The transcript levels for each HKG were calculated using the CT values obtained from the amplification curves generated by the analysis software (Biorad CFX Manager, Biorad Laboratories). HKGs listed in order of their transcript levels averaged across all samples are as follows (CT values in parentheses): 18S rRNA (10.69±0.15) > Tubb3 (22.33±0.13) ≈ GAPDH (23.62±0.17) > actin (27.17±0.15) ≈ Tubb5 (28.21±0.15) ≈ HPRT1 (28.69±0.13) > MAPK6 (32.33±0.19). HKG transcript levels in DRGs from control and SNL animals at different time points are shown in Figure 1, represented as fold difference using day 3 control DRG samples as an arbitrary reference.
Figure 1.
Transcript levels of 7 HKGs in DRG at 3, 7 and 21 days after injury. The amount of transcript expression of each gene in each sample is calculated using the comparative CT method. Fold differences of transcript levels in 3, 7 and 21-day control and post-injury samples are calculated by comparison to day 3 controls. The data represent mean ± SEM. The brackets represent significant differences in the expression of all the HKGs taken together for each sample compared to the same day controls. The samples include Control (C), the 4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation (SNL L4), and the axotomized 5th lumbar dorsal root ganglion after SNL (SNL L5), at 3, 7 and 21 days after SNL.
Reference gene evaluation using geNorm
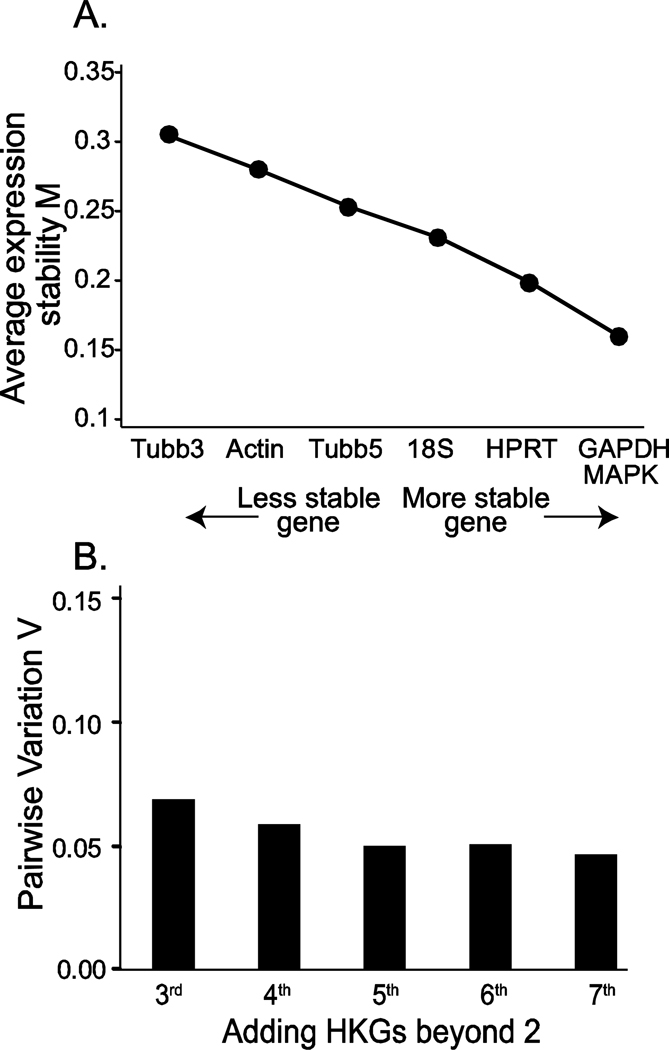
To rank HKG gene stability, we first employed geNorm, which compares gene stability on the basis of average pairwise variation of a particular gene with all other control genes. Figure 2A shows the order of stability of the 7 HKGs analyzed for the 3, 7 and 21-day SNL and control samples using geNorm software. The ranking of gene expression stability values for the 7 genes was MAPK6/GAPDH > HPRT1 > 18S rRNA > Tubb5 > actin > Tubb3. Using this analysis method, MAPK6 and GAPDH were the most stable transcripts across all the samples studied in our experiment. Furthermore, pairwise variation values (V) demonstrated that the criterion for a sufficient number of reference genes (V<0.15) is achieved at the point of comparing two versus three genes (V = 0.069, Figure 2B). Our data thus confirms that analyzing two distinct housekeeping genes, namely MAPK6 and GAPDH, would be statistically sufficient to provide reliable normalization of gene expression changes in specific genes using our experimental conditions.
Figure 2.
A. Determination of stable and required number of HKGs with geNorm software. The stability value M was determined from the data for all the 7 HKGs presented in Figure 1 using geNorm and are plotted in sequence from the most stable (low M value) on the right to the least stable on the left. B. The pairwise variation value (V) for incremental addition of genes shows that the threshold level for sufficiency (V < 0.15) is achieved with the minimum two HKGs.
Reference gene evaluation using NormFinder
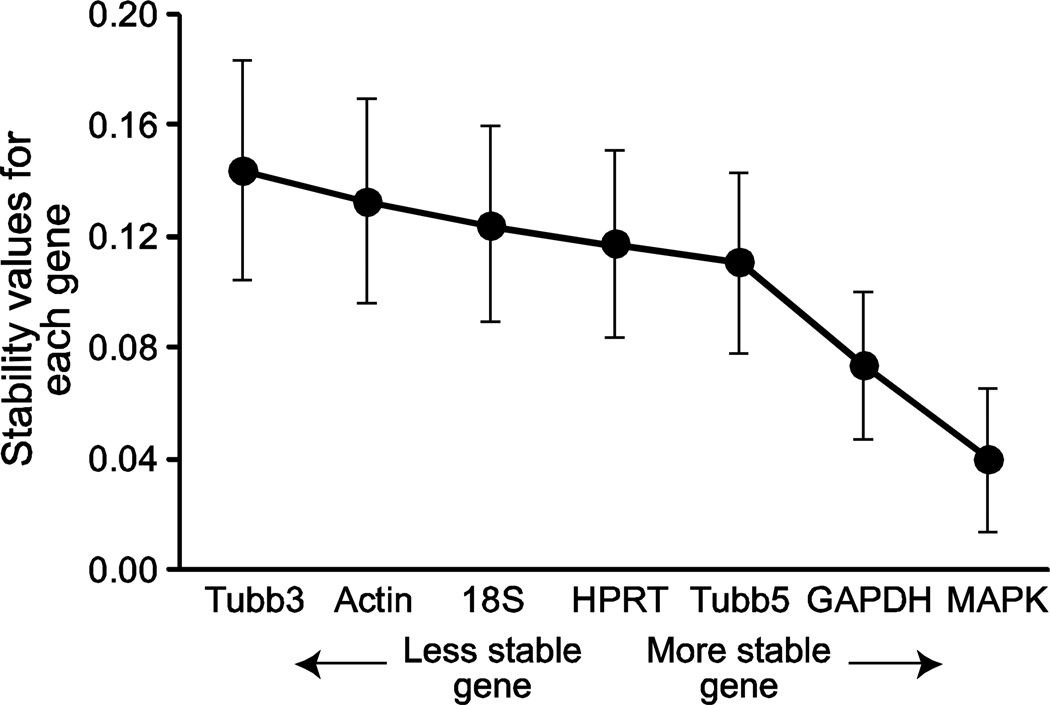
We used a second statistical program, NormFinder program, to identify the most stable reference genes by calculating inter- and intra-group variations. The most to least stable HKGs from our panel using NormFinder (Figure 3) was calculated as follows: MAPK6 > GAPDH > Tubb5 > HPRT1 > 18S rRNA > actin > Tubb3. These findings correlate extremely well with the results obtained from our analysis using the geNorm program (Figure 3).
Figure 3.
NormFinder analysis for identifying the most stable HKGs, showing the 7 HKGs in the sequence of their stability.
Effect of reference gene choice on measured target gene expression
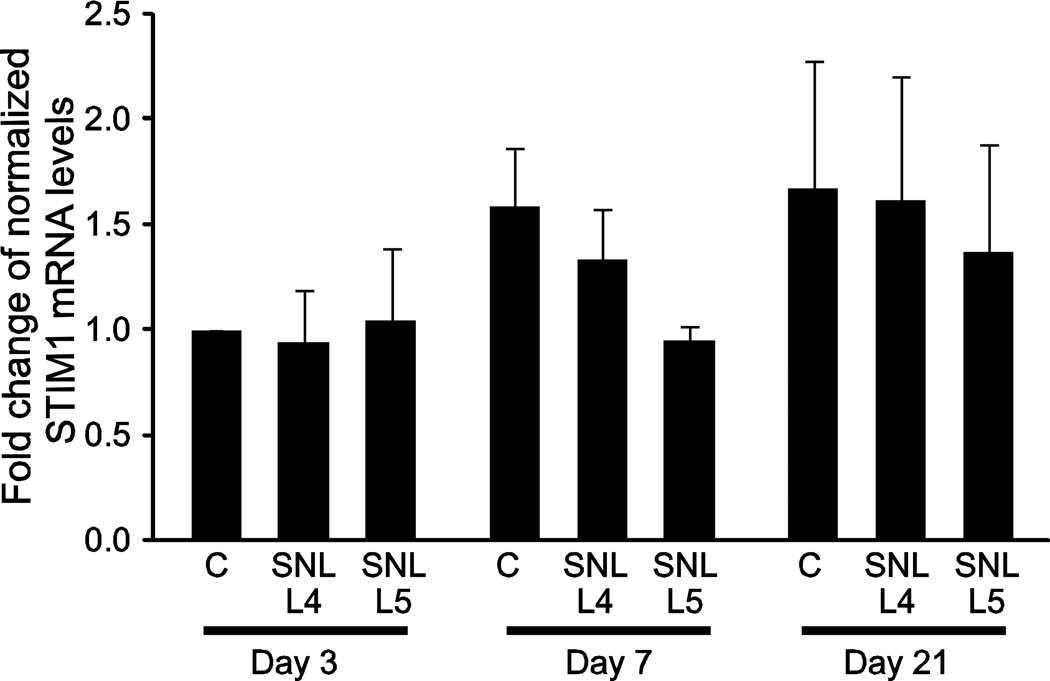
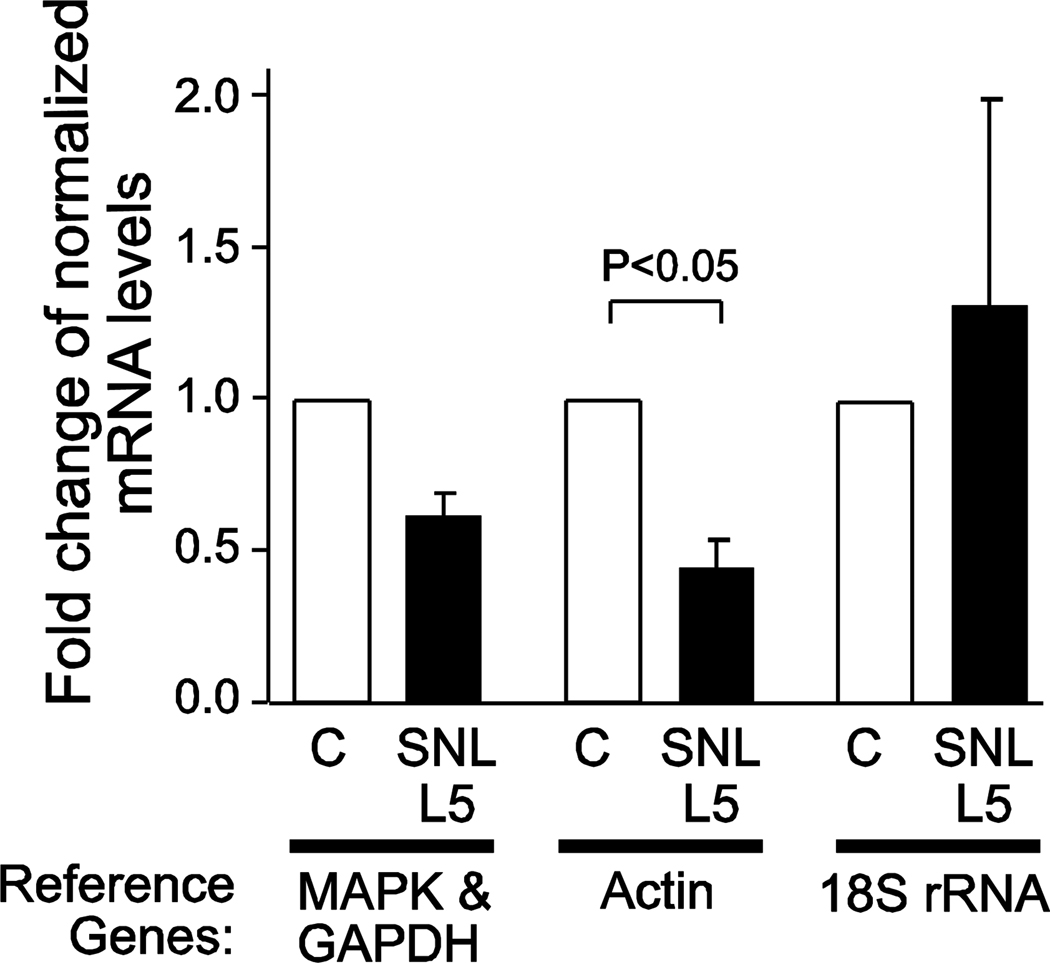
In order to identify if the choice of a particular reference gene may influence the calculated level of expression of a gene of interest, we analyzed a sample target gene, STIM1, using various HKGs for qRT-PCR quantification. Using the geometric mean of the CT values of MAPK6 and GAPDH for normalization of the CT values obtained for STIM1, transcript levels showed no significant differences between the day 3, 7 and 21 samples with and without injury, compared to the day 3 controls (Figure 4). However, when we compared the relative expression level of STIM1 in the day 7 SNL L5 samples using actin or 18s rRNA for normalization, rather than MAPK6/GAPDH, significant differences are seen (Figure 5), with the appearance of diminished STIM1 expression using actin and high variance using 18s rRNA. Thus, dynamic changes in common HKG transcript levels used to normalize qRT-PCR data can confound in the interpretation of a gene of interest, indicating that changes in reference genes must be carefully considered using the SNL-injury model. Although we did not formally evaluate the stability of STIM1 compared to the other reference genes, the fact that STIM1 did not appear to change with injury suggests that this mRNA product may also have qualities of a reference gene in this injury model.
Figure 4.
Stromal interaction molecule-1 (STIM1) gene expression after injury. The tissue samples analyzed for STIM1 expression included skin incision control (C), the 4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation (SNL L4), and the axotomized 5th lumbar dorsal root ganglion after SNL (SNL L5), at 3, 7 and 21 days after surgery. Expression of STIM1 was calculated using the comparative CT method following qRT-PCR. Fold differences of transcript levels in 3, 7 and 21-day control and post-injury samples were calculated by comparison to day 3 controls after normalization with the two most stable reference genes, MAPK6 and GAPDH. The data is shown as mean ± SEM. No significant differences were identified between groups using 2-way ANOVA.
Figure 5.
Effect of reference genes on Stromal interaction molecule-1 (STIM1) gene expression. The expression level of STIM1 was measured at day 7, and the fold difference was calculated between injured versus control DRG using various normalizing genes. The data represent mean ± SEM. Brackets represent significant differences by Mann-Whitney U test.
Global influence of day and injury
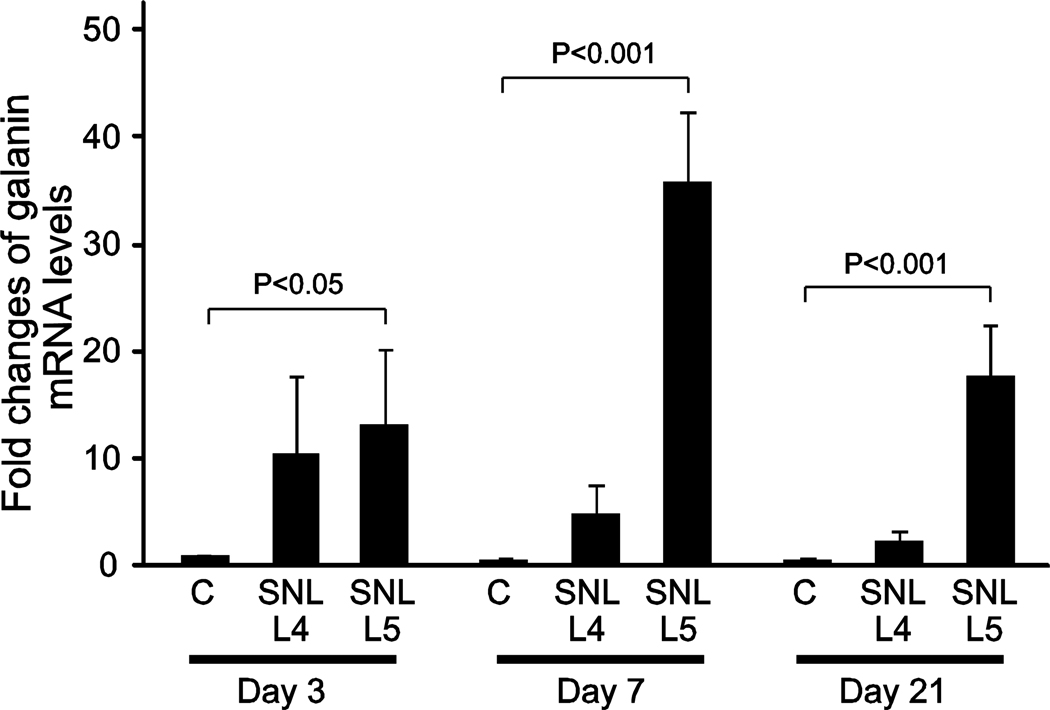
HKG expression (Figure 1) suggested a generalized depression of levels on day 3 in the SNL DRGs. We therefore analyzed the overall influence of day and injury on HKG expression by 2-way ANOVA of the HKGs grouped together. There was a significantly reduced level of global HKG transcript on day 3 in both the L4 DRGs (1.4±0.23 fold or 30±8.1% reduction; P<0.05) and L5 DRGs (2.9±0.23 fold or 66±2.6% reduction; P<0.001) from SNL animals compared to the control DRGs. This difference resolved on days 7 and 21, at which time there was no significant difference between control and SNL-treated DRG at either L4 or L5. Primer dysfunction or inhibition of the reverse transcriptase reaction cannot explain this anomaly, since the same primer sets as well as reverse transcriptase reaction mix did not produce this effect on samples from other days. The same pattern was exhibited in three different experiments (n=3). A loading error is also an unlikely explanation because this finding was consistent in three different experiments for each of the affected experimental groups. Another possibility could be accelerated degradation of those samples during RNA isolation. To determine whether quantification of all genes is similarly affected at day 3 after SNL-injury, we analyzed the expression of galanin, which is a transcript that is known to be elevated following injury (Hokfelt et al. 1994; Valder et al. 2003). We measured a significantly higher expression of galanin (13.3±7 fold; P<0.05) in L5 DRG compared to the day 3 controls (Figure 6). The expression of galanin further increased to 35.9±6.7 fold (P<0.001) at day 7, and then returned back towards day 3 SNL-injury levels (17.7±4.8 fold; P<0.001) by day 21. Thus, we conclude that transcript levels are not depressed universally by the SNL-injury.
Figure 6.
Gene expression of galanin following injury to the DRG. The tissue samples analyzed for galanin expression included skin incision control (C), the 4th lumbar dorsal root ganglion after 5th lumbar spinal nerve ligation (SNL L4), and the axotomized 5th lumbar dorsal root ganglion after SNL (SNL L5), at 3, 7 and 21 days after surgery. Galanin expression was calculated using the comparative CT method following qRT-PCR. Fold differences of transcript levels in 3, 7 and 21 control and post-injury samples were calculated by comparison to day 3 controls. Data are shown as mean ± SEM. Statistical analysis was performed by 2-way ANOVA. Brackets indicate significant paired comparisons to control groups for each day (Bonferroni’s correction).
The comparative evaluation of HKGs stability using either of the analytical programs was unaffected by removal of the day 3 data (data not shown). Therefore, the decrease in the day 3 SNL L4 and L5 transcript levels did not adversely affect the performance of the geNorm and NormFinder programs in determining the optimal reference genes.
Discussion
Our study examined samples across a time series and between three injury conditions (skin incision, SNL L4, and SNL L5) to evaluate the stability of HKGs after peripheral nerve injury. The set of reference genes examined in our study were chosen, because of their previous use to normalization changes in gene expression. For example, MAPK6 and GAPD have been found to be stable normalization genes in rat brain tissue (Cai et al. 2007). Use of 18S RNA and actin have been highly published in the literature. On the other hand, in situ hybridization and Northern blotting experiments have shown that β tubulin isoforms may change depending on the subtype. Specifically, β-tubulin I is unchanged after injury whereas β- tubulin III is increased by axotomy (Hoffman and Cleveland 1988; Moskowitz et al. 1993). To confirm these previous findings, we included both β- tubulin isoforms in our analysis, using the more sensitive qRT-PCR method.
The analysis using geNorm and NormFinder identified MAPK6 and GAPDH as the two HKGs with the most stable expression across our experimental conditions, and therefore predicts that these are optimal among those in this panel for the normalization of a target gene in this setting. In contrast, some commonly used housekeeping genes such as Tubb3 and actin would be undesirable choices for normalization. There was a confirmatory concordance between geNorm and NormFinder in rating the stability for the seven HKGs, and both identified MAPK6 and GAPDH as the optimal reference genes for normalization of real time data in this peripheral nerve injury model. As expected, our exercise with a test gene STIM1 revealed a strong dependency of relative expression levels upon the choice of the reference gene.
An unexpected finding was substantial and generalized reduction of expression for all tested HKGs 3 days after axotomy in the SNL L5 samples and to a lesser extent in the L4 samples at that time. This difference may result from the more severe injury of the L5 neurons, which suffer complete axotomy, whereas there is minimal direct injury of the neurons in L4. This effect resolved by day 7 after injury, but it makes the use of even the most reliable reference genes for normalization 3 days after nerve injury problematic. Specifically, decreased reference gene levels may produce an artifactual apparent elevation of target gene expression. Therefore, transcript level determinations should be interpreted cautiously in the immediate post-injury time interval. Quantifying the expression of genes of interest at least 7 days after injury should be more reliable.
The etiology of generalized suppression of HKG transcript levels 3 days after peripheral axotomy is uncertain. Transcript degradation during isolation can be considered to explain the findings, but this is unlikely to happen in all 3-day SNL samples from 3 different experiments and not in others, and selectively for only HKGs. The observation that galanin expression is elevated in the same day 3 samples for which other HKGs are depressed indicates that there is not an absolute transcriptional impediment. In addition to regulation of transcription, there is growing evidence that a variety of mechanisms modulate the rate of mRNA decay (Garneau et al. 2007), both of which might play a role. Furthermore, since we started each determination with comparable amounts of total RNA, the observed results need not result from a decrease in the examined HKG RNA, but could just as well reflect an injury-induced increase in other RNAs in the fixed mass of the sample. Large increases in transcripts have been noted after injury for various genes, including galanin, neuropeptide Y, and vasoactive intestinal peptide (Valder et al. 2003). However, elevated expression in the DRG after injury persists for most genes up to 2 weeks (Kim et al. 2009), although examples of rapid recovery within 48hrs also exist (Boeshore et al. 2004). Finally, the observed day 3 fall in apparent HKG expression could result from a change in the relative contribution of RNA from different populations of DRG cell types, attributable to glial activation and proliferation or inflammatory cell recruitment after injury. In contrast to our findings using SNL, other reports using sciatic nerve transection in the thigh have not observed low HKG transcript levels 3 days after injury (Costigan et al. 2002; Xiao et al. 2002). However, only approximately 50% of DRG neurons project to the sciatic nerve at the level of the thigh (Devor et al. 1985), so a diminished effect may result from this factor. Furthermore, that lesion is more distant from the DRG and may produce delayed changes relative to the very proximal SNL injury. In contrast to our findings in DRGs, a lesion made close to the superior cervical ganglion increases galanin mRNA but without any obvious decrease in GAPDH mRNA (Sun and Zigmond 1996).
There may be a benefit in using a neuron-specific HKG gene as reference for a target gene that is also only expressed in neurons, since this will normalize in a fashion that ignores changes in the mass or makeup of non-neuronal RNA. β Tubulin-III (Tubb3) is an example of a reference gene with expression restricted to neurons (Moskowitz et al. 1993), but we found this to be the least stable gene in our panel of HKGs under the conditions of peripheral nerve injury. Similarly, expression of other neuron-specific proteins including growth associated protein 43 (GAP-43) (Nahin et al. 1994), microtubule associated protein 2 (MAP-2) (Boeshore et al. 2004) and neuron specific enolase (NSE) (Kirino et al. 1983) have been shown to be altered after nerve injury. A neuron-specific marker that is stable following peripheral nerve injury has not yet been identified.
Our findings provide an insight into a possible source of error during quantification of genes involved in neuropathic pain processes after peripheral nerve injury. However, other HKGs have been identified as optimal normalizing genes when examining neuronal tissue in other settings (Bonefeld et al. 2008; Nelissen et al. 2010; Langnaese et al. 2008; Santos and Duarte 2008), and it is likely that a similar exercise evaluating stability of reference genes in different chronic pain models, such as those for inflammatory pain or cancer pain, would support the use of different HKGs for normalization. Even in models of neuropathic pain based on trauma at sites other than the lumbar spinal nerves, optimal employment of qRT-PCR may require independent determination of the most stable reference genes.
Conclusion
MAPK6 and GAPDH are the most stable reference genes for use in normalizing transcript level of a target gene in the context of nerve injury determined by the geNorm and NormFinder program analysis. The reduced transcript levels of HKGs in samples 3 days after axotomy indicate that measuring genes of interest using even these HKGs for normalization may be inaccurate, and determinations at 7 days or more after injury may be preferable for identifying the effect of nerve injury. Our work extends previous findings in other tissues that show the importance of validating reference gene stability for reliable qRT-PCR analysis.
Acknowledgments
This study was supported by NIH grant NS42150 (to Q. Hogan). We thank Gregory Fischer, Hsiang-En Wu, Vasiliki Zoga, and Qingbo Tang for assistance in experimentation, and Daniel Eastwood for statistical advice.
List of Abbreviations
- SNL
spinal nerve ligation
- HKG
housekeeping gene
- qRT-PCR
quantitative real-time polymerase chain reaction
Footnotes
Competing Interests: The authors claim no conflict of interest.
Authors’ contributions: QH, FP, MLYB, and AH conceived and designed the experiments. QH, FP and MLYB were involved in writing the manuscript. MLYB also performed the experiments and analyzed the data. JBM was involved in designing the HKG primers.
Contributor Information
Madhavi Latha Yadav Bangaru, Email: mbangaru@mcw.edu.
Frank Park, Email: fpark@mcw.edu.
Andy Hudmon, Email: ahudmon@iupui.edu.
J. Bruce McCallum, Email: bmccallum@mcw.edu.
References
- Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol. 2004;59(2):216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- Bonefeld BE, Elfving B, Wegener G. Reference genes for normalization: a study of rat brain tissue. Synapse. 2008;62(4):302–309. doi: 10.1002/syn.20496. [DOI] [PubMed] [Google Scholar]
- Cai JH, Deng S, Kumpf SW, Lee PA, Zagouras P, Ryan A, Gallagher DS. Validation of rat reference genes for improved quantitative gene expression analysis using low density arrays. Biotechniques. 2007;42(4):503–512. doi: 10.2144/000112400. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R, Frank I, Raber P. Proliferation of primary sensory neurons in adult rat dorsal root ganglion and the kinetics of retrograde cell loss after sciatic nerve section. Somatosens Res. 1985;3(2):139–167. doi: 10.3109/07367228509144581. [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8(2):113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, Koopmeiners AS, Cruikshank JM, Kwok WM, Hogan QH. Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci. 2011;31(10):3536–3549. doi: 10.1523/JNEUROSCI.5053-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS. Spinal nerve ligation: what to blame for the pain and why. Pain. 2000;84(2–3):117–120. doi: 10.1016/s0304-3959(99)00309-7. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW. Neurofilament and tubulin expression recapitulates the developmental program during axonal regeneration: induction of a specific beta-tubulin isotype. Proc Natl Acad Sci U S A. 1988;85(12):4530–4533. doi: 10.1073/pnas.85.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101(2):476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17(1):22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors. Pain. 2009;143(1–2):114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kirino T, Brightman MW, Oertel WH, Schmechel DE, Marangos PJ. Neuron-specific enolase as an index of neuronal regeneration and reinnervation. J Neurosci. 1983;3(5):915–923. doi: 10.1523/JNEUROSCI.03-05-00915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langnaese K, John R, Schweizer H, Ebmeyer U, Keilhoff G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol Biol. 2008;9:53. doi: 10.1186/1471-2199-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LM, Machado VM, McQuarrie IG. Increased beta-actin and tubulin polymerization in regrowing axons: relationship to the conditioning lesion effect. Exp Neurol. 2002;178(2):306–312. doi: 10.1006/exnr.2002.8034. [DOI] [PubMed] [Google Scholar]
- Moskowitz PF, Smith R, Pickett J, Frankfurter A, Oblinger MM. Expression of the class III beta-tubulin gene during axonal regeneration of rat dorsal root ganglion neurons. J Neurosci Res. 1993;34(1):129–134. doi: 10.1002/jnr.490340113. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Ren K, De Leon M, Ruda M. Primary sensory neurons exhibit altered gene expression in a rat model of neuropathic pain. Pain. 1994;58(1):95–108. doi: 10.1016/0304-3959(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Smeets K, Mulder M, Hendriks JJ, Ameloot M. Selection of reference genes for gene expression studies in rat oligodendrocytes using quantitative real time PCR. J Neurosci Methods. 2010;187(1):78–83. doi: 10.1016/j.jneumeth.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Santos AR, Duarte CB. Validation of internal control genes for expression studies: effects of the neurotrophin BDNF on hippocampal neurons. J Neurosci Res. 2008;86(16):3684–3692. doi: 10.1002/jnr.21796. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996;67(4):1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- Takagi S, Ohashi K, Utoh R, Tatsumi K, Shima M, Okano T. Suitable reference genes for the analysis of direct hyperplasia in mice. Biochem Biophys Res Commun. 2008;377(4):1259–1264. doi: 10.1016/j.bbrc.2008.10.137. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Ohashi K, Taminishi S, Okano T, Yoshioka A, Shima M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem Biophys Res Commun. 2008;374(1):106–110. doi: 10.1016/j.bbrc.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87(3):560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114(3):529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11(3):280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99(12):8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]