Abstract
Wiskott-Aldrich syndrome (WAS) is a rare X-linked disorder caused by mutations in the WAS gene. Glomerulonephritis is a frequent complication, however, histopathological data from affected patients is scarce because the thrombocytopenia that affects most patients is a contraindication to renal biopsies. We found that WASp-deficient mice develop proliferative glomerulonephritis reminiscent of human IgA nephropathy (IgAN). We examined whether increased aberrant IgA production is associated with the development of glomerulonephritis in WASp-deficient mice. Serum IgA and IgA production by splenic B cells was increased in WASp-deficient mice compared to wild-type (WT) mice. A lectin-binding study revealed a reduced ratio of sialylated and galactosylated IgA in the sera from old WASp-deficient mice. Circulating IgA-containing immune complexes showed significantly higher titers in WASp-deficient mice compared to WT mice. These results indicate that the increased IgA production and aberrant glycosylation of IgA may be critically involved in the pathogenesis of glomerulonephritis in WAS.
1. Introduction
Wiskott-Aldrich syndrome (WAS) is a rare X-linked disorder caused by mutations in the WAS gene and is characterized by the triad of thrombocytopenia, eczema, and susceptibility to infection(1). Autoimmune complications are also exceedingly common in WAS and affect 40–70% of patients according to retrospective cohort studies. These associated disorders increasingly complicate the clinical management of WAS as affected patients live longer due to more effective prophylaxis and treatment of infectious complications(2–4). Kidney disease is a frequent complication of WAS. It is found in 3.5–19% of cases(3,5) and can progresses to chronic renal failure, requiring renal transplantation(6,7). However, histopathological data regarding kidney disease is scarce because renal biopsies are often contraindicated due to thrombocytopenia in WAS patients. Membranoproliferative glomerulonephritis and interstitial nephritis have been reported in a few studies, however, in the majority of cases, IgA nephropathy (IgAN) has been diagnosed(5–11).
IgAN is characterized by immune deposits with dominant or codominant IgA component in the glomerular mesangium(12). Circulating immune complexes (CIC) containing aberrantly O-glycosylated IgA1 play a pivotal role in the pathogenesis of IgAN(13–22). It is generally accepted that CIC are formed in IgAN as galactose-deficient IgA1 are produced in affected patients and recognized by anti-glycan IgG or IgA1 antibodies(14,15). In turn, the production of CIC results in deposits in the renal mesangium and glomerular injury.
In WAS patients, increased basal activity of β1,6-N-acetylglucosamine transferase (an O-linked glycosyltransferase) results in the expression of highly O-glycosylated sialophorin or core 2 O-glycans(23). Sialophorin is a functional regulator in T-cell activation(24). Therefore, the abnormal O-glycosylation of sialophorin may have a role in immune dysfuction of WAS patients(25,26). It has been shown that retroviral-mediated genetic correction can restore the activity of β1,6-N-acetylglucosamine transferase and improve glycosylation in lymphoblastoid WAS B-cells(27). Furthermore, aberrant O-glycosylation of serum IgA1 with characteristics similar to that observed in IgAN, was detected in a WAS-carrier patient who presented with Henoch-Schönlein purpura(28).
A hyper-IgA (HIGA) mouse, a model for human IgAN with high serum IgA levels, shows reduced galactosylation of N-glycans on serum IgA(29). It has been recently reported that NZWxC57BL/6 F1-bcl-2 transgenic mice that are known to develop both IgAN-like and autoimmune lupus-like diseases, show reduced levels of galactosylation and sialylation of serum IgA(30). Altogether, these observations support the notion that aberrant N-glycosylation of IgA is involved in the pathogenesis of IgAN.
We have investigated the renal histopathology in 129/SvEv WASp-deficient mice and found that WASp-deficient mice produce autoantibodies and develop proliferative glomerulonephritis with immune complex deposition(31). In this report, we extend our characterization of glomerulonephritis in WASp-deficient mice and evaluated the role of aberrant IgA production in the development of glomerulonephritis in WASp-deficient mice.
2. Methods
2.1 Animals
WASp-deficient (Was-KO) mice on a 129 background (129S6/SvEvTac-Wastm1Sbs/J) were obtained from Jackson Labs. Mice were maintained in SPF conditions and experiments conducted according to the guidelines of the NHGRI Animal Care and Use Committee.
2.2 Renal histology, immunofluorescence studies, and electron microscopic analysis
Wild-type (WT) control and Was-KO mice were euthanized at the specified ages and 5-micron sections of renal tissue were fixed in buffered formalin, stained with periodic acid-Schiff (PAS), and processed for light microscopic evaluation. Specimens that contained >30 glomeruli were used for histopathologic analysis and quantitation of glomeruli with segmental and global sclerosis and/or mesangial cell proliferation and/or an increase in mesangial matrix. Each specimen was assigned a score representative of the calculated percentages of affected glomeruli (0: 0%; 1: 1–24%; 2: 25–49%; 3: >50%). The total maximal score for each specimen was 9 using this scoring system(32). Specimens were evaluated in a triple-blinded manner by 3 nephrologists. Immunofluorescence was performed on four micron cryostat sections of the other cryopreserved kidney with the use of goat anti-mouse FITC-conjugated polyclonal antibodies to IgG, IgA, IgM (K&P Laboratories, Gaithersberg, MD), and rat anti-mouse FITC-conjugated monoclonal antibody to C3 (Cedarlane, Hornby, Ontario, Canada). For electron microscopic examination, the samples were fixed with glutaraldehyde and osmium tetraoxide, embedded in EPON™ resin. Sections (100 nm) were stained with uranyl acetate and lead citrate, and examined under the electron microscope.
2.3 Measurement of urine albumin, creatinine, and serum IgA
Urine albumin and creatinine were measured using enzyme-linked immunosorbent assay (ELISA) quantitation kits (Alpha Diagnostic International, San Antonio, TX, and R&D Systems, Inc., Minneapolis, MN, USA, respectively), according to the manufacturer’s protocol.
Serum IgA was measured using a mouse IgA ELISA quantitation kit, according to the manufacturer’s protocol (Bethyl Laboratories, Inc., Montgomery, TX, USA).
2.4 In vitro IgA production of splenic B cells
Resting B cells were negatively selected from splenocytes by immunomagnetic depletion using a mouse B cell isolation kit, (Miltenyi Biotech Inc., Auburn, CA, USA). Cells (1×106 cells/mL) were stimulated in 24-well plates with 10 μg/mL lipopolysaccharide (LPS; Sigma, St. Louis, MO, USA) in combination with the following cytokines: 0.5 ng/mL transforming growth factor(TGF)-beta1 (R&D Systems, Minneapolis, MN, USA), 8 ng/mL IL-4 (PeproTech Inc., Rocky Hill, NJ, USA), and 0.25 ng/mL IL-5 (PeproTech). Cells were cultured in RPMI-1640 medium or Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 0.1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, and1 mM sodium pyruvate. After 4 d of culture, supernatants were collected and tested for IgA levels using a mouse IgA ELISA Quantitation kit (Bethyl Laboratories, Inc.), according to the manufacturer’s protocol.
2.5 Detection of circulating IgA-containing immune complexes
Levels of IgA–IgG- or IgA–C3-containing immune complexes in serum were determined by ELISA. Microtiter plate wells were coated with 10 μg/ml anti-mouse IgG (Bethyl Laboratories, Inc.) or 10 μg/ml anti-mouse C3 (MP Biomedicals, LLC, Solon, OH, USA), washed and blocked with bovine serum albumin (BSA; Sigma Chemical Company, St Louis, MO, USA) in phosphate buffered saline (PBS, pH 7.4) containing 0.05%Tween-20 (BSA–PBS–Tween). Serum samples were diluted 100-fold with the same buffer were then applied. After 3 h incubation at room temperature, test samples were removed, wells washed with BSA–PBS–Tween, and incubated for1 h at room temperature with HRP-labelled goat anti-mouseIgA (Bethyl Laboratories, Inc.) diluted 1:100. After washing with the same buffer, wells were incubated with ABTS substrate (Zymed Laboratories Inc., South San Francisco, CA, USA) and optical density (OD) was read at 405 nm.
2.6 Measurement of lectin-binding serum IgA
To examine terminal galactosylation and sialylation of IgA molecules, lectin-binding assays were designed using biotinylated elderberry (Sambucus nigra) bark lectin (SNA; Vector Laboratories, Burlingame, CA, USA) and Ricinus communis agglutinin I (RCA-I; Vector Laboratories), which recognize terminal sialic acid (specifically Neu5Aca2-6Gal) and galactose residues, respectively(29,33). Microtiter plates coated with goat anti-mouse IgA (Bethyl Laboratories, Inc.) were incubated with these samples and biotinylated SNA or RCA-I was then applied. Horseradish peroxidase (HRP)-conjugated streptavidin (Vector Laboratories) was also applied and binding to lectin and IgA were measured. Ratios between of IgA bound to SNA or RCA-I and total IgA were also calculated.
2.7 Statistical analysis
Results are expressed as means ± SD. Statistical significance was determined using the Mann–Whitney test. Differences were considered significant if P values were <0.05.
3. Results
3.1 Development of immune complex mediated glomerulonephritis in Was-KO mice
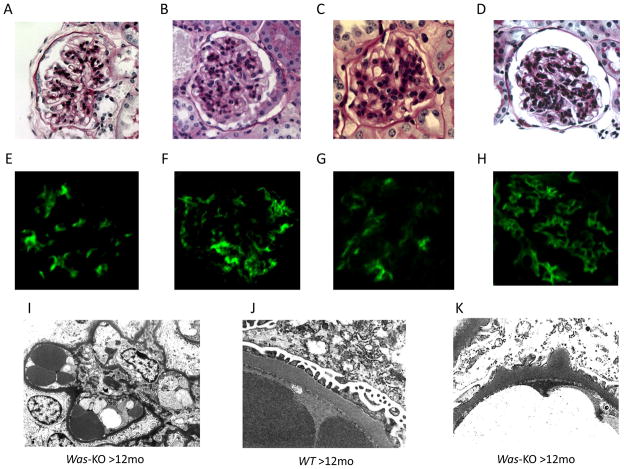
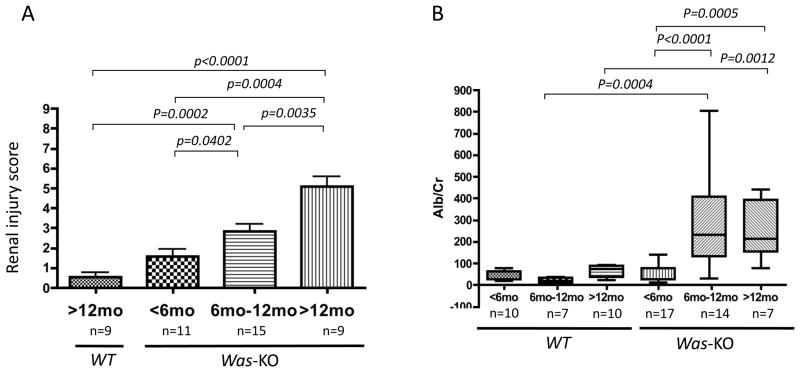
Increased mesangial cellularity and matrix deposition with or without endocapillary proliferation were observed in PAS-stained sections from Was-KO mice older than 6 months of age, but not in samples from WT control mice (Figures 1A–D). Indirect immunofluorescence showed significantly more intense glomerular IgA, IgG, IgM and C3 complement deposition in the kidneys of WASp deficient mice (Figure 1E–H). Electron microscopic evaluation showed the presence of hump-like deposits, mesangial and paramesangial deposits in Was-KO mice older than 6 months of age (Figure 1I, K), while there were no deposits in WT mice older than 6 months of age (Figure 1J). Renal injury score increased in severity with advancing age in Was-KO mice and was significantly higher than in WT mice starting at 6 months of age (Figure 2A). Proteinuria in Was-KO mice over 6 months of age was significantly elevated compared to WT mice and younger Was-KO mice (Figure 2B).
Figure 1. Immune complex deposition and mesangial cell proliferation in WASp deficient mice.
Biopsy specimens from WT controls and Was-KO mice were examined pathologically at different time points. PAS staining of A: WT (>12months) and B: Was-KO (<6months), C: Was-KO (6–12months), D: Was-KO (>12months). Immunofluorescence studies showed immune complex deposition in Was-KO mice. E: IgG, F: IgA, G: IgM, H: C3. Electron microscopic examinations showed mesangial and paramesangial deposits (I), and hump-like deposits (K) in Was-KO mice and no deposits in WT mice (J).
Figure 2. Development of proliferative glomerulonephritis increasing in severity with age.
The degree of renal injuries was evaluated with PAS staining and scored (A). The columns represent the mean and bars show SD of samples for each age. Urinary albumin/creatinine ratio was determined in WT and Was-KO mice at different time points (B). The bars represent the mean ± SD of samples for each age.
3.2 Elevated serum IgA, increased IgA production of B cells and IgA-containing immune complexes in WASp-deficient mice
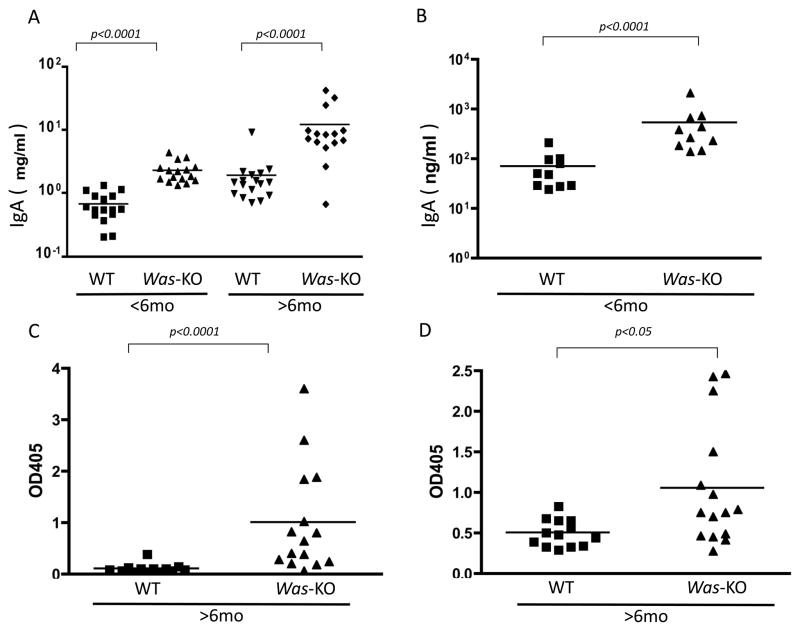
Determination of serum IgA levels showed significantly higher titers in Was-KO mice compared to WT controls (Figure 3A). Such differences were noted both in young and older Was-KO mice. Surface staining of splenic B-lymphocytes showed no differences in the numbers of IgA positive cells between WT and Was-KO mice before stimulation (data not shown). After stimulation with LPS, TGF-β1, IL-4, and IL-5 for 4 d, IgA levels in supernatants were significantly greater in Was-KO mice compared to WT controls (Figure 3B).
Figure 3. Increased serum IgA, IgA production by splenic B cells and circulating IgA containing immune complexes in WASp-deficient mice.
A: Serum IgA; serum IgA was determined by mouse IgA ELISA quantitation kit. Data were compared between WT control and Was-KO mice at different age groups.
B: In vitro IgA production; splenic B cells were isolated by immunomagnetic isolation kit and the cells were cultured in vitro in the presence of LPS and various cytokines. After 4 d, supernatant samples were harvested and IgA levels determined. Data were compared between WT control and Was-KO mice. Circulating IgA containing immune complexes were also determined by ELISA. Data were compared between WT and Was-KO mice and expressed as OD′ at 405 nm.
C: Serum circulating IgA-containing immune complex (IgA–C3)
D: Serum circulating IgA-containing immune complex (IgA–IgG)
ELISA determination of IgA-containing immune complexes in serum samples from Was-KO mice older than 6 month of age showed significantly increased leves of IgA–C3 (Figure 3C) and IgA–IgG (Figure 3D) immune complexes compared to WT littermates.
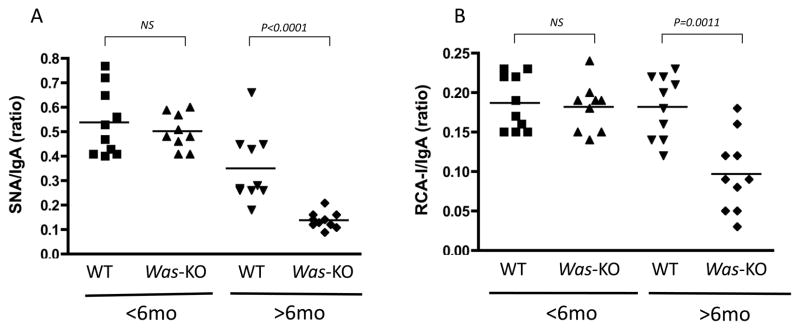
3.3 Abnormal IgA glycosylation in Was-KO mice
Samples from Was-KO mice older than 6 month of age contained IgA molecules with significantly lower binding to lectin SNA and RCA-I (Figure 4A–B) compared to WT mice. This abnormality seemed to be age-dependent, as no clear differences in glycosylation were noted among younger WT and Was-KO mice.
Figure 4. Lectin-binding IgA in sera.
Lectin-binding IgA levels were determined by the specific lectin-binding assays as described in Methods. Data were compared between WT control and Was-KO mice at different time points. The data are shown as the ratios of specific lectin-binding IgA to total IgA for SNA (A) and RCA-I (B).
4. Discussion
IgAN is the most common form of glomerulonephritis in humans and a significant proportion of patients affected with this disorder progress to renal failure(12). Renal disease is a frequent and severe complication of WAS that can lead to kidney failure and consequent need for renal transplantation(3–7). Findings from the limited number of renal biopsies performed in WAS patients have shown variable renal histological features, including membranoproliferative glomerulonephritis and interstitial nephritis. However, in the majority of cases, IgAN has been diagnosed(5–11). Among the speculative mechanisms invoked to explain mesangial IgA deposition in WAS patients, it has been suggested that immune complex deposition and insufficient immune complex clearance may occur after recurrent respiratory infections that often complicate the clinical picture of WAS(8). IgAN, however, has also been described in X-linked thrombocytopenia, and variant forms of WAS without overt immunodeficiency(32).
Studies in the general population affected with IgAN, have pointed to abnormal features of serum IgA, namely the aberrant galactosylation and sialylation of O-glycans in the IgA1 hinge region, as contributory factors to the pathogenesis of the disease(13–22). O-linked sugars are composed of N-acetylgalactosamine (GalNAc) O-linked to serine or threonine residues. The chain may be extended with the addition of galactose to GalNAc and may carry 1 or 2 sialic acids. β1,3 galactosyltransferase catalyzes the transfer of galactose to GalNAc. Defective galactosylation of IgA1 results in increased exposure of GalNAc. It has been reported that IgG–IgA1 and IgA1–IgA1 circulating complexes can form where the IgA1 hinge region O-glycans acting as antigenic moiety(13–16). Circulating immune complexes are known to deposit in the renal mesangium and induce glomerular injury; although molecular mechanisms of IgAN are poorly understood, a role of IgA-containing immune complexes cannot be ruled out.
Mouse IgA molecules differ from human IgA for the presence of N-glycans instead of O-glycans. Human IgA1 has two N-glycosylation sites and several O-glycosylation sites, while mouse IgA has two N-glycosylation sites without O-glycosylation site. Recent evidence has shown that mice lacking β-1,4-galactosyltransferase (β4GalT)-I, which transfers galactose to the terminal N-acetylglucosamine of N- and O-linked glycans, develop glomerular lesions with IgA deposition and expanded mesangial matrix, features that resemble the IgAN pathological findings in humans(35). Also similar to IgAN in humans, β4GalT-I-deficient mice show high serum IgA levels with increased polymeric forms. These findings add to other lines of evidence such as the features of the HIGA mouse model of human IgAN in which high serum IgA levels and reduced galactosylation of N-glycans on serum IgA have been demonstrated(29) and the reduced levels of galactosylation and sialylation of serum IgA found in NZWxC57BL/6 F1-bcl-2 transgenic mice that develop both IgAN-like and autoimmune lupus-like diseases(30). These data, together with the findings in human IgAN, indicate that aberrant N- or O-linked glycosylation of IgA is involved in the pathogenesis of IgAN, both in mice and humans.
Our results show that Was-KO mice develop immune complex nephritis. The pathological features and renal impairment observed in these mice are reminiscent of IgAN features reported in WAS(5–11). Our previous investigations have shown intense glomerular IgA, IgG, and C3 complement deposition in the kidneys of Was-KO mice compared and the presence of anti-dsDNA and antinuclear antibodies that may play a role in the development of immune complex nephritis in these mice(31). We now show that levels of sialylation and galactosylation of N-glycans on serum IgA in Was-KO mice are significantly reduced, which support a role of aberrant N-glycosylation of IgA in the pathogenesis of immune complex nephropathy that develops in these mice. While in previous studies we have not observed differences in the production of autoantibodies in Was-KO mice on the 129SvEv, C3H/HeJ and C57Bl/6J backgrounds(31), we have limited the present study to Was-KO mice on the 129SvEv background and we cannot conclude that WASp deficiency would result in similar quantitative and qualitative defects of IgA synthesis in other mouse strains. To this regard, the recent observations by Becker-Herman et al. indicate, in the C57Bl/6J background, severe kidney pathology can develop in the absence of IgA glomerular deposition (36). Further studies are therefore required to reveal how genetic determinants may be associated with increased IgA production and aberrant glycosylation of IgA might in the pathogenesis of glomerulonephritis in 129SvEv Was-KO mice.
Altered oligosaccharide biosynthesis in lymphocytes from WAS patients has been extensively reported(37), however, the specific mechanisms responsible for the aberrant glycosylation of IgA is unclear. Our results show reduced galactosylation of N-glycans on serum IgA only in Was-KO mice older than 6 months of age and suggest that the B cell populations that produce aberrant IgA may increase with age. Another possibility is that IgA molecules may be influenced by other factors induced by WASp deficiency, including changes in cytokine balance. Of note, Th2 cytokines are known to affect terminal glycosylation on IgA molecules(33) and Th2 cytokine predominance occurs in Was-KO mice(38), which could contribute to the IgA glycosylation defect noted in these mice.
Increased synthetic rate of IgA is a known characteristic of WAS patients(39). In this report, serum IgA levels were increased in 129SvEv Was-KO mice regardless of their age and in vitro production of IgA by WASp-deficient splenic B cells was increased compared to WT controls. Further studies are necessary to clarify the mechanisms responsible for the increased IgA production by WASp-deficient B cells, however, these findings may play a role in the pathogenesis of the renal disease observed in 129SvEv Was-KO mice, as increased production of abnormally glycosylated IgAs can result in IgA-containing immune complexes and consequent glomerular injury.
In conclusion, we show that 129SvEv Was-KO mice produce IgA with defective glycosylation and develop nephritis that is reminiscent of human IgAN, a frequent complication of WAS. Therefore, 129SvEv Was-KO mice represent a useful model for the investigation of the role of IgA carbohydrates in the development of IgAN and the physiopathology and of IgAN in WAS, and offer opportunities for developing and testing novel therapeutic strategies.
Acknowledgments
The Authors would like to thank the NHGRI animal program for excellent and human care of research animals. This work was supported by the NIH intramural research programs of NHGRI and NIAMS.
Abbreviations
- WAS
Wiskott-Aldrich syndrome
- WASp
WAS protein
- IgAN
IgA nephropathy
- WT
wild type
- CIC
circulating immune complexes
- HIGA mouse
a hyper-IgA mouse
- PAS
periodic acid-Schiff
- ELISA
enzyme-linkedimmunosorbent assay
- SNA
Sambucus nigra bark
- RCA-I
Ricinus communis agglutinin I
Footnotes
Disclosures
The authors have no financial conflicts of interest to disclose.
References
- 1.Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia RM. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–6295. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, Blanche S, Fischer A. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single center cohort of 55 patients. Pediatrics. 2003;111:e622–627. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 4.Schurman SH, Candotti F. Autoimmunity in Wiskott-Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446–453. doi: 10.1097/00002281-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 6.Webb MC, Andrews PA, Koffman CG, Cameron JS. Renal transplantation in Wiskott-Aldrich syndrome. Transplantation. 1993;56:1585. [PubMed] [Google Scholar]
- 7.Fischer A, Binet I, Oertli D, Bock A, Thiel G. Fatal outcome of renal transplantation in a patient with the Wiskott-Aldrich syndrome. Nephrol Dial Transplant. 1996;11:2077–2079. doi: 10.1093/oxfordjournals.ndt.a027102. [DOI] [PubMed] [Google Scholar]
- 8.DeSanto NG, Sessa A, Capodicasa G, Meroni M, Capasso G, Esposito L, Ferrara M, Torri Tarelli L, Annunziata S, Giordano C. IgA glomerulonephritis in Wiskott-Aldrich syndrome. Child Nephrol Urol. 1988;9:118–120. [PubMed] [Google Scholar]
- 9.Gutenberger J, Trygstad CW, Stiehm ER, Opitz JM, Thatcher LG, Bloodworth JM. Familial thrombocytopenia, elevated serum IgA levels and renal disease. A report of kindred. Am J Med. 1970;49:729–741. doi: 10.1016/s0002-9343(70)80055-9. [DOI] [PubMed] [Google Scholar]
- 10.Standen GR, Lillicrap DP, Matthews N, Bloom AL. Inherited thrombocytopenia, elevated serum IgA and renal disease. Identification of a variant of the Wiskott-Aldrich syndrome. Q J Med. 1986;59:401–408. [PubMed] [Google Scholar]
- 11.Spitler LE, Wray BB, Mogerman S, Miller JJ, O’Reilly RJ, Lagios M. Nephropathy in the Wiskott-Aldrich syndrome. Pediatrics. 1980;66:391–398. [PubMed] [Google Scholar]
- 12.Berger J, Hinglais N. Les depots intercapillaires d’IgA-IgG (Intercapillary deposits of IgA-IgG) J Urol Nephrol. 1968;74:694–695. [PubMed] [Google Scholar]
- 13.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgAN patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 14.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgAN consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestecky J, Tomana M, Crowley-Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgAN. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 18.Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M. Interactions of human mesangial cells with IgA and IgA-containing circulating immune complexes. Kidney Int. 2002;62:465–475. doi: 10.1046/j.1523-1755.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 19.Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J. IgA1-containing immune complexes in IgAN differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 20.Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgAN. Nephrology (Carlton) 2007;12:275–284. doi: 10.1111/j.1440-1797.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 21.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J. Patients with IgAN have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 22.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins EA, Siminovitch KA, Zhuang DL, Brockhausen I, Dennis JW. Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. J Biol Chem. 1991;266:6280–6290. [PubMed] [Google Scholar]
- 24.Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, Sperling AI. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med. 2004;199:1277–83. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siminovitch KA, Greer WL, Axelsson B, Rubin LA, Novogrodsky A, Peacocke M. Selective impairment of CD43-mediated T cell activation in the Wiskott-Aldrich syndrome. Immunodeficiency. 1993;4:99–108. [PubMed] [Google Scholar]
- 26.Khan S, Holding S, Doré PC, Sewell WA. Abnormal O-glycosylation of CD43 may account for some features of Wiskott-Aldrich syndrome. Med Hypotheses. 2008;70:269–72. doi: 10.1016/j.mehy.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 27.Huang MM, Tsuboi S, Wong A, Yu XJ, Oh-Eda M, Derry JM, Francke U, Fukuda M, Weinberg KI, Kohn DB. Expression of human Wiskott-Aldrich syndrome protein in patients’ cells leads to partial correction of a phenotypic abnormality of cell surface glycoproteins. Gene Ther. 2000;7:314–320. doi: 10.1038/sj.gt.3301085. [DOI] [PubMed] [Google Scholar]
- 28.Lasseur C, Allen AC, Deminière C, Aparicio M, Feehally J, Combe C. Henoch-Schöenlein purpura with immunoglobulin A nephropathy and abnormal IgA in Wiskott-Aldrich syndrome carrier. Am J Kidney Dis. 1997;29:285–287. doi: 10.1016/s0272-6386(97)90043-3. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi I, Nogaki F, Kusano H, Ono T, Miyawaki S, Yoshida H, Muso E. Interleukin-12 alters the physicochemical characteristics of serum and glomerular IgA and modifies glycosylation in a ddY mouse strain having high IgA levels. Nephrol Dial Transplant. 2002;17:2108–2116. doi: 10.1093/ndt/17.12.2108. [DOI] [PubMed] [Google Scholar]
- 30.Marquina R, Díez MA, López-Hoyos M, Buelta L, Kuroki A, Kikuchi S, Villegas J, Pihlgren M, Siegrist CA, Arias M, Izui S, Merino J, Merino R. Inhibition of B cell death causes the development of an IgA nephropathy in (New Zealand white x C57BL/6)F(1)-bcl-2 transgenic mice. J Immunol. 2004;172:7177–7185. doi: 10.4049/jimmunol.172.11.7177. [DOI] [PubMed] [Google Scholar]
- 31.Nikolov NP, Shimizu M, Cleland S, Bailey D, Aoki J, Strom T, Schwartzberg PL, Candotti F, Siegel RM. Systemic autoimmunity and defective Fas ligand secretion in the absence of the Wiskott-Aldrich syndrome protein. Blood. 2010;116:740–7. doi: 10.1182/blood-2009-08-237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, Kanou T, Tsukaguchi H, Novak J, Horikoshi S, Tomino Y. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–2395. doi: 10.1681/ASN.2007121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327–2333. [PubMed] [Google Scholar]
- 34.Matsukura H, Kanegane H, Miya K, Ohtsubo K, Higuchi A, Tanizawa T, Miyawaki T. IgA nephropathy associated with X-linked thrombocytopenia. Am J Kidney Dis. 2004;43:e7–12. doi: 10.1053/j.ajkd.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Nishie T, Miyaishi O, Azuma H, Kameyama A, Naruse C, Hashimoto N, Yokoyama H, Narimatsu H, Wada T, Asano M. Development of Immunoglobulin A nephropathy-like disease in β-1,4-Galactosyltransferase-I deficient mice. Am J Pathol. 2007;170:447–456. doi: 10.2353/ajpath.2007.060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, Khim S, Liggitt D, Song W, Silverman GJ, Alpers CE, Rawlings DJ. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med. 2011 doi: 10.1084/jem.20110200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durand G, Seta N. Protein glycosylation and diseases: blood and urinary oligosaccharides as markers for diagnosis and therapeutic monitoring. Clin Chem. 2000;46:795–805. [PubMed] [Google Scholar]
- 38.Nguyen DD, Maillard MH, Cotta-de-Almeida V, Mizoguchi E, Klein C, Fuss I, Nagler C, Mizoguchi A, Bhan AK, Snapper SB. Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein. Gastroenterology. 2007;133:1188–1197. doi: 10.1053/j.gastro.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaese RM, Strober W, Levy AL, Waldmann TA. Hypercatabolism of IgG, IgA, IgM, and albumin in the Wiskott-Aldrich syndrome. A unique disorder of serum protein metabolism. J Clin Invest. 1971;50:2331–2338. doi: 10.1172/JCI106731. [DOI] [PMC free article] [PubMed] [Google Scholar]