Abstract
Smoking is highly correlated with enhanced likelihood of atherosclerosis by inducing endothelial dysfunction. In endothelial cells, various cell-adhesion molecules including E-selectin, are shown to be upregulated upon exposure to nicotine, the addictive component of tobacco smoke; however, the molecular mechanisms underlying this induction are poorly understood. Here we demonstrate that nicotine induced E-selectin transcription in human aortic endothelial cells (HAECs) could be significantly blocked by α7-nAChR subunit inhibitor, α-BT, Src-kinase inhibitor, PP2, or siRNAs against Src or β-Arrestin-1 (β-Arr1). Further, chromatin immunoprecipitations show that E-selectin is an E2F1 responsive gene and nicotine stimulation results in increased recruitment of E2F1 on E-selectin promoter. Inhibiting E2F1 activity using RRD-251, a disruptor of the Rb-Raf-1 kinase interaction, could significantly inhibit the nicotine induced recruitment of E2F1 to the E-selectin promoter as well as E-selectin expression. Interestingly, stimulation of HAECs with nicotine results in increased adhesion of U937 monocytic cells to HAECs and could be inhibited by pre-treatment with RRD-251. Similarly, depletion of E2F1 or Src using RNAi blocked the increased adhesion of monocytes to nicotine stimulated HAECs. These results suggest that nicotine stimulated adhesion of monocytes to endothelial cells is dependent on the activation of α7-nAChRs, β-Arr1 and cSrc regulated increase in E2F1-mediated transcription of E-selectin gene. Therefore, agents such as RRD-251 that can target activity of E2F1 may have potential therapeutic benefit against cigarette-smoke induced atherosclerosis.
Keywords: E-selectin, atherosclerosis, monocyte adhesion, RRD-251, β-arrestin-1, Src, Rb
Introduction
Cardiovascular diseases are the principal causes of death in the United States, Europe, and much of Asia [1]. Extensive statistical and clinical studies have identified cigarette smoking as one of the major independent risk factor of heart diseases. According to the American Heart Association, smokers are 2-4 times more at risk of developing heart diseases than nonsmokers. Among smokers, atherosclerosis is the major form of heart disease [2,3]. Atherosclerosis is an inflammatory process that involves various cellular and molecular events and is characterized by the progressive accumulation of lipid, fibrous tissues and cell components in large and medium-sized elastic and muscular arteries [4]. This severely reduces blood flow and leads to ischemia of the heart, brain, or extremities. Among all the components of tobacco smoke, nicotine is found to have the maximum pro-atherosclerotic effects [5-9].
Exposure to risk factors, including tobacco smoke, causes endothelial dysfunction that results in the recruitment of circulating leukocytes, mainly monocytes and some T-cells to the sites of injured endothelial cells [4,10]. These monocytes adhere to the endothelial cells and migrate into the sub-endothelial space, where they differentiate into activated macrophages that are efficient scavengers of oxidized low-density lipoprotein (LDL) [10]. In the presence of increased amounts of oxidized LDL, these macrophages accumulate large amounts of cholesteryl esters in lipid droplets and become “foam cells” that form “fatty streaks”; these then have the potential to develop into complicated atherosclerotic plaques [4,10]. Studies in mouse models have shown that the atherogenesis is greatly attenuated in the absence of monocyte and macrophage recruitment to injured endothelium, even in the presence of high lipid levels [11]. This suggests that leukocyte-endothelial cell interactions are needed for the initiation and progression of atherosclerosis.
In the presence of several atherogenic stimuli, increased adhesiveness of the circulating leukocytes on the injured endothelium depends on the expression of specific cellular adhesion molecules [12,13]. These include several selectins and intercellular adhesion molecules which act as receptors for glycoconjugates and integrins present on monocytes and T-cells [12,14]. Knockout of E-selectin resulted in decreased atherosclerosis in in-vivo models [15,16]. Moreover, increased expression of E-selectin is found in the endothelium of human atherosclerotic lesions [14,17-19]. Thus, reducing the expression of E-selectin may lead to a reduction in the leukocyte-endothelial cell interactions and may ultimately lead to a decreased initiation of atherosclerosis. Our earlier studies had shown that cytokines like TNF-α could induce apoptosis of human aortic endothelial cells [20], while inducing proliferation of aortic smooth muscle cells in an E2F1 dependent manner [21]. In the present study, we report the E2F1 mediated transcriptional upregulation of E-selectin expression upon nicotine exposure and the reduction of E-selectin expression through inhibition of E2F1 mediated transcription through a small molecule inhibitor RRD-251. This results in reduced adhesion of monocytic U937 cells to HAECs. These results show for the first time that the Rb-E2F transcriptional regulatory pathway contributes to the expression of genes involved in promoting atherosclerosis.
2. Materials and Methods
2.1 Cell Culture and reagents
Primary Human Aortic Endothelial Cells (Lonza) were cultured in endothelial growth medium (EGM) supplemented with endothelial growth factors and 5% FBS (Cambrex). Experiments were done on cells that were within passages 2 to 7. Human monocytic cell line U937 [22] was purchased from ATCC and cultured in RPMI-1640 medium supplemented with 10% FBS and used as surrogate for monocytes. Src-kinase inhibitor, PP2 and α7-nAChRs inhibitor α-bungarotoxin (α-BT), were purchased from Sigma Chemical Company. RRD-251 was synthesized as described earlier [23]. Nicotine, at a concentration (1 μM) that typically found in the blood stream of a heavy smoker, was used as stimulus [24]. Unless otherwise noted, 20μM of RRD-251 was used in the experiments.
2.2 siRNA Transfections
siRNA for cSrc (Src), β-Arrestin-1 (β-Arr1) and E2F1 were purchased from Santa Cruz Biotechnology Inc. 20 picomoles of siRNAs were transfected in each well of a standard 96 well plate and 100 picomoles in 60mm dishes using Oligofectamine reagent (Invitrogen) as per the manufacturer’s instructions [25]. A non-targeting siRNA sequence was used as control.
2.3 Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were conducted on 2×107control, nicotine or serum stimulated cells as indicated following published protocols [25-27]. Following primer sequences were used to amplify the region spanning the E2F binding sites on E-selectin promoter: Forward 5′-TCTCCCCAGGAAAGTATTTCAAGCC-3′ and Reverse 5′-GGACAGCCCCAGACAAGCAA -3′.
2.4 RNA extraction and real-time reverse transcription-PCR
Total RNA was extracted and purified according to manufacturer’s instructions using RNeasy Mini Kit (Qiagen). Reverse Transcription reaction was then carried out to synthesize cDNA using iScript (Bio-Rad). Real-time PCR was done with 1 μL of the reverse transcription product in a MyiQ real-time PCR detection system (Bio-Rad) by using iQ SYBR Green PCR Supermix (Bio-Rad) as described before [25,28,29]. The PCR cycling conditions used were as follows: 40 cycles of 15 seconds at 95°C, 15 seconds at 55°C and 20 seconds at 72°C. Fold inductions were calculated using the formula 2−(ddCt) using GAPDH as internal control genes. The gene-specific primer pairs were as follows. E-selectin-F 5′TGAAGCTCCCACTGAGTCCAA3′, E-selectin-R 5′-GGTGCTAATGTCAGGAGGGAGA-3′, GAPDH-F 5′-GGTGGTCTCCTCTGACTTCAACA-3′ and GAPDH-R 5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
2.5 Monocyte Adhesion Assay
CytoSelect™ leukocyte-endothelium adhesion assay kit was purchased from Cell BioLabs. U937 cells were labeled with LeukoTracker™ according to manufacturer’s instructions. The endothelial cells were grown to full confluency in a 96 well plate and were rendered quiescent by culturing in EGM containing 0.5% FBS for 24 hrs; subsequently, the cells were treated with 1 μM nicotine for 3 hrs. Following treatment, the endothelial layer was washed with serum-free EGM and 105 labeled U937 cells were added per well. The co-culture was incubated for 60 minutes, unadhered cells washed off. Adhesion was visualized using an inverted florescence microscope and three different fields were counted.
2.6 Statistical analysis
Experiments were done in triplicate and the statistical significance evaluated using the Student’s t test.
3. Results
3.1 Nicotine induces E-selectin expression in β-Arr1-Src-regulated manner
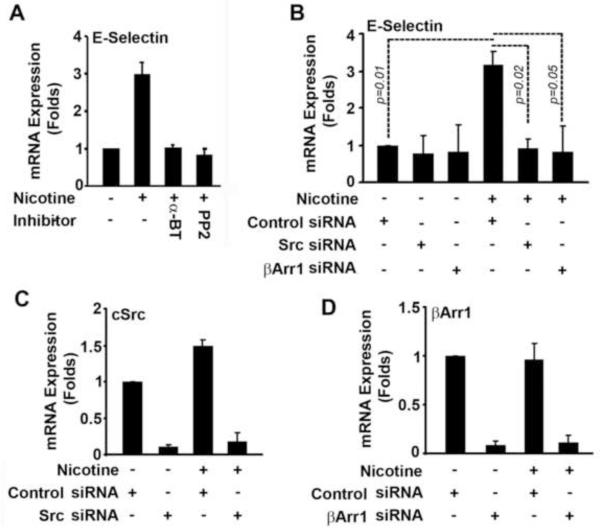
Our earlier studies have shown that nicotine stimulation of lung cancer cells induced the expression of E2F-regulated proliferative genes in α7-nicotinic acetylcholine receptor (α7-nAChR), β-Arr1 as well as Src dependent manner [24,25]. Since nicotine has been reported to induce E-selectin expression, we examined whether similar molecular mechanisms are involved in its induction in HAECs. Towards this purpose, HAECs were rendered quiescent by culturing in 0.5% serum for 24hrs followed by three hours of treatment with nicotine (1μM) alone or in presence of α7-nAChR inhibitor α-BT (1 μM) or Src-kinase inhibitor PP2 (1 μM). As shown in figure 1A, nicotine treatment resulted in approximately 3-fold increase in the expression of E-selectin mRNA, as seen by RT-PCR. In agreement with previous results, we also found a significant inhibition of nicotine induced E-selectin expression in α-BT treated cells [6,30] confirming its dependence on α7-nAChR mediated signaling. Interestingly, inhibition of Src by its inhibitor PP2 could completely inhibit the induction of E-selectin transcription by nicotine. Our earlier studies had shown a role for the scaffolding protein, β-Arr1, in the nicotine-mediated activation of Src and proliferation of lung cancer cells [24]. To ascertain whether a similar pathway was involved, siRNA to β-Arr1 or Src were transiently transfected into HAECs. Cells transfected with siRNA were rendered quiescent for 24hrs followed by three hours of treatment with nicotine (1μM). As demonstrated in figure 1B, nicotine mediated induction of E-selectin transcription was significantly decreased in Src as well as β-Arr1 depleted cells. Depletion of Src and β-Arr1 was confirmed by RT-PCR analysis (Fig. 1C and D). This suggests that nicotine-mediated induction of E-selectin in HAECs involve α-7 nAChR, β-Arr1 and Src dependent signaling.
Fig. 1. Signal transduction dependent induction of E-selectin transcription in response to nicotine stimulation.
(A) Nicotine induced E-selectin transcription was inhibited by of α7-nAChR inhibitor α-BT or Src inhibitor PP2. Data represent the mean±SD (p < 0.05). (B) Nicotine induced E-selectin transcription was suppressed by β-Arr1 or Src siRNA. (C and D) Suppression of Src expression by Src siRNA (C) and suppression of β-Arr1 expression by β-Arr1 siRNA was evaluated by RT-PCR analysis.
3.2 Nicotine induced E-selectin expression is E2F1 regulated
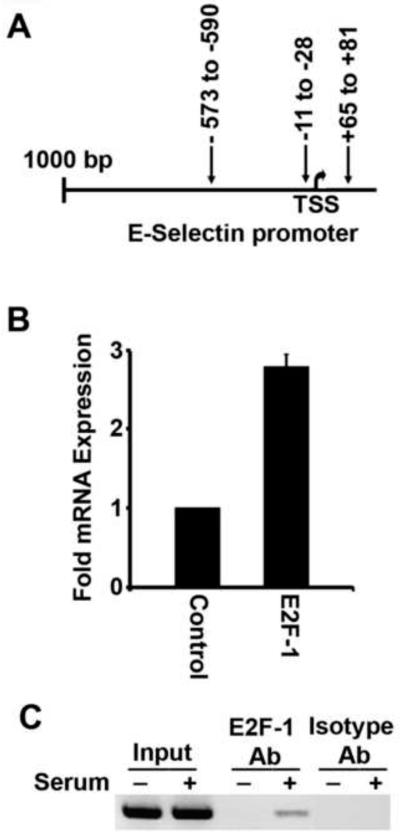
Our earlier studies had shown that nicotine stimulated β-Arr1-Src activation, results in the enhanced interaction between Raf-1 kinase and retinoblastoma tumor suppressor protein, Rb. This led to the phosphorylation-mediated inactivation of Rb, resulting in enhanced E2F1-mediated transcription [24,25]. To assess if similar events are involved in the nicotine-mediated induction of E-selectin in HAECs, we first examined the E-selectin promoter for E2F1-bidning sites. Analysis of the promoter regions of human E-selectin gene using the MatInspector program (Genomatix Software Inc) revealed three putative-E2F-binding sites very close to the transcription start site (TSS), as shown in figure 2A.
Fig. 2. E-selectin promoter is E2F1 responsive.
(A) Three putative E2F binding sites are shown in relation to TSS. (B) E-selectin was significantly upregulated at transcriptional level upon transient overexpression of E2F1 in HAECs (p<0.05). (C) ChIP assay showing the binding of endogenous E2F1 on E-selectin promoter in serum stimulated HAECs.
To examine the possible regulation of E-selectin promoter by E2F1 transcription factor, HAECs were transiently transfected with E2F1-expression vector or a control vector. The expression of endogenous E-selectin mRNA was found to be upregulated by approximately 3 fold as compared to mock-transfected cells (Fig. 2B). This suggests that E2F1 can regulate E-selectin gene transcription in HAECs. Next, the binding of endogenous E2F1 on E-selectin promoter was determined by ChIP assays. Quiescent HAECs were serum stimulated for 3 hrs and ChIP assay was performed as described in materials and methods. As shown in figure 2C, E2F1 was recruited on E-selectin promoter upon serum stimulation, correlating with the transcriptional induction.
3.3 Inhibition of E2F1 activity by RRD-251 results in decreased E-selectin expression
The transcriptional activity of E2F1 is regulated by its physical interaction with the Rb protein [24,31,32]. Our laboratory had shown that in response the signaling kinase Raf-1 can physically interact with Rb upon nicotine stimulation, leading to its phosphorylation and inactivation in HAECs [24,25]. Furthermore, the disruption of Rb-Raf-1 interaction by a small molecule inhibitor of Rb-Raf-1 interaction, RRD-251, inhibits the expression of E2F1 target genes [23,32]. This suggests that preventing the binding of Raf-1 to Rb eventually activates Rb and results in E2F repression [31]. The possibility of using RRD-251 as an inhibitor of E2F1 mediated E-selectin expression in HAECs was explored.
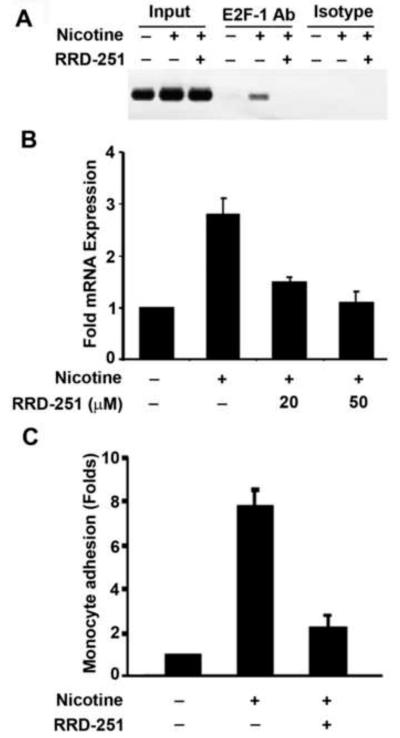
Quiescent HAECs were stimulated with 1μM nicotine in the presence or absence of 20μM RRD-251 for 3 hrs. Nicotine induced recruitment of E2F1 on E-selectin promoter was analyzed by ChIP assay. As shown in figure 3A, E2F1 was recruited on E-selectin promoter upon nicotine stimulation. Interestingly, this recruitment was completely suppressed in RRD-251 treated cells. Real-time PCRs were conducted to examine whether the inhibition of E2F1 binding to the promoter upon RRD-251 treatment correlated with a repression of E-selectin expression; treatment with RRD-251 significantly suppressed the nicotine induced expression of E-selectin gene (Fig. 3B), correlating with the lack of E2F1 recruitment to the promoter.
Fig. 3. RRD-251 inhibits nicotine mediated induction of E-selectin and monocyte binding.
(A) Nicotine stimulated E2F1 binding on E-selectin promoter was suppressed by RRD-251 as seen in a ChIP assay. (B) Nicotine stimulated expression of E-selectin was significantly suppressed by RRD-251 in dose dependent manner. (C) U937 monocytic cells adhered on nicotine stimulated HAECs, which was significantly blocked by RRD-251 (p < 0.05).
3.4 Suppressed adhesion of monocytes on endothelial cells by E2F1 and Src inhibition
Attempts were made to assess whether induction of E-selectin by nicotine had an effect on the adhesion of monocytes on HAECs. Specifically, monocyte adhesion assay was performed on nicotine stimulated HAECs in presence or absence of RRD-251 at indicated doses. There was a significant increase in the number of monocytes that bound to the nicotine-stimulated endothelial cells (Fig. 3C). Interestingly, the adhered monocytes were significantly reduced in presence of RRD-251 (Fig. 3C). These results suggest that repressing the transcriptional activity of E2F1 by RRD-251 leads to a decrease in the expression of E-selectin which suppressed monocyte binding on nicotine stimulated HAECs.
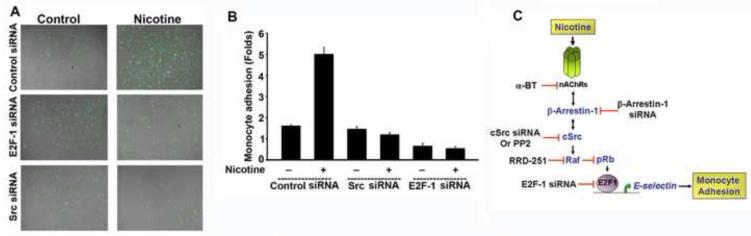
The direct role of E2F1 and Src in monocyte-endothelial cell interaction was confirmed by targeting the endogenous expression of these proteins using siRNA approach. Monocyte adhesion assays were performed on nicotine stimulated HAECs, after transient transfection of a non-targeting control siRNA, E2F1 siRNA or Src siRNA. Upon Nicotine stimulation, HAECs, transfected with Src1 siRNA or E2F1 siRNA showed a significant decrease in the number of adhered monocytes as compared to control siRNA transfected cells. The representative images from two different experiments are shown in figure 4A. The average number of adhered monocyes from three different fields of two different experiments were counted and represented as histogram in figure 4B. These results revealed a pivotal role Src-kinase and E2F1 functions in the monocyte adherence on HAECs.
Fig. 4. Adhesion of Monocytic cells on HAECs is dependent on Src and E2F1 activity.
(A and B) siRNA mediated depletion of Src or E2F1 results in suppressed adhesion of U937 cells on nicotine stimulated HAECs. The image is a composite of fluorescent U937 cells adhered on a confluent monolayer of endothelial cells. (B) Bar graph representing the average number of U937 adhered on the HAECs. (C) Schematic of nicotine induced monocyte adhesion on endothelial cells. Nicotine stimulation results in α7-nAChR/β-Arr-1/Src/Raf-1-Rb/E2F1 mediated transcriptional upregulation of E-selectin in HAECs. Targeting β-Arr1, Src or E2F1 suppressed the expression of E-selectin which results in decreased monocyte adhesion.
4. Discussion
Pathophysiological effects of cigarette smoke components are associated with cardiovascular diseases like atherosclerosis. While recent studies have demonstrated that the expression of cell-adhesion molecules is regulated by cigarette smoke condensate as well as its addictive component, nicotine [23,30,31,33-40], the underlying mechanisms are not completely understood. The elevated expression of these adhesion molecules at the site of inflammation is mainly due to the transcriptional induction of their respective genes. Mainly, transcription factor NF-κB has been reported as a dominant regulator of transcription for adhesion molecules in response to various cytokines [41,42]. NF-κB is found to upregulate the adhesion molecules ICAM-1 and VCAM-1 expression in nicotine stimulated human umbilical vein endothelial cells (HUVECs). Nicotine is also shown to transcriptionally induce the expression of E-selectin in HUVECs [43]; however, the transcription factors involved in E-selectin gene regulation has not been studied earlier.
Our laboratory had previously reported the physiological effect of nicotine stimulation on human microvascular endothelial cells from lung (HMECLs) as well as HAECs [24]. Upon treatment with 1 μM nicotine we have shown a robust increase in Src phosphorylation, Rb-Raf-1 interaction and E2F1 activation which in-turn results in transcriptional upregulation of E2F1 target genes involved in cell proliferation as well as angiogenic tubule formation [24]. Since, previous studies form our laboratory have elaborated the nAChRs-β-Arr1-Src dependent activation of E2F1 in nicotine stimulated cells, we hypothesized that E-selectin upregulation may also be E2F1 mediated in HAECs. Therefore, we explored the role of nicotine-stimulated E2F1 transcriptional activity in the regulation of E-selectin expression and whether or not it can be targeted to inhibit the endothelial cells-monocytes interaction.
The transcriptional activity of E2Fs is regulated by its physical interaction with the Rb family members [23,24,30]. In quiescent endothelial cells, E2Fs 1, 2 and 3 are bound to Rb and their transcriptional activity is suppressed. However, in response to growth factors or nicotine stimulation, Raf-1 interacts with Rb within 2 hrs that initiates a priming phosphorylation of Rb in HAECs [23,24]. This priming phosphorylation facilitates subsequent hyper-phosphorylation and complete inactivation of Rb and disassociation from E2F1 [23,24], enabling free E2F1 to transcribe its target genes. Disruption of Rb-Raf-1 interaction by RRD-251 is associated with downregulation of trascriptional targets of E2F1 involved in cell proliferation and angiogenesis [24,32,44,45]. While we found hat RRD-251 prevents the dissociation of Rb from E2F1 on proliferative promoters, we find that RRD-251 could block the recruitment of E2F1 on E-selectin promoter.
Increase in expression of various cell adhesion molecules such as E-selectin leads to increased binding of monocytes to endothelial cells, which is an important event in the initial stages of atherosclerosis [4,10]. Here we show that repressing the level of E2F1 through an agent such as RRD-251 was able to decrease the number of bound monocytes to the endothelial layer (Fig. 3C). Our recent studies also showed that RRD-251 could affect TNF-α mediated induction of aortic smooth muscle proliferation, which contributes to the formation of atheroscleoric plaques [21]. Further, E2F1 was also found to be necessary for TNF-α to induce apoptosis in aortic endothelial cells, through the induction of p73 gene [20]. Therefore, it thus appears that the Rb-E2F pathway can also contribute to multiple facets of atherosclerotic process, by affecting TNF-α mediated apoptosis of HAECs and proliferation of AOSMCs, as well as by affecting nicotine mediated induction of E-selectin gene and monocyte-endothelial cell interaction.
In conclusion, we show that E2F1 contributes to nicotine induced expression of E-selectin in HAECs in a α7-nAChR/β-Arr1/Src/Raf-1-Rb/E2F1 dependent manner, as shown in figure 4C. Results further suggest that repressing the activity of E2F1 or Src might be a novel therapeutic approach for cigarette smoke induced atherogenesis. Therefore, novel agents like RRD-251, which can target the transcriptional activity of the E2F1 may have potential benefits as therapeutic agents against atherosclerosis as well, in addition to cancer.
Highlights.
Nicotine induces E-selctin gene through the E2F1 transcription factor
Inhibiting E2F1 activity prevents nicotine-induced E-selectin expression
E2F1 activity was needed for nicotine-induced adhesion of monocytes to HAECs
Targeting the Rb-E2F pathway might be an avenue to combat atherosclerosis
Acknowledgements
This study was supported by the grant CA127725 from the NCI. Support of the Core Facilities at Moffitt Cancer Center is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- [2].Aldoori MI, Rahman SH. Smoking and stroke: a causative role. Heavy smokers with hypertension benefit most from stopping. B. M. J. 1998;317:962–963. doi: 10.1136/bmj.317.7164.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. J. A. M. A. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- [4].Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J. Biol. Chem. 1996;271:8837–8842. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- [5].Li JM, Cui TX, Shiuchi T, Liu HW, Min LJ, Okumura M, Jinno T, Wu L, Iwai M, Horiuchi M. Nicotine enhances angiotensin II-induced mitogenic response in vascular smooth muscle cells and fibroblasts. Arterioscler. Thromb. Vasc. Biol. 2004;24:80–84. doi: 10.1161/01.ATV.0000104007.17365.1c. [DOI] [PubMed] [Google Scholar]
- [6].Zhang S, Day I, Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis. 2001;154:277–283. doi: 10.1016/s0021-9150(00)00475-5. [DOI] [PubMed] [Google Scholar]
- [7].Cirillo P, Pacileo DERS,M, Gargiulo A, Leonardi A, Angri V, Formisano S, Chiariello M. Nicotine induces tissue factor expression in cultured endothelial and smooth muscle cells. J. Thromb. Haemost. 2006;4:453–458. doi: 10.1111/j.1538-7836.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- [8].Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- [9].Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J. Appl. Physiol. 1998;84:2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- [10].Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am. J. Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- [13].Chia MC. The role of adhesion molecules in atherosclerosis. Crit. Rev. Clin. Lab. Sci. 1998;35:573–602. doi: 10.1080/10408369891234282. [DOI] [PubMed] [Google Scholar]
- [14].Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J. Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- [15].Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J. Exp. Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van der Wal AC, Das PK, Tigges AJ, Becker AE. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am. J. Pathol. 1992;141:1427–1433. [PMC free article] [PubMed] [Google Scholar]
- [18].Wood KM, Cadogan MD, Ramshaw AL, Parums DV. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993;22:437–444. doi: 10.1111/j.1365-2559.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]
- [19].Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. F.E.B.S. Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- [20].Rastogi S, Rizwani W, Joshi B, Kunigal S, Chellappan SP. TNF-alpha response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Davis R, Pillai S, Lawrence N, Sebti S, Chellappan S. TNF-α mediated proliferation of vascular smooth muscle cells involves Raf-1 mediated inactivation of Rb and transcription of E2F1 regulated genes. Cell Cycle. 2012;11 doi: 10.4161/cc.11.1.18473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int. J. Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- [23].Kinkade R, Dasgupta P, Carie A, Pernazza D, Carless M, Pillai S, Lawrence N, Sebti SM, Chellappan S. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–3818. doi: 10.1158/0008-5472.CAN-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J. Clin. Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dasgupta P, Rizwani W, Pillai S, Davis R, Banerjee S, Hug K, Lloyd M, Coppola D, Haura E, Chellappan SP. ARRB1-mediated regulation of E2F target genes in nicotine-induced growth of lung tumors. J. Natl. Cancer Inst. 2011;103:317–333. doi: 10.1093/jnci/djq541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- [27].Pillai S, Dasgupta P, Chellappan SP. Chromatin immunoprecipitation assays: analyzing transcription factor binding and histone modifications in vivo. Methods in molecular biology. 2009;523:323–339. doi: 10.1007/978-1-59745-190-1_22. [DOI] [PubMed] [Google Scholar]
- [28].Pillai S, Kovacs M, Chellappan S. Regulation of vascular endothelial growth factor receptors by Rb and E2F1: role of acetylation. Cancer Res. 2010;70:4931–4940. doi: 10.1158/0008-5472.CAN-10-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ, Bepler G, Haura E, Coppola D, Chellappan S. ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol. and Cell. Bio. 2011;31:3052–3067. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cirillo P, Pacileo M, De Rosa S, Calabro P, Gargiulo A, Angri V, Prevete N, Fiorentino I, Ucci G, Sasso L, Petrillo G, Musto D′Amore S, Chiariello M. HMG-CoA reductase inhibitors reduce nicotine-induced expression of cellular adhesion molecules in cultured human coronary endothelial cells. J. Vasc. Res. 2007;44:460–470. doi: 10.1159/000106464. [DOI] [PubMed] [Google Scholar]
- [31].Davis RK, Chellappan S. Disrupting the Rb-Raf-1 interaction: a potential therapeutic target for cancer. Drug News Perspect. 2008;2:331–335. doi: 10.1358/dnp.2008.21.6.1246832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Singh S, Johnson J, Chellappan S. Small molecule regulators of Rb-E2F pathway as modulators of transcription. Biochim. Biophys. Acta. 2010;1799:788–794. doi: 10.1016/j.bbagrm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Nicotine enhances human vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-kappaB, and AP-1. Cardiovasc. Toxicol. 2006;6:39–50. doi: 10.1385/ct:6:1:39. [DOI] [PubMed] [Google Scholar]
- [34].Wang Y, Wang L, Ai X, Zhao J, Hao X, Lu Y, Qiao Z. Nicotine could augment adhesion molecule expression in human endothelial cells through macrophages secreting TNF-alpha, IL-1beta. Int. Immunopharmacol. 2004;4:1675–1686. doi: 10.1016/j.intimp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- [35].Albaugh G, Bellavance E, Strande L, Heinburger S, Hewitt CW, Alexander JB. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann. Vasc. Surg. 2004;18:302–307. doi: 10.1007/s10016-004-0030-9. [DOI] [PubMed] [Google Scholar]
- [36].Kalra VK, Ying Y, Deemer K, Natarajan R, Nadler JL, Coates TD. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J. Cell Physiol. 1994;160:154–162. doi: 10.1002/jcp.1041600118. [DOI] [PubMed] [Google Scholar]
- [37].Itier V, Bertrand D. Neuronal nicotinic receptors: from protein structure to function. F.E.B.S. Lett. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- [38].Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann. N. Y. Acad. Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- [39].Ng MK, Wu J, Chang E, Wang BY, Katzenberg-Clark R, Ishii-Watabe A, Cooke JP. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 2007;27:106–112. doi: 10.1161/01.ATV.0000251517.98396.4a. [DOI] [PubMed] [Google Scholar]
- [40].Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW, Gazdar AF, Lam WK, Minna JD. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- [41].Kouzarides T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- [42].Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr. Opin. Cell. Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- [43].Wang Y, Wang Z, Zhou Y, Liu L, Zhao Y, Yao C, Wang L, Qiao Z. Nicotine stimulates adhesion molecular expression via calcium influx and mitogen-activated protein kinases in human endothelial cells. Int. J. Biochem. Cell. Biol. 2006;38:170–182. doi: 10.1016/j.biocel.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [44].Johnson JL, Pillai S, Pernazza D, Sebti SM, Lawrence NJ, Chellappan SP. Regulation of Matrix Metalloproteinase Genes by E2F transcription factors: Rb-Raf-1 interaction as a novel target for metastatic disease. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Singh S, Davis R, Alamanda V, Pireddu R, Pernazza D, Sebti S, Lawrence N, Chellappan S. Rb-Raf-1 interaction disruptor RRD-251 induces apoptosis in metastatic melanoma cells and synergizes with dacarbazine. Mol. Cancer. Ther. 2010;9:3330–3341. doi: 10.1158/1535-7163.MCT-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]