Abstract
Vasoactive intestinal peptide (VIP) and ciliary neurotrophic factor (CNTF) are identified as autocrines of human corneal endothelial (CE) cells working in concert to maintain the differentiated state and promote the survival of the corneal endothelium. From VIP gene knockdown study, endogenous VIP is shown to maintain the level of the differentiation marker, the adhesion molecule N-cadherin , CE cell size, shape, and retention, in situ in the human donor corneoscleral explants. Exogenous VIP protects the corneal endothelium against the killing effect of oxidative stress, in part by upholding ATP levels in CE cells dying of oxidative stress-induced injury, allowing them to die of an apoptotic death instead of an acute necrotic one. The switch from the acute necrosis to the programmed cell death (apoptosis) may have allowed the injured CE cell to be rescued by the VIP-up-regulated pathways, including those of Bcl-2 and N-cadherin, and resulted in long-term CE cell survival. The endogenous VIP in CE cells is upregulated by CNTF, which is released by CE cells surviving the oxidative stress. The CNTF receptor (CNTFRα) is expressed in CE cells in human donor corneoscleral explant and gradually becomes lost during corneal storage. VIP treatment (10−8M, 37°C, 30 min) prior to storage of freshly dissected human donor corneosceral explants increases their CE cell CNTFRα level and responsiveness to CNTF in upregulating the gap junctional protein connexin-43 expression. VIP treatment of both fresh and preserved corneoscleral explants reduces CE damage in the corneoscleral explants and in the corneal buttons trephined from them. CE cell loss is a critical risk factor in corneal graft failure at any time in the life of the graft, which can be as late as 5–10 years after an initially successful transplant. A new procedure, Descemet’s stripping automated endothelial keratoplasty (DSAEK), which is superior to the traditional full thickness transplantation in many aspects, nevertheless subjects the corneal endothelium to extensive mechanical forces, resulting in even more pronounced CE cell loss than the traditional technique. Whereas it is known that cells transduce mechanical stress through N-cadherin, stimulation of the N-cadherin pathway increases the anti-apoptotic protein Bcl-2 expression. Since N-cadherin and Bcl-2 in the corneal endothelium are both upregulated by VIP, we aim to strengthen the CE sheet by VIP treatments of the corneoscleral explants for full thickness traditional corneal transplantation and pre-cut corneas for DSAEK.
We have identified vasoactive intestinal peptide (VIP) and ciliary neurotrophic factor (CNTF) as autocrines of human corneal endothelial (CE) cells working in concert to maintain the differentiated state and promote the survival of the corneal endothelium. Thus, the human corneal endothelium, a neural crest-derived tissue with very limited regenerative capacity (Joyce, 2005), plays an active role in maintaining its own differentiated state and survival. To allow the human corneal endothelium to play such a role in stored corneas for transplantation and in transplanted corneas in the recipients’ eyes will enhance corneal endothelial (CE) cell survival, which is the most critically important issue in the success of corneal transplantation (Claerhout et al., 2008). The identification of CE autocrine trophic factors has laid the foundation for the first mechanism-based strategy for enhancing CE integrity and reducing CE damage in human donor corneoscleal explants stored for transplantation, including those for Descemet’s stripping automated endothelial keratoplasty (DSAEK).
I. Identification of VIP and CNTF as autocrine trophic factors of the corneal endothelium
A. Endogenous VIP maintains the differentiated state of the human corneal endothelium in donor human corneoscleral explant
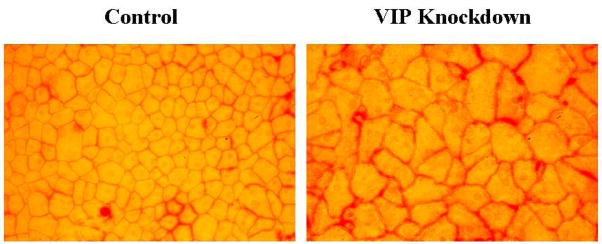
VIP, a 28 amino acid neuropeptide, is widely distributed in the central and peripheral nervous systems, where its neuroprotective role is observed in a variety of in vitro and in vivo systems (Brenneman, 2007; Moody et al., 2011). VIP is produced by the corneal endothelium (Koh et al., 2007; Koh and Waschek, 2000) and VIP-immunoreactivity is present in the aqueous humor of a variety of species including the human (Koh et al., 2005; Taylor et al., 1994). The VIP gene and protein expressions in the corneal endothelium of corneoscleral explants (including those that have been preserved in Optisol) is up-regulated by CNTF (Koh et al., 2007), an injury factor that was discovered in the ciliary body and iris (Adler et al., 1979) and is also an autocrine of CE cells (Koh, 2002). The endogenous VIP maintains the differentiated state of the corneal endothelium in that knocking down VIP gene expression results in dramatic deterioration of the CE mosaic (Koh et al., 2008; Fig. 1) in which hexagonal cells are replaced by irregularly shaped ones and in a diminishing level of the CE cell intercellular junction protein N-cadherin (a differentiation marker of the corneal endothelium) and CE cell density (Koh et al., 2008).
Fig.1.
Alizarin red S-stained, flat-mounted, paired human donor corneas (Koh et al., 2008) demonstrating that VIP gene knockdown results in deterioration of the hexagonal CE cell shape and diminishing density.
B. Exogenous VIP protects the corneal endothelium against the killing effect of severe oxidative stress and up-regulates the expression of the CE differentiation marker N-cadherin and the anti-apoptotic protein BCL-2 in corneoscleral explants
VIP binds to two types of adenylyl cyclase stimulatory heterotrimeric G protein-coupled receptors (VPAC1 and VPAC2) and transduces signals through cAMP-dependent and -independent pathways (Langer and Robberecht, 2007). The VPAC1 (not VPAC2) receptor is expressed in bovine (Koh et al., 2009b) and human CE cells (Koh et al., 2011). In CE cells, VIP stimulates production of large amounts of cAMP (Koh and Yue, 2002), phosphorylation of the cAMP-responsive element binding protein (CREB), and up-regulation of the anti-apoptotic protein Bcl-2 (Koh et al., 2009b). CREB phosphorylation mediates cell survival through induction of the anti-apoptotic Bcl-2 (Xiang et al., 2006). The role of Bcl-2 in protecting CE cells against staurosporine-induced apoptosis has been demonstrated in cultured bovine CE cells transfected with Bcl-2 cDNA (Joo et al., 1999). Furthermore, up-regulation of Bcl-2 is associated with the cytopreotective effect of minocycline in human CE cells (Kernt et al., 2010). CREB phosphorylation may play a role in cAMP-induced CNTFRα gene expression (MacLennan et al., 1996). VIP up-regulates the CE differentiation marker N-cadherin (Koh et al., 2008).
VIP protects the corneal endothelium against the acute killing effect of oxidative stress (Koh and Waschek, 2000) in agreement with the known properties of VIP as a singlet oxygen scavenger (Misra and Misa, 1990) at the molecular level, an inhibitor of lipid peroxidation (Caraglia et al., 2006) at the cellular level, and a protective agent in ischemic and reperfused myocardium (Kalfin et al., 1993). VIP switches the death mode from the inflammatory necrosis to the inflammation neutral apoptosis in oxidative stress-injured CE cells in corneoscleral explants by upholding the ATP level, which may have resulted from VIP-stimulated glycogen breakdown (Koh et al., 2009b). VIP up-regulates the expression of anti-apoptotic protein Bcl-2 and the differentiation marker N-cadherin in a kinase A-dependent manner during recovery from the oxidative stress-induced injury (Koh et al., 2009b). Whether the upregulated Bcl-2 and N-cadherin rescue the apoptotic CE cells remains to be determined. Nevertheless, VIP promotes the long-term survival of CE cells under oxidative stress (Koh et al., 2009b). VIP, either endogenous (Koh et al., 2008) or exogenous (unpublished data) does not modulate the CE gap junctional protein, whereas CNTF does.
C. Endogenous CNTF in the corneal endothelium
CNTF was discovered in an extract of ciliary body, iris, and choroid (Adler et al., 1979). CNTF, a cytokine that does not have a classic secretory signal peptide sequence, is released only after injury through an unknown mechanism (Sendtner et al., 1992). We previously demonstrated that CNTF is produced by the corneal endothelium and released in a complex with the CN TF-binding CNTFRα by CE cells surviving the oxidative stress (Koh, 2002).
D. Exogenous CNTF
The CNTF-binding subunit of the CNTF receptor, i.e. CNTFRα is expressed in bovine (Koh, 2002) and human CE cells (Koh et al., 2009a). On binding of the exogenous CNTF to the endogenous membrane-bound CNTFRα, the two β subunits (gp130 and LIFRβ) of the receptor dimerize, forming a trimeric receptor complex that activates the Janus family of tyrosine kinases Jak1/Jak2. This activation leads to tyrosine phosphorylation and nuclear translocation of signal transducer and activator of transcription STAT3, which then bind to specific responsive elements in the promoter regions of the CNTF-responsive genes (Ip and Yancopoulo, 1996; Segal and Greenberg, 1996). CNTF-responsive genes included genes of vasoactive intestinal peptide (Symes et al., 1993; Symes et al., 1994) and the gap junctional protein connexin-43 (Ozog et al., 2002; Ozog et al., 2004). We have demonstrated CNTF induction of the VIP gene and protein in CE cells of human donor corneoscleral explants preserved in storage medium Optisol-GS (Koh et al., 2007) and those of connexin-43 (Koh et al., 2009a). The beneficial effects of CNTF treatment observed in a variety of animal models of photoreceptor cell degeneration (reviewed in Wen et al., 2006) led to phase I, II, and III human clinical trials, in which encapsulated cells engineered to release CNTF were implanted into the vitreous of the retinitis pigmentosa and macular degeneration patients (Clinical trials; Sieving et al., 2006; Emerich et al., 2008; Zhang et al., 2011).
II. CE cell loss and graft failure
CE cell loss and immunologic rejection are the causes of graft failure and the need for a second transplant (Claerhout et al., 2008; Chong et al., 2008), which has a 50% success rate (Hori et al., 2007). After an initially successful transplant, late CE failure in the absence of rejection episodes can occur 5–10 years later (Bell et al. 2000; Bourne, 2001; Claerhout et al., 2008; Nishimura et al., 1999). Grafts that develop late CE failure demonstrate lower CE cell numbers immediately following the transplantation than those that do not develop late CE failure and not an in creased rate of chronic postoperative CE cell loss (Bourne, 2001; Claerhout et al., 2008; Nishimura et al., 1999). The Cornea Donor Study Investigator Group (2008) concludes that “the CE cell loss rates highlight the importance of continued research into ways to improve overall corneal health through advances in corneal preservation and postoperative management. This may become increasingly relevant with the advent of endothelial keratoplasty, which is potentially even more traumatic to the endothelium at the time of surgery than standard penetrating keratoplasty”.
III. Preservation of corneal endothelial integrity in human donor corneoscleral explants in storage for transplantation: effect of a brief VIP treatment of the explants
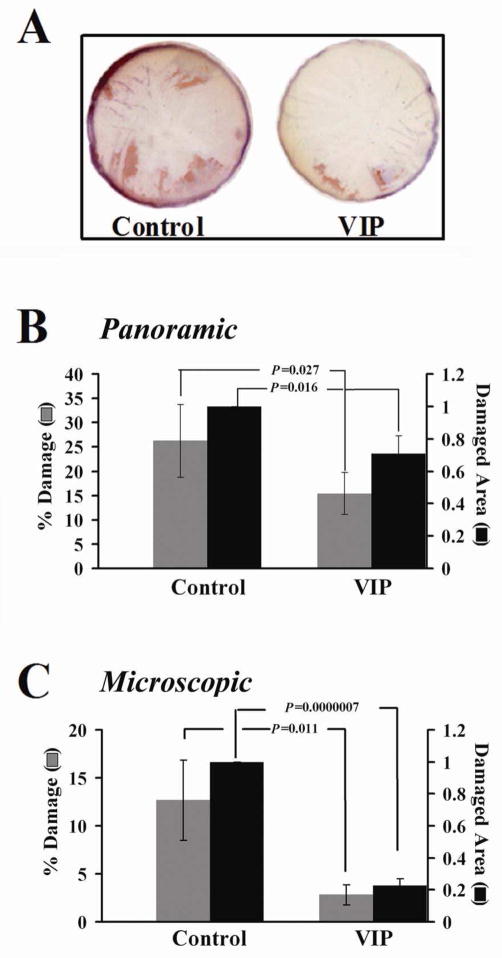
Significant CE cell loss in corneal storage in Optisol (at 4°C) is well recognized. Over the years, studies are conducted in which supplements are added to Optisol to improve CE cell survival (Kitzmann et al., 2008; Lindstrom, 1990). No study was designed to maintain the differentiation state of CE cell, partly because the mechanism of CE cell differentiation has been unknown. We have demonstrated that the α subunit of the receptor for CNTF, i.e. CNTFRα, in CE cells gradually becomes lost during human corneoscleral explants storage for transplantation and the functional CNTFRα can be restored by recombinant CNTFRα (Koh et al. 2009a). A brief VIP treatment of the fresh human donor corneoscleral explants prior to their storage prevents CNTFRα from being lost, which can increase CE cell responsiveness to CNTF in up-regulating the gap junctional protein connexin-43 as demonstrated in VIP- treated corneoscleral explants that have been previously stored in Optisol-GS (Koh et al., 2011). Following CNTF treatment of stored corneoscleral explants, reduced CE damages were found in those that have received a brief VIP treatment either during their storage (Koh et al., 2011) or prior to their storage (data not shown). In human donor corneoscleral explants in long-term storage, a brief intermittent VIP treatment increased the level of CE cell retention (Koh et al., 2011). Corneal buttons trephined from VIP-treated corneoscleral explants, including those freshly dissected demonstrated lower level of CE damage than those from the paired control corneoscleral explants (Fig. 2). As previously discussed, CNTF is present in abundance in ciliary body, iris, and choroid (Adler et al., 1979) and released only after injury through an unknown mechanism (Sendtner et al., 1992). Therefore, after being transplanted to the recipient eye, the donor cornea with better preserved CE CNTFRα and CNTF responsiveness (by VIP) will likely better preserve its CE cells.
Fig. 2.
(Copyright to ARVO, Koh et al., 2011). Reduced CE damage in corneal buttons (8.5 mm in diameter) trephined from human donor corneoscleral explants that experienced a brief intermittent VIP treatment during their storage. Following the VIP treatment (30 min, 37°C, 10−8 M) explants were kept in Optisol-GS storage for five additional days before corneal buttons (8.5 mm in diameter) were trephined from them. The buttons were incubated at 37°C for 1 h and then in the presence of CNTF (0.83 nM) for 20–25 h before the CE damage was assessed. (A) Panoramic view of the whole corneal buttons (from one pair of human corneoscleral explants freshly dissected) following alizarin red S staining. (B) Quantification of damaged (alizarin red S-stained) areas in the panoramic photographs of the whole corneal buttons demonstrating the beneficial effect of the VIP treatment. The percentages of CE damage of the whole buttons (
 ) were (mean+/−sem) (26.3+/−7.5) and (15.4+/−4.3) %, in buttons from the control and VIP-treated explants, respectively (p= 0.027, N=9 pairs). The damaged CE areas (■) found in buttons from VIP-treated explants were (mean+/−sem) (71.0+/−11.0) % of those in the respective controls (p= 0.016, N=9 pairs). (C) Quantification of the microscopic damage in the corneal endothelium of flat-mounted buttons (corneal endothelium side down) revealed under an inverted microscope (magnification=200X). The percentages of CE damage (
) were (mean+/−sem) (26.3+/−7.5) and (15.4+/−4.3) %, in buttons from the control and VIP-treated explants, respectively (p= 0.027, N=9 pairs). The damaged CE areas (■) found in buttons from VIP-treated explants were (mean+/−sem) (71.0+/−11.0) % of those in the respective controls (p= 0.016, N=9 pairs). (C) Quantification of the microscopic damage in the corneal endothelium of flat-mounted buttons (corneal endothelium side down) revealed under an inverted microscope (magnification=200X). The percentages of CE damage (
 ) were (mean+/−sem) (12.7+/−4.2) and (2.8+/−1.0) %, in buttons from the control and VIP-treated explants, respectively (p= 0.011, N=7 pairs). The damaged CE areas (■) found in buttons from VIP-treated explants were (mean+/−sem) (23.0+/− 4.0) %) of those in the respective controls (p= 6.8X10−7, N=7 pairs). Both freshly dissected and preserved human donor corneoscleral explants from the eye banks were used.
) were (mean+/−sem) (12.7+/−4.2) and (2.8+/−1.0) %, in buttons from the control and VIP-treated explants, respectively (p= 0.011, N=7 pairs). The damaged CE areas (■) found in buttons from VIP-treated explants were (mean+/−sem) (23.0+/− 4.0) %) of those in the respective controls (p= 6.8X10−7, N=7 pairs). Both freshly dissected and preserved human donor corneoscleral explants from the eye banks were used.
IV. Enhancement of the integrity and reduction in damage of the corneal endothelium in human donor corneoscleral explants for DSAEK by VIP
Endothelial keratoplasty is performed by stripping the diseased CE sheet plus Descemet’s membrane and replacing it with the same cut from the donor cornea. When the donor cornea is cut by a microkeratome in preparation of the CE sheet the procedure is termed Descemet’s stripping automated endothelial keratoplasty (DSAEK). Donor corneas pre-cut at eye banks and those cut by surgeons intra-operatively are comparable in outcomes including CE cell loss (Chen et al., 2008; Price and Price, 2006; Price et al., 2008; Terry et al., 2009a). Endothelial keratoplasty offers advantages over the full thickness grafting, including rapid visual recovery and minimal induced astigmatism and ocular surface problems (Chen et al., 2008; Price and Price, 2007, 2008a, 2008b). However, CE cell loss in endothelial keratoplasty is very pronounced with a reduction averaging 37% at six months and 40–50% at 6–12 months after surgery (2008 Cornea donor study investigator group; Lee et al., 2009; Price et al., 2008; Price and Price, 2009; Terry et al., 2008a,2009a; Price et al., 2010). In the full thickness grafts, CE cell loss of 16 % at two months (Nishimura et al., 1999) and 12 % at six months after surgery are reported (Lindstrom et al., 1992). Using donor corneas with higher CE cell densities does not lead to a higher CE cell density one year after DSAEK (Terry et al., 2008b), thus what is needed is to prevent CE cell loss in DSAEK.
A. Modulation of CE cells to increase their survivability in DSAEK
Whereas the techniques for endothelial keratoplasty are continuously being refined (Price and Price, 2009.), an interesting finding indicates that as long as an established procedure is strictly adhered to, the amount of experience the surgeon has is not a factor in affecting the extent of CE cell loss (Chen et al., 2009). Thus, development of new surgical devices and training of surgeons to become capable of strictly adhering to established procedures will improve the survivability of CE cells in DSAEK. However, one possible way to enhance the survivability of CE cells in DSAEK that has so far been overlooked is to strengthen the CE sheet through biochemical and physiological manipulations of CE cells. We have found that CE cell survival and the differentiated state maintenance in human donor and bovine corneas are promoted by its autocrine factor VIP.
B. Microkeratome dissection of human donor corneoscleral explants for DSAEK at eye banks: CE Bcl-2 and N-cadherin upregulation by VIP in CE cells
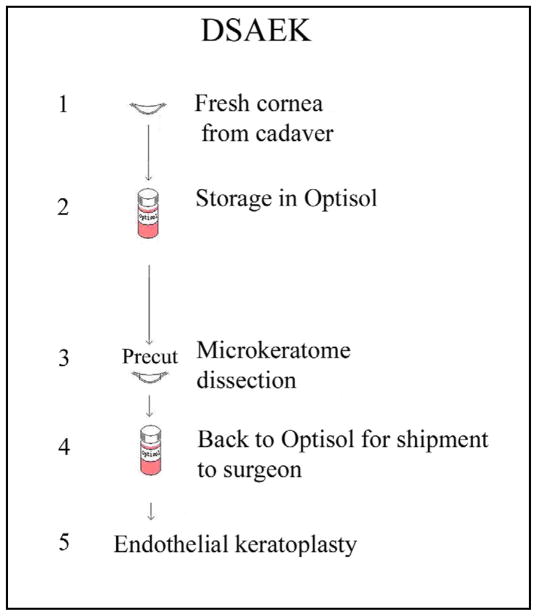
The current eye bank procedure of providing pre-cut corneas for DSAEK is depicted in Fig.3: In Optisol for 4–5 days, donor corneoscleral explants for DSAEK are microkeratome re-dissected to excise the anterior lamellar corneal tissue, which is then reattached to the posterior cornea before storage of the whole corneosceral explant in fresh Optisol (4°C) (Rieck et al., 2003) for shipment to surgeons, who then trephine the cornea to obtain the CE sheet with attached Descemet’s membrane (plus some stroma) intra-operatively. Strengthening CE sheets when CE cells experience mechanical force in pre-cut corneas in steps 1, 3, and 5 likely would reduce CE damage. In the corneal endothelium, N-cadherin is up-regulated by VIP (Koh et al., 2008), whereas cells transduce mechanical stress through N-cadherin (Schwartz et al., 2008), the cell-cell junctional protein mediating cell adhesion (Ganz et al., 2006) that plays important roles in shaping cells (Derycke et al., 2004; Hayashi et al., 2004) and in strengthening intercellular adhesion (Carthew, 2005; Chan et al., 2004; El Sayegh et al., 2004), functions as an adhesion-activated receptor capable of initiating distinctive signaling pathways (Ganz et al., 2006). Stimulation of the N-cadherin pathway increases the anti-apoptotic protein Bcl-2 expression (Tran et al., 2002) and promotes cell survival (Koutsouki et al., 2005). In vivo cadherin inhibition interrupts junction formation, intercellular adhesion, and increases apoptosis (Tinkle et al., 2008). In mechanical stretch-induced apoptosis the level of the anti-apoptotic protein Bcl-2 decreases (Hammerschmidt et al., 2007; Leri et al., 1998). Thus, increasing N-cadherin and Bcl-2 expression in CE cells by VIP treatment of human donor corneosceral explants likely will strengthen cell-cell adhesion and promote CE cell survival.
Fig. 3.
Steps in eye bank preparation for pre-cut cornea for DSAEK.
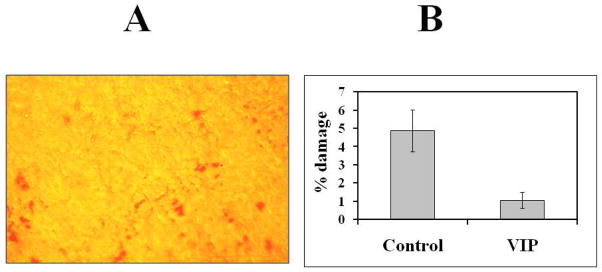
There is evidence suggesting that under force, adhesions strengthen in order to maintain stable adhesion (Ide et al., 2008). We aim to strengthen the CE sheet in pre-cut corneas by VIP treatments, followed by demonstration of the superior quality of these corneas in an established in vitro model of endothelial keratoplasty (Mehta et al., 2008b). The preliminary results (Fig.4) indicated CE cell damage in pre-cut cornea for DSAEK was reduced by VIP treatment of the freshly dissected human donor corneoscleral explants before its storage.
Fig.4.
CE damage in pre-cut cornea reduced by a brief (30 min, 37°C) VIP (10−8 M) treatment of the corneoscleral explants prior to their storage in Optisol-GS. (A) CE damage revealed by intense alizarin red staining of the exposed Descemet’s membrane in areas of denuded corneal endothelium of a flat-mounted control corenoscleral explants under an inverted microscope (Koh et al., 2011). Original magnification=200X. (B) Quantification of the damaged areas in the corneal endothelium of the paired corneoscleral explants. Analysis using Photoshop computer software (Koh et al., 2011; Saad et al., 2008) of 15 CE images taken from the control and VIP-treated corneoscleral explants demonstrated the damaged areas were 4.9+/−1.2 % and 1.1+/−0.4 %, respectively (p=0.002).
V. Conclusion
The studies represent the first attempt to prevent CE cell loss in corneoscleral explants in storage for transplantation by manipulating CE cells themselves. By treating CE cells in human donor corneas with VIP, which we have demonstrated to be the differentiated state-maintaining and oxidative stress-protecting autocrine of CE cells, our studies thus provide the first mechanism–based CE cell protective protocol. Finally, we have demonstrated the usefulness of basic science knowledge on a clinically relevant stage (storage and pre-cut of human donor corneas for transplantation).
Autocrine VIP ensures survival of the corneal endothelium in human donor corneas for transplantation.
CNTF, also an autocrine, upregulates VIP, which prevents loss of CNTF receptor during corneal storage.
In oxidative stress, VIP upholds ATP, increases N-cadherin, Bcl-2, and survival.
VIP gene knock down diminishes N-cadherin, hexagonal cells, and cell density.
CNTF, released by corneal endothelium surviving oxidative stress, upregulates connexin-43.
Acknowledgments
Grant Support:
National Institutes of Health grant RO1EY-11607 and Research to Prevent Blindness, Inc.
The authors thank the Lions Eye Institute for Transplant & Research, Inc. (Tampa, FL) and the Anatomy Board of the State of Maryland (Baltimore, MD) for the human donor corneoscleral explants for research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler R, et al. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204:1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Bell KD, et al. Pathology of late endothelial failure: late endothelial failure of penetrating keratoplasty: study with light and electron microscopy. Cornea. 2000;19:40–46. doi: 10.1097/00003226-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001;20:560–69. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–1726. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Caraglia M, et al. Effects of VIP and VIP-DP on proliferation and lipid peroxidation metabolism in human KB cells. Ann NY Acad Sci. 2006;1070:167–172. doi: 10.1196/annals.1317.007. [DOI] [PubMed] [Google Scholar]
- Carthew RW. Adhesion proteins and the control of cell shape. Curr Opion Genetics & Development. 2005;15:358–363. doi: 10.1016/j.gde.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Chan MWC, et al. Regulation of intercellular adhesion strength in fibroblasts. J Biol Chem. 2004;279:41047–41057. doi: 10.1074/jbc.M406631200. [DOI] [PubMed] [Google Scholar]
- Chen ES, et al. Precut tissue in Descemet's stripping automated endothelial keratoplasty donor characteristics and early postoperative complications. Ophthalmology. 2008;115:497–502. doi: 10.1016/j.ophtha.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Chen ES, et al. Endothelial keratoplasty: Vision, endothelial survival, and complications in a comparative case series of fellow vs attending surgeons. Am J Ophthalmol. 2009;148:26–31. doi: 10.1016/j.ajo.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- Choudhary R, et al. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol. 2008;215:172–81. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- Claerhout I, et al. Gaft failure: I. endothelial cell loss. Int Ophthal. 2008;28:165–73. doi: 10.1007/s10792-007-9087-0. [DOI] [PubMed] [Google Scholar]
- ClinicalTrial.gov 1. NCT00277134.; 2: NCT00447954; 3. NCT00447980; 4. NCT00447993.
- Lass JH, et al. Cornea Donor Study Investigator Group. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular microscopy ancillary study results. Ophthalmology. 2008;115:627–632. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derycke LDM, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- El Sayegh TY, et al. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sc. 2004;117:5117–5131. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Thanos CG. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008;10:506–515. [PubMed] [Google Scholar]
- Ganz A, et al. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, et al. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol. 2007;37:699–705. doi: 10.1165/rcmb.2006-0429OC. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hori J, Niederkorn JY. Immunogenicity and immune privilege of corneal allografts. Chem Immunol Allergy. 2007;92:290–299. doi: 10.1159/000099279. [DOI] [PubMed] [Google Scholar]
- Ide T, et al. Descemet-stripping automated endothelial keratoplasty: effect of anterior lamellar corneal tissue-on/-off storage condition on Descemet-stripping automated endothelial keratoplasty donor tissue. Cornea. 2008;27:754–757. doi: 10.1097/ICO.0b013e31816a6266. [DOI] [PubMed] [Google Scholar]
- Ip NY, Yancopoulos GD. The neurotrophins and CNTF: two families of collaborative neurotrophic factors. Annu Rev Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- Joo C, et al. Protective role for bcl-2 in experimentally induced cell death of bovine corneal endothelial cells. Ophthalmic Res. 1999;31:287–296. doi: 10.1159/000055549. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Cell cycle status in human corneal endothelium. Exp Eye Res. 2005;81:629–38. doi: 10.1016/j.exer.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kalfin, et al. Protective role of intracoronary VIP in Ischemic and reperfused myocardium. J Pharmcol Exp Ther. 1993;268:952–968. [PubMed] [Google Scholar]
- Kernt M, et al. Minocycline is cytoprotective in human corneal endothelial cells and induces anti-apoptotic B-cell CLL/lymphoma 2 (Bcl-2) and X-linked inhibitor of apoptosis (XIAP) Br J Ophthalmol. 2010;94:940–946. doi: 10.1136/bjo.2009.165092. [DOI] [PubMed] [Google Scholar]
- Kitzmann AS, et al. Eye bank survey of surgeons using precut donor tissue for descemet stripping automated endothelial keratoplasty. Cornea. 2008;27:634–639. doi: 10.1097/QAI.0b013e31815e4011. [DOI] [PubMed] [Google Scholar]
- Koh SW, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthamol Vis Sci. 2000;41:4085–4092. [PubMed] [Google Scholar]
- Koh SW. Ciliary neurotrophic factor released by corneal endothelium surviving oxidative stress ex vivo. Invest Ophthalmol Vis Sci. 2002;43:2887–2896. [PubMed] [Google Scholar]
- Koh SW, Yue BY. VIP stimulation of cAMP production in corneal endothelial cells in tissue and organ cultures. Cornea. 2002;21:270–274. doi: 10.1097/00003226-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Koh SW, et al. VIP immunoreactivity in human aqueous humor. Curr Eye Res. 2005;30:189–194. doi: 10.1080/02713680490908715. [DOI] [PubMed] [Google Scholar]
- Koh SW, et al. Vasoactive intestinal peptide induction by ciliary neurotrophic factor in donor human corneal endothelium in situ. Neurosci Lett. 2007;423:89–94. doi: 10.1016/j.neulet.2007.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SW, et al. VIP and VIP gene silencing modulation of differentiation marker N-cadherin and cell shape of corneal endothelium in human cornea ex vivo. Invest Ophthalmol Vis Sci. 2008;49:491–3918. doi: 10.1167/iovs.07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SWM. Vasoactive intestinal peptide acting in concert with ciliary neurotrophic factor to promote the survival of corneal endothelium under oxidative stress. In: Troger J, Kieselbach G, editors. Neuropeptides in the Eye. Research Signpost; Kerala, India: 2009. pp. 55–67. [Google Scholar]
- Koh SWM, et al. Restoration of functional CNTF receptor α subunit (CNTFRα) in corneal endothelial cells in stored human donor corneas by recombinant CNTFRα: connexin-43 up-regulation. Invest Ophthalmo Vis Sci. 2009a;50:1801–1807. doi: 10.1167/iovs.08-2590. [DOI] [PubMed] [Google Scholar]
- Koh SWM, et al. VIP Down-regulates the inflammatory potential and promotes survival of dying (neural crest-derived) corneal endothelial cells ex vivo: necrosis to apoptosis switch and up-regulation of Bcl-2 and N-cadherin. J Neurochem. 2009b;109:792–806. doi: 10.1111/j.1471-4159.2009.06012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SW, et al. Corneal endothelial autocrine VIP enhances its integrity in stored human donor corneoscleral explant. Invest Ophthalmol Vis Sci. 2011;52:5632–5640. doi: 10.1167/iovs.10-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouki E, et al. N-cadherin dependent cell-cell contacts promote human saphenous vein smooth muscle cell survival. Arterioscler Thromb Vasc Biol. 2005;25:982–988. doi: 10.1161/01.ATV.0000163183.27658.4b. [DOI] [PubMed] [Google Scholar]
- Langer I, Robberecht P. Molecular mechanisms involved in vasoactive intestinal peptide receptor activation and regulation: current knowledge, similarities to and differences from the A family of G-protein-coupled receptors. Biochem Soc Trans. 2007;35:724–728. doi: 10.1042/BST0350724. [DOI] [PubMed] [Google Scholar]
- Lee WB, et al. Descemet’s stripping endothelial kertoplasty:sfty and outcomes. A report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Leri A, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101:1326–42. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom RL. Advances in corneal preservation. Trans Am Ophthalmol Soc. 1990;88:555–648. [PMC free article] [PubMed] [Google Scholar]
- Lindstrom RL, et al. Optisol corneal storage medium. Am J Ophthalmol. 1992;114:345–56. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, et al. Ciliary neurotrophic factor receptor alpha mRNA in NB41A3 neuroblastoma cells: regulation by cAMP. Eur J Pharmacol. 1996;295:103–108. doi: 10.1016/0014-2999(95)00622-2. [DOI] [PubMed] [Google Scholar]
- McCulloch CAG. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sci. 2005;117:5117–5131. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- Means TL, et al. Viability of human corneal endothelium following optisol-GS storage. Arch Ophthalmol. 1995;113:805–809. doi: 10.1001/archopht.1995.01100060131047. [DOI] [PubMed] [Google Scholar]
- Mehta JS, et al. Comparison of donor insertion techniques for descemet stripping automated endothelial keratoplasty. Arch Ophthalmol. 2008b;126:1383–1388. doi: 10.1001/archopht.126.10.1383. [DOI] [PubMed] [Google Scholar]
- Misra RB, Misa PH. Vasoactive intestinal peptide, a singlet oxygen quencher. J Biol Chem. 1990;265:15371–15374. [PubMed] [Google Scholar]
- Moody TW, Ito T, Osefo N, Jensen RT. VIP and PACAP: recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr Opin Endocrinol Diabetes Obes. 2011;18:61–7. doi: 10.1097/MED.0b013e328342568a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano N, et al. Corneal endothelial cell damage by free radicals associated with ultrasound oscillation. Arch Ophthalmol. 2008;126:816–21. doi: 10.1001/archopht.126.6.816. [DOI] [PubMed] [Google Scholar]
- Nishimura JK, et al. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmology. 1999;106:1962–1965. doi: 10.1016/S0161-6420(99)90409-8. [DOI] [PubMed] [Google Scholar]
- Ozog MA, et al. Ciliary neurotrophic factor (CNTF) in combination with its soluble receptor (CNTFRα) increases connexin-43 expression and suppresses growth of C6 glioma cells. Cancer Res. 2002;62:3544–3548. [PubMed] [Google Scholar]
- Ozog MA, et al. The complex of ciliary neurotrophic factor-ciliary neurotrophic factor receptor α upregulates connexin-43 and intercellular coupling in astrocytes via the Janus tyrosine kinase/signal transducer and activator of transcription pathway. Mol Biol Cell. 2004;15:4761–4774. doi: 10.1091/mbc.E04-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MO, et al. Randomized, prospective comparison of precut vs surgeon-dissected grafts for descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008;146:36–41. doi: 10.1016/j.ajo.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Price MO, et al. Descemet's stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117:438–44. doi: 10.1016/j.ophtha.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MO, Price FW. Descemet's stripping with endothelial keratoplasty. Comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology. 2006;113:1936–1942. doi: 10.1016/j.ophtha.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Price MO, Price FW. Descemet’s sripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18:290–294. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- Price MO, Price FW., Jr Endothelial cell loss after descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology. 2008a;115:857–865. doi: 10.1016/j.ophtha.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Price FW, Price MO. Adult keratoplasty: has the prognosis improved in the last 25 years? Int Ophthalmol. 2008b;28:141–6. doi: 10.1007/s10792-007-9183-1. [DOI] [PubMed] [Google Scholar]
- Price FW, Price MO. Does endothelial cell survival differ between DSEK and standard PK? Ophthalmology. 2009;116:367–8. doi: 10.1016/j.ophtha.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Rangon CM, et al. VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. J Pharmacol Exp Ther. 2005;314:745–752. doi: 10.1124/jpet.105.086405. [DOI] [PubMed] [Google Scholar]
- Reneker LW, et al. Formation of corneal endothelium is essential for anterior segment development- a transgenic mouse model of anterior segment dysgenesis. Development. 2000;127:533–542. doi: 10.1242/dev.127.3.533. [DOI] [PubMed] [Google Scholar]
- Rieck PW, et al. Fibroblast growth factor-2 protects endothelial cells from damage after corneal storage at 4 degrees C. Graefes Arch Clin Exp Ophthalmol. 2003;241:757–64. doi: 10.1007/s00417-003-0687-8. [DOI] [PubMed] [Google Scholar]
- Saad HA, et al. An easy and inexpensive method for quantitative analysis of endothelial damage by using vital dye staining and Adobe Photoshop software. Cornea. 2008;27:818–24. doi: 10.1097/ICO.0b013e3181705ca2. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Sendtner M, et al. Synthesis and localization of ciliary neurotrophic factor in sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992;118:1436–1453. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes A, et al. Ciliary neurotrophic factor coordinately activates transcription of neuropeptide genes in a neuroblastoma cell line. Proc Natl Acad Sci U S A. 1993;90:572–576. doi: 10.1073/pnas.90.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes A, et al. STAT proteins participate in the regulation of the vasoactive intestinal peptide gene by the ciliary neurotrophic factor family of cytokines. Mol Endocrinol. 1994;8:1750–1763. doi: 10.1210/mend.8.12.7708062. [DOI] [PubMed] [Google Scholar]
- Taylor AW, et al. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- Terry MA, et al. Endothelial cell loss after Descemet's stripping endothelial keratoplasty in a large prospective series. Ophthalmology. 2008a;115:488–496. doi: 10.1016/j.ophtha.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Terry MA, et al. Endothelial keratoplasty: The influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell count. Cornea. 2008b;27:1131–1137. doi: 10.1097/ICO.0b013e3181814cbc. [DOI] [PubMed] [Google Scholar]
- Terry MA, et al. Precut tissue for Descemet’s stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology. 2009a;116:248–256. doi: 10.1016/j.ophtha.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Terry MA, et al. Endothelial keratoplasty: the influence of insertion techniques and incision size on donor endothelial survival. Cornea. 2009b;28:24–31. doi: 10.1097/ICO.0b013e318182a4d3. [DOI] [PubMed] [Google Scholar]
- Tinkle CL, et al. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NL, et al. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–32914. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- Troger J, et al. Different concentrations of vasoactive intestinal peptide in aqueous humor of patients with proliferative vitreoretinopathy and cataract patients. Ger J Ophthalmol. 1994;3:245–247. [PubMed] [Google Scholar]
- Wen R, et al. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci. 2006;26:13523–30. doi: 10.1523/JNEUROSCI.4021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, et al. Role of the cyclic AMP response element in the bcl-2 promoter in the regulation of endogenous Bcl-2 expression and apoptosis in murine B cells. Mol Cell Biol. 2006;26:8599–8606. doi: 10.1128/MCB.01062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]