Abstract
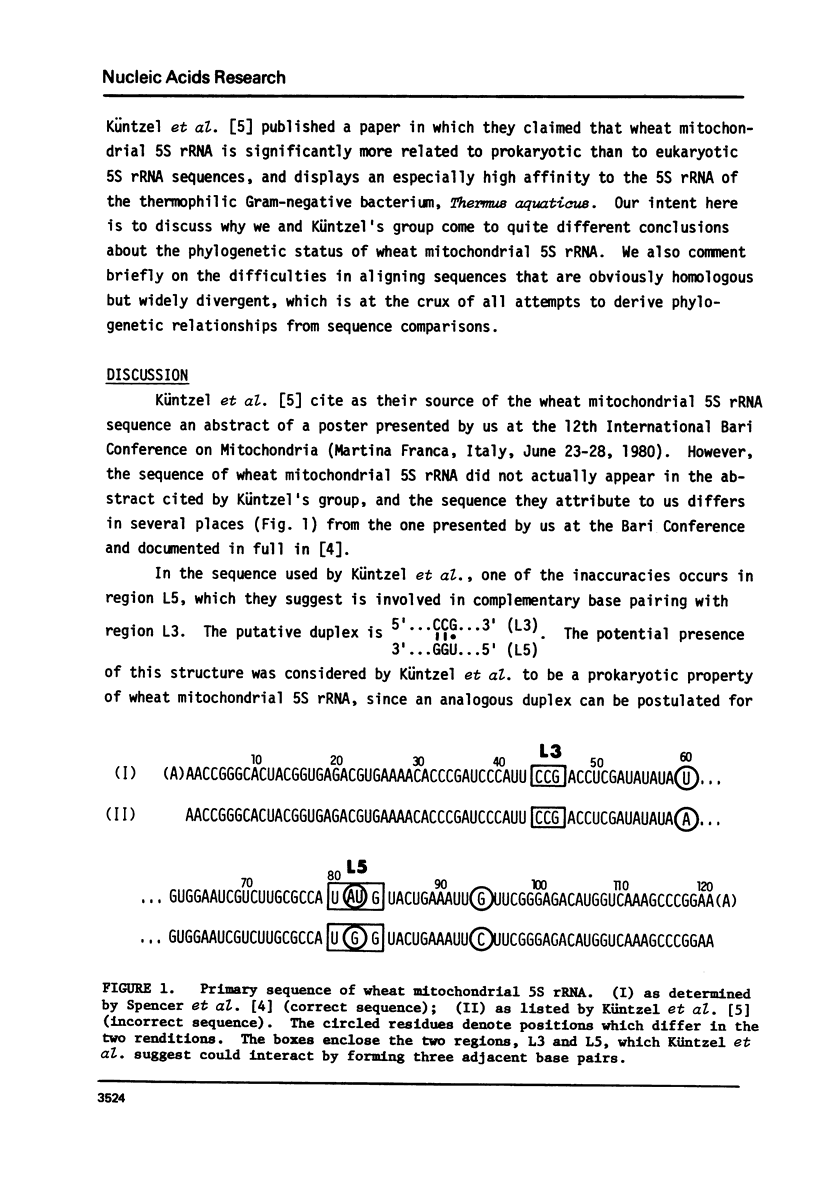
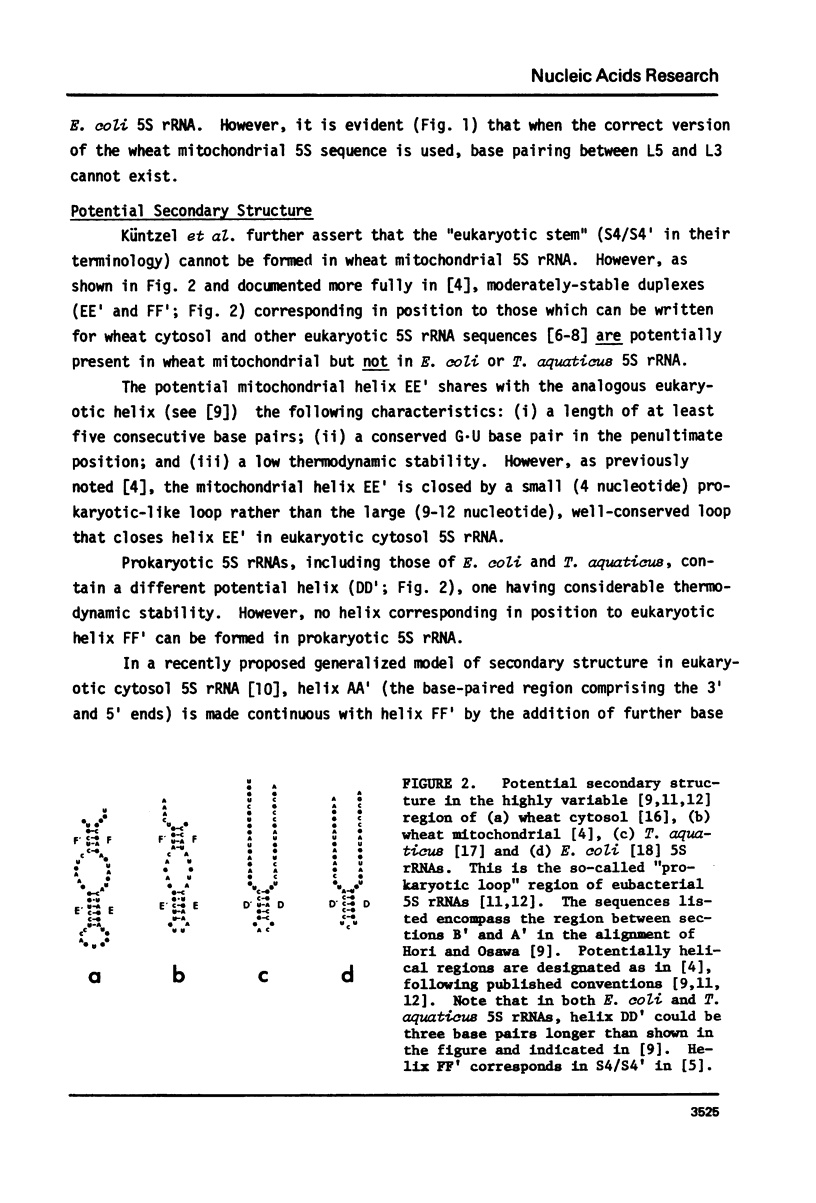
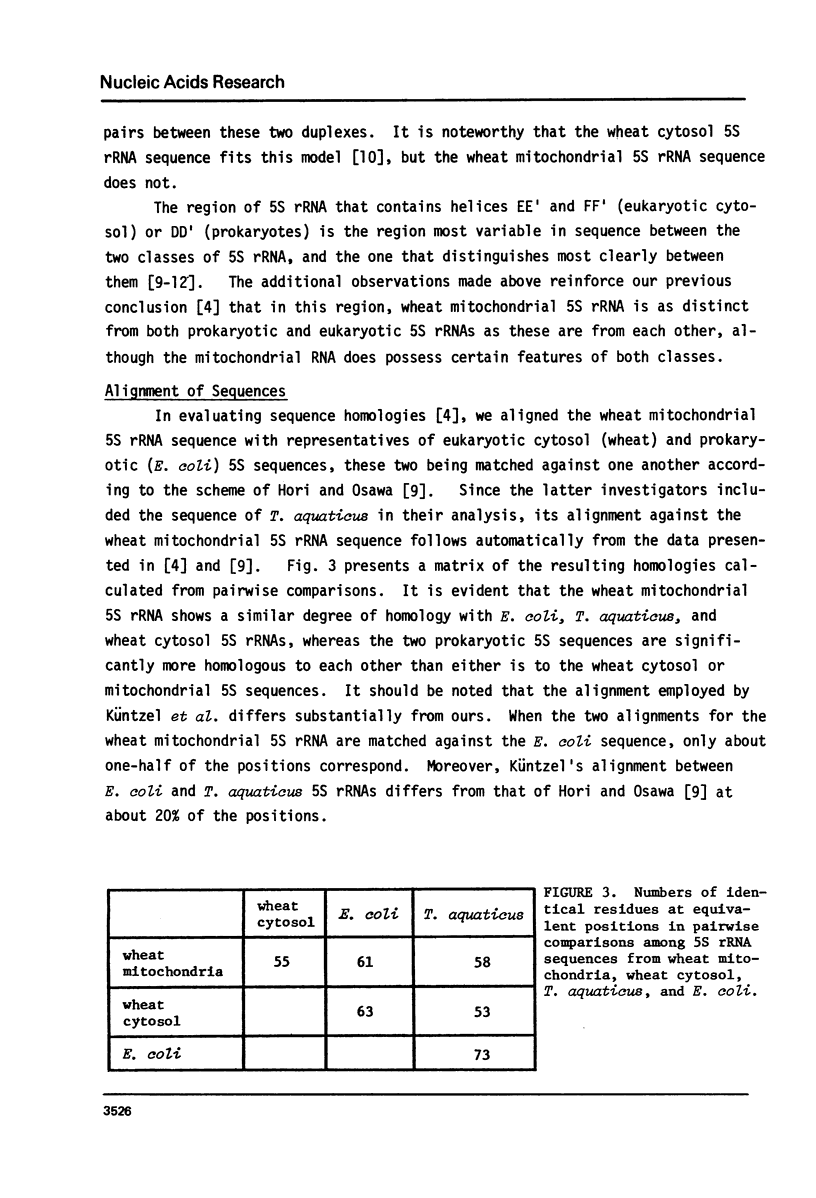
Küntzel et al. (1981) (Nucleic Acids Res. 9, 1451-1461) recently concluded that the sequence of wheat mitochondrial 5S rRNA is significantly more related to prokaryotic than to eukaryotic 5S rRNA sequences, and displays an especially high affinity to that of the thermophilic Gram-negative bacterium, Thermus aquaticus. However, the sequence on which this conclusion was based, although attributed to us, differs in several places from the one determined by us. We show here that the correct sequence (Spencer, D.F., Bonen, L. and Gray, M.W. (1981) Biochemistry, in press) does not support the conclusions of Küntzel et al. about potential secondary structure in wheat mitochondrial 5S rRNA and its phylogenetic significance. We further show that when the wheat mitochondrial 5S rRNA sequence is matched against published alignments for E. coli, T. aquaticus, and wheat cytosol 5S rRNAs, the mitochondrial sequence shows no greater homology to the T. aquaticus sequence than to the E. coli sequence, and only slightly more homology to these two sequences than to wheat cytosol 5S rRNA. This analysis confirms our original view (Biochemistry, in press) that wheat mitochondrial 5S rRNA is neither obviously prokaryotic nor eukaryotic in nature, but shows characteristics of both classes of 5S rRNA, as well as some unique features.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonen L., Cunningham R. S., Gray M. W., Doolittle W. F. Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 1977 Mar;4(3):663–671. doi: 10.1093/nar/4.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. Nucleotide sequence of 5S-ribosomal RNA from Escherichia coli. Nature. 1967 Aug 12;215(5102):735–736. doi: 10.1038/215735a0. [DOI] [PubMed] [Google Scholar]
- Cunningham R. S., Bonen L., Doolittle W. F., Gray M. W. Unique species of 5S, 18S, and 26S ribosomal RNA in wheat mitochondria. FEBS Lett. 1976 Oct 15;69(1):116–122. doi: 10.1016/0014-5793(76)80666-7. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. The architecture of 5S rRNA and its relation to function. J Mol Evol. 1975 Oct 3;6(1):61–76. doi: 10.1007/BF01732674. [DOI] [PubMed] [Google Scholar]
- Hori H. Molecular evolution of 5S RNA. Mol Gen Genet. 1976 May 7;145(2):119–123. doi: 10.1007/BF00269583. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H., Heidrich M., Piechulla B. Phylogenetic tree derived from bacterial, cytosol and organelle 5S rRNA sequences. Nucleic Acids Res. 1981 Mar 25;9(6):1451–1461. doi: 10.1093/nar/9.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Harmey M. A. Higher-plant mitochondrial ribosomes contain a 5S ribosomal ribonucleic acid component. Biochem J. 1976 Jul 1;157(1):275–277. doi: 10.1042/bj1570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Fox G. E. Secondary structure of eukaryotic cytoplasmic 5S ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2150–2154. doi: 10.1073/pnas.78.4.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay R. M., Spencer D. F., Doolittle W. F., Gray M. W. Nucleotide sequences of wheat-embryo cytosol 5-S and 5.8-S ribosomal ribonucleic acids. Eur J Biochem. 1980 Dec;112(3):561–576. doi: 10.1111/j.1432-1033.1980.tb06122.x. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Matheson A. T. Nucleotide sequence of Thermus aquaticus ribosomal 5 S ribonucleic acid. Sequence homologies in thermophilic organisms. J Biol Chem. 1977 Jun 25;252(12):4256–4261. [PubMed] [Google Scholar]
- Nishikawa K., Takemura S. Structure and function of 5S ribosomal ribonucleic acid from Torulopsis utilis. II. Partial digestion with ribonucleases and derivation of the complete sequence. J Biochem. 1974 Nov;76(5):935–947. [PubMed] [Google Scholar]
- Vigne R., Jordan B. R. Partial enzyme digestion studies on Escherichia coli, Pseudomonas, Chlorella, Drosophila, HeLa and yeast 5S RNAs support a general class of 5S RNA models. J Mol Evol. 1977 Sep 20;10(1):77–86. doi: 10.1007/BF01796136. [DOI] [PubMed] [Google Scholar]
- Whatley F. R. The establishment of mitochondria: Paracoccus and Rhodopseudomonas. Ann N Y Acad Sci. 1981;361:330–340. doi: 10.1111/j.1749-6632.1981.tb46529.x. [DOI] [PubMed] [Google Scholar]



