Abstract
Arthrobacter sp. strain G1 is able to grow on 4-fluorocinnamic acid (4-FCA) as sole carbon source. The organism converts 4-FCA into 4-fluorobenzoic acid (4-FBA) and utilizes the two-carbon side-chain for growth with some formation of 4-fluoroacetophenone as a dead-end side product. We also have isolated Ralstonia sp. strain H1, an organism that degrades 4-FBA. A consortium of strains G1 and H1 degraded 4-FCA with Monod kinetics during growth in batch and continuous cultures. Specific growth rates of strain G1 and specific degradation rates of 4-FCA were observed to follow substrate inhibition kinetics, which could be modeled using the kinetic models of Haldane–Andrew and Luong–Levenspiel. The mixed culture showed complete mineralization of 4-FCA with quantitative release of fluoride, both in batch and continuous cultures. Steady-state chemostat cultures that were exposed to shock loadings of substrate responded with rapid degradation and returned to steady-state in 10–15 h, indicating that the mixed culture provided a robust system for continuous 4-FCA degradation.
Keywords: Organofluorine compounds, Arthrobacter, Ralstonia, Biodegradation, Defluorination, 4-Fluorocinnamic acid
Introduction
Fluorinated chemicals are prominent xenobiotics that are used in pharmaceutical and agricultural applications because of their bioactivity, biological stability, high lipophilicity and ability to resist metabolic conversion (Natarajan et al. 2005; Tavener and Clark 2003). Fluoro-substitution can cause significant biological effects, such as enhanced enzyme inhibition, changes in cell-to-cell communication, disruption of transport over the membrane and inhibition of energy generation (Key et al. 1997). Industrial applications of fluoroorganics are widespread. For example, polymers of 4-fluorophenol and 4-fluorocinnamic acid (4-FCA) are used in electronic industries (Gerus et al. 2004), which can cause environmental contamination if industrial effluent is discharged untreated. The use of organofluorines in open applications and improper disposal of waste have led to their occurrence as ubiquitous environmental contaminants (Key et al. 1997).
Even though the synthesis and use of fluoroorganics is growing and they often inevitably end up in the environment, knowledge about their biodegradation is scarce. Biodegradation studies with halogenated chemicals have mostly been focused on brominated and chlorinated chemicals (Fetzner 1998; Hardman 1991; Janssen et al. 2001), and few papers describe the biodegradation of fluorinated compounds (Hughes et al. 2011; Murphy 2010; Murphy et al. 2011).
Treatment of 4-FCA containing waste streams from electronic industries is necessary to preserve environmental quality. Among the various techniques available for removing xenobiotic chemicals, biological treatment often is the most economical and versatile approach, as it leads to complete mineralization (Parales et al. 2002). For the design of processes for treatment of toxic waste streams, an understanding of the degradation kinetics is very important. Models that include bacterial growth and substrate utilization have been developed to describe the kinetics of contaminant biodegradation. The microbial growth rate is most often described using Monod kinetics. However, high substrate concentrations may become inhibitory to growth. Several models have been developed to quantify inhibitory effects of toxic substrates on growth and degradation. The Haldane–Andrew (Andrew 1968; Haldane 1930) and Luong–Levenspiel (Levenspiel 1980; Luong 1987) models are the most commonly used ways to describe microbial metabolism with inhibition kinetics.
Recently, we have demonstrated the possibility of partial degradation of 4-FCA by Arthrobacter sp. strain G1 and complete degradation by a mixed culture of strain G1 and Ralstonia sp. strain H1 (Hasan et al. 2011). Several strains of the genus Arthrobacter are known to utilize similar aromatic compounds which include p-hydroxybenzoic acid (Johnson et al. 1999), gentisic acid (Gerus et al. 2004), 4-chlorobenzoate (Marks et al. 1984; Muller et al. 1988; Zaitsev et al. 1991), mono- and dichlorinated biphenyls (Furukawa and Chakrabarty 1982), 3-aminophenol (Lechner and Straube 1988), phenol (Karigar et al. 2006), 4-fluorophenol (Ferreira et al. 2008) and a mixture of phenols (Unell et al. 2008). Members of the genus Ralstonia are also known for their potential to mineralize aromatic compounds (Baggi et al. 2004; Chen et al. 2004, 2007; Leonard and Lindly 1999; Salehi et al. 2010). The aerobic degradation of 4-FCA was reported earlier to proceed via 4-fluoro-acetophenone to form 4-fluorobenzoic acid (4-FBA) as the end product (Creaser et al. 2002; dos Santos et al. 2001; New et al. 2000). These studies were performed with activated sludge that was used for the treatment of a pharmaceutical waste. We came to an alternative degradation pathway for 4-FCA by the consortium of strain G1 and strain H1 through studying metabolites formed by whole cells and testing partially purified enzymes (Hasan et al. 2011). We found that strain G1 utilizes 4-FCA for growth by cleaving off and mineralizing the two-carbon side-chain with release of 4-FBA as the major product. Traces of 4-fluoroacetophenone were formed as a side product.
In the current study, we further evaluate the mineralization of 4-FCA by a pure and mixed cultures under batch and continuous cultivation conditions in shake flask and chemostat. We also provide the information about the degradation kinetics and the effect of shock loadings. The consortium established by the symbiotic relationship of these strains may be useful to understand and improve bioremediation of organofluorine-contaminated streams.
Materials and methods
Growth conditions
Cells of Arthrobacter sp. strain G1 and Ralstonia sp. strain H1 were grown aerobically at 30°C in flasks under rotary shaking or in a 2.5 l fermentor. Growth medium (MMY) contained per liter: 5.37 g of Na2HPO4·12H2O, 1.36 g of KH2PO4, 0.5 g of (NH4)2SO4, 0.2 g of MgSO4·7H2O. The media were supplemented with a trace elements solution (5 ml l−1) (Hasan et al. 2011) and 10 mg yeast extract (Difco Laboratories).
In batch culture experiments, cells of strain G1 and H1 were grown separately on 4-FCA and 4-FBA, respectively, as sole carbon source. For biodegradation experiments with cell suspensions, cells were harvested (4,000×g for 10 min) at mid log phase at an optical density at 450 nm of approximately 0.5. Cells were washed twice with 100 mM potassium phosphate buffer (pH 6.8) and resuspended in the same buffer. To follow biodegradation of 4-FCA, cell suspensions were added to 250 ml flasks containing 100 ml of MMY medium supplemented with 4-FCA at a concentration ranging from 0.2 to 15 mM. To study biodegradation of 4-FBA, cells were incubated similarly in MMY medium supplemented with 2 mM 4-FBA. Cells were incubated in a rotary shaker at 30°C and 150 rpm. Samples were taken with suitable time intervals, centrifuged at 16,000×g for 2 min, and analyzed immediately by HPLC, LC-MS and ion chromatography. To study the kinetics of complete mineralization of 4-FCA, mixed cultures of strains G1 and H1 were inoculated into MMY medium containing 0.1–30 mM 4-FCA.
Optical densities were monitored using a spectrophotometer at a wavelength of 450 nm. The OD450 values were then converted to dry cell mass (OD450 of 1 corresponds to 0.16 g l−1 dry weight).
Growth kinetics
For studying growth kinetics of strain G1 with 4-FCA as a limiting substrate, we used the Monod equation:
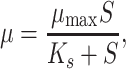 |
1 |
where μ is the specific growth rate (h−1), μmax the maximum specific growth rate (h−1), S the substrate concentration (mM), and K s the half saturation substrate concentration (mM).
For describing growth of microorganisms with inhibition at high substrate concentrations, the Haldane–Andrew model (Andrew 1968) was used, which is given by the following equation:
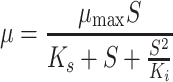 |
2 |
Where K i is the substrate inhibition constant (mM). Luong (1987) applied the equation of Levenspiel (1980), which is an extended Monod type model that can also be applied to describe growth inhibition at high substrate concentrations:
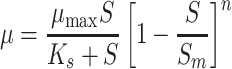 |
3 |
Where S m is the critical inhibitor concentration above which growth stops and n is an empirical constant.
In order to establish the effect of the 4-FCA concentration on growth and degradation, specific growth rates and substrate degradation rates at different 4-FCA concentrations were obtained from time course measurements of the OD450 and substrate concentration in cultures started with different 4-FCA concentrations.
Continuous culture
For growth of strains in continuous culture, we used a 3 l fermentor filled with 2.5 l of MMY medium. The pH was maintained at 7.0 with a sterile solution of 2 M NaOH added by a pump connected to a pH controller. The reactor was kept at 30°C by a temperature sensor and controller, and the agitator speed was adjusted to 250 rpm. Culture medium was supplied to the reactor vessel with a peristaltic pump. Sterile air was supplied to the fermentor by passing it through a 0.45 μm pore size filter. The airflow rate was controlled with a mass flow controller. The purity of the cultures during operation of the fermentor was regularly checked by plating culture samples on NB and LB plates, which were incubated at 30°C. When a culture attained steady state (after 5 volume changes), samples were collected for estimation of dry cell mass, fluoride release and residual 4-FCA. The values of maximum specific growth rate (μmax) were determined by the method of washout, i.e. when D > μmax. The biomass decreases according to Eq. 4 (Molin 1983),
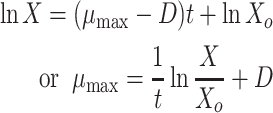 |
4 |
where X o is the initial biomass concentration and X is the biomass concentration at time t (h).
The Monod half-saturation constant (K s) was determined by analysis of substrate concentrations at different dilution rates using the Monod equation.
Chemostat pulse experiments
The effect of a pulse addition of 4-FCA on the growth of the consortium was studied by following the response after a sudden supply of 10 mM 4-FCA to a continuous culture that was operated at a dilution rate of 0.033 h−1. Steady-state conditions were disturbed by injecting 4-FCA through a septum directly into the reactor vessel to a final concentration of 10–16 mM. One sample was taken immediately after 4-FCA injection, centrifuged, and the concentrations of 4-FCA, 4-FBA and fluoride were measured. Subsequent samples were taken with suitable intervals until the entire amount of 4-FCA added was removed due to dilution and conversion by the cells. The time course of substrate concentration is given by supply to the culture, washout by dilution, and biodegradation, according to differential equation 5:
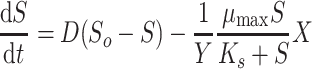 |
5 |
The rate of change of biomass concentration of the bacterial consortium in continuous culture may be described by Eq. 6:
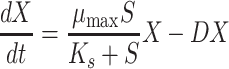 |
6 |
The rate of fluoride formation during a pulse experiment in continuous culture is described by Eq. 7:
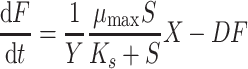 |
7 |
In Eqs. 5–7, S o is the 4-FCA concentration in the chemostat reservoir (mM), S is the concentration of 4-FCA in the chemostat culture at time t (mM), F is the fluoride concentration in the effluent (mM), μ max is the maximum specific rate of 4-FCA conversion (mmol 4-FCA transformed per gram biomass in 1 h, h−1), K s is the Monod constant for the conversion of 4-FCA and formation of fluoride (mM), D is the dilution rate (h−1), X is the biomass concentration at time t (g l−1), and Y is the yield coefficient.
Parameter estimation for Eqs. 5, 6 and 7 was carried out using the program Scientist (MicroMath Inc., Salt Lake City, UT).
Analytical methods
Concentrations of 4-FCA, 4-FBA and their metabolites in culture supernatants were determined by reverse phase HPLC (Jasco PU-2086 pump and Jasco AS-2051 autosampler), using a Lichrosorb C18 column (250 × 4.6 mm, 5 μm particle size). The mobile phase was 0.02 M ammonium acetate adjusted to pH 4.5 with 70/30 (v/v) acetic acid/methanol. The injection volume was 10 μl, the flow rate was 0.8 ml min−1, and detection was at 254 nm with a variable UV-absorbance detector (Jasco UV-2075). The reproducibility of these assays was within 5%, which is better than the experiment-to-experiment variability, and therefore data that are given are representative examples of individual experiments.
LC-MS was carried out using a MicroMass ZMD equipped with a 996 Waters photodiode array detector and an Alliance 2690 separation module. HPLC conditions were as described in the previous paragraph. The mass spectrometer scan range was from m/z 50 to 600 and detection was in the negative ion mode. The source and desolvation temperatures were set to 125 and 150°C, respectively. The cone and capillary voltages were set to 30 and 2.25 V, respectively.
Fluoride measurements were performed by IC using a DX 120 ion chromatograph (Dionex, Sunnyvale, CA, USA) connected to an autosampler. This was equipped with an Alltech A-2 anion (100 × 4.6 mm, 7 μm) column and an Alltech guard (50 × 4 mm) column. The injection volume was 50 μl. The column temperature was set to 30°C. The eluent used was a mixture of NaHCO3 and Na2CO3 in deionized water at a flow rate of 1.2 ml min−1.
Results and discussion
Kinetics of 4-FCA degradation
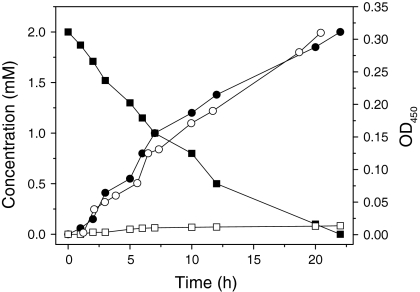
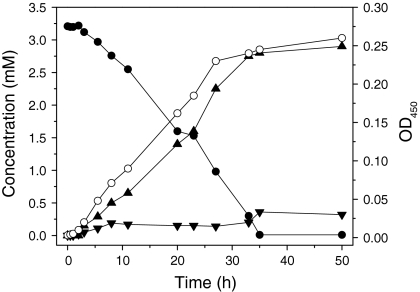
In order to investigate the kinetic properties of Arthrobacter sp. strain G1 and Ralstonia sp. strain H1, we performed a number of growth experiments in batch cultures. In MMY medium, strain G1 grew exponentially between 3 and 16 h with specific growth rate of 0.22 h−1 (Fig. 1) when 2 mM 4-FCA was supplied as the sole source of carbon and energy. Both 4-FBA and 4-fluoroacetophenone (small amounts) were observed as products by LS-MS. Strain H1 degraded 4-FBA, forming 4-fluorocatechol (4-FC) which led to ring fission with fluoride release (Fig. 2). Traces of 4-FC remained in the culture medium for a prolonged period.
Fig. 1.
Growth of strain G1 in MMY medium supplemented with 2 mM 4-FCA. Symbols: filled square 4-FCA concentration; filled circle 4-FBA concentration; open circle optical density at 450 nm; and open square 4-fluoroacetophenone concentration
Fig. 2.
Growth of Ralstonia sp. H1 in MMY supplemented with 3.25 mM 4-FBA. Symbols: filled circle 4-FBA concentration; open circle optical density at 450 nm; filled triangle F− concentration; inverted triangle 4-FC concentration
In order to examine the toxic effects of 4-FCA and its degradation products on growth, cells of strain G1 were incubated in MMY medium containing increasing concentrations (0.2–15 mM) of 4-FCA. For comparison, mixed cultures of strains G1 and H1 were incubated in separate flasks containing MMY medium supplemented with 0.4–30 mM 4-FCA. For each batch culture with a certain 4-FCA concentration, cell growth was measured at a function of time. The specific growth rates at different 4-FCA concentrations were calculated by measuring the slope of increasing OD450 and average cell density of every data set obtained at different 4-FCA concentrations.
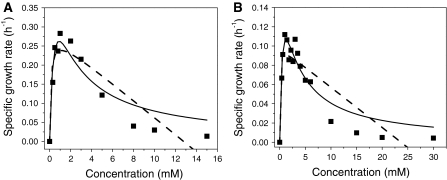
From the results, it is quite clear that the specific growth rate increases with an increase in substrate concentration until a maximum value is reached. However, above 2 mM in pure culture (Fig. 3A) and above 4 mM in case of the mixed culture (Fig. 3B), the specific growth rate started to decrease, indicating substrate inhibition of growth. This may be due to cell damage or disruption of membrane integrity at higher 4-FCA concentrations (Sikkema et al. 1995; Weber and de Bont 1996).
Fig. 3.
Specific growth rates of (A) Arthrobacter sp. G1 and (B) a mixed culture of strain G1 and Ralstonia sp. H1 at different 4-FCA concentrations and fitting with the kinetic models. Symbols: filled square experimental data for [4-FCA]; solid line Haldane–Andrew regression curve; and dashed line Luong–Levenspiel regression curve
The Monod model was used to determine the growth parameters and the Haldane–Andrew and Luong–Levenspiel models were used to quantify the effects of substrate inhibition on growth rates. To fit the data and estimate the values of the biokinetic constants of these models, non-linear regression was done with Microcal Origin 7. The parameters estimated by using these models are summarized in Table 1. The Haldane–Andrew model give K i values that indicate the sensitivity of the culture to substrate inhibition above a concentration where which the specific growth rate declines. The higher K i value of the mixed culture (4.1 mM) compared to the K i of the pure culture (2.5 mM) indicated a higher tolerance to substrate of the mixed culture. This higher resistance to increased substrate levels of the mixed culture was not accompanied by a higher growth rate. Maybe the sequential nature of the degradation process or the need to accumulate 4-FBA before growth of strain H1 can start is responsible for this lower apparent maximum growth rate. Alternatively, strain H1 may form transient metabolites that reduce the 4-FCA consumption rate.
Table 1.
Kinetic parameters with Monod, Haldane–Andrew, and Luong–Levenspiel models for batch conditions with (a) a pure culture of Arthrobacter sp. strain G1 and (b) a mixed culture of strains G1 and Ralstonia sp. strain H1, growing in MMY supplemented with 4-FCA
| Kinetic parameter | (a) 4-FCA growth kinetics in pure culture | (b) 4-FCA growth kinetics in mixed culture | ||||
|---|---|---|---|---|---|---|
| Monod model | Haldane–Andrew model | Luong–Levenspiel model | Monod model | Haldane–Andrew model | Luong–Levenspiel model | |
| μ max (h−1) | 0.37 | 0.42 | 0.40 | 0.10 | 0.16 | 0.15 |
| K s (mM), K m (mM) | 0.11 | 0.52 | 0.14 | 0.11 | 0.60 | 0.30 |
| K i (mM) | – | 2.50 | – | – | 4.10 | – |
| S m (mM), n | – | – | 13.1, n = 1 | – | – | 24.8, n = 1 |
The Luong–Levenspiel model uses a critical substrate concentration (S m), at which the growth rate falls to zero. A high S m (24 mM) was found for the mixed culture, indicating that it can survive and grow at quite high levels of 4-FCA. In case of the pure culture of strain G1, a two-fold lower S m for 4-FCA was found. Of the inhibition models, the Haldane–Andrew model gave a somewhat better fit to our data than the Luong–Levenspiel model.
Kinetics of 4-FCA degradation and growth of strain G1 in chemostat culture
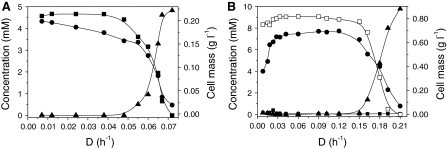
In order to evaluate the kinetics of strain G1 in continuous culture, we measured growth and 4-FCA removal in a chemostat bioreactor that was inoculated to an OD450 of 0.013 with a batch culture of strain G1 pregrown on 5 mM 4-FCA. Growth of strain G1 with 5 mM 4-FCA was followed in batch mode to an OD450 of 0.81. As soon as the stationary growth phase was reached, the culture was switched to continuous mode. Growth kinetics were subsequently studied at various dilution rates, starting with 0.007 h−1 and increasing until the critical dilution rate was reached (Fig. 4A).
Fig. 4.
Utilization of 4-FCA in continuous culture at different dilution rates. A 5 mM 4-FCA with a pure culture of Arthrobacter sp. G1 and B 10 mM 4-FCA with a consortium of Arthrobacter sp. G1 and Ralstonia sp. H1. Symbols: filled square 4-FBA concentration; filled circle biomass; filled triangle 4-FCA concentration; open square F− concentration
The μ max of strain G1 on 4-FCA determined under washout conditions (using Eq. 4) was 0.042 h−1. Steady states were established at dilution rates between 0.007 and 0.038 h−1. The growth of strain G1 followed Monod kinetics. The K s was 41 μM as calculated using the Monod equation. Cell mass was almost constant up to a dilution rate of 0.038 h−1, but at higher dilution rates it decreased sharply. The growth yield was almost constant during steady states and it was determined as 18.8 g of cell dry-mass per mole of substrate. No wall growth of cells was observed and no formation of fluoride occurred during the course of the experiment, but the concentration of 4-FBA was 4.8 ± 0.1 mM up to the maximum dilution rate of 0.06 h−1. The concentration of 4-FBA in the fermentor decreased sharply beyond 0.06 h−1 and was reduced to almost zero at a higher dilution rate.
To study the complete biodegradation of 4-FCA, a consortium of strains G1 and H1 was inoculated in a chemostat with a continuous supply of 10 mM 4-FCA. Cell mass and concentrations of 4-FCA, 4-FBA and F− were measured at different dilutions rates. The culture density was almost constant up to a dilution rate of 0.15 h−1, but decreased sharply with higher dilution rates (Fig. 4B). The residual concentration of 4-FCA was below 0.05 mM from 0.008 to 0.15 h−1, and increased sharply at higher dilution rates. The apparent K s value of the consortium was 47 μM and the μ max obtained was 0.11 h−1, which is higher than what is obtained in batch culture or with a pure culture of strain G1 in the chemostat.
Irrespective of the main product of 4-FCA degradation, the low values of K s indicate that under both pure and mixed culture conditions the bacterial cultures are capable of efficient degradation of 4-FCA. A low cell yield was noted during operation of the reactor when a pure culture of strain G1 was employed to degrade 4-FCA at dilution rates 0.007–0.038 h−1. At a two-fold higher substrate concentration, the cell yield was four-fold higher for the consortium of strains G1 and H1 between dilution rates of 0.033–0.12 h−1 (Fig. 4), which is in agreement with strain H1 being responsible for mineralization of 4-FBA, the end product of 4-FCA side-chain degradation by strain G1.
The μ max values determined for strain G1 (using Eq. 4, Fig. 4A) and for the consortium of strains G1 and H1 (Fig. 4B) were lower than the μ max values determined under wash-out conditions. In contrast, an early wash-out at D < μ max was reported for a multi-species culture growing aerobically on 6-aminonaphthalene-2-sulphonic acid (Diekmann et al. 1988) and another one growing anaerobically on phenol (Khoury et al. 1992). Although there is no clear explanation for the latter, a possible cause could be early wash-out of a stabilizing escorting species which is not directly involved in the degradation process. In our system, both strains of the mixed culture are involved in the degradation of substrate and metabolites. The possibility to increase the dilution rate to values exceeding the µmax determined by the wash-out experiment may be due to adaptation of the cells to higher flow rates if they are kept in the chemostat in the chemostat for a prolonged period.
Effect of shock loadings of 4-FCA to the chemostat culture
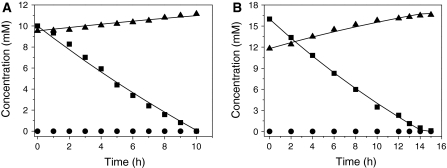
The capability of the consortium of strains G1 and H1 to degrade shock loadings of 4-FCA in continuous mode was tested by pulse additions of 10 and 16 mM of 4-FCA to a steady-state culture growing on 10 mM 4-FCA at a dilution rate of 0.033 h−1 (Fig. 5). Before the pulse, the steady-state concentration of 4-FCA was below the detection limit (<0.02 mM) and the fluoride concentration was 9.5 mM. After a pulse, the consortium completely removed the extra added 4-FCA in 10 or 15 h, respectively. During consumption of pulsed 4-FCA, cell growth resumed and the same steady-state conditions as before the pulse addition of 4-FCA were obtained.
Fig. 5.
Effect of 4-FCA shock loadings on substrate depletion and fluoride formation by a consortium of Arthrobacter sp. G1 and Ralstonia sp. H1 growing in a bioreactor that is continuously supplied with 10 mM 4-FCA at a rate of 0.033 h−1. A A pulse of 10 mM 4-FCA was injected directly into the chemostat vessel. 4-FCA (filled square) was depleted (maximal rate 2.8 mmol h−1 g−1 cells) and fluoride (filled triangle) was released (1.23 mmol h−1 g−1). B A pulse of 16 mM, followed by depletion of 4-FCA (filled square) at a maximal rate of 3.0 mmol h−1 g−1 cells and fluoride (filled triangle) formation occurred at 1.22 mmol h−1 g−1 cells. The residual concentration of 4-FBA (filled circle) in the reactor remained below 0.01 mM. The solid lines show the fit with the Monod equation (5 and 7) for the removal of 4-FCA and release of fluoride
A model based on Monod kinetics (Eqs. 5–7) was appropriate for describing the conversion of 4-FCA during the pulse experiments (Fig. 5), because no inhibition of growth or degradation was observed. Only 53 and 60% of the expected liberated fluorine was detected as free fluoride after the 10 and 16 mM pulses of 4-FCA, respectively. This indicates formation of some intermediate organofluorines that were washed out and remained undetected in the effluent. Earlier, we have shown that 4-fluoroacetophenone may be formed as a side product, and transient accumulation of 4-FBA, 4-FC, and other products may occur (Hasan et al. 2011).
The maximum 4-FCA conversion rates (μ max) in the case of 10 and 16 mM pulses of 4-FCA were estimated as 2.8 and 3.0 mmol h−1 g−1 cell dry weight, respectively. The maximum fluoride formation rate for 10 and 16 mM of 4-FCA could be estimated to 1.23 and 1.22 mmol h−1 g−1 cells, respectively.
The removal of a high 4-FCA concentration and the final return to the initial conditions indicated that the consortium of strains G1 and H1 is highly resistant to shock loadings and can withstand a temporary exposure to a high concentration of 4-FCA in continuous culture. The increasing biomass concentration and the quick depletion of 4-FCA from the bioreactor indicates that the metabolites formed by side-chain cleavage of 4-FCA, especially 4-FBA, are not toxic for strain H1, and the consortium readily consumes these metabolites.
Conclusion
The data reported here show that Arthrobacter sp. strain G1 which can degrade 4-FCA by removing the side-chain of two-carbon atoms, can form a consortium with Ralstonia sp. strain H1. The mixed culture has a high tolerance to 4-FCA toxicity both in batch mode and continuous culture. It can also absorb shock loadings of higher concentrations of 4-FCA. This feature potentially enables the consortium to be used for the treatment of industrial wastewater or bioaugmentation of contaminated soils.
Acknowledgments
We thank Theodora Tiemersma for help in LC-MS analysis. S. A. Hasan gratefully acknowledges the Higher Education Commission (HEC), Government of Pakistan, for financial support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Andrew JF. A mathematical model for continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol Bioeng. 1968;10:707–723. doi: 10.1002/bit.260100602. [DOI] [Google Scholar]
- Baggi G, Cavalca L, Francia P, Zangrossi M. Chlorophenol removal from soil suspensions: effects of a specialized microbial inoculum and a degradable analogue. Biodegradation. 2004;15:153–160. doi: 10.1023/B:BIOD.0000026479.12672.d0. [DOI] [PubMed] [Google Scholar]
- Chen W–M, Chang J-S, Wu C-H, Chang S-C. Characterization of phenol and trichloroethene degradation by the rhizobium Ralstonia taiwanensis. Res Microbiol. 2004;155:672–680. doi: 10.1016/j.resmic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Chen B-Y, Chen W-M, Chang J-H. Optimal biostimulation strategy for phenol degradation with indigenous rhizobium Ralstonia taiwanensis. J Hazard Mater B1. 2007;39:232–237. doi: 10.1016/j.jhazmat.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Creaser C, dos Santos LF, Lamarca DG, New A, Wolff JC. Biodegradation studies of 4-fluorobenzoic acid and 4-fluorocinnamic acid: an evaluation of membrane inlet mass spectrometry as an alternative to high performance liquid chromatography and ion chromatography. Anal Chim Acta. 2002;454:137–145. doi: 10.1016/S0003-2670(01)01514-8. [DOI] [Google Scholar]
- Diekmann RB, Nortemann B, Hempel DC, Knackmuss H-J. Degradation of 6-aminonaphthalene-2-sulfonic acid by mixed cultures: kinetic analysis. Appl Microbiol Biotechnol. 1988;29:85–88. doi: 10.1007/BF00258356. [DOI] [Google Scholar]
- dos Santos LMF, Spicq A, New AP, Biundo GL, Wolff JC, Edwards A. Aerobic biotransformation of 4-fluorocinnamic acid to 4-fluorobenzoic acid. Biodegradation. 2001;12:23–29. doi: 10.1023/A:1011973824171. [DOI] [PubMed] [Google Scholar]
- Ferreira MIM, Marchesi JR, Janssen DB. Degradation of 4-fluorophenol by Arthrobacter sp. strain IF1. Appl Microbiol Biotechnol. 2008;78:709–717. doi: 10.1007/s00253-008-1343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzner S. Bacterial dehalogenation. Appl Microbiol Biotechnol. 1998;50:633–657. doi: 10.1007/s002530051346. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Chakrabarty AM. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982;44:619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerus I, Glushchenko A, Kurioz Y, Reznikov Y, Tereshchenko O. Sticking of liquid crystal on photosensitive polymer layers. Opto-Electron Rev. 2004;12:281–284. [Google Scholar]
- Haldane JSB (1930) Enzymes, longmans, green, UK. Republished by MIT Press, Cambridge, MA (1965)
- Hardman DJ. Biotransformation of halogenated compounds. Crit Rev Biotechnol. 1991;11:1–40. doi: 10.3109/07388559109069182. [DOI] [PubMed] [Google Scholar]
- Hasan SA, Ferreira MIM, Koetsier MJ, Arif MI, Janssen DB. Complete biodegradation of 4-fluorocinnamic acid by a consortium comprising Arthrobacter sp. strain G1 and Ralstonia sp. strain H1. Appl Environ Microbiol. 2011;77:572–579. doi: 10.1128/AEM.00393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D, Clark BR, Murphy CD. Biodegradation of polyfluorinated biphenyl in bacteria. Biodegradation. 2011;22:741–749. doi: 10.1007/s10532-010-9411-7. [DOI] [PubMed] [Google Scholar]
- Janssen DB, Oppentocht JE, Poelarends GJ. Microbial dehalogenation. Curr Opin Biotechnol. 2001;12:254–258. doi: 10.1016/S0958-1669(00)00208-1. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Sims GK, Ellsworth TR, Balance AR. Effects of moisture and sorption on bioavailability of p-hydroxybenzoic acid to Arthrobacter sp. in soil. Microbiol Res. 1999;153:349–353. doi: 10.1016/S0944-5013(99)80049-4. [DOI] [PubMed] [Google Scholar]
- Karigar C, Mahesh A, Nagenahalli M, Yun DJ. Phenol degradation by immobilized cells of Arthrobacter citreus. Biodegradation. 2006;17:47–55. doi: 10.1007/s10532-005-3048-y. [DOI] [PubMed] [Google Scholar]
- Key BD, Howell RD, Criddle CS. Fluorinated organics in the biosphere. Environ Sci Technol. 1997;31:2445–2454. doi: 10.1021/es961007c. [DOI] [Google Scholar]
- Khoury N, Wolfgang D, Kampfer P. Anaerobic degradation of phenol in batch and continuous cultures by a denitrifying bacterial consortium. Appl Microbiol Biotechnol. 1992;37:524–528. [Google Scholar]
- Lechner U, Straube G. Degradation of 3-aminophenol by Arthrobacter sp. mA3. J Basic Microbiol. 1988;28:629–637. doi: 10.1002/jobm.3620280918. [DOI] [PubMed] [Google Scholar]
- Leonard D, Lindly NA. Growth of Ralstonia eutropha on inhibitory concentration of phenol: diminished growth can be attributed to hydrophilic perturbation of phenol hydroxylase activity. Enzym Microb Technol. 1999;25:271–277. doi: 10.1016/S0141-0229(99)00039-3. [DOI] [Google Scholar]
- Levenspiel O. The Monod equation: a revisit and a generalization to product inhibition situations. Biotechnol Bioeng. 1980;22:1671–1687. doi: 10.1002/bit.260220810. [DOI] [Google Scholar]
- Luong JHT. Generalization of Monod kinetics for analysis of growth data with substrate inhibition. Biotechnol Bioeng. 1987;29:242–248. doi: 10.1002/bit.260290215. [DOI] [PubMed] [Google Scholar]
- Marks TS, Wait R, Smith AR. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984;124:669–674. doi: 10.1016/0006-291X(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Molin G. Measurement of the maximum specific growth-rate in chemostat of Pseudomonas sp. with different abilities for biofilm formation. Eur J Appl Microbiol Biotechnol. 1983;18:303–307. doi: 10.1007/BF00500496. [DOI] [Google Scholar]
- Muller R, Oltmanns RH, Lingens F. Enzymatic dehalogenation of 4-chlorobenzoate by extracts from Arthrobacter sp. SU DSM 20407. Biol Chem Hoppe-Seyler. 1988;369:567–571. doi: 10.1515/bchm3.1988.369.2.567. [DOI] [PubMed] [Google Scholar]
- Murphy CD. Biodegradation and biotransformation of organofluorine compounds. Biotechnol Lett. 2010;32:351–359. doi: 10.1007/s10529-009-0174-3. [DOI] [PubMed] [Google Scholar]
- Murphy CD, Clark BR, Amadio J. Metabolism of fluoroorganic compounds in microorganisms: impacts for the environment and the production of fine chemicals. Appl Microbiol Biotechnol. 2009;84:617–629. doi: 10.1007/s00253-009-2127-0. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Azerad R, Badet B, Copin E. Microbial cleavage of C–F bond. J Fluor Chem. 2005;126:425–436. doi: 10.1016/j.jfluchem.2004.12.001. [DOI] [Google Scholar]
- New AP, dos Santos LMF, Biundo GL, Spicq A. Analytical techniques used for monitoring the biodegradation of fluorinated compounds in waste streams from pharmaceutical production. J Chromatogr A. 2000;889:177–184. doi: 10.1016/S0021-9673(00)00571-9. [DOI] [PubMed] [Google Scholar]
- Parales RE, Bruce NC, Schmid A, Wackett LP. Biodegradation, biotransformation and biocatalysis (B3) Appl Environ Microbiol. 2002;68:4699–4709. doi: 10.1128/AEM.68.10.4699-4709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi Z, Sohrabi M, Vahabzadeh F, Fatemi S, Kawase Y. Modeling of p-nitrophenol biodegradation by Ralstoniaeutropha via application of the substrate inhibition concept. J Hazard Mater. 2010;177:582–585. doi: 10.1016/j.jhazmat.2009.12.072. [DOI] [PubMed] [Google Scholar]
- Sikkema J, de Bont FA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavener SJ, Clark FH. Can fluorine chemistry be green chemistry? J Fluor Chem. 2003;123:31–36. doi: 10.1016/S0022-1139(03)00140-4. [DOI] [Google Scholar]
- Unell M, Nordin K, Jernberg C, Stenstrom J, Jansson JK. Degradation of mixtures of phenolic compounds by Arthrobacter chlorophenolicus A6. Biodegradation. 2008;19:495–505. doi: 10.1007/s10532-007-9154-2. [DOI] [PubMed] [Google Scholar]
- Weber FJ, de Bont JA. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta. 1996;1286:225–245. doi: 10.1016/s0304-4157(96)00010-x. [DOI] [PubMed] [Google Scholar]
- Zaitsev GM, Tsoi TV, Grishenkov VG, Plotnikova EG, Boronin AM. Genetic control of degradation of chlorinated benzoic acids in Arthrobacterglobiformis, Corynebacterium sepedonicum and Pseudomonas cepacia strains. FEMS Microbiol Lett. 1991;65:171–176. doi: 10.1111/j.1574-6968.1991.tb04742.x. [DOI] [PubMed] [Google Scholar]