Abstract
Purpose
The Roux en Y method has rarely been performed due to longer operation time and high risk of complication, despite several merits including prevention of bile reflux. We conducted a retrospective review of the result of Roux en Y reconstruction using two circular staplers after subtotal gastrectomy.
Materials and Methods
From December 2008 to May 2009, a total of 26 patients underwent Roux en Y reconstruction using two circular staplers after subtotal gastrectomy, and seventy-two patients underwent Billroth-I reconstruction. Roux en Y anastomosis was performed using two circular staplers without hand sewing anastomosis. We compared clinicopathologic features and surgical outcomes between the two groups. All patients underwent gastrofiberscopy between six and twelve months after surgery to compare the bile reflux.
Results
No significant differences in clinicopathologic findings were observed between the two groups, except for the rate of minimal invasive surgery (P=0.004) and cancer stage (P=0.002). No differences in the rate of morbidity (P=0.353) and admission duration (P=0.391) were observed between the two groups. Gastrofiberscopic findings showed a significant reduction of bile reflux in the remnant stomach in the Roux en Y group (P=0.019).
Conclusions
When compared with Billroth-I reconstruction, Roux en Y reconstruction using the double stapler technique was found to reduce bile reflux in the remnant stomach without increasing postoperative morbidity. Based on these results, we planned to begin a randomized controlled clinical trial for comparison of Roux en Y reconstruction using this method with Billroth-I anastomosis.
Keywords: Stomach neoplasms, Gastrectomy, Reconstructive surgical procedure
Introduction
Gastric cancer is one of the most common malignant tumors in the world, and surgical resection is the only proven modality that can lead to a significant increase of the survival rate.(1) As the proportion of gastric cancer diagnosed at the early stage has shown a recent increase, the survival time of gastric cancer patients after surgery is expected to be longer than that of the past decade.(2) Therefore, interest in postoperative quality of life has increased, and it is strongly associated with the reconstruction method after gastric resection.(2)
The traditional reconstruction method followed with distal gastrectomy included only one anastomosis site, like gastroduodenostomy or gastrojejunostomy.(3) Although these procedures have obvious demerits, such as chemical stimulation in the remnant stomach by bile reflux, due to their simplicity and safety, surgeons have shown a preference for these procedures over the Roux en Y reconstruction method. Meanwhile, the Roux en Y procedure, which has typically been used as a method for reconstruction after total gastrectomy, was rarely used after distal gastrectomy.(4) In selection of Roux en Y reconstruction method after distal gastrectomy, the possibility of increased complications followed by a longer operation time for two anastomosis and complicated procedures has taken precedence over considerations for postoperative quality of life. However, recent interest in postoperative quality of life would lead to increased selection of Roux en Y reconstruction after distal gastrectomy.
An anastomotic device that will allow simplification of the procedure, which has in the past been considered complex, has recently been developed. Here, we wish to introduce a novel procedure for performance of Roux en Y reconstruction after distal gastrecotomy. In addition, to evaluate the safety and efficacy of this procedure, we compared this procedure with the conventional reconstruction method of gastroduodenostomy in terms of surgical outcome and the degree of bile reflux.
Materials and Methods
From December 2008 to May 2009, twenty-six patients who were diagnosed with gastric adenocarcinoma were enrolled in this study. All patients underwent distal subtotal gastrectomy with proper lymph node dissection, and Roux en Y reconstruction with two circular staplers. We conducted a retrospective investigation of the clinicopathologic features, surgical outcomes, and results of follow up gastrofiberscopy, and compared them with those of seventy two patients who underwent Billroth-I reconstruction during the same period.
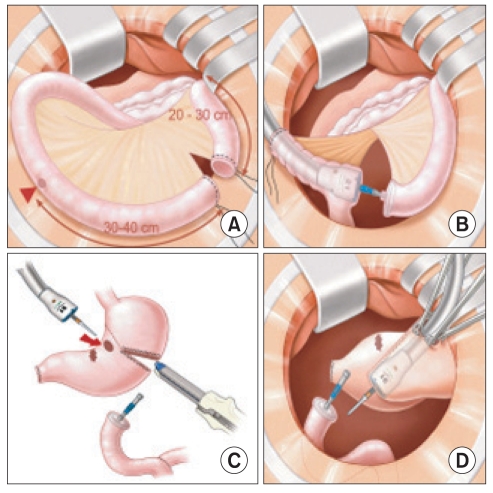
Lymph node dissection with omentectomy was performed using either open laparotomy or laparoscopy; however, the reconstruction was performed through a vertical minilaparotomy, mean 6 cm size in upper abdomen, in the laparoscopy assisted approach. The distal 20 to 30 cm portion of the jejunum from the Treitz ligament was divided by two purse-string cramps accompanied by mesenteric dissection (Fig. 1A). The envil of a 21 mm circular stapler was inserted into the proximal part of the divided jejunum, and the body of the 21 mm stapler was then introduced into the distal part of the divided jejunum, and the end of the boy lead to the 30 to 40 cm distal (Fig. 1B). After combining the body and envil, a reinforcement suture was performed and the body of the stapler was removed. The envil (29 mm) of the larger circular stapler was inserted into the distal part of the previously divided jejunum (Fig. 1C). Gastrojejunostomy was performed using a circular stapler without hand-sewing, as previously reported for gastroduodenostomy (Fig. 1D).(5) After resection of the distal stomach, including the cancerous lesion and opening site for insertion of the stapler body, the anastomosis was completed (Fig. 2). In the Billroth-I group, gastroduoenostomy also was performed as previously reported.(5)
Fig. 1.
Surgical procedure for Roux en Y reconstruction. (A) Division of the proximal jejunum. (B) Insertion of the 21 mm envil into the distal end of the divided jejunum and introduction of the body of a 21 mm stapler into 30 to 40 cm distal. (C) Insertion of another envil into the distal end of the resected jejunum. (D) Stapling for gastrojejunostomy.
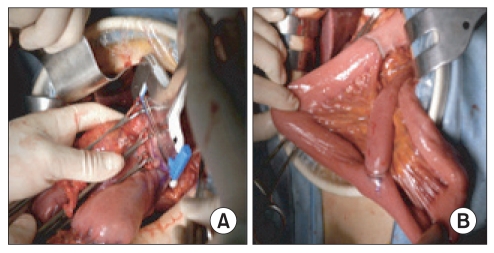
Fig. 2.
Completion of Roux en Y reconstruction using two circular staplers after distal gastrectomy.
We investigated surgical outcomes, including operative complications, operation time, and length of hospital stay, and clinicopathologic features, including age, gender, comorbidity, and so on in the two groups. All patients underwent follow-up gastrofiberscopy six to nine months after surgery; residual food, gastritis, and bile reflux in the remnant stomach were evaluated as a classification of a previous report by Japanese surgeons.(6) Regarding residual food, Grade 0 is no residual food; Grade 1 is small amount; Grade 2 is moderate amount possible to observe entire surface of remnant stomach; Grade 3 is hider observation of remnant stomach; Grade 4 is impossible to observe the remnant stomach. Grade 0 of gastritis is normal mucosa; Grade 1 is mild redness; Grade 2 is intermediate; Grade 3 is severe redness; Grade 4 is apparent erosion. When residual food and gastritis scored over grade 3, we decided those were positive finding. Bile reflux is graded into grade 0 (Absence) or 1 (Present). We reviewed all gastrofiberscopic finding with endoscopist. Results were compared between the two groups.
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Analysis of continuous variables was performed with an un-paired t-test, and categorical variables with a chi-square test to investigate differences between the two groups. Continuous variables were expressed as the mean±standard deviation, and differences were considered statistically significant when P-values were less than 0.05.
Results
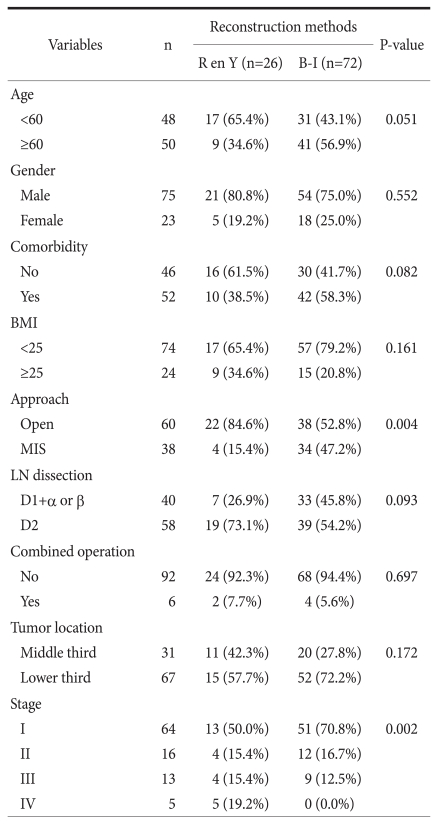
Mean age of all patients was 55.6±14.2 years; the study included 75 males and 50 females. Patient characteristics, including age distribution, gender, comorbidity, and obesity represented by body mass index, did not differ significantly between the Roux en Y and Billroth-I groups (Table 1). Most common comorbidity was hypertension, and it was 39% of all comorbidity. In terms of surgical methods, the extent of lymph node dissection and the rate of combined operation (cholecystectomy was most common combined operation) were not significantly different between the two groups, except the proportion of minimally invasive surgery. Pathologic results showed that patients of the Roux en Y group had gastric cancer of a relatively earlier stage.
Table 1.
Clinicopathologic features according to reconstruction methods (n=98)
R en Y = Roux en Y reconstruction; B-I = Billroth-I reconstruction; BMI = body mass index; MIS = minimally invasive surgery; LN = lymph node.
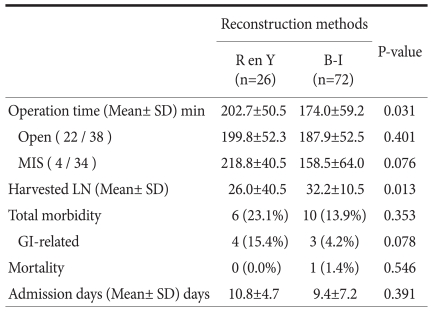
Surgical outcomes showed that operation time for patients in the Roux en Y group was longer than that in the Billroth-I group (P=0.031). The cause of this difference was assumed to be the longer operation time for patients of minimally invasive surgery in the Roux en Y group. Total postoperative morbidity (23.1% vs. 13.9%, P=0.353), mortality (0.0% vs. 1.4%, P=0.546), and length of hospital stay (10.8±4.7 vs. 9.4±7.2 days, P=0.391) did not differ between the Roux en Y and Billroth-I groups. One mortality case was caused by a sepsis following with anastomosis leakage in Billroth-I group. Morbidities related to the gastrointestinal tract, like delayed emptying, ileus, and resection line bleeding occurred in patients who underwent Roux en Y reconstruction, and this complication rate also did not show difference between two groups (P=0.078). Other complications of Roux en Y group were pulmonary complication and urinary tract infection (Table 2).
Table 2.
Surgical outcomes of both groups
R en Y = Roux en Y reconstruction; B-I = Billroth-I reconstruction; SD = standard deviation; MIS = minimally invasive surgery; LN = lymph node; GI = gastroin testinal.
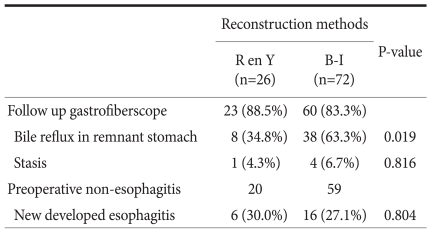
Gastrofiberscopy findings at postoperative 6 to 9 months showed a significant reduction in the proportion of bile reflux in the remnant stomach in patients who underwent Roux en Y reconstruction, compared with Billroth-I reconstruction (P=0.019). The proportion of stasis (P=0.816) and gastritis (P=0.100) did not differ between the two groups. Development of postoperative esophagitis was also similar within the two groups (Table 3).
Table 3.
Gastrofiberscopy results
R en Y = Roux en Y reconstruction; B-I = Billroth-I reconstruction.
Discussion
Despite its effectiveness in prevention of bile reflux, Roux en Y reconstruction has been known to have a higher complication rate than that of Billroth-I or Billroth-II.(3) In our study, we wanted to try a novel procedure using two circular staplers, and confirmed a lower rate of bile reflux in the remnant stomach without increased postoperative morbidity.
With the Roux limb of 30 to 40 cm, superiority of Roux en Y reconstruction after distal gastrectomy in gastric cancer surgery to Billroth-I or Billroth-II reconstruction in prevention of bile reflux into the remnant stomach has been demonstrated.(3,7) These reports confirmed reduction of bile reflux by 24 hour bilirubin monitoring and biliary scintigraphy. Bile reflux in the remnant stomach can damage the gastric mucosa and increase the opportunity for exposure to carcinogens, such as nitrosamines and pathologic bacteria.(8,9) In addition, stimulation of reflux in the remnant stomach could be deemed as a major cause of various symptoms after distal gastrectomy, and can exert a negative influence on postoperative quality of life.(10) Therefore, Roux en Y reconstruction is a very useful reconstruction method. In our study, endoscopic findings were used for evaluation of bile reflux, gastritis, and stasis, and its usefulness had already been validated in previous studies by Japanese researchers.(6,11) As in previous studies comparing Roux en Y and other reconstructions, our study showed the benefit in terms of bile reflux in postoperative endoscopic findings for the remnant stomach.
Compared with hand suturing, the stapling device has allowed easier, faster, and more reliable performance of gastrointestinal anastomosis. In particular, recent development of the device has led to reduced complications, like leakage and bleeding, and its range of use has been extended.(12-14) However, anastomosis using staples for jejunojejunostomy in Roux en Y reconstruction is not easy, and most surgeons have used a hand-sewn technique. In this study, we developed a new technique in which jejunojejunostomy was easily performed by passage of a 21 mm diameter circular stapler through the Roux limb 30 to 40 cm from the divided distal end of the jejunum in order to make the anastomosis. The proper length of Roux limb is important because a long limb prevents passage of a circular stapler, and makes folds in jejunal limb, and needs more mesenteric division. In our study, complications such as bleeding from the circular staple line or leakage were not observed in patients of the Roux en Y group. In addition, other surgical complications were not increased in the Roux en Y group, compared with the Billroth-I group.
Some investigators have expressed concerns over the fact that stasis syndrome by the Roux limb developed in 10% to 50% patients of Roux en Y reconstruction after distal gastrectomy.(15-17) Vagotomy during lymph node dissection and division of jejunal mesentery as the cause of mesentery are assumed. Although some studies have recommended uncut Roux en Y reconstruction or postoperative medication for improvement of postoperative stasis, the results have been controversial. However, because a vagotomy is also required, stasis in the remnant stomach is also possible with Billroth-I reconstruction. In addition, Gustavsson et al.(18) reported that the length of the Roux en Y limb of over 40 cm could have an effect on Roux limb stasis. Therefore, for comparison of surgical outcomes between the Roux en Y and Billroth-I groups in this study, we limited the length of the Roux limb to 30 cm to 40 cm. As a result, postoperative stasis between the two groups did not show a significant difference. One patient who complained of stasis was readmitted, and made a full recovery by conservative treatment.
In our series, none of the patients included in the Roux en Y group showed postoperative complications related to anastomosis. One study of patients who underwent LADG with Billroth-I anastomosis using a circular stapler reported a leakage rate of 14%.(19) The high rate of leakage was assumed to be caused by excessive duodenal stump devascularization and tension on the anastomosis. In Roux en Y reconstruction, a 30 to 40 cm length of the Roux limb is sufficient for creation of an gastrojejunostomy without tension, in spite of even ante-colic fashion. Abundant vascularization of the stomach can induce healing of anastomosis after gastrojejunostomy. Some surgeons may have concerns with regard to bleeding from the anastomosis line; however, using our technique, we can confirm non-bleeding from the anastomosis line through the insertion site of the stapler.
Our study has several limitations. First, we did not evaluate symptomatic differences between the two groups. Therefore, we plan to conduct a randomized controlled clinical trial in which quality of life as well as reflux symptoms will be evaluated between Roux en Y and Billroth-I reconstruction. Second, although we expected that the Roux en Y method using double staplers would not differ from the Billroth-I method in terms of operation time, the results described a longer operation time in the Roux en Y group. We assumed that the reconstruction procedure through minilaparotomy in laparoscopy-assisted surgery might effect on the overall operation time. The small window of minilaparotomy might make division of jejunal mesentry and anastomosis difficult. However, the experience of several cases could lead to conquest of the learning curve in our study.
In conclusion, Roux en Y reconstruction using the double stapler technique was found to be feasible and safe, and reduced bile reflux in the remnant stomach when compared with B-I reconstruction. Based on these results, we planned to begin a randomized controlled clinical trial for comparison of Roux en Y reconstruction using this method with Billroth-I anastomosis.
Acknowledgments
This work was supported by a grant of the Korea Healthcare technology R&D project, Ministry of Health, Welfare, & Family Affairs, Republic of Korea (1020410).
References
- 1.Kim JP. Current status of surgical treatment of gastric cancer. J Surg Oncol. 2002;79:79–80. doi: 10.1002/jso.10050. [DOI] [PubMed] [Google Scholar]
- 2.Roukos DH. Current advances and changes in treatment strategy may improve survival and quality of life in patients with potentially curable gastric cancer. Ann Surg Oncol. 1999;6:46–56. doi: 10.1007/s10434-999-0046-z. [DOI] [PubMed] [Google Scholar]
- 3.Fukuhara K, Osugi H, Takada N, Takemura M, Higashino M, Kinoshita H. Reconstructive procedure after distal gastrectomy for gastric cancer that best prevents duodenogastroesophageal reflux. World J Surg. 2002;26:1452–1457. doi: 10.1007/s00268-002-6363-z. [DOI] [PubMed] [Google Scholar]
- 4.Lehnert T, Buhl K. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg. 2004;91:528–539. doi: 10.1002/bjs.4512. [DOI] [PubMed] [Google Scholar]
- 5.Yang HK, Lee HJ, Ahn HS, Yoo MW, Lee IK, Lee KU. Safety of modified double-stapling end-to-end gastroduodenostomy in distal subtotal gastrectomy. J Surg Oncol. 2007;96:624–629. doi: 10.1002/jso.20883. [DOI] [PubMed] [Google Scholar]
- 6.Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg. 2008;247:962–967. doi: 10.1097/SLA.0b013e31816d9526. [DOI] [PubMed] [Google Scholar]
- 7.Shinoto K, Ochiai T, Suzuki T, Okazumi S, Ozaki M. Effectiveness of Roux-en-Y reconstruction after distal gastrectomy based on an assessment of biliary kinetics. Surg Today. 2003;33:169–177. doi: 10.1007/s005950300039. [DOI] [PubMed] [Google Scholar]
- 8.Lorusso D, Linsalata M, Pezzolla F, Berloco P, Osella AR, Guerra V, et al. Duodenogastric reflux and gastric mucosal polyamines in the non-operated stomach and in the gastric remnant after Billroth II gastric resection. A role in gastric carcinogenesis? Anticancer Res. 2000;20:2197–2201. [PubMed] [Google Scholar]
- 9.Miwa K, Hasegawa H, Fujimura T, Matsumoto H, Miyata R, Kosaka T, et al. Duodenal reflux through the pylorus induces gastric adenocarcinoma in the rat. Carcinogenesis. 1992;13:2313–2316. doi: 10.1093/carcin/13.12.2313. [DOI] [PubMed] [Google Scholar]
- 10.Svensson JO. Duodenogastric reflux after gastric surgery. Scand J Gastroenterol. 1983;18:729–734. doi: 10.3109/00365528309182087. [DOI] [PubMed] [Google Scholar]
- 11.Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002;5:83–89. doi: 10.1007/s101200200014. [DOI] [PubMed] [Google Scholar]
- 12.Berman S, Hashizume M, Yang Y, DuPree J, Matsumoto T. Intraoperative hemostasis and wound healing in intestinal anastomoses using the ILA stapling device. Am J Surg. 1988;155:520–525. doi: 10.1016/s0002-9610(88)80127-2. [DOI] [PubMed] [Google Scholar]
- 13.Shoji Y, Nihei Z, Hirayama R, Mishima Y. Experiences with the linear cutter technique for performing Roux-en-Y anastomosis following total gastrectomy. Surg Today. 1995;25:27–31. doi: 10.1007/BF00309381. [DOI] [PubMed] [Google Scholar]
- 14.Yo LS, Consten EC, Quarles van Ufford HM, Gooszen HG, Gagner M. Buttressing of the staple line in gastrointestinal anastomoses: overview of new technology designed to reduce perioperative complications. Dig Surg. 2006;23:283–291. doi: 10.1159/000096648. [DOI] [PubMed] [Google Scholar]
- 15.Britton JP, Johnston D, Ward DC, Axon AT, Barker MC. Gastric emptying and clinical outcome after Roux-en-Y diversion. Br J Surg. 1987;74:900–904. doi: 10.1002/bjs.1800741010. [DOI] [PubMed] [Google Scholar]
- 16.Herrington JL, Jr, Scott HW, Jr, Sawyers JL. Experience with vagotomy--antrectomy and Roux-en-Y gastrojejunostomy in surgical treatment of duodenal, gastric, and stomal ulcers. Ann Surg. 1984;199:590–597. doi: 10.1097/00000658-198405000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hocking MP, Vogel SB, Falasca CA, Woodward ER. Delayed gastric emptying of liquids and solids following Roux-en-Y biliary diversion. Ann Surg. 1981;194:494–501. doi: 10.1097/00000658-198110000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustavsson S, Ilstrup DM, Morrison P, Kelly KA. Roux-Y stasis syndrome after gastrectomy. Am J Surg. 1988;155:490–494. doi: 10.1016/s0002-9610(88)80120-x. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara M, Kodera Y, Kasai Y, Kanyama Y, Hibi K, Ito K, et al. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg. 2003;196:75–81. doi: 10.1016/s1072-7515(02)01539-9. [DOI] [PubMed] [Google Scholar]