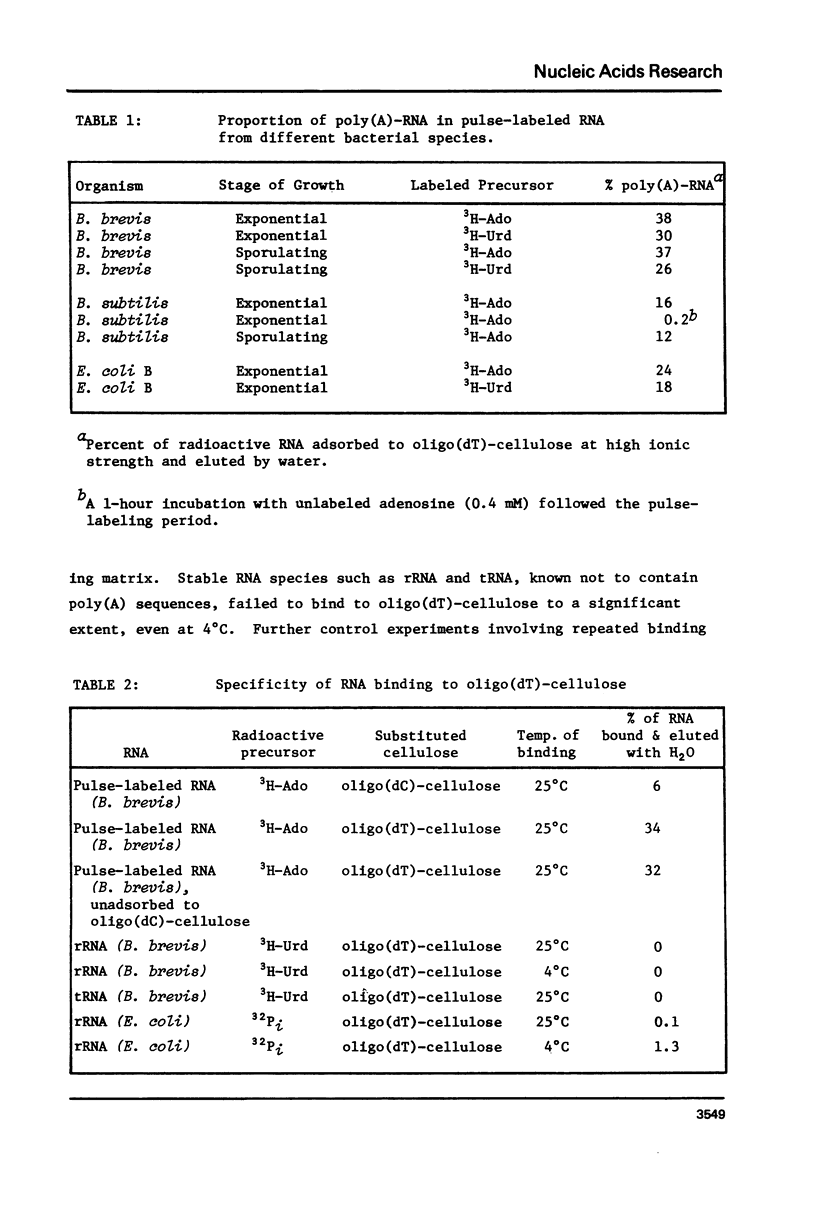
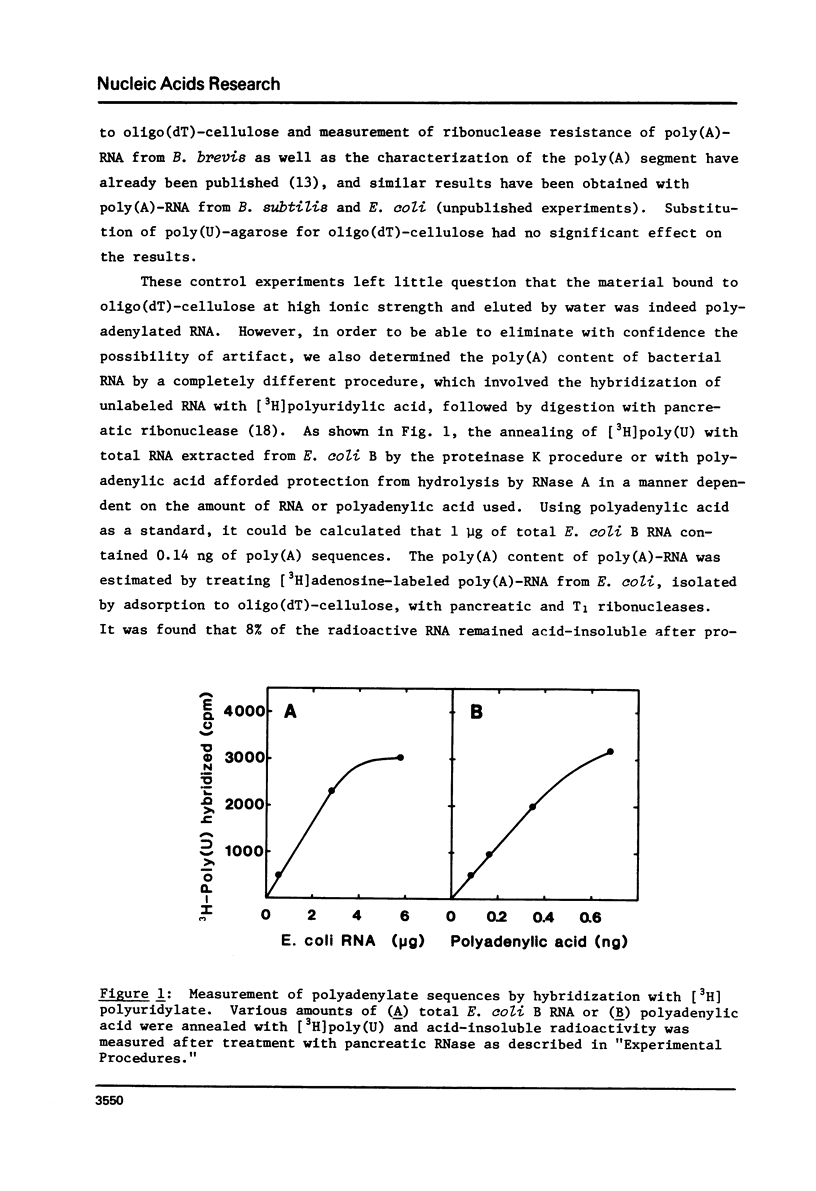
Abstract
A new one-step procedure for the isolation of bacterial RNA, involving lysis by proteinase K in the presence of sodium dodecyl sulfate, is described. Pulse-labeled RNA isolated by this procedure for Bacillus brevis, Bacillus subtilis, and Escherichia coli B has been found to contain a substantial fraction (15-40%) of polyadenylated RNA as determined by adsorption to oligo(dT)-cellulose. This contrasts with RNA isolated by procedures involving phenol extraction, a process which appears to lead to the selective loss of polyadenylated RNA. The presence of polyadenylated RNA in E. coli was confirmed by an independent method which involved hybridization with [3H]polyuridylic acid. Using the proteinase K method for RNA isolation, it was possible to demonstrate the in vitro synthesis of polyadenylated RNA by toluene-treated cells of B. brevis, B. subtilis, and E. coli.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI V . THE ROLE OF METHIONINE IN THE PRODUCTION OF HOST SPECIFICITY. J Mol Biol. 1965 Feb;11:247–256. doi: 10.1016/s0022-2836(65)80055-9. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann I. E., Brawerman G. Loss of the polyadenylate segment from mammalian messenger RNA. Selective cleavage of this sequence from polyribosomes. J Mol Biol. 1980 May 25;139(3):439–454. doi: 10.1016/0022-2836(80)90140-0. [DOI] [PubMed] [Google Scholar]
- Bina M., Feldmann R. J., Deeley R. G. Could poly(A) align the splicing sites of messenger RNA precursors? Proc Natl Acad Sci U S A. 1980 Mar;77(3):1278–1282. doi: 10.1073/pnas.77.3.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Synthesis and degradation of poly(A) in permeable cells of Escherichia coli. J Biol Chem. 1978 Aug 25;253(16):5579–5584. [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Haselkorn R. Messenger RNA. Annu Rev Biochem. 1969;38:647–676. doi: 10.1146/annurev.bi.38.070169.003243. [DOI] [PubMed] [Google Scholar]
- Kaur S., Jayaraman K. Appearance of polyadenylated RNA species during sporulation in Bacillus polymyxa. Biochem Biophys Res Commun. 1979 Jan 30;86(2):331–339. doi: 10.1016/0006-291x(79)90870-2. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. Isolation and characterization of polyadenylated RNA species from sporulating cells of Bacillus subtilis. Biochem Biophys Res Commun. 1980 Mar 13;93(1):201–208. doi: 10.1016/s0006-291x(80)80266-x. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- Mendelsohn S. L., Young D. A. Inhibition of ribonuclease. Efficacy of sodium dodecyl sulfate, diethyl pyrocarbonate, protein ase K and heparin using a sensitive ribonuclease assay. Biochim Biophys Acta. 1978 Jul 24;519(2):461–473. doi: 10.1016/0005-2787(78)90099-0. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Venkatesan S., Edmonds M. Polyadenylic acid sequences in E. coli messenger RNA. Nature. 1975 Jul 10;256(5513):144–146. doi: 10.1038/256144a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Ohta N., Sanders M., Newton A. Characterization of unstable poly (A)-RNA in Caulobacter crescentus. Biochim Biophys Acta. 1978 Jan 26;517(1):65–75. doi: 10.1016/0005-2787(78)90034-5. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1437–1443. doi: 10.1128/jb.97.3.1437-1443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Isolation and characterization of polyadenylate-containing RNA from Bacillus brevis. Biochemistry. 1978 Aug 22;17(17):3468–3474. doi: 10.1021/bi00610a007. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Paulus H. A guanosine 3':5'-monophosphate-sensitive nuclease from Bacillus brevis. J Biol Chem. 1975 Jan 25;250(2):684–690. [PubMed] [Google Scholar]
- Schultz G. A., Chaconas G., Moore R. L. Polyadenylic acid sequences in the RNA of Hyphomicrobium. J Bacteriol. 1978 Feb;133(2):569–575. doi: 10.1128/jb.133.2.569-575.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P. R., Ramanarayanan M., Rabbani E. Presence of polyriboadenylate sequences in pulse-labeled RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2910–2914. doi: 10.1073/pnas.72.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Klett H. Measurement of polyadenylic acid by hybridization with polyuridylic acid: a source of error due to the lability of tritiated polyuridylic acid in trichloroacetic acid. Anal Biochem. 1978 Nov;91(1):173–179. doi: 10.1016/0003-2697(78)90828-x. [DOI] [PubMed] [Google Scholar]



