Abstract
A series of 20 pentamidine analogs were prepared using 2 general Schemes that evaluated heteroatoms, sulfobenzene and alkanediamide groups in the aliphatic linker and methoxy substituents attached to the benzene rings for efficacy against the fungal pathogen, Pneumocystis carinii in an ATP bioassay. All but one of the 20 bisamidines reduced the ATP content of the P. carinii over the 72 hr of the assay period. The highest activities were associated with the lack of methoxy groups and the presence of the O, N and S heteroatoms. Activity (IC50) for compounds 1, 5, 6, 10 ranged from 1.1 to 2.13 µM. The compound 11 with similar activity (1.33 µM), bears a sulfobenzene group at a nitrogen in the aliphatic linker. The alkanediamide-linked bisbenzamidines showed a moderate inhibition of ATP. Generally, the inclusion of a heteroatom in the aliphatic linker and absence of methoxy groups at the benzene rings were associated with higher activities in this assay. Of note, most of the compounds had little to no cytotoxicity in mammalian cell cultures. Although not quite as potent as other pentamidine derivatives, these compounds hold promise for decreased side effects within the mammalian host.
Keywords: Pentamidine analogs, anti-Pneumocystis carinii activity, in vitro ATP bioluminescent assay
1. Introduction
Treatment of Pneumocystis pneumonia (PCP) remains a challenge due to limited therapeutic choices, potential evolving mutations in the targets of standard anti-Pneumocystis compounds including trimethoprim-sulfamethoxazole and atovaquone, and toxicity associated with second line therapies such as pentamidine isethionate [1]. Few drugs are in the development pipeline due to elimination of programs supported within the pharmaceutical industry and shifting priorities of national research foundations. Concomitantly, infection with Pneumocystis jirovecii (the species infecting humans) is expanding into new patient populations besides the frankly immunocompromised host. PCP is a significant cause of morbidity and mortality in patients with rheumatoid arthritis or other chronic conditions requiring anti-TNF alpha therapies [2,3], while colonization with P. jirovecii is associated with a poorer outcome and more severe disease in patients with Chronic Obstructive Pulmonary Disease (COPD) [4]. Standard antifungal therapies such as the azoles or amphotericin B are not effective against PCP, likely due to the lack of ergosterol biosynthesis by these fungi [5]. Therefore, a common approach to identify new effective therapies for PCP has been to use compounds with known efficacies, such as trimethoprim, sulfamethoxazole or pentamidine, and chemically modify these parent compounds to increase efficacy and reduce toxicity [6–9].
In the present report, we have undertaken an analysis of linear pentamidine analogs for the purpose of identifying candidate anti-Pneumocystis therapy. The antimicrobial activity of aromatic bisamidines is well known, but only pentamidine is clinically used. Its high activity is associated with toxicity and low bioavailability, indicating a need for new derivatives that provide increased efficacy with no toxicity. The mechanism of biological action of the bisamidines is not clear, but their ability to bind to the AT minor groove has been demonstrated [10–14].
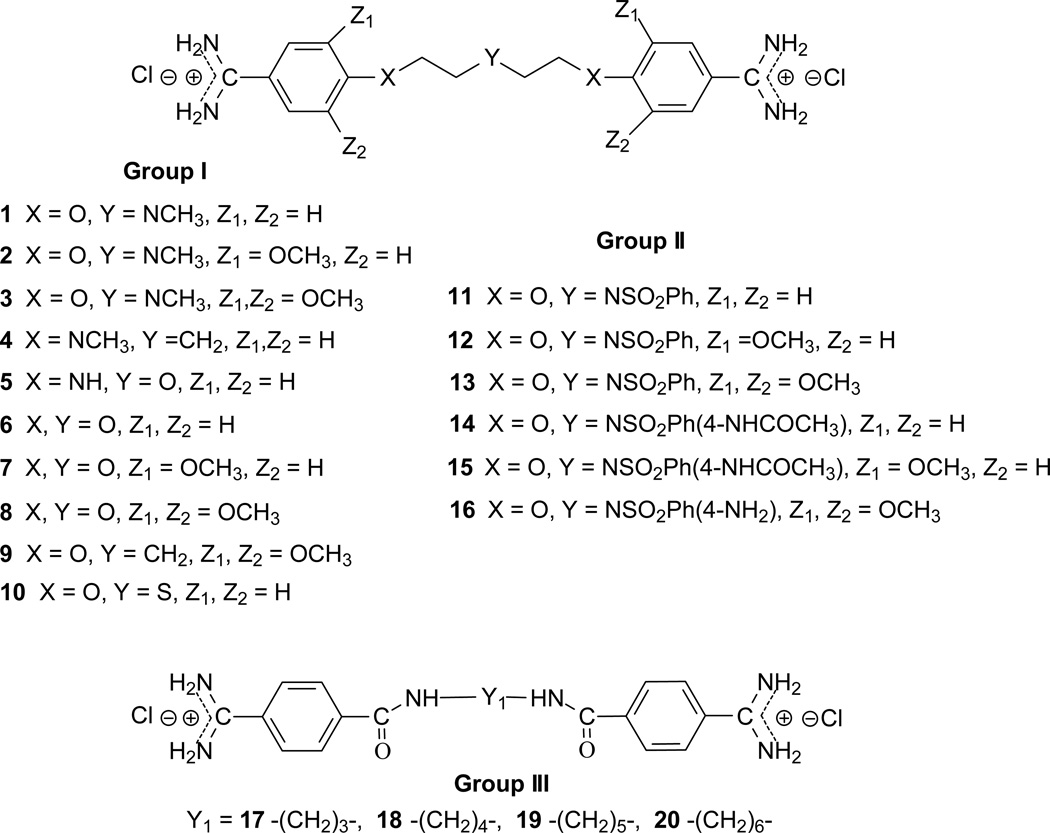
The first group of tested pentamidine analogs include 10 compounds 1 – 10 with different heteroatoms in the aliphatic linker (O, N, and S), and varying numbers of methoxy groups on the benzene rings (0, 2 or 4) (Group I in Figure 1). We have planned to develop derivatives which could combine both, pentamidine and trimethoprim potency. The second group (Group II in Figure 1) of pentamidine analogs were synthesized to include sulfonamide substituents, which were hypothesized to increase anti-Pneumocystis activity given the known efficacy of the sulfonamide group against Pneumocystis . Group II includes 6 compounds 11 – 16, with the N atom bearing sulfobenzene substituents in the middle of the aliphatic linker, and with 0-, 2- and 4-methoxy substituents at the benzene rings. The third group includes the bisamide linkers type of Ph-CONH-Rn-NHOC-Ph, with n = 3 to 6 (Group III in Figure 1). Activity of this series of bisamides 17 – 20 was compared to previous studies of similar compounds where the carbonyl groups were switched with amino groups giving compounds of the type of Ph-NHOC-Rn-CONH-Ph [15].
Figure 1.
Chemical structures of tested pentamidine analogs divided in Group I, II and III.
2. Result and Discussion
2.1. Chemistry
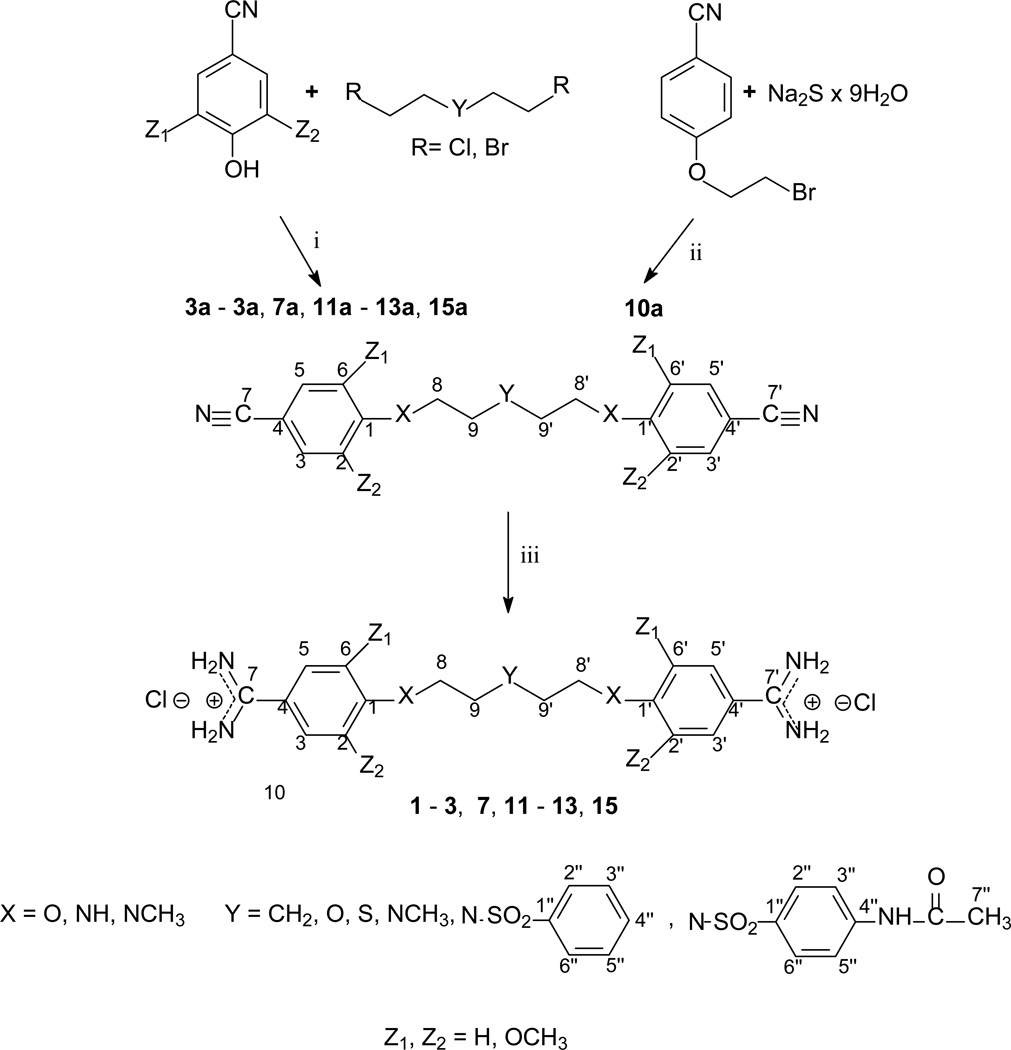
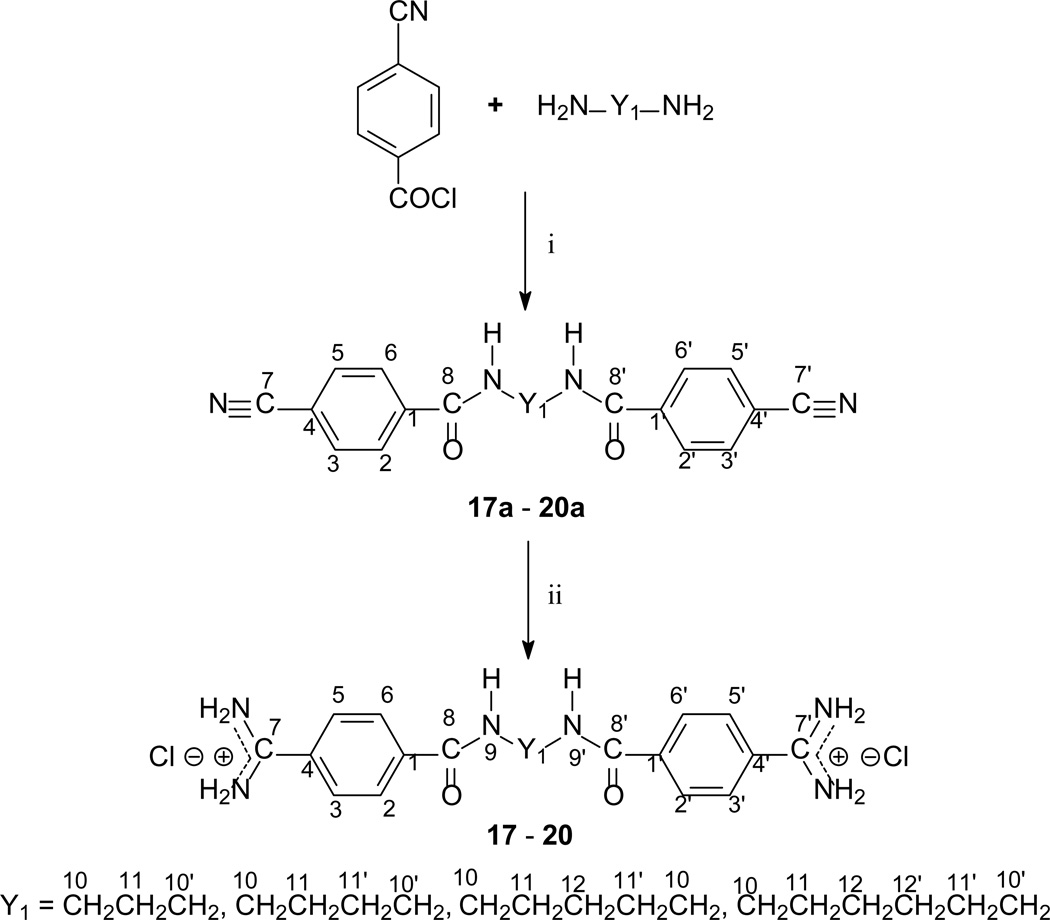
The method of synthesis for these bisamidines generally followed established procedures [16–18], which involves the preparation of the bisnitriles and their conversion into bisamidines. Compounds 1 – 3, 7 – 9, and 11 – 13, and 15 were obtained in the course of a three-step synthesis (Schemes 1), which involved O-alkylation of 4-hydroxybenzonitrile with bis(2-chloroethyl)ether, N,N-bis(2-chloroethyl)-N-methylamine or N,N-bis(2-chloroethyl)sulfonamides, then conversion of the formed bisnitriles 1a – 3a, 7a, 11a – 13a, and 15a to bisamidines by the subsequent reactions with ethanol and ammonia. Only the synthesis of compound 10 required the use of 4-(2-chloroethoxy)benzonitrile and sodium sulfide for preparation of bisnitrile 10a. Bisnitriles which are essential for preparation of compounds 17 – 20, were obtained by substitution of 4-cyanobenzoyl chloride with the appropriate alkyldiamines. Their transformation into bisamidines followed the procedure for the rest of the bisnitriles (Scheme 2). Syntheses of the bisamidines 4 – 6, 14 and 16 and bisnitriles 1a, 4a – 6a, 8a – 9a, 14a, 16a have been published by us [19–23] together with their structural analysis, and synthesis of the bisnitrile 18a was given in paper [24].
Scheme 1.
Synthetic routes to bisnitriles 1a – 3a, 7a, 11a – 13a, 15a and bisamidines 1 – 3, 7, 11 – 13, 15 with atom numbering; i: K2CO3, N-methyl-2-pyrrolidone; ii: DMSO; iii: 1) HCl/EtOH, 2) NH3/EtOH.
Scheme 2.
Synthetic routes to bisnitriles 17a – 20a and bisamidines 17 – 20 with atom numbering; i: TEA, CH2Cl2; ii: 1) HCl/EtOH, 2) NH3/EtOH.
2.2. Anti-Pneumocystis Activity and Cytotoxicity
The biological data are reported in Table 1. This series of compounds was remarkable for 2 reasons. First, there was very little toxicity associated with any of the compounds and all but 1 of the 20 compounds showed some efficacy. This is in contrast to previous studies where efficacy was highly variable and toxicity was common [8]. Four of the 10 compounds in Group I showed marked activity; 5 had moderate activity and only 1 had no activity in the ATP bioassay. While their potency was 2–3 fold less than that of pentamidine, (IC50 = 0.50 µM; 0.30µg/ml), there was little to no toxicity associated with their anti-P. carinii activity. Within Group I, greater inhibitory activity was associated with the presence of a heteroatom within the aliphatic linker and the absence of methoxy groups at the benzene rings. The introduction of S atom increased the activity of bisamidine 10 two-fold as compared to 6, in which the O atom is in the middle of the aliphatic chain. While the presence of the N atom in the aliphatic linker with the sulfobenzene group and the absence of methoxy groups in compound 11 correlated with marked activity, addition of methoxy groups to this structure reduced efficacy (compounds 12, 13). A lack of activity in sulfonamide group was associated with the lack of methyl group and the addition of N-acetyl group to the sulfobenzene group (14). Activity could be reinstated with the addition of methoxy groups to the benzene rings (15) or elimination of the N-acetyl group on the sulfobenzene group (16). The alkanediamide-linked bisbenzamidines 17 –20 were generally less active than the other compounds tested. The compounds 17 and 18 linked with shorter alkanediamide chains were more active than those with longer chains (compounds 19, 20). The activities of these compounds were much less than the alkanediamide-linked bisamidines in which the alkane chain was bound to carbonyl groups [15].
Table 1.
Anti-Pnemocystis Activity (IC50), A549 and L2 toxicity of compounds 1 – 13, 15 – 20.
| Name | Chemical structure | IC50 (µg/ml) ± SD for P. carinii |
IC50 (µM) |
Activity ranking |
A549 Toxicity IC50 (µg/ml) |
L2 Toxicity IC50 (µg/ml) |
|---|---|---|---|---|---|---|
| 1 | 0.82 ± 0.44 | 1.73 | Marked | >100 | >100 | |
| 2 | 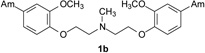 |
2.10 ± 1.24 | 3.41 | Moderate | >100 | >100 |
| 3 | 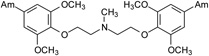 |
45.21 ± 16.9 | 6.26 | Slight | >100 | >100 |
| 4 | 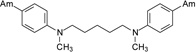 |
1.02 ± 0.51 | 1.99 | Moderate | >100 | 47.1 |
| 5 | 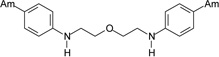 |
0.47 ± 0.09 | 1.11 | Marked | >100 | 36.9 |
| 6 | 0.96 ± 0.32 | 2.13 | Marked | >100 | >100 | |
| 7 | 2.00 ± 1.65 | 4.21 | Moderate | >100 | >100 | |
| 8 | 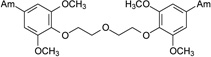 |
7.66 ± 1.67 | 12.99 | Moderate | >100 | >100 |
| 9 | 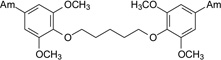 |
1.68 ± 0.37 | 3.00 | Moderate | >100 | 17.8 |
| 10 | 0.52 ± 0.04 | 1.18 | Marked | >100 | >100 | |
| 11 | 0.81 ± 0.12 | 1.33 | Marked | >100 | >100 | |
| 12 | 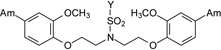 |
1.80 ± 0.76 | 2.66 | Moderate | >100 | >100 |
| 13 | 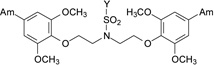 |
3.13 ± 0.99 | 4.40 | Moderate | >100 | >100 |
| 15 | 2.59 ± 0.83 | 3.71 | Moderate | >100 | >100 | |
| 16 | 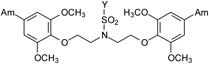 |
3.24 ± 0.88 | 4.16 | Moderate | >100 | >100 |
| 17 | 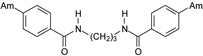 |
1.53 ± 0.90 | 2.99 | Moderate | >100 | >100 |
| 18 | 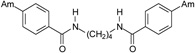 |
1.85 ± 0.01 | 3.58 | Moderate | >100 | >100 |
| 19 | 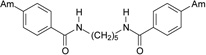 |
7.40 ± 0.22 | 14.33 | Moderate | >100 | >100 |
| 20 | 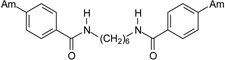 |
4.90 ± 1.20 | 9.15 | Moderate | >100 | >100 |
3. Conlusions
All but one (14) of the 20 bisamidines tested in the ATP assay at the screening concentration of 100 µg/ml reduced the ATP content of the P. carinii over the 72 hr of the assay period. After titration to determine the IC50 of the remaining compounds, 4 of the 5 compounds with the highest activities were found in the Group I, which explored the role of heteroatoms in the aliphatic linker and the addition of methoxy substituents to the benzene rings of the bisamidine. Activity was associated with the lack of methoxy substituents and the presence of the heteroatoms, O, N and S (1, 5, 6 and 10). The other compound with similar activity is 11 (from Group II), which added a sulfobenzene substituent to a nitrogen in the aliphatic linker. Addition of methoxy groups to the benzene rings also reduced efficacy in this group as well as in the first scheme. The alkanediamide-linked bisbenzamidines from Group III only showed a moderate inhibition of ATP pools in the P. carinii bioassay. These results were in contrast to a previous study for compounds in which carbonyl groups were switched with amino groups. Generally, the inclusion of a heteroatom in the aliphatic linker and absence of methoxy groups on the benzene groups was associated with higher activities in this assay. Most of the compounds had little to no toxicity in the cell line assay typically used to evaluate this characteristic. Although not as quite as potent as other pentamidine derivatives, these compounds hold promise for decreased side effects within the mammalian host and can next be evaluated in a mouse model of Pneumocystis pneumonia.
4. Experimental Section
4.1. Assessment of Anti-Pneumocystis Activity
P. carinii were obtained from the lungs of corticosteroid-immunosuppressed male CD rats (Charles River, Portage, MI housed at the Cincinnati Veteran’s Affairs Veterinary Medical Unit under barrier conditions. Their immune system suppression was induced by weekly injections of 20 mg/kg methylprednisolone (Depo-Medrol, Pfizer Pharmacia, New York, NY). After two weeks of suppression, rats were inoculated by intratracheal or intranasal installation of 2 ×107 cryopreserved P. carinii by nuclei count. Immunosuppression continued for 8 weeks and the fungi were purified from rodent lung tissue and cryopreserved as previously described [25]. The assay plates were set up by rapidly thawing of the cryopreserved P. carinii at 37°C, which were then resuspended at 5 × 107 nuclei/ml in RPMI-1640 containing 20 % calf serum (Atlanta Biologicals) with or without the bisamidines. Each drug concentration was assayed in triplicate wells in Costar #3548 multiwell plates (Fisher Scientific, Cincinnati, OH) in 3 different suspension assays using two different batches of P. carinii. Media without drug, with P. carinii, and with 10 µg of ampicillin/ml (Fisher Scientific, FairLawn, NJ) served as negative controls; pentamidine isethionate (Sigma-Aldrich) at 1 µg/ml served as the positive drug activity control. Plates were incubated at 5 % CO2, 37 °C. At, 6, 24, 48, and 72 hours post inoculation, 50 µl samples were transferred to opaque white plates (USA Scientific, Ocala, FL) and assessed for ATP content using ATPlite-M (Perkin-Elmer, Waltham, MA) [25]. Each of the 20 bisamidines was screened at 100µg/ml in 2 assays and if the ATP was decreased by at least 50 % vs untreated control P. carinii, a titered series was then performed.
4.1.1. ATP assay
The in vitro ATP bioluminescent assay to evaluate the efficacy of compounds against P. carinii was conducted as previously described [26–28]. The linear range of the ATP assay is 1 µM to 100 fM (~20,000,000 to 2,000 RLU). Samples removed from the suspension cultures were lysed, placed in opaque white plates and the ATP content measured with a luciferin-luciferase kit (ATPlite, Perkin-Elmer, Inc.) for light emission at 562 nm with a FluoSTAR Optima plate reader (BMG Labtechnologies, Inc.). A quench control to evaluate effects on the enzyme-substrate reaction was run for every drug tested and no inhibition was observed with any compound.
4.1.2. Data analysis
The IC50 for each bisamidine was calculated using linear regression of the percent decrease in ATP content of compound vs the log drug concentrations (GraphPad Software v2 for Science; GraphPad, San Diego, Calif.). Based on the IC50 values, each agent was classified by using an activity scale of 5 rankings ranging from “Highly active” (compounds with an IC50 of < 0.010 µg/ml), “Very marked” (IC50s of 0.011 to 0.099 µg/ml), “Marked” (IC50s from 0.10 to 0.99 µg/ml), “Moderate” (IC50s from 1.0 to 9.99 µg/ml), “Slight” (IC50s from 10.0 to 49.9 µg/ml), and “None” (i.e., inactive; IC50s of ≥50 µg/ml) [1].
4.2. Chemistry
All chemicals were purchased from major chemical suppliers as high or highest purity grade and used without any further purification. Melting points were determined with an Electrothermal 9001 Digital Melting Point. The chemical structure of the synthesized compounds were confirmed by their spectral data (1H NMR and 13C NMR 1D and 2D spectra in solution were recorded with Varian 300 V NMR S or with a Bruker Avance DMX 400, and chemical shifts δ (ppm) in solutions were referenced to TMS). Their purity was verified by elemental analyses using C, H, N, S Elementar GmbH Vario EL III apparatus. For thin layer chromatography (TLC) prepared plates Merck Kieselgel 60 F254 were used (toluene/dioxane/ethanol 6.0/3.2/0.5). For column chromatography Merck Silicagel 60, 230–400 mesh ASTM (0.040–0.063 mm) was used.
4.2.1. General procedure for synthesis of bisamidines
An appropriate bisnitrile (2–5 mmol) was stirred in a sealed flask with the saturated solution of HCl in anhydrous ethanol (25–50 ml) for 20–40 h at room temperature. The solvent was evaporated in vacuum or dry diethyl ether was added until complete precipitation was attained. The precipitate was quickly filtered off and dried under reduced pressure over anhydrous CaCl2 for 2–6 hours giving the very hygroscopic HCl salt in nearly quantitative yield. The crude diiminoester was immediately added to a saturated solution of NH3 in anhydrous ethanol (25–50 ml). The resulting mixture was stirred at room temperature for 24–48 h in a sealed vessel, the solvent was then evaporated in vacuum or refluxed for 3 h, and subsequently poured into a 10% aqueous solution of NaOH (10–20 ml). The precipitate of free bisamidine was filtered, washed with water and acetone, then dried under reduced pressure over anhydrous CaCl2. The solid of free bisamidine was suspended in anhydrous ethanol (5–10 ml) then an ethanolic solution of HCl (2–5 ml) was added and heated to boiling for a few minutes, to obtain the appropriate hydrochlorides.
The preparation of bisnitrile, the substrate leading to bisamidine, is given before appropriate bisamidine, if it was not published.
4.2.1.1. 1,5-Bis(4-amidinophenoxy)-N-methyl-3-azapentane trihydrochloride (1)
A white solid of 1 was obtained (Yield 44 %). M.p.= 262–265 °C (decomp.). 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 2.94–2.95 (m; 3H; H-11), 3.60–3.74 (m; 4H; H-9,H-9’), 4.58–4.59 (m; 4H; H-8,H-8’), 7.20–7.22 (m; 4H; H-2,H-6, H-2’, H-6’), 7.91–7.93 (m; 4H; H-3, H-5, H-3’, H-5’), 9.15 (broad s; 4H, 2 × 2NH), 9.36 (broad s; 4H; 2 × 2NH), 11.52 (broad s; 1H; N × HCl). 13C NMR (100.61 MHz, DMSO–d6) δ in ppm: 40.9 (C-11), 54.2 (C-9, C-9’), 62.8 (C-8, C-8’), 115.0 (C-2, C-6, C-2’, C-6’), 120.2 (C-4, C-4’), 130.2 (C-3, C-5, C-3’, C-5’), 161.8 (C-1, C-1’), 164.7 (C-7, C-7’). C19H25N5O2 × 3HCl × ½H2O (473.82 g/mol). Calcd. (%) C=48.16; H= 6.12; N=14.77; Cl=22.45; found (%): C=47.80; H=6.20; N=15.10; Cl=22.46.
4.2.1.2. 1,5-Bis(4-cyano-2-methoxyphenoxy)-N-methyl-3-azapentane (2a)
N,N-bis(2-chloroethyl)-N-methylamine hydrochloride (0.96 g; 5 mmol), 3-methoxy-4-hydroxybenzonitrile (1.49 g; 10 mmol), anhydrous K2CO3 (4.14 g; 30 mmol) and N-methyl-2-pyrrolidone (20 ml) was stirred together at 115 °C for 3 hours (progress of the reaction was followed by TLC) and then poured into ice water (200 ml). The precipitated solid was filtered, washed with water, and dried to obtain 1.86 g (Yield: 98 %) of a white solid of 2a. M.p.= 84.5–88 °C. 1H NMR (299.86 MHz, CDCl3) δ in ppm: 2.56 (s; 3H; H-11), 3.09 (t; J=5.7Hz; 4H; H-9, H-9’), 3.85 (s; 6H; H-10, H-10’), 4.23 (t; J=5.7Hz; 4H; H-8, H-8’), 6.92 (d; J=8.4Hz; 2H; H-6, H-6’), 7.07 (d; J=1.5Hz; 2H; H-3, H-3’), 7.25 (dd; J1=8.4Hz J2=1.5Hz; 2H; H-5, H-5’). 13C NMR (75.40 MHz, CDCl3) δ in ppm: 43.73 (C-11), 56.3 (C-10, C-10’), 56.3 (C-9, C-9’), 67.2 (C-8, C-8’), 104.5 (C-4, C-4’), 112.9 (C-6, C-6’), 114.5 (C-3, C-3’), 119.3 (C-7, C-7’), 126.5 (C-5, C-5’), 149.7 (C-2, C-2’), 151.2 (C-1, C-1’). C21H23N3O4 (381.44 g/mol): calcd (%): C=66.13; H=6.08; N=11.02; found (%): C=65.87; H=6.24; N=10.91.
4.2.1.3. 1,5-Bis(4-amidino-2-methoxyphenoxy)-N-methyl-3-azapentane (2)
A light yellow solid of 2 (Yield: 58 %) was obtained. M.p.= 255.5–257,5 °C (decomp). 1H NMR (299.86 MHz, DMSO-d6) δ in ppm: 3.00 (broad s, 3H; H-11), 3.63–3.81 (m; 4H; H-9, H-9’), 3.87 (s; 6H; H-10, H10’), 4.59 (t; J=4.8Hz; 4H; H-8, H-8’), 7.26 (d; J=8.4Hz; 2H; H-6, H-6’), 7.55–7.58 (m; 4H; H-3, H-5, H-3’, H-5’), 9.13 (broad s; 4H; 2 × 2NH), 9.40 (broad s; 4H; 2 × 2NH), 11.62 (broad s; 1H; N × HCl). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 41.5 (C-11), 54.2 (C-9, C-9’), 56.1 (C-10, C10’), 63.7 (C-8, C-8’), 111.7 (C3, C3’), 113.0 (C6, C6’), 120.3 (C4, C4’), 121.7 (C5, C5’), 148.7 (C2, C2’), 151.4 (C1, C1’), 164.6 (C7, C7’). C21H29N5O4 × 3HCl × 5H2O (614.98 g/mol): calcd. (%): C=41.02; H=6.88; N=11.39 %; found (%): C=40.89; H=6.87; N=11.28.
4.2.1.4. 1,5-Bis(4-cyano-2,6-dimethoxyphenoxy)-N-methyl-3-azapentane (3a)
To N,N-bis (2-chloroethyl)-N-methylamine hydrochloride (0.48 g, 2.5 mmol) and anhydrous K2CO3 (2.07 g, 15 mmol) in N-methyl-2-pyrrolidone (15 ml), 3,5-dimethoxy-4-hydroxybenzonitrile (0.90 g, 5 mmol) was added and the entire reaction was stirred together at 80 °C for 90 minutes (progress of the reaction was followed by TLC), then poured into water (400 ml). The precipitated solid was filtered, washed with water and purified using column chromatography (eluent CH2Cl2/MeOH 99.5/0.5) to give a light beige solid of 3a (Yield: 74%). M.p.= 186.0–189.0 °C (decomp). 1H NMR (400.13 MHz, CDCl3) δ in ppm: 2.45 (s; 3H; H-11), 2.92 (t; J=6 Hz; 4H; H-9, H-9’), 3.85 (s; 12H; H-10, H-10’), 4.14 (t; J=6 Hz; 4H; H-8, H-8’), 6.85 (s; 4H; H-3, H-5, H-3’, H-5’). 13C NMR (100.61 MHz, CDCl3) δ in ppm: 42.9 (C-11), 56.5 (C-10, C10’), 57.3 (C-9, C-9’), 71.3 (C-8, C-8’), 106.9 (C-4, C-4’), 109.5 (C-3, C-5, C-3’, C-5’), 119.2 (C-7, C-7’), 141.7 (C-1, C-1’), 153.9 (C-2, C-6, C-2’, C-6’). C23H27N3O6 (441.49 g/mol): calcd (%): C=62.57, H=6.16, N=9.52; found (%): C=62.28, H=6.19, N=9.26.
4.2.1.5. 1,5-Bis(4-amidino-2,6-dimethoxyphenoxy)-N-methyl-3-azapentane trihydrochloride (3)
A white solid of 3 was obtained (Yield: 72%). M.p.= 206.5–208 °C (decomp). 1H NMR (299.87 MHz, DMSO-d6) δ in ppm: 3.06–3.08 (m; 3H; H-11), 3.57–3.77 (broad m; 4H; H-9, H-9’), 3.92 (s; 12H; H-10, H10’), 4.37 (t; J=4.2Hz; 4H; H-8, H-8’), 7.34 (s; 4H; H-3, H-5, H-3’ ,H-5’), 9.26 (broad s; 4H; 2 × 2NH), 9.56 (broad s; 4H; 2 × 2NH), 10.81 (broad s; 1H; N × HCl). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 40.2 (C-11), 54.9 (C-9, C-9’), 56.5 (C-10, C-10’), 67.0 (C-8, C-8’), 105.9 (C-3, C-5, C-3’, C-5’), 123.3 (C-4, C-4’), 139.4 (C-1, C-1’), 152.7 (C-2, C-6, C-2’, C-6’), 164.6 (C-7, C-7’). C23H33N5O6 × 3HCl × 4H2O (657.01 g/mol): calcd. (%): C=42.05, H=6.75, N=10.66; found (%): C=41.76, H=6.28, N=10.45.
4.2.1.6. 1,5-Bis(4-cyano-2-methoxyphenoxy)-3-oxapentane (7a)
To a solution of 4-hydroxy-3-methoxybenzonitrile 1.49 g (10 mmol) in N-methyl-2-pyrrolidone (15 ml) with anhydrous K2CO3 2.76 g (20 mmol), 0.72 g (5 mmol) of bis(2-chloroethyl)ether was added and stirred at 130 °C for 3 hours (progress of the reaction was followed by TLC). The hot reaction mixture was poured into ice water (300 ml), and the precipitated solid was filtered, washed with water and dried at 60 °C. Recrystalisation from ethanol gave 1.64 g (Yield: 89 %) of a white solid of 7a. M.p.= 137–139 °C. 1H NMR (400.13 MHz, CDCl3) δ in ppm: 3.86 (s; 6H; H- 10, H-10’), 3.98 (t; J=4.7Hz; 4H; H-8, H-8’), 4.24 (t; J=4.7Hz; 4H; H-9, H-9’), 6.93 (d; J=8.4Hz; 2H; H-6, H-6’), 7.08 (broad s; 2H; H-3, H-3’), 7.24 (dd; J1=1.2Hz J2=8.4Hz; 2H; H-5, H-5’). 13C NMR (100.61 MHz, DMSO-d6) δ in ppm: 56.3 (C-10, C-10’), 68.8 (C-9, C-9’), 69.9 (C-8, C-8’), 104.5 (C-4, C-4’), 113.2 (C-6, C-6’), 114.6 (C-3, C-3’), 119.4 (C-7, C-7’), 126.5 (C-5, C-5’), 149.8 (C-2, C-2’), 152.4 (C-1, C-1’). C20H20N2O5 (368.39 g/mol): calcd. (%): C=65.21, H=5.47, N=7.60; found (%): C=65.38, H=5.52, N=7.37.
4.2.1.7. 1,5-Bis(4-amidino-2-methoxyphenoxy)-3-oxapentane dihydrochloride (7)
A yellow solid was obtained, filtered, dissolved in water (25 ml) and the insoluble residue was filtered. Dry acetone was added to the water solution until the solid started to precipitate. The resultant solid was filtered and dried to give a pure beige solid of 7 (Yield: 67 %). M.p.=280–282 °C (decomp.). 1H NMR (299.87 MHz, DMSO-d6) δ in ppm: 3.86 (broad s; 10H, H-9, H-10, H-9’, H-10’), 4.23 (t; J=4.5Hz; 4H; H-8, H-8’), 7.20 (d; J=8.4Hz; 2H; H-6, H-6’), 7.48–4.51 (m; 4H; H-3, H-5, H-3’, H-5’), 8.95 (broad s; 4H; 2 × 2NH), 9.24 (broad s; 4H; 2 × 2NH). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 55.9 (C-10, C-10’), 68.2 (C-8, C-8’), 68.8 (C-9, C-9’), 111.4 (C-3, C-3’), 112.4 (C-6, C-6’), 119.4 (C-4, C-4’), 121.8 (C-5, C-5’), 148.6 (C-2, C-2’), 152.5 (C-1, C-1’), 164.6 (C-7, C-7’). C20H26N4O5 × 2HCl (475.37 g/mol): calcd. (%): C=50.53, H=5.94, N=11.79; found (%): C=50.49, H=6.06, N=11.61.
4.2.1.8. 1,5-Bis(4-amidino-2,6-dimethoxyphenoxy)-3-oxapentane dihydrochloride (8)
Ethanol was evaporated in vacuo. The resultant brown crystals were dissolved in a small amount of water and dry acetone was added until the solid started to precipitate. The resultant solid was filtered, washed with acetone, and dried at room temp. to give a beige solid of 8 (Yield: 75%). M.p.= 142.5–145 °C (decomp). 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 3.70 (t; J=5.6Hz, 4H; H-9, H-9’), 3.86 (s; 12H; H-10, H-10’), 4.06 (t; J=5.6Hz, 4H; H-8, H-8’), 7.25 (s; 4H; H-3, H-5, H-3’, H-5’), 9.18 (broad s; 4H; 2 × 2NH), 9.44 (broad s; 4H; 2 × 2NH). 13C NMR (100.61 MHz, DMSO-d6) δ in ppm: 56.4 (C-10, C10’), 69.7 (C-9, C-9’), 71.9 (C-8, C-8’), 106.0 (C-3, C-5, C-3’, C-5’), 122.3 (C-4, C-4’), 140.9 (C-1, C-1’), 152.8 (C-2, C-6. C-2’, C-6’), 164.8 (C-7, C-7’). C22H30N4O7 × 2HCl × 3H2O (589.49 g/mol): calcd. (%): C=44.83, H=6.50, N=9.50; found (%): C=44.72, H=6.35, N=9.07.
4.2.1.9. 1,5-Bis(4-amidino-2,6-dimethoxyphenoxy)pentane dihydrochloride (9)
Ethanol was evaporated in vacuo. The resultant solid was dissolved in a small amount of water and dry acetone was added until the solid started to precipitate. The resultant solid was filtered, washed with acetone and dried at room temp. to yield a beige solid of 9 (Yield: 58%). M.p.= 225.5–227.0 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.57–1.58 (m; 2H; H-11), 1.66–1.68 (m; 4H; H-9, H-9’), 3.86 (s; 12H; H-10, H-10’), 3.95 (t; J=6 Hz; 4H; H-8, H-8’), 7.27 (s; 4H; H-3, H-5, H-3’, H-5’), 9.20 (broad s; 4H; 2 × 2NH), 9.47 (broad s; 4H; 2 × 2NH). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 21.7 (C-11), 29.2 (C-9, C-9’), 56.4 (C-10, C-10’), 72.5 (C-8, C-8’), 106.0 (C-3, C-5, C-3’, C-5’), 122.2 (C-4, C-4’), 141.0 (C-1, C-1’), 153.0 (C-2, C-6, C-2’, C-6’), 164.8 (C-7, C-7’). C23H32N4O6 × 2HCl × 1½H2O (560.48 g/mol): calcd. (%): C=49.28, H=6.60, N=9.99; found (%): C=49.07, H=6.72, N=9.74.
4.2.1.10. 1,5-Bis(4-cyanophenoxy)-3-thiapentane (10a)
4-(2-bromoethoxy)benzonitrile (6,78 g; 30 mmol) and Na2S˙9H2O (3.60 g; 15 mmol) were stirred with DMSO (30 ml) for 2 h at 115–120 °C. The mixture was poured into ice water (150 ml) and left for 24 h. The precipitate was filtered, washed with cold water, and recrystalized from ethanol to give 3.89 g (Yield: 80 %) of 10a. M.p.= 106–107 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 3.01 (t; J=6.3 Hz; 4H: H-9, H-9’), 4.26 (t; J=6.3 Hz; 4H: H-8, H-8’), 7.11 (d; J=8.5 Hz; 4H; H-2, H-6, H-2’, H-6’), 7.76 (d; J=8.5 Hz; 4H; H-3, H-5, H-3’, H-5’). 13C NMR (100.62; DMSO-d6) δ in ppm: 30.4 (C-9, C-9’), 68.0 (C-8, C-8’), 103.0 (C-4, C-4’), 115.6 (C-2, C-6, C-2’, C-6’), 119.1 (C-7, C-7’), 134.2 (C-3, C-5, C-3’, C-5’), 161.7 (C-1, C-1’).
4.2.1.11. 1,5-Bis(4-amidinophenoxy)-3-thiapentane dihydrochloride (10)
The obtained solution was cooled and diluted with an excess of anhydrous diethyl ether. The precipitated solid was filtered, washed with diethyl ether and dried at 100 °C for 3–4 h to obtain an almost anhydrous white solid of 10 (Yield: 65 %). M.p.= 235.5–236.5 °C. 1H NMR (299.86 MHz, DMSO-d6) δ in ppm: 3.04 (t; J=6.5 Hz; 4H; H-8, H-8’), 4.30 (t; J=6.5 Hz; 4H; H-9, H-9’), 7.16 (m; 4H, H-2,6, H-2’6’), 7.89 (m; 4H, H-3,5, H-3’5’), 9.12 (broad s; 4H; 2 × 2NH), 9.30 (broad s; 4H; 2 × 2NH). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 30.5 (C-9, C-9’), 68.0 (C-8, C-8’), 114.8 (C-2, C-6, C-2’, C-6’), 119.6 (C-4, C-4’), 130.2 (C-3, C-5, C-3’, C-5’), 162.6 (C-1, C-1’), 164.7 (C-7, C-7’).
4.2.1.12. N,N-Bis[2(4-cyanophenoxy)ethyl]benzenesulfonamide (11a)
4-hydroxybenzonitrile 1.19 g (10 mmol), N,N-bis (2-chloroethyl)benzenesulfonamide 1.41 g (5 mmol), and anhydrous K2CO3 2.76 g (20 mmol) were heated to 130 °C with stirring in N-methyl-2-pyrrolidone (15 ml). Progress of the reaction was followed by TLC and after 14 h the hot reaction mixture was poured into ice water (200 ml) and extracted with ethyl acetate. The combined ethyl acetate layers were dried (MgSO4) and the solvent was evaporated in vacuo. Crude product was crystallized from ethanol to obtain 0.78 g of crystals of 11a (Yield: 35 %). M.p.= 136–137 °C. 1H NMR (299.86 MHz, CDCl3) δ in ppm: 3.68 (t; J=5.7 Hz; 4H; H-9, H-9’), 4.23 (t; J=5.7 Hz; 4H; H-8, H-8’), 6.83–6.86 (m; 4H;H-2, H-6, H-2’, H-6’), 7.48–7.62 (m; 7H; H-3, H-5, H-3’, H-5’, H-3”, H-4”, H-5”), 7.82–7.85 (m; 2H; H-2”-, H-6”). 13C NMR (75.40 MHz, CDCl3) δ in ppm: 49.2 (C-9, C-9’), 67.6 (C-8, C-8’), 104.9 (C-4, C-4’), 115.3 (C-2, C-6, C-2’, C-6’), 119.0 (C-7, C-7’), 127.2 (C-2”, C-6”), 129.5 (C-3”, C-5”), 133.2 (C-4”), 134.3 (C-3, C-5, C-3’, C-5’), 139.3 (C-1”), 161.5 (C-1, C-1’). C24H21N3O4S (477.52 g/mol): calcd. (%): C=64.41, H=4.73, N=9.39, S=7.16; found (%): C=64.16, H=4.87, N=9.11, S=7.02.
4.2.1.13. N,N-Bis[2(4-amidinophenoxy)ethyl]benzenesulfonamide dihydrochloride (11)
The solvent was evaporated in vacuo to give a yellow solid of 11 (Yield: 82 %). M.p.= 246–249 °C (decomp.). 1H NMR (299.86 MHz, DMSO-d6) δ in ppm: 3.67 (t; J=5.3 Hz; 4H; H-9, H-9’), 4.27 (t; J=5.3 Hz; 4H; H-8.H-8’), 7.03–7.06 (m; 4H; H-2, H-6, H-2’, H-6’), 7.58–7.63 (m; 2H; H-3”, H-5”), 7.66–7.71 (m; 1H; H-4”), 7.84–7.90 (m; 6H; H-3, H-5, H-3’, H-5’, H-2”, H-6”), 9.10 (broad s; 4H; 2 × 2NH), 9.30 (broad s; 4H; 2 × 2NH). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 47.7 (C-9, C-9’), 66.8 (C8, C-8’), 114.6 (C-2, C-6, C-2’, C-6’), 119.7 (C-4, C-4’), 126.9 (C-2”, C-6”), 129.4 (C-3”, C-5”), 130.2 (C-3, C-5, C-3’, C-5’), 133.0 (C-4”), 138.8 (C-1”), 162.3 (C-1, C-1’), 164.6 (C-7, C-7’). C24H27N504S × 2HCl × 3H2O (608.56 g/mol): calcd. (%): C=47.37, H=5.80, N=11.51, S=5.27; found (%): C=47.79, H=5.88, N=11.45, S=5.50.
4.2.1.14. N,N-Bis[2(4-cyano-2-methoxyphenoxy)ethyl]benzenesulfonamide (12a)
3-methoxy-4-hydroxybenzonitrile (1.49 g, 10 mmol), N,N-bis (2-chloroethyl)benzenesulfonamide (1.41 g, 5 mmol) and anhydrous K2CO3 (2.76 g, 20 mmol) in N-methyl-2-pyrrolidone (15 ml) were stirred together at 120 °C for 10 h (progress of the reaction was followed by TLC). The hot reaction mixture was poured into ice water (250 ml), and the precipitated solid was filtered, washed with water and dried. After recrystalisation from ethanol with hot filtering, the white solid of 12a was obtained (Yield: 54%). M.p.= 151.5–153.5 °C. 1H NMR (400.13 MHz, CDCl3) δ in ppm: 3.63 (t; J=6.0 Hz; 4H; H-9, H-9’), 3.69 (s; 6H; H-10, H10’), 4.25 (t; J=6.0 Hz; 4H; H-8, H-8’), 6.81–6.83 (ps d; 2H; H-6, H-6’), 6.98 (d; J=1.2Hz; 2H; H-3, H-3’), 7.17–7.19 (m; 2H; H-5, H-5’), 7.41–7.44 (m; 2H; H-3”, H-5”), 7.50–7.53 (m; 1H; H-4”), 7.77–7.79 (m; 2H; H-2”, H-6”). 13C NMR (100.62 MHz, CDCl3) δ in ppm: 49.9 (C-9, C-9’), 56.1 (C-10, C-10’), 68.3 (C-8, C-8’), 104.7 (C-4, C-4’), 112.7 (C-6, C-6’), 114.4 (C-3, C-3’), 119.2 (C-7, C-7’), 126.5 (C-5, C-5’), 127.3 (C-2”, C-6”), 129.5 (C-3”, C-5”), 133.2 (C-4”), 139.0 (C-1”), 149.5 (C-2, C-2’), 151.8 (C-1, C-1’). C26H25N3O6S (507.57 g/mol): calcd. (%): C=61.53, H=4.96, N=8.28, S=6.32; found (%): C=61.28, H=5.14, N=8.51, S=6.12.
4.2.1.15. N,N-Bis[2(4-amidino-2-methoxyphenoxy)ethyl]benzenesulfonamide dihydrochloride (12)
Ethanol was evaporated in vacuo to give a yellow solid of 12 (Yield: 78). M.p.= 166,0–170,0 °C. 1H NMR (299.87 MHz, DMSO-d6) δ in ppm: 3.68 (t; J=5.7Hz; 4H; H-9, H-9’), 3.79 (s, 6H; H-10, H-10’), 4.28 (t; J=5.7Hz; 4H; H-8, H-8’), 7.14 (d; J=8.1Hz; 2H; H-6, H-6’), 7.49–7.52 (m; 4H; H-3, H-5, H-3’, H-5’), 7.56–7.61 (m; 2H; H-3”, H-5”), 7.65–7.70 (m; 1H; H-4”), 7.88–7.91 (m; 2H; H-2”, H-6”), 8.98 (broad s; 4H; 2 × 2NH), 9.28 (broad s; 4H; 2 × 2NH). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 48.4 (C-9, C-9’), 55.9 (C-10, C-10’), 67.6 (C-8, C-8’), 111.4 (C-3, C-3’), 112.2 (C-6, C-6’), 119.6 (C-4, C-4’), 121.8 (C-5, C-5’), 126.9 (C-2”, C-6”), 129.5 (C-3”, C-5”), 133.1 (C-4”), 138.5 (C-1”), 148.5 (C-2, C-2’), 152.0 (C-1, C-1’), 164.5 (C-7, C-7’). C26H31N5O6S × 2HCl × 3 ½ H2O (677.62 g/mol): calcd. (%): C=46.08, H=5.90, N=10.33, S=4.73; found (%): 46.08, H=5.80, N=10.25, S=4.68.
4.2.1.16. N,N-Bis[2(4-cyano-2,6-dimethoxyphenoxy)ethyl]benzenesulfonamide (13a)
3,5-dimethoxy-4-hydroxybenzonitrile (1.79 g, 10 mmol), N,N-bis (2-chloroethyl)benzenesulfonamide (1.41 g, 5 mmol), anhydrous K2CO3 (2.76 g, 20 mmol) and N-methyl-2-pyrrolidone (30 ml) were heated at 120 °C for 3 h while stirring (progress of the reaction was followed by TLC) and then poured into ice water (250 ml). The resultant solid was filtered, washed with water and dried at 60 °C. Recrystalisation from a large volume of ethanol gave a white solid of 13a (Yield: 64 %). M.p.= 194–195 °C. 1H NMR (299.86 MHz, CDCl3) δ in ppm: 3.71 (t; J=8.0 Hz, 4H; H-9, H-9’), 3.80 (s; 12H; H-10, H-10’), 4.22 (t; J=8.0 Hz, 4H; H-8, H-8’), 6.82 (s; 4H; H-3, H-5, H-3’, H-5’), 7.44–7.48 (m; 2H; H-3”, H-5”), 7.52–7.57 (m; 1H; H-4”), 7.83–7.85 (m; 2H; H-2”, H-6”). 13C NMR (75.40 MHz, CDCl3) δ in ppm: 49.2 (C-9, C-9’), 56.4 (C-10, C-10’), 72.4 (C-8, C-8’), 107.1 (C-4, C-4’), 109.5 (C-3, C-5, C-3’, C-5’), 119.0 (C-7, C-7’), 127.4 (C-2”, C-6”), 129.2 (C-3”, C-5”), 132.7 (C-4”), 140.1 (C-1”), 141.4 (C-1, C-1’), 153.7 (C-2, C-6, C-2’, C-6’). C28H29N3O8S (567.62 g/mol): calcd. (%): C=59.25, H=5.15, N=7.40, S=5.65; found (%): C=59.06, H=5.23, N=7.16, S=5.89.
4.2.1.17. N,N-Bis[2(4-amidino-2,6-dimethoxyphenoxy)ethyl]-benzenesulfonamide (13)
The solvent was evaporated in vacuo and the resultant brown solid was recrystalised from a small amount of ethanol to give a light brown solid of 13 (Yield: 51%). M.p.=207.5–210.0 °C (decomp). 1H NMR (299,86 MHz, DMSO-d6) δ in ppm: 3.62 (t; J=5.7Hz; 4H; H-9, H-9’), 3.81 (s; 12H; H-10, H-10’), 4.08 (t; J=5.7Hz; 4H; H-8, H-8’), 7.21 (s; 4H; H-3, H-5, H-3’, H-5’), 7.56–7.61 (m; 2H; H-3”, H-5”), 7.63–7.68 (m; 1H; H-4”), 7.79–7.82 (m; 2H; H-2”, H-6”), 9.11 (broad s; 4H; 2 × 2NH), 9.39 (broad s; 4H; 2 × 2NH). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 48.3 (C-9, C-9’), 56.3 (C-10, C-10’), 71.4 (C-8, C-8’), 105.8 (C-3, C-5, C-3’, C-5’), 122.5 (C-4, C-4’), 126.8 (C-2”, C-6”), 129.3 (C-3”, C-5”), 132.8 (C-4”), 139.2 (C-1”), 140.5 (C-1, C-1’), 152.6 (C-2, C-6, C-2’, C-6’), 164.7 (C-7, C-7’). C28H35N5O8S × 2HCl × 2H2O (710.64 g/mol): calcd. (%): C=47.33, H=5.82, N=9.86, S=4.51; found (%): C=47.48, H=6.11, N=9.66, S=4.60.
4.2.1.18. N,N-Bis[2(4-cyano-2-methoxyphenoxy)ethyl]-4-acetamidobenzenesulfonamide (15a)
3-methoxy-4-hydroxybenzonitrile (1.49 g, 10 mmol), N,N-bis (2-chloroethyl)-4-acethyloaminobenzenesulfonamide (1.69 g, 5 mmol), and anhydrous K2CO3 (3.46 g, 25 mmol) in N-methyl-2-pyrrolidone (20 ml) were stirred together at 130 °C for 7 h (progress of the reaction was followed by TLC). The hot reaction mixture was poured into ice water (250 ml), the resultant solid was filtered, washed with water and dried at room temp. After recrystalisation from ethanol, a white solid of 15a was obtained (50%). M.p.= 106.5–108.0 °C. 1H (299.86 MHz, CDCl3) δ in ppm: 2.23 (s; 3H; H-13), 3.71 (t; J=6.0 Hz, 4H; H-9, H-9’), 3.78 (s; 6H; H-10, H-10’), 4.30 (t; J=6.0 Hz; 4H; H-8, H-8’), 6.87 (d; J=8.4 Hz; 2H; H-6, H-6’), 7.04 (d; J=1.8Hz; 2H; H-3, H-3’), 7.24 (dd; J1=8.4Hz J2=1.8Hz; 2H; H-5. H-5’), 7.48 (broad s; 1H; NH-11), 7.58–7.61 (m; 2H; H-3”, H-5”), 7.75–7.78 (m; 2H; H-2”, H-6”). 13C NMR (75.40 MHz, CDCl3) δ in ppm: 24.9 (C-13), 49.7 (H-9, H-9’), 56.2 (C-10, C-10’), 68.3 (C-8, C-8’), 104.6 (C-4, C-4’), 112.8 (C-6, C-6’), 114.5 (C-3, C-3’), 119.3 (C-7, C-7’), 119.5 (C-3”, C-5”), 126.5 (C-5, C-5’), 128.6 (C-2”, C-6”), 133.9 (C-1”), 142.3 (C-4”), 149.6 (C-2, C-2’), 151.9 (C-1, C-1’), 168.8 (C-12). C28H28N4O7S (564.62 g/mol): calcd. (%): C=59.56, H=5.00, N=9.92, S=5.68; found (%): C=59.48, H=4.87, N=10.06, S=5.79.
4.2.1.19. N,N-Bis[2(4-amidino-2-methoxyphenoxy)ethyl]-4-acetamidobenzenesulfonamide (15)
A light yellow solid of 15 was obtained (Yield: 72%). M.p.=262.0-264.0 °C. 1H (299.86 MHz, DMSO-d6) δ in ppm: 2.09 (s; 3H; H-13), 3.65 (t; J=5.7Hz; 4H; H-9. H-9’), 3.80 (s; 6H; H-10, H-10’), 4.26 (t; J=5.7Hz; 4H; H-8, H-8’), 7.12 (d; J=8.7Hz; 2H; H-6, H-6’), 7.46–7.52 (m; 4H; H-3, H-5, H-3’, H-5’), 7.75–7.81 (m; 4H; H-2”, H-3”, H-5”, H-6”), 9.03 (broad s; 4H; 2 × 2NH), 9.32 (broad s; 4H; 2 × 2NH), 10.54 (s; 1H; NH-11). 13C NMR (75.40 MHz, DMSO-d6) δ in ppm: 23.7 (C-13), 47.9 (C-9, C-9’), 55.5 (C-10, C-10’), 67.2 (C-8, C-8’), 111.0 (C-3, C-3’), 111.8 (C-6, C-6’), 118.3 (C-3”, C5”), 119.2 (C-4, C-4’), 121.4 (C-5, C-5’), 127.7 (C-2”, C-6”), 131.7 (C-1”), 143.0 (C-4”), 148.1 (C-2, C-2’), 151.6 (C-1, C-1’), 164.2 (C-7, C-7’), 168.7 (C-12). C28H34N6O7S × 2HCl × 1½H2O (698.60 g/mol): calcd. (%): C=48.14, H=5.64, N=12.02, S=4.59; found (%): C=48.13, H=5.71, N=11.72, S=4.31.
4.2.2. General procedure for synthesis of bisnitriles 17a – 20a
A solution of an appropriate aliphatic diamine (10 mmol) in dichloromethane (30 mL) and triethylamine (2.8 mL) was added dropwise to a stirred, ice cooled solution of 4-cyanobenzoyl chloride (3.31 g, 20 mmol) in dichloromethane (30 mL). The reaction mixture was stirred at room temperature for 12 h (progress of the reaction was followed by TLC), then the solvent was evaporated under reduced pressure and the residue was washed with 1 M NaHCO3, 1 M HCl and water, then dried over anhydrous CaCl2. Analytical samples were obtained after recrystalisation from DMSO-water mixtures (50 – 90 % DMSO) .
4.2.2.1. N,N’-Propane-1,3-diylbis(4-cyanobenzamide) (17a)
A white solid of 17a was obtained (Yield: 94%). M.p.=212.5–213.5 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.81 (quintet; J=6.8; 2H; H-11), 3.34 (quartet; J=6.3 Hz; 4H; H-10, H-10’), 7.97 (m; 8H; H-2, H-3, H-5, H-6, H-2’, H-3’, H-5’, H-6’), 8.73 (m; 2H; H-9, H-9’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 28.8 (C-11), 37.3 (C-10, C-10’), 113.5 (C-4, C-4’), 118.3 (C-7, C-7’), 128.0 (C-2, C-6, C-2’, C-6’), 132.4 (C-3, C-5, C-3’, C-5’), 138.5 (C-1, C-1’), 164.8 (C-8, C-8’). C19H16N4O2 × 0.75H2O (345,87 g/mol). Calcd. (%) C=65.99; H=5.07; N=16.21; found (%): C=66.05; H=4.84; N=16.00.
4.2.2.2. N,N’-Propane-1,3-diylbis(4-amidinobenzamide) dihydrochloride (17)
A beige solid of 17 was obtained (Yield: 37%). M.p.=302.5–304.5 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.82 (quintet; J=6.3; 2H; H-11), 3.38 (quartet; J=6.2 Hz; 4H; H-10, H-10’), 7.95 (d; J=8.4 Hz; 4H; H-3, H-5, H-3’, H-5’), 8.12 (d; J=8.4 Hz; 4H; H-2, H-6, H-2’, H-6’), 9.09 (t; J=5.6 Hz; 2H; H-9, H-9’), 9.40 (s; 4H; H-7, H-7’), 9.58 (s; 4H; H-7, H-7’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 28.8 (C-11), 37.0 (C-10, C-10’), 127.6 (C-2, C-6, C-2’, C-6’), 128.2 (C-3, C-5, C-3’, C-5’), 130.2 (C-4, C-4’), 138.9 (C-1, C-1’), 164.9 (C-8, C-8’), 165.2 (C-7, C-7’). C19H22N6O2 × 2HCl × 4H2O (511.40 g/mol). Calcd. (%) C=44.62; H=6.26; N=16.44; found (%): C=44.61; H=6.06; N=16.21.
4.2.2.3. N,N’-Butane-1,4-diylbis(4-cyanobenzamide) (18a) [24]
A white solid of 18a was obtained (Yield: 97%). M.p.=266.5–268.5 °C (Ref. [24] 264–265 °C).
4.2.2.4. N,N’-Butane-1,4-diylbis(4-amidinobenzamide) dihydrochloride (18)
A beige solid of 18 was obtained (Yield: 52%). M.p.=295.0–296.0 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.61 (m; 4H; H-11, H-11’), 3.32 (m; 4H; H-10, H-10’), 7.92 (d; J=8.4 Hz; 4H; H-3, H-5, H-3’, H-5’), 8.05 (d; J=8.7 Hz; 4H; H-2, H-6, H-2’, H-6’), 8.82 (t; J=5.6 Hz; 2H; H-9, H-9’), 9.28 (s; 4H; H-7, H-7’), 9.51 (s; 4H; H-7, H-7’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 26.6 (C-11, C-11’), 39.1 (C-10, C-10’), 127.6 (C-2, C-6, C-2’, C-6’), 128.2 (C-3, C-5, C-3’, C-5’), 130.2 (C-4, C-4’), 139.0 (C-1, C-1’), 164.8 (C-8, C-8’), 165.1 (C-7, C-7’). C20H24N6O2 × 2HCl × 3½5H2O (516.42 g/mol). Calcd. (%) C=46.51; H=6.40; N=16.28; found (%): C=46.67; H=6.56; N=15.95.
4.2.2.5. N,N’-Pentane-1,5-diylbis(4-cyanobenzamide) (19a)
White solid of 19a was obtained from 1,5-diaminopentane dihydrochloride after following the general procedure for synthesis of bisnitriles 17a – 20a (Yield: 72%). M.p.=174.0–174.5 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.35 (m; 2H; H-12), 1.56 (quintet; J=7.2 Hz; 4H; H-11, H-11’), 3.27 (quartet; J=6.5 Hz; 4H; H-10, H-10’), 7.95 (m; 8H; H-2, H-3, H-5, H-6, H-2’, H-3’, H-5’, H-6’), 8.68 (t; J=5.4 Hz; 2H; H-9, H-9’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 23.9 (C-12), 28.6 (C-11, C-11’), 39.3 (C-10, C-10’), 113.4 (C-4, C-4’), 118.3 (C-7, C-7’), 128.0 (C-2, C-6, C-2’, C-6’), 132.3 (C-3, C-5, C-3’, C-5’), 138.6 (C-1, C-1’), 164.7 (C-8, C-8’). C21H20N4O2 × 0.25H2O (364.91 g/mol). Calcd. (%) C=69.14; H=5.62; N=15.36; found (%): C=68.98; H=5.60; N=15.19.
4.2.2.6. N,N’-Pentane-1,5-diylbis(4-amidinobenzamide) dihydrochloride (19)
A beige solid of 19 was obtained (Yield: 44%). M.p.=101.0–104.0 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.37 (m; 2H; H-12), 1.61 (quintet; J=7.1 Hz; 4H; H-11, H-11’), 3.30 (m; 4H; H-10, H-10’), 7.94 (d; J=8.1 Hz; 4H; H-3, H-5, H-3’, H-5’), 8.06 (d; J=8.4 Hz; 4H; H-2, H-6, H-2’, H-6’), 8.83 (t; J=5.4 Hz; 2H; H-9, H-9’), 9.37 (s; 4H; H-7, H-7’), 9.57 (s; 4H; H-7, H-7’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 24.0 (C-12), 28.7 (C-11, C-11’), 39.3 (C-10, C-10’), 127.6 (C-2, C-6, C-2’, C-6’), 128.2 (C-3, C-5, C-3’, C-5’), 130.1 (C-4, C-4’), 139.1 (C-1, C-1’), 164.8 (C-8, C-8’), 165.2 (C-7, C-7’). C21H26N6O2 × 2HCl × 3H2O (516.42 g/mol). Calcd. (%) C=48.37; H=6.53; N=16.12; found (%): C=48.73; H=6.84; N=15.94.
4.2.2.7. N,N’-Hexane-1,6-diylbis(4-cyanobenzamide) (20a)
A white solid of 20a was obtained (Yield: 98%). M.p.=230.0–231.0 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.34 (m; 4H; H-12, H-12’), 1.53 (m; 4H; H-11, H-11’), 3.26 (quartet; J=6.4 Hz; 4H; H-10, H-10’), 7.96 (m; 8H; H-2, H-3, H-5, H-6, H-2’, H-3’, H-5’, H-6’), 8.68 (t; J=5.0 Hz; 2H; H-9, H-9’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 26.2 (C-12, C-12’), 28.9 (C-11, C-11’), 39.2 (C-10, C-10’), 113.4 (C-4, C-4’), 118.3 (C-7, C-7’), 128.0 (C-2, C-6, C-2’, C-6’), 132.4 (C-3, C-5, C-3’, C-5’), 138.6 (C-1, C-1’), 164.7 (C-8, C-8’). C22H22N4O2 × ½H2O (383,44 g/mol). Calcd. (%) C=68.93; H=6.01; N=14.62; found (%): C=68.93; H=5.80; N=14.43.
4.2.2.8. N,N’-Hexane-1,6-diylbis(4-amidinobenzamide) dihydrochloride (20)
A beige solid of 20 was obtained (Yield: 83%). M.p.=253.0–256.0 °C. 1H NMR (400.13 MHz, DMSO-d6) δ in ppm: 1.34 (m; 2H; H-12, H-12’), 1.53 (m; 4H; H-11, H-11’), 3.26 (quartet; J=6.4 Hz; 4H; H-10, H-10’), 7.92 (d; J=8.7 Hz; 4H; H-3, H-5, H-3’, H-5’), 8.05 (d; J=8.7 Hz; 4H; H-2, H-6, H-2’, H-6’), 8.79 (t; J=5.7 Hz; 2H; H-9, H-9’), 9.32 (s; 4H; H-7, H-7’), 9.52 (s; 4H; H-7, H-7’). 13C NMR (100.62 MHz, DMSO-d6) δ in ppm: 26.2 (C-12, C-12’), 29.0 (C-11, C-11’), 39.2 (C-10, C-10’), 127.6 (C-2, C-6, C-2’, C-6’), 128.2 (C-3, C-5, C-3’, C-5’), 130.1 (C-4, C-4’), 139.0 (C-1, C-1’), 164.8 (C-8, C-8’), 165.1 (C-7, C-7’). C22H28N6O2 × 2HCl × 3H2O (535.46 g/mol). Calcd. (%) C=49.35; H=6.73; N=15.70; found (%): C=49.32; H=7.12; N=15.61.
Research highlights.
A series of 20 pentamidine analogs were prepared with high yield and purity
All were evaluated for efficacy against Pneumocystis carinii in an ATP bioassay
Heteroatoms in linker and absence of methoxy groups on benzene were associated with higher activities
These compounds hold promise for decreased side effects within the mammalian host
Acknowledgment
MTC/MSC would like to recognize the support of the NIH (through the contract mechanism N01-A1-25647) for development of the in vitro methods used for the screening of candidate anti-Pneumocystis agents.
Funding Sources
MTC/MSC were supported by a grant from the National Institutes of Health, NIAID R01 AI076104 and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cushion MT, Walzer PD. Preclinical drug discovery for new anti-neumocystis compounds. Curr. Med. Chem. 2009;16:2514–2530. doi: 10.2174/092986709788682038. [DOI] [PubMed] [Google Scholar]
- 2.Kaur N, Mahl TC. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for crohn's disease. Dig. Dis. Sci. 2004;49:1458–1460. doi: 10.1023/b:ddas.0000042246.58984.98. [DOI] [PubMed] [Google Scholar]
- 3.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: A review of 84 cases. Dig. Dis. Sci. 2007;52:1481–1484. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 4.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and pneumocystis colonization. Am. J. Respir. Crit. Care Med. 2004;170:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 5.Joffrion TM, Cushion MT. Sterol biosynthesis and sterol uptake in the fungal pathogen pneumocystis carinii. FEMS Microbiol. Lett. 2010;311:1–9. doi: 10.1111/j.1574-6968.2010.02007.x. [DOI] [PubMed] [Google Scholar]
- 6.Tidwell RR, Jones SK, Geratz JD, Ohemeng KA, Bell CA, Berger BJ, Hall JE. Development of pentamidine analogues as new agents for the treatment of pneumocystis carinii pneumonia. Ann. N. Y. Acad. Sci. 1990;616:421–441. doi: 10.1111/j.1749-6632.1990.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 7.Donkor IO, Tidwell RR, Jones SK. Pentamidine congeners. 2. 2-butene-bridged aromatic diamidines and diimidazolines as potential anti-pneumocystis carinii pneumonia agents. J. Med. Chem. 1994;37:4554–4557. doi: 10.1021/jm00052a014. [DOI] [PubMed] [Google Scholar]
- 8.Cushion MT, Walzer PD, Ashbaugh A, Rebholz S, Brubaker R, Vanden Eynde JJ, Mayence A, Huang TL. In vitro selection and in vivo efficacy of piperazine- and alkanediamide-linked bisbenzamidines against pneumocystis pneumonia in mice. Antimicrob. Agents Chemother. 2006;50:2337–2343. doi: 10.1128/AAC.00126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushion MT, Walzer PD, Collins MS, Rebholz S, Vanden Eynde JJ, Mayence A, Huang TL. Highly active anti-pneumocystis carinii compounds in a library of novel piperazine-linked bisbenzamidines and related compounds. Antimicrob. Agents Chemother. 2004;48:4209–4216. doi: 10.1128/AAC.48.11.4209-4216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenidge PA, Jenkins TC, Neidle S. DNA minor groove recognition properties of pentamidine and its analogs: a molecular modeling study. Mol. Pharmacol. 1993;43:982–988. [PubMed] [Google Scholar]
- 11.Simpson IJ, Michael I, Kumar A, Boykin DW, Neidle S. DNA minor groove interactions and biological activity of 2,5-bis-[4-(N-alkylamidino)phenyl]furans. Bioorg. Med. Chem. Lett. 2000;10:2593–2597. doi: 10.1016/s0960-894x(00)00511-4. [DOI] [PubMed] [Google Scholar]
- 12.De Oliveira AM, Custodio FB, Donnici CL, Montanari CA. QSAR and molecular modeling studies of B-DNA recognition of minor groove binders. Eur. J. Med. Chem. 2003;38:141–155. doi: 10.1016/s0223-5234(02)01419-8. [DOI] [PubMed] [Google Scholar]
- 13.Żołek T, Maciejewska D. Theoretical models of pentamidine analogs based on their DNA minor groove complexes. Eur. J. Med. Chem. 2010;45:1991–1999. doi: 10.1016/j.ejmech.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen B, Lee MPH, Hamelberg D, Joubert A, Bailly Ch, Brun R, Neidle S, Wilson WD. Strong binding in DNA minor goove by an aromatic diamidine with a shape that does not match the curvature of the groove. J. Am. Chem. Soc. 2002;124:13680–13681. doi: 10.1021/ja027953c. [DOI] [PubMed] [Google Scholar]
- 15.Huang TL, Vanden Eynde JJ, Mayence A, Collins MS, Cushion MT, Rattendi D, Londono I, Mazumder L, Bacchi CJ, Yarlett N. Synthesis and SAR of alkanediamide-linked bisbenzamidines with anti-trypanosomal and antipneumocystis activity. Bioorg. Med. Chem. Lett. 2009;19:5884–5886. doi: 10.1016/j.bmcl.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley JN, Barber HJ, Ewins AJ, Newbery G, Self ADH. A chemiotherapeutic comparison of the trypanocidal actin of some aromatic diamidines. J. Chem. Soc. 1942:103–116. [Google Scholar]
- 17.Berg SS, Newbery G. The search for chemiotherapeutic amidines. Part X. Substituted 4: 4’-diamidino-αω-diphenoxyalkanes and –diphenyl ethers. J. Chem. Soc. 1949:642–648. [Google Scholar]
- 18.Francesconi I, Wilson WD, Tanious FA, Hall JE, Bender BC, Tidwell RR, McCurdy D, Boykin DW. 2,4-Diphenyl furan diamidines as novel anti-Pneumocystis carinii pneumonia agents. J. Med. Chem. 1999;42:2260–2265. doi: 10.1021/jm990071c. [DOI] [PubMed] [Google Scholar]
- 19.Maciejewska D, Kaźmierczak P, Żabinski J, Wolska I, Popis S. Pentamidine Analogs: Syntheses, structures In solid state by 13C CP/MAS NMR spectroscopy, and X-ray crystallography and their preliminary biological screening against human cancer. Monatsh. f. Chem. 2006;137:1225–1240. [Google Scholar]
- 20.Żabinski J, Maciejewska D, Kaźmierczak P. Structural analysis of bis-nitriles and bisamidines in solid state by combining NMR spectroscopy and molecular modeling. J. Mol. Struct. 2009;923:132–140. [Google Scholar]
- 21.Żabinski J, Maciejewska D, Wolska I. Solid state structural analysis of new pentamidine analogs designed as chemotherapeutics that targed DNA by X-ray diffraction and 13C, 15N CP/MAS NMR methods. J. Mol. Struct. 2010;984:68–74. [Google Scholar]
- 22.Maciejewska D, Wolska I, Żabinski J. Examination of the structure in solid state of amino analogs of 4,4’[1,5-pentanediylbis(oxy)]bisbenzonitrile by means of X-ray diffraction, 13C CP/MAS NMR, and theoretical calculations. J. Mol. Struct. 2008;879:53–59. [Google Scholar]
- 23.Żabiński J, Wolska I, Maciejewska D. Comparsion of structure in solid state of new 1,5-bis(4-cyano-2,6-dimethoxyphenoxy)alkanes by means of 13C CP/MAS NMR and X-Ray diffraction. J. Mol. Struct. 2007;883:74–81. [Google Scholar]
- 24.Cui J, Crich D, Wink D, Lam M, Rheingold AL, Case DA, Fu W, Zhou Y, Rao M, Olson AJ, Johnson ME. Desing and synthesis of highly constrained factor Xa inhibitors: amidine-substituted bis(benzoyl)-[1,3]-diazepan-2-ones and bis(benzylidene)-bis(gem-dimethyl)cycloketones. Bioorg. & Med. Chem. 2003;11:3379–3392. doi: 10.1016/s0968-0896(03)00332-8. [DOI] [PubMed] [Google Scholar]
- 25.Collins MS, Cushion MT. Standardization of an in vitro drug screening assay by use of cryopreserved and characterized pneumocystis carinii populations. J. Eukaryot. Microbiol. 2001;48 Suppl.:178S–179S. doi: 10.1111/j.1550-7408.2001.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 26.Cushion MT, Chen F, Kloepfer N. A cytotoxicity assay for evaluation of candidate anti-pneumocystis carinii agents. Antimicrob. Agents Chemother. 1997;41:379–384. doi: 10.1128/aac.41.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneshiro ES, Collins MS, Cushion MT. Inhibitors of sterol biosynthesis and amphotericin B reduce the viability of pneumocystis carinii f. sp. carinii. Antimicrob. Agents Chemother. 2000;44:1630–1638. doi: 10.1128/aac.44.6.1630-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cushion MT, Collins MS, Hazra B, Kaneshiro ES. Effects of atovaquone and diospyrin-based drugs on the cellular ATP of pneumocystis carinii f. sp. carinii. Antimicrob. Agents Chemother. 2000;44:713–719. doi: 10.1128/aac.44.3.713-719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]