Summary
Background
Neonatal interventions are largely focused on reduction of mortality and progression towards Millennium Development Goal 4 (child survival). However, little is known about the global burden of long-term consequences of intrauterine and neonatal insults. We did a systematic review to estimate risks of long-term neurocognitive and other sequelae after intrauterine and neonatal insults, especially in low-income and middle-income countries.
Methods
We searched Medline, Cumulative Index to Nursing and Allied Health Literature, the Cochrane Library, and Embase for studies published between Jan 1, 1966, and June 30, 2011, that reported neurodevelopmental sequelae after preterm or neonatal insult. For unpublished studies and grey literature, we searched Dissertation Abstracts International and the WHO library. We reviewed publications that had data for long-term outcome after defined neonatal insults. We summarised the results with medians and IQRs, and calculated the risk of at least one sequela after insult.
Findings
Of 28 212 studies identified by our search, 153 studies were suitable for inclusion, documenting 22 161 survivors of intrauterine or neonatal insults. The overall median risk of at least one sequela in any domain was 39·4% (IQR 20·0–54·8), with a risk of at least one severe impairment in any insult domain of 18·5% (7·7–33·3), of at least one moderate impairment of 5·0% (0·0–13·3%), and of at least one mild impairment of 10·0% (1·4–17·9%). The pooled risk estimate of at least one sequela (weighted mean) associated with one or more of the insults studied (excluding HIV) was 37·0% (95% CI 27·0–48·0%) and this risk was not significantly affected by region, duration of the follow-up, study design, or period of data collection. The most common sequelae were learning difficulties, cognition, or developmental delay (n=4032; 59%); cerebral palsy (n=1472; 21%); hearing impairment (n=1340; 20%); and visual impairment (n=1228; 18%). Only 40 (26%) studies included data for multidomain impairments. These studies included 2815 individuals, of whom 1048 (37%) had impairments, with 334 (32%) having multiple impairments.
Interpretation
Intrauterine and neonatal insults have a high risk of causing substantial long-term neurological morbidity. Comparable cohort studies in resource-poor regions should be done to properly assess the burden of these conditions, and long-term outcomes, such as chronic disease, and to inform policy and programme investments.
Funding
The Bill & Melinda Gates Foundation, Saving Newborn Lives, and the Wellcome Trust.
Introduction
Nearly 140 million children per year are born worldwide, with 3·6 million neonatal deaths and 2·6 million stillbirths.1–3 More than 90% of neonatal deaths occur in resource-poor countries, mostly in rural areas.4 Worldwide, an increasing proportion (currently more than 40%) of mortality in children younger than 5 years occurs in the neonatal period (aged 0–28 days), which has led to increased attention to neonatal mortality. The common causes of neonatal mortality include preterm birth complications, intrapartum-related factors such as hypoxic ischaemic encephalopathy, infections (notably sepsis, meningitis, and neonatal tetanus), and other conditions such as jaundice and congenital infections (cytomegalovirus, toxoplasma, syphilis, and rubella).4–6 However, the prevalences of these insults and the long-term consequences for neonates who survive, particularly in resource-poor regions, are unclear.4
Many neonates survive major insults without any evidence of impairment because of the plasticity of the developing brain and improvements in medical care. However, in some newborn babies, insults can cause varying degrees of long-term neurodevelopmental impairment.5–8 These impairments cause a major socioeconomic burden, especially in resource-poor countries. Intrauterine and neonatal insults substantially affect the global burden of disease, measured in disability-adjusted life-years, because they contribute to both premature mortality and long-term disability.9 However, little is known about the severity and distribution of long-term impairments after intrauterine or neonatal insults. As a result, sequelae from intrauterine and neonatal insults have not been adequately captured in estimates of the global burden of disease.10
We reviewed published data for the long-term consequences of intrauterine and neonatal insults. The questions addressed were: what are the long-term outcomes after intrauterine and neonatal insults (neonatal sepsis, neonatal meningitis, hypoxic ischaemic neonatal encephalopathy, neonatal jaundice, preterm birth, neonatal tetanus, congenital infections [cytomegalovirus, toxoplasma, syphilis, rubella], and HIV)? What is the risk and severity of at least one sequela and of multiple sequelae reported after these insults? And what is the risk and severity of sequelae after multiple insults for one infant?
Methods
Search strategy and selection criteria
We included studies that reported neurological outcomes after an identifiable and well defined neonatal insult (webappendix). We searched Medline, Cumulative Index to Nursing and Allied Health Literature, the Cochrane Library, and Embase for studies published between Jan 1, 1966, and June 30, 2011. For unpublished studies and grey literature, we searched Dissertation Abstracts International and WHO library. The initial search strategy used the words “neonate” and “outcome”, and each of the exposures (eg, “jaundice”) listed in webappendix pp 6–7. These searches were refined by the addition of terms such as “complications” or “diagnosis” (webappendix p 7). Further searches were done by replacement of “outcome” with “sequela”, which were then refined by addition of specific outcomes, for example, “neurological impairment” (webappendix 8–9). The reference list of all identified reports and articles were manually searched for additional studies. No language restrictions were used.
We reviewed the online abstracts of studies identified in the database searches and obtained reprints of potentially eligible studies. Disagreements about eligibility were resolved by discussion in a final review with CRJCN. Inclusion criteria were: occurrence of the insult during the intrauterine or neonatal period (up to 28 days of life); insults were verified with the appropriate diagnostic method or criteria (webappendix); follow-up of at least 6 months after the neonatal insult to exclude transient impairments and capture sequelae that become evident later (eg, epilepsy); use of standardised tests or controls in neurodevelopmental assessment; at least 80% of neonates surviving the insult identified for follow-up for at least the first 6 months; and study publication between Jan 1, 1966, and June 30, 2011 inclusive. We excluded case series, single case reports, and reviews.
Data extraction
Two reviewers (MKM and MA) examined the titles, abstracts, and studies independently with identical case definitions and study selection criteria (webappendix). Data were organised into broad domains for each insult: learning difficulties, cognition or developmental delay; seizures, convulsion, or epilepsy; behavioural problems; cerebral palsy; and hearing and vision. Additionally, a category for gross motor and coordination disorders was included to capture data from studies that did not explicitly identify cerebral palsy but reported gross motor function or coordination difficulties. When a single study described multiple insults, data from the various insults were extracted separately.
To assess the severity of the reported sequelae, we used the definitions of severity of sequelae from the Global Burden of Disease, Disease Control Priorities Project.9 We developed a table to include commonly reported mild to moderate impairments. To do so, we took into account the functional range when a standardised test was applied, and the degree of difficulty in doing routine age-appropriate self-care and motor activities (table 1).
Table 1.
Grading of severity of impairment across multiple domains
| Mild | Moderate | Severe | |
|---|---|---|---|
| Cognition | Cognitive Z score <−1 for more than one test, or mean Z score <−1 for a construction task and non-verbal task, or mean Z score <−1 for verbal tasks | Cognitive Z score <−2 for more than one test, or mean Z score <−2 for a construction task and non-verbal task, or mean Z score <−2 for verbal tasks | Z score <−3 for more than one severe test, or mean Z score <−3, or mean Z score <−3 for verbal tasks |
| Motor | Difficulty in everyday motor activities appropriate for age but able to move around without help | Difficulty in holding implements, dressing, and sitting upright. Able to move around with help | Inability to walk and absence of functional use of hands |
| Hearing | Audiometric hearing threshold level of 26–30 dB HL in children aged <15 years and 26–40 dB HL in adults | Audiometric hearing threshold level of 31–60 dB HL in children aged <15 years and 41–60 dB HL in adults. Hearing aid not normally used. Or, audiometric hearing threshold level of 31–60 dB HL in children aged <15 years and 41–60 dB HL in adults. Hearing aid normally used | Audiometric hearing threshold level of 61 dB HL or greater. Hearing aid not normally used. Or, audiometric hearing threshold level of 61 dB HL or greater. Hearing aid normally used |
| Vision | Visual acuity in the best eye of <6/12 but ≥6/18, or corresponding visual field loss | Visual acuity in the best eye of <6/18 but ≥6/60; or, corresponding visual field loss | Visual acuity in the best eye of <6/60 but >3/60; or, visual field loss with blindness (visual acuity in the best eye of <3/60); or, corresponding visual field loss |
| Seizure disorder | More than one non-febrile seizure per year | More than one non-febrile seizure per month | More than one non-febrile seizure per week |
| Behavioural* | Any reported | .. | .. |
| Multidomain | One or two domains mildly affected | Three or more domains mildly affected, or one moderately affected and more than one mildly affected | If any domain is severely affected, or two or more are moderately affected |
HL=hearing level.
Definitions of behavioural problems could not be standardised or assessed.
Statistical analysis
We summarised the results by calculation of the medians and IQRs of the proportions of individual with any sequela resulting from each insult independently and overall. Similarly, we recorded the proportions of impairments that were severe, moderate, or mild. Most of the studies reported the number of patients for each impaired domain separately (eg, number or percentage with hearing loss), omitting to systematically assess all domains and describe those with multiple impairments. Thus, we could not easily derive mutually exclusive categories for each domain (eg, cerebral palsy only, or learning difficulties only); therefore some overlap was unavoidable.
To investigate the effect of covariates (WHO region, period of data collection, study design [prospective or retrospective], and duration of follow-up after discharge from hospital) on the risk of at least one sequel, each covariate was categorised into subgroups. We did a random effect meta-analysis of all available studies in each subgroup to calculate estimates of risk for each covariate—eg, for duration of follow-up we calculated estimates for less than 12 months, 12–35 months, 36–60 months, and more than 60 months. We examined each covariate fitted singly and compared estimates with the most prevalent subgroup by number of survivors followed up and assessed (bivariate meta-regression). Finally, we included all the four covariates in a multivariable meta-regression analysis. We calculated heterogeneity with I2.11 All analyses were done with Stata (version 11.0).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Our search identified 28 212 publications. From the titles we selected 1330 (5%) for review of the abstract and finally selected 949 studies for detailed review (figure 1). We excluded 796 (84%) after assessment of full text because they did not meet the inclusion criteria. The main reasons for exclusion were that numbers of survivors with sequelae could not be extracted; no clear description or diagnosis of the neonatal insult was provided; less than 80% of survivors were followed up after definite exposure in the first 6 months; and no appropriate test to establish the nature and extent of the sequelae or impairment was done (figure 1). Additional reports were excluded because they were reviews, case series, or commentaries.
Figure 1.
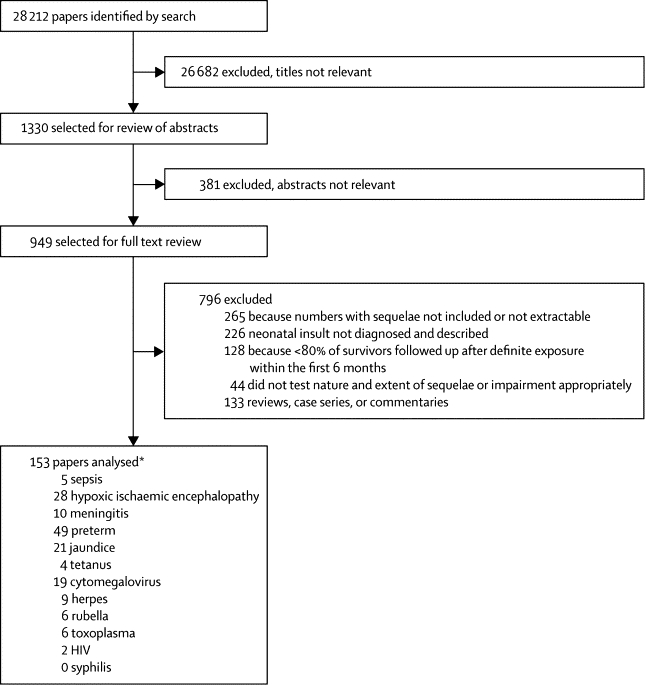
Literature review
*Six studies included data for more than one insult.
We were unable to identify any unpublished data that met our inclusion criteria. 153 studies were included (figure 1). 49 (32%) presented data for preterm birth, 28 (18%) for hypoxic ischaemic encephalopathy, 21 (14%) for neonatal jaundice, ten (6%) for meningitis, and five (3%) for sepsis. Five studies presented data for more than one insult. 68 papers (44%) were from the Americas (65 from North America and three from South America), 59 from Europe, 15 from the western Pacific, five from southeast Asia, three from Africa, and one from the eastern Mediterranean. Two studies covered several regions. The webappendix shows the references of these studies.
Table 2 summarises the prevalence of each impairment per insult and overall. 22 161 (82%) of the survivors (excluding those with HIV) were followed up and assessed. The median number of survivors per study was 50 (IQR 24–113). The median percentage loss to follow-up was 8·1% (0·0–23·9). 6851 (31%) of survivors assessed had an impairment. The most common impairments were learning difficulties, cognition, or developmental delay (n=4032; 59%); cerebral palsy (1472; 21%); hearing impairment (1340; 20%); and visual impairment (1228; 18%). Behavioural problems were reported in 718 patients (11%, IQR 5–25; table 3). The overall median risk of at least one sequela in any domain was 39·4% (IQR 20·0–54·8), with a risk of at least one severe impairment in any one domain of 18·5% (7·7–33·3), at least one moderate impairment of 5·0% (0·0–13·3), and at least one mild impairment of 10·0% (1·4–17·9).
Table 2.
Summary of sequelae risk after intrauterine and neonatal insults
| Neonates assessed (n) | Sequelae (n; %) |
Type of sequelae (n; % of total with impairment) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognition, general developmental delay, or learning difficulties | Cerebral palsy | Deaf or hearing loss | Impaired vision or blind | Gross motor and coordination* | Epilepsy | Behavioural problems | |||
| Sepsis (5 studies) | 2442 | 977 (40%) | 720 (74%) | 353 (36%) | 97 (10%) | 317 (32%) | 5 (1%) | .. | .. |
| Meningitis (11 studies) | 501 | 209 (42%) | 209 (100%) | 43 (21%) | 25 (12%) | 84 (40%) | 10 (5%) | 24 (11%) | 3 (1%) |
| Hypoxic ischaemic encephalopathy (27 studies) | 2708 | 1002 (37%) | 453 (45%) | 291 (29%) | 95 (9%) | 258 (26%) | 167 (17%) | 122 (12%) | 15 (1%) |
| Preterm birth (47 studies) | 6558 | 2006 (31%) | 1209 (60%) | 539 (27%) | 148 (7%) | 224 (11%) | 202 (10%) | 20 (1%) | 25 (1%) |
| Jaundice (20 studies) | 7212 | 1311 (18%) | 985 (75%) | 190 (14%) | 146 (11%) | .. | 36 (3%) | 40 (3%) | 57 (4%) |
| Tetanus (4 studies) | 109 | 28 (26%) | 28 (100%) | 1 (4%) | .. | .. | 4 (14%) | .. | 6 (21%) |
| Cytomegalovirus (17 studies) | 918 | 377 (41%) | 250 (66%) | 23 (6%) | 251 (67%) | 13 (3%) | 60 (16%) | 23 (6%) | 7 (2%) |
| Herpes (9 studies) | 311 | 116 (37%) | 109 (94%) | 32 (28%) | 2 (2%) | 55 (47%) | 34 (29%) | 27 (23%) | 2 (2%) |
| Rubella (6 studies) | 890 | 720 (81%) | 42 (6%) | .. | 576 (80%) | 191 (27%) | 44 (6%) | .. | 2 (<1%) |
| Toxoplasmosis (5 studies) | 512 | 105 (21%) | 27 (26%) | .. | .. | 86 (82%) | 1 (1%) | 12 (11%) | .. |
| Syphilis (0 studies) | .. | .. | .. | .. | .. | .. | .. | .. | .. |
| Overall | 22 161 | 6851 (31%) | 4032 (59%) | 1472 (21%) | 1340 (20%) | 1228 (18%) | 563 (8%) | 268 (4%) | 117 (2%) |
Impairment by sequelae type greater than 100% because of overlap.
Cerebral palsy was not defined and classified but motor and coordination impairments were reported.
Table 3.
The risk of impairment sequelae by insult and overall
| Overall | Sepsis | Meningitis | Hypoxic ischaemic encephalopathy | Preterm birth | Jaundice | Tetanus | Cytomegalovirus | Herpes | Rubella | Toxoplasmosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| At least one sequela in any domain | 39·4% (20·0–54·8) | 48·9% (39·1–59·2) | 46·4% (36·9–68·4) | 44·2% (20·6–53·9) | 27·9% (18·6–46·4) | 25·3% (13·2–39·2) | 20·8% (11·5–45·0) | 48·3% (20·8–73·8) | 51·5% (31·3–56·5) | 82·0% (68·5–100·0) | 55·6% (51·2–85·7) |
| Cognitive or learning difficulties or developmental delay | 26·0% (14·3–43·4) | 30·0% (26·4–44·4) | 33·3% (26·7–36·8) | 29·7% (13·0–40·7) | 20·9% (12·5–39·5) | 16·1% (11·7–23·9) | 20·8% (10·5–95·8) | 27·8% (9·5–57·1) | 12·5% (5·2–17·4) | 22·7% (7·1–23·6) | 33·3% (19·1–70·8) |
| Cerebral palsy | 15·5% (9·2–28·6) | 12·4% (11·1–14·9) | 11·7% (6·2–29·0) | 28·3% (19·1–42·0) | 11·6% (8·0–17·7) | 10·5% (3·7–14·4) | .. | 24·3% (11·4–35·8) | 29·9% (20·0–39·1) | .. | .. |
| Hearing loss | 13·4% (5·0–28·6) | 18·7% (8·9–36·1) | 8·6% (2·6–13·1) | 10·0% (5·0–11·6) | 2·9% (1·2–8·7) | 11·1% (5·7–23·0) | .. | 25·0% (16·1–50·0) | .. | 56·9% (28·6–69·9) | .. |
| Visual disturbance | 12·9% (3·5–33·4) | 12·5% (11·1–21·9) | 20·0% (13·3–36·8) | 12·0% (3·3–24·3) | 2·8% (1·4–11·3) | .. | .. | 7·1% (2·3–21·8) | 48·7% (40·8–57·2) | 37·3% (31·3–65·2) | 55·6% (51·2–85·7) |
| Epilepsy | 14·7% (7·2–44·4) | .. | 15·8% (5·4–50·0) | 10·9% (3·4–23·1) | 31·3% (29·4–33·3) | 11·9% (3·5–16·7) | .. | 11·1% (10·7–21·7) | 32·5% (30·2–36·1) | .. | 14·3% (11·1–16·7) |
| Gross motor and coordination problems* | 16·9% (10·0–29·2) | .. | 6·7% (2·6–10·6) | 20·1% (5·6–33·3) | 18·9% (11·8–25·6) | 20·1% (12·8–39·4) | .. | 10·9% (2·2–58·2) | 25·0% (22·2–37·5) | .. | 6·6% (4·8–8·3) |
| Behavioural problems | 10·7% (5·5–25·0) | .. | .. | 13·1% (2·9–23·3) | 9·4% (4·9–17·9) | .. | .. | .. | .. | .. | .. |
| Severe sequela in at least one domain | 18·5% (7·7–33·3) | 39·9% (32·1–44·8) | 15·0% (10·8–26·3) | 27·1% (20·6–38·5) | 9·6% (6·6–18·2) | 9·3% (2·3–22·7) | .. | 11·1% (5·6–27·3) | 34·8% (25·0–40·6) | 82·3% (63·6–96·1) | 22·2% (3·2–25·0) |
| Moderate sequelae in at least one domain | 5·0% (0·0–13·3) | 9·3% (0·0–21·8) | 6·7% (1·1–13·3) | 2·9% (0·0–8·3) | 5·5% (0·0–13·4) | 1·5% (0·0–12·7) | .. | 11·1% (0·0–27·3) | 5·8% (0·0–12·5) | .. | 22·8% (2·1–29·2) |
| Minor or mild sequelae in at least one domain | 10·0% (1·4–17·9) | .. | 10·0% (0·0–20·0) | 8·5% (1·4–12·5) | 13·9% (4·7–25·0) | 4·2% (2·6–10·9) | .. | 11·1% (8·0–19·1) | 12·5% (5·2–17·4) | 13·1% (4·1–31·7) | 11·1% (5·0–37·5) |
| Severe cognitive or learning difficulties | 17·3% (6·3–26·9) | 26·4% (22·2–30·0) | 11·4% (5·6–18·9) | 25·0% (17·9–40·7) | 7·0% (4·1–18·7) | .. | .. | 19·6% (5·9–37·8) | 36·4% (6·3–40·6) | .. | 10·7% (4·8–16·7) |
| Moderate cognitive or learning difficulties | 7·1% (0·0–12·5) | .. | .. | 6·9% (0·0–11·5) | 11·0% (5·6–13·9) | .. | .. | .. | 7·5% (4·3–10·0) | .. | 6·5% (4·8–8·3) |
| Mild cognitive or learning difficulties | 10·0% (0·7–15·0) | .. | 14·4% (0·0–15·8) | 2·4% (0·0–10·0) | 14·0% (7·4–22·7) | .. | .. | 13·2% (3·6–14·3) | 9·1% (3·0–15·0) | .. | 8·9% (8·3–9·5) |
| Severe cerebral palsy | 11·4% (7·4–24·0) | .. | 10·0% (2·7–11·4) | 24·0% (16·7–36·5) | 9·2% (4·1–12·1) | .. | .. | .. | .. | .. | .. |
| Moderate cerebral palsy | 2·4% (0·0–6·6) | .. | .. | 1·4% (0·0–4·3) | 5·5% (3·5–7·5) | .. | .. | .. | .. | .. | .. |
| Mild cerebral palsy | 1·5% (0·0–4·1) | .. | 1·8% (0·0–12·8) | 0·9% (0·0–1·6) | 4·1% (2·0–6·7) | .. | .. | .. | .. | .. | .. |
| Deaf or severe hearing loss | 4·9% (1·1–17·4) | 11·1% (4·1–19·4) | 0·6% (0·0–1·5) | .. | 1·8% (0·9–4·3) | .. | .. | 9·5% (5·6–17·4) | .. | 37·7% (21·4–60·2) | .. |
| Mild to moderate hearing loss | 10·4% (0·0–15·2) | 4·8% (0·0–21·4) | 6·5% (0·9–13·1) | .. | 0·3% (0–4·3) | .. | .. | 13·6% (11·1–16·7) | .. | 13·1% (5·4–17·9) | .. |
| Blind or severe visual loss | 2·4% (0·6–10·0) | 11·1% (5·6–12·5) | 4·5% (1·1–12·3) | .. | 1·9% (0·5–2·8) | .. | .. | .. | .. | .. | 22·2% (3·2–25·0) |
| Mild to moderate visual impairment | .. | .. | 14·5% (4·5–30·0) | .. | 0·7% (0·0–4·0) | .. | .. | .. | .. | .. | 33·3% (7·1–66·7) |
| Multiple impairments | 13·8% (8·0–33·3) | .. | .. | 20·5% (14·7–37·8) | 8·1% (3·7–10·2) | 12·4% (5·5–19·4) | .. | 22·6% (8·3–33·3) | .. | .. | .. |
Data are median (IQR). No data are given when the numbers were too few, domain was not assessed, or the impairment was graded.
Cerebral palsy was not defined and classified but motor and coordination impairments were reported.
In nine studies most infants had more than one insult. Most (2405; 98%) survivors who had sepsis were born prematurely. For survivors with preterm birth as the only insult, quantification of the degree of impairment by gestational age at birth was a challenge because of overlapping gestations in the studies. 13 studies specifically examined newborn babies who weighed 1500 kg or less. The risk of at least one sequela in this subgroup was 26·7% (23·9–44·4). The concentration of total serum bilirubin can affect the likelihood of brain impairment. Therefore, we separately analysed data from studies of neonates who had bilirubin concentrations of ≥400 μmol/L or more. 395 neonates were followed up, and 117 (30%) had impairments. The most common impairments were gross motor (n=72, 62%) and sensorineural hearing loss (n=23, 20%). The overall risk of impairments in this subgroup was 26·9% (IQR 16·7–61·5), with 18·0% (3·1–33·3) having severe impairments. 505 preterm neonates had hyperbilirubinaemia, of whom 488 (97%) were followed up and assessed, and 140 (28%) had impairments. Of those impaired, 60 (43%) had learning difficulties, 39 (28%) cerebral palsy, and 33 (24%) sensorineural hearing loss (eight had mixed impairments). The median risk of at least one sequela in any domain in preterm newborns with hyperbilirubinaemia was 29·4% (IQR 27·3–45·0).
Only 40 (26%) studies presented data for multidomain impairments. These studies included 2815 individuals, of whom 1048 (37%) had impairments, with 334 (32%) having multiple impairments. In those with multiple impairments, 175 (52%) had three or more sequelae, all with cerebral palsy and learning difficulties plus a third or fourth impaired domain such as vision or hearing. 64 (19%) patients had a combination of cerebral palsy plus learning difficulties only, whereas 34 (10%) had cerebral palsy plus epilepsy. Learning difficulties or cerebral palsy in combination with another impaired domain (excluding each other or epilepsy) was documented in 39 (12%) patients.
Two studies documented HIV infection during the neonatal period.12,13 36 neonates were infected before the end of the neonatal period, of whom 15 (42%) had learning difficulties compared with 13 of 56 (23%) of those infected after the neonatal period. We excluded HIV from the final pooled estimate because of the small sample sizes in the two studies.
The pooled risk estimate of development of at least one sequela (weighted mean) in any domain for all insults (excluding HIV) was 37·0% (95% CI 27·0–48·0). The risk was highest after rubella infection, and lowest for survivors of neonatal jaundice; figure 2).
Figure 2.
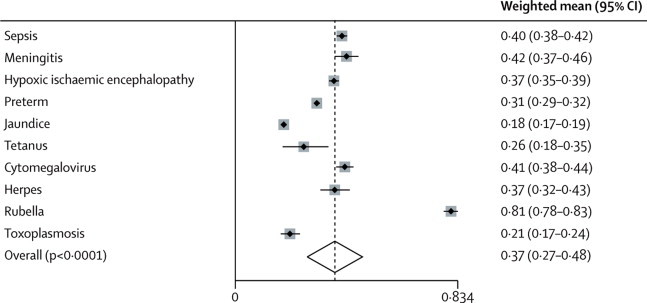
Random effect meta-analysis of the risk of development of one sequela by insult
Overall, heterogeneity was high between the studies used to estimate the risk of at least one sequela irrespective of the type of insult (figure 2). However, the risks did not differ with any of the four covariates tested: WHO region, period of data collection, duration of follow-up, and study design (prospective or retrospective; table 4). Other covariates—eg, socioeconomic status—were rarely reported.
Table 4.
Meta-regression analysis of the effect of key variables on the risk of sequelae
| Participants (number of studies) |
Bivariate meta-regression* |
Multivariate meta-regression† |
|||
|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| WHO region | |||||
| Americas | 13 951 (68) | 1·00 | .. | 1·00 | .. |
| European | 6716 (59) | 1·03 (0·92–1·16) | 0·58 | 0·93 (0·67–1·30) | 0·68 |
| Western Pacific | 1187 (15) | 0·97 (0·85–1·12) | 0·72 | 0·86 (0·61–1·21) | 0·38 |
| Southeast Asia | 158 (5) | 0·90 (0·71–1·14) | 0·36 | 0·78 (0·52–1·18) | 0·24 |
| Africa | 115 (3) | 1·06 (0·80–1·39) | 0·70 | 0·93 (0·61–1·41) | 0·71 |
| East Mediterranean | 34 (1) | 0·85 (0·53–1·35) | 0·49 | 0·74 (0·42–1·29) | 0·28 |
| Data collection period (year) | |||||
| <1980 | 7452 (38) | 1·00 | .. | 1·00 | .. |
| 1980–89 | 6559 (48) | 0·94 (0·86–1·03) | 0·18 | 0·87 (0·54–1·42) | 0·59 |
| 1990–99 | 6108 (47) | 1·09 (1·00–1·19) | 0·06 | 0·97 (0·59–1·57) | 0·89 |
| ≥2000 | 2042 (19) | 0·94 (0·82–1·08) | 0·37 | 0·86 (0·52–1·41) | 0·55 |
| Follow-up (months) | |||||
| <12 | 1631 (29) | 0·96 (0·86–1·06) | 0·40 | 1·06 (0·90–1·24) | 0·49 |
| 12–35 | 8184 (52) | 1·01 (0·89–1·08) | 0·85 | 1·07 (0·93–1·24) | 0·33 |
| 36–60 | 1315 (18) | 0·92 (0·88–1·06) | 0·21 | 1·01 (0·87–1·18) | 0·88 |
| >60 | 10 002 (39) | 1·00 | .. | 1·00 | .. |
| Mixed (across the age ranges) | 1029 (15) | 1·12 (1·01–1·33) | 0·03 | 1·15 (0·96–1·38) | 0·13 |
| Study design | |||||
| Prospective | 20 095 (124) | 1·00 | .. | 1·00 | .. |
| Retrospective | 2066 (29) | 1·06 (1·00–1·17) | 0·28 | 1·05 (0·94–1·18) | 0·39 |
Estimates compared to most prevalent group within the subgroup.
Multivariate regression approach (WHO region with the Americas as the baseline, data collection period with <1980 as the baseline, follow-up with >60 months as the baseline).
Discussion
In our study, the median overall risk of sequelae in survivors of intrauterine and neonatal insults was very high. Our Article provides supportive evidence that intrauterine and neonatal insults result in significant long-term neurological morbidity and that these insults have a high risk of affecting more than one domain (eg, cognitive impairment, motor impairment and hearing and vision loss). Despite the fact that we initially identified many studies, few had data that were suitable for analysis, which is common in reviews of global estimates. For example, in a series of reviews of child mortality data,14 only 308 studies were included from more than 17 000 abstracts. Data for morbidity and impairment are even worse, which is indicative of the low value placed on the collection of disability data.
Although we recorded significant heterogeneity, the decade in which data were collected did not significantly affect the risk of development of sequelae, which suggests that the risk of long-term impairment after intrauterine and neonatal insults has not changed over time. Alternatively, advances in technologies for care of sick newborn babies might have increased survival in neonates who would have otherwise died, with the increased proportions of impairments in this group of survivors cancelling out any reduction in impairments gained in less severely afflicted newborn babies. Large cohort studies in a range of health-care settings but with consistent definitions of exposure, outcome, mortality, and sequelae are needed to advance understanding of this issue. Most of the studies were from Europe or North America, where the standard of care differs greatly to that in low-income settings. The predominance of studies from only two regions might be why WHO region did not affect the risk of development of sequelae. However, combination of studies of different insults and with varying outcomes could be an important contributor to the recorded heterogeneity.
We did not identify any acceptable studies of syphilis. Although long-term effects of maternal syphilis are well known,15 liberal use of penicillin both in the antenatal period and as treatment for neonatal sepsis might have substantially reduced this burden. Tetanus was estimated to cause only about 60 000 neonatal deaths in 2009, a dramatic reduction from the previous decade.16–19 However, the data we extracted for impairments after neonatal tetanus are not robust because of the few studies that were acceptable and low numbers of patients. The very high inpatient and immediate mortality after discharge20–22 of tetanus might limit the number of suitable studies. However, the focus might have been on neonatal deaths, and the impairment burden of this disorder has been poorly assessed.
Vertical HIV transmission is a substantial burden:23 4% or more of pregnant women were infected in the studies that we identified. 24–27 With the expansion of antiretroviral treatment for children in many countries, including those in resource-poor regions,28,29 many HIV-infected neonates survive into adulthood. Importantly, the timing of HIV infection is thought to be one of the factors that affects long-term neurological and developmental outcome.30–32 Despite this scenario, only two studies met our criteria and documented the timing of HIV infection in the neonatal period and long-term sequelae.12,13 Although these two studies had small sample sizes they do indicate that HIV infection in the intrauterine or early neonatal period might be associated with a worse long-term neurological outcome than later HIV infections. More studies with larger follow-up cohorts are required to further understand the effect of HIV on long-term neurological outcomes.
Neonates commonly present with multiple insults. Because the median impairment after preterm birth alone was 28%, and that of septic premature neonates was 49%, sepsis probably increases the likelihood of neurological impairment in preterm neonates. Moreover, the degree of impairment was more likely to be severe in septic preterm neonates than in nonseptic neonates. However, because the studies of sepsis that we identified were mostly of premature neonates, the sequelae resulting from septicaemia in term newborn babies are still unclear and few studies exist about the outcome of neonatal sepsis in term infants. The degree of impairment in preterm neonates with hyperbilirubinaemia did not differ from the background impairment due to preterm birth alone. Because jaundice in preterm births is very common,33,34 a proportion of neonates in the studies that had data only for long-term sequelae after preterm birth might also have developed hyperbilirubinaemia, hence the almost equal degree of impairment in the two groups.
Although early-onset brain damage, especially of the prefrontal cortex, could result in behavioural problems, assessment is very difficult during the early years, especially when other severe impairments (eg, of motor and cognition) are present. At least a tenth of survivors have behavioural problems; however, in view of the above limitations, this proportion is likely to be an underestimate.
Our review has several limitations. First, nearly 20% of survivors were lost to follow-up. Loss to follow-up can bias results in two ways. Survivors with minor or no sequelae might be more prone to loss to follow-up because they perceive little benefit of being re-examined.35 This effect might skew the estimates towards more severe sequelae. However, emotional stress and stigma might also reduce follow-up and affect the results.35,36 Additionally, those with severe impairments are likely to have a higher mortality than are those with mild to moderate sequelae during follow-up.36 This effect might leave survivors with spuriously low prevalences of impairment, or a preponderance of minor sequelae. Second, all the studies we reviewed were based in hospitals, which might not be a major constraint in high-income countries where access to care is almost universal, but in low-income countries might introduce a bias towards the inclusion of patients of relatively high social and economic status, who have better access to both acute and long-term health care and tend to have a lower risk of impairment.37 Third, we were able to extract data for multidomain impairments from only 25% of studies; thus, the number of multiple impairments might have been underestimated. Furthermore, data after multiple insults were sparse. Fourth, we used strict criteria, which excluded many studies, particularly those of preterm birth and HIV. Finally, our review shows the paucity of studies from resource-poor countries, especially sub-Saharan Africa, where only three studies met the inclusion criteria. Many factors make the situation and outcomes for neonates in low-income and middle-income countries very different to those in high-income settings: infections and intrapartum complications are more prevalent, many sick neonates do not present for care or present late to health-care facilities, inpatient treatment might not be optimum, and postdischarge care is often rudimentary.3,18,38 More than 98% of neonatal morbidity occurs in these countries1,3,38 and yet the least data are available. The absence of adequate data from these countries is the major constraint to understanding the burden of long-term impairments after neonatal insults, particularly in middle-income countries where neonatal care is increasing survival rates, but follow-up data lag behind the health-care systems.
Our Article has key implications for policy and research. First, in view of the poor outcome, prevention and improved treatment of complications during birth and in the neonatal period need to be emphasised, along with research into adjunct treatment approaches that might have neuroprotective effects and that are feasible in low-income settings. Second, more resources should be allocated for follow-up and rehabilitation of neonates who survive insults. Additionally, urgent attention is needed to improve multidomain, longer term assessment of these survivors.
Attention has only recently been given to worldwide newborn survival, and most efforts are directed at prevention of mortality. This focus is important in view of the short time to the Millennium Development Goals in 2015. However, insults during the intrauterine and neonatal period lead to substantial impairments, with major burdens on families and societies, and shortened life expectancy. Cohort studies, mainly from high-income countries, show that exposures during pregnancy and birth are major risk factors for chronic disease. As neonatal care improves in middle-income and low-income countries, might we recreate the epidemics of impairment because of prematurity, such as retinopathy, seen in the mid-20th century in Europe and North America? We need better data to clarify this issue.
Our results draw attention to the importance of both primary and secondary prevention measures. Many relevant insults such as intrapartum complications, neonatal tetanus, and neonatal jaundice can be prevented by timely and highly cost-effective interventions. Complications of preterm birth can be reduced by cost-effective interventions such as antenatal steroids, which are infrequently used in low-income and middle-income countries.39 Importantly, effective care, including attention to appropriate use of oxygen, could reduce the severity of sequelae. Rehabilitation and supportive care might improve quality of life. However, changes to policy and programmes are unlikely without a more cohesive, interagency, proactive approach to improving data.
Acknowledgments
Acknowledgments
This study was funded by a grant from The Bill & Melinda Gates Foundation to the Child Health Epidemiology Reference Group and by Saving Newborn Lives (a programme of Save the Children) and the Wellcome Trust. CN is funded by the Wellcome Trust. This report was submitted for publication with the permission of the director of the Kenya Medical Research Institute.
Contributors
MKM designed the study, reviewed abstracts, selected studies for inclusion, extracted data, analysed data, and wrote the draft. MA helped design literature searches, extracted data, assisted in analysis, and wrote the draft. JEL conceived and designed the study, contributed to the literature searches, and wrote the report. CRJCN conceived the study, designed the study, designed the literature searches, extracted data, and approved the final report.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3 6 million neonatal deaths—what is progressing and what is not? Semin Perinatol. 2010;34:3710–3786. doi: 10.1053/j.semperi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Cousens S, Blencowe H, Stanton C. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377:1319–1330. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- 3.Oestergaard MZ, Inoue M, Yoshida S, for the United Nations Inter-Agency Group for Child Mortality Estimation. the Child Health Epidemiology Reference Group Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8:e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 5.Crichton JU, Dunn HG, McBurney AK, Robertson AM, Tredger E. Long-term effects of neonatal jaundice on brain function in children of low birth weight. Pediatrics. 1972;49:656–670. [PubMed] [Google Scholar]
- 6.Gordon AL, English M, Tumaini Dzombo J, Karisa M, Newton CR. Neurological and developmental outcome of neonatal jaundice and sepsis in rural Kenya. Trop Med Int Health. 2005;10:1114–1120. doi: 10.1111/j.1365-3156.2005.01496.x. [DOI] [PubMed] [Google Scholar]
- 7.Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107:480–484. doi: 10.1542/peds.107.3.480. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Adams-Chapman I. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 9.WHO . The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Harvard University Press; Cambridge, MA: 2001. [Google Scholar]
- 10.Olusanya BO. “The right stuff”: the global burden of disease. PLoS Med. 2007;4:e84. doi: 10.1371/journal.pmed.0040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R, Malee K, Charurat M. Timing of perinatal human immunodeficiency virus type 1 infection and rate of neurodevelopment. The Women and Infant Transmission Study Group. Pediatr Infect Dis J. 2000;19:862–871. doi: 10.1097/00006454-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 13.McGrath N, Fawzi WW, Bellinger D. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J. 2006;25:47–52. doi: 10.1097/01.inf.0000195638.80578.e0. [DOI] [PubMed] [Google Scholar]
- 14.Rudan I, Lawn J, Cousens S. Gaps in policy-relevant information on burden of disease in children: a systematic review. Lancet. 2005;365:2031–2040. doi: 10.1016/S0140-6736(05)66697-4. [DOI] [PubMed] [Google Scholar]
- 15.Blencowe H, Cousens S, Kamb M, Berman S, Lawn JE. Lives Saved Tool supplement detection and treatment of syphilis in pregnancy to reduce syphilis related stillbirths and neonatal mortality. BMC Public Health. 2011;11(suppl 3):S9. doi: 10.1186/1471-2458-11-S3-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947–1959. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 17.Eregie CO. Epidemiological factors associated with neonatal tetanus mortality: observations from a cluster survey in Nigeria. East Afr Med J. 1993;70:434–437. [PubMed] [Google Scholar]
- 18.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 19.Black RE, Cousens S, Johnson HL. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 20.Barlow JL, Mung'Ala-Odera V, Gona V, Newton CR. Brain damage after neonatal tetanus in a rural Kenyan hospital. Trop Med Int Health. 2001;6:305–308. doi: 10.1046/j.1365-3156.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- 21.Teknetzi P, Manios S, Katsouyanopoulos V. Neonatal tetanus—long-term residual handicaps. Arch Dis Child. 1983;58:68–69. doi: 10.1136/adc.58.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anlar B, Yalaz K, Dizmen R. Long-term prognosis after neonatal tetanus. Dev Med Child Neurol. 1989;31:76–80. doi: 10.1111/j.1469-8749.1989.tb08414.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeremiah TA, Frank-Peterside N, Jeremiah ZA. HIV seropositivity and CD4 T-lymphocyte counts among infants and children in Port Harcourt, Nigeria. East Afr J Public Health. 2009;6:17–21. doi: 10.4314/eajph.v6i1.45807. [DOI] [PubMed] [Google Scholar]
- 24.Swai RO, Somi GG, Matee MI. Surveillance of HIV and syphilis infections among antenatal clinic attendees in Tanzania-2003/2004. BMC Public Health. 2006;6:91. doi: 10.1186/1471-2458-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offor E, Onakewhor JU, Okonofua FE. Maternal and neonatal seroprevalence of human immunodeficiency virus antibodies in Benin City, Nigeria. J Obstet Gynaecol. 2000;20:589–591. doi: 10.1080/0144361001396. [DOI] [PubMed] [Google Scholar]
- 26.Theng T, Sok P, Pugatch D. HIV-1 cord-blood seroprevalence of parturient women in Sihanoukville, Cambodia. Int J STD AIDS. 2004;15:419–421. doi: 10.1258/095646204774195290. [DOI] [PubMed] [Google Scholar]
- 27.Chopra M, Piwoz E, Sengwana J, Schaay N, Dunnett L, Saders D. Effect of a mother-to-child HIV prevention programme on infant feeding and caring practices in South Africa. S Afr Med J. 2002;92:298–302. [PubMed] [Google Scholar]
- 28.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in Lesotho. Pediatr Infect Dis J29: 340–45. [DOI] [PubMed]
- 29.Vreeman RC, Nyandiko WM, Ayaya SO, Walumbe EG, Marrero DG, Inui TS. Factors sustaining pediatric adherence to antiretroviral therapy in western Kenya. Qual Health Res. 2009;19:1716–1729. doi: 10.1177/1049732309353047. [DOI] [PubMed] [Google Scholar]
- 30.Thomas PA, Weedon J, Krasinski K. Maternal predictors of perinatal human immunodeficiency virus transmission. The New York City Perinatal HIV Transmission Collaborative Study Group. Pediatr Infect Dis J. 1994;13:489–495. doi: 10.1097/00006454-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Diaz C, Hanson C, Cooper ER. Disease progression in a cohort of infants with vertically acquired HIV infection observed from birth: the Women and Infants Transmission Study (WITS) J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:221–228. doi: 10.1097/00042560-199807010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Dickover RE, Dillon M, Leung KM. Early prognostic indicators in primary perinatal human immunodeficiency virus type 1 infection: importance of viral RNA and the timing of transmission on long-term outcome. J Infect Dis. 1998;178:375–387. doi: 10.1086/515637. [DOI] [PubMed] [Google Scholar]
- 33.Melamed N, Klinger G, Tenenbaum-Gavish K. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114:253–260. doi: 10.1097/AOG.0b013e3181af6931. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Huang C, Lou S. The clinical outcomes of late preterm infants: a multi-center survey of Zhejiang, China. J Perinat Med. 2009;37:695–699. doi: 10.1515/JPM.2009.130. [DOI] [PubMed] [Google Scholar]
- 35.Ganz ML, Tendulkar SA. Mental health care services for children with special health care needs and their family members: prevalence and correlates of unmet needs. Pediatrics. 2006;117:2138–2148. doi: 10.1542/peds.2005-1531. [DOI] [PubMed] [Google Scholar]
- 36.WHO Global literature review of Haemophilus influenzae type b and Streptococcus pneumoniae invasive disease among children less than five years of age: 1980–2005 [WHO/IVB/09.02] 2009. http://whqlibdoc.who.int/hq/2009/WHO_IVB_09.02_eng.pdf (accessed June 10, 2010).
- 37.Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev. 2003;43:57–69. doi: 10.1016/s0165-0173(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 38.Paul VK. The current state of newborn health in low income countries and the way forward. Semin Fetal Neonatal Med. 2006;11:7–14. doi: 10.1016/j.siny.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol. 2010;39(suppl 1):i122–i133. doi: 10.1093/ije/dyq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


