Summary
Background
In patients with suspected coronary heart disease, single-photon emission computed tomography (SPECT) is the most widely used test for the assessment of myocardial ischaemia, but its diagnostic accuracy is reported to be variable and it exposes patients to ionising radiation. The aim of this study was to establish the diagnostic accuracy of a multiparametric cardiovascular magnetic resonance (CMR) protocol with x-ray coronary angiography as the reference standard, and to compare CMR with SPECT, in patients with suspected coronary heart disease.
Methods
In this prospective trial patients with suspected angina pectoris and at least one cardiovascular risk factor were scheduled for CMR, SPECT, and invasive x-ray coronary angiography. CMR consisted of rest and adenosine stress perfusion, cine imaging, late gadolinium enhancement, and MR coronary angiography. Gated adenosine stress and rest SPECT used 99mTc tetrofosmin. The primary outcome was diagnostic accuracy of CMR. This trial is registered at controlled-trials.com, number ISRCTN77246133.
Findings
In the 752 recruited patients, 39% had significant CHD as identified by x-ray angiography. For multiparametric CMR the sensitivity was 86·5% (95% CI 81·8–90·1), specificity 83·4% (79·5–86·7), positive predictive value 77·2%, (72·1–81·6) and negative predictive value 90·5% (87·1–93·0). The sensitivity of SPECT was 66·5% (95% CI 60·4–72·1), specificity 82·6% (78·5–86·1), positive predictive value 71·4% (65·3–76·9), and negative predictive value 79·1% (74·8–82·8). The sensitivity and negative predictive value of CMR and SPECT differed significantly (p<0·0001 for both) but specificity and positive predictive value did not (p=0·916 and p=0·061, respectively).
Interpretation
CE-MARC is the largest, prospective, real world evaluation of CMR and has established CMR's high diagnostic accuracy in coronary heart disease and CMR's superiority over SPECT. It should be adopted more widely than at present for the investigation of coronary heart disease.
Funding
British Heart Foundation.
Introduction
Coronary heart disease is a leading cause of death and disability.1 Various techniques are used to diagnose coronary heart disease and assess the need for revascularisation. Increasingly, imaging tests have replaced exercise treadmill testing, with single-photon emission computed tomography (SPECT) being the most common. However, although a negative SPECT result provides reassuring prognostic information, it exposes patients to ionising radiation, and estimates of its accuracy vary widely.2–4
Cardiovascular magnetic resonance (CMR) imaging could be an alternative to SPECT. Theoretical advantages of CMR include the lack of ionising radiation, high spatial resolution, and its multiparametric nature—ie, its ability to assess multiple aspects of pathology in a single examination (eg, ventricular function, myocardial perfusion, viability, and coronary artery anatomy).5–10 Small single7,11–15 and multicentre16,17 studies have tested the accuracy of stress-perfusion CMR alone for the detection of coronary heart disease and some studies showed equal12 or improved15 results compared with SPECT. Stress-perfusion CMR and SPECT performed equally well in a subanalysis of a multicentre study (MR-IMPACT).18 However, this study was underpowered and the population was highly selected (the entry criteria were previous coronary angiography or a positive SPECT). Evidence from large, prospective studies comparing the two techniques is lacking. Additionally, multiparametric CMR protocols might increase the diagnostic yield compared with stress perfusion alone,9 but no large studies have investigated their clinical role. The inclusion of coronary MR angiography is especially relevant, because this component is time-consuming and the image quality is more variable than that of other CMR methods.
The Clinical Evaluation of MAgnetic Resonance imaging in Coronary heart disease (CE-MARC) study was designed to establish the diagnostic accuracy of multiparametric CMR in a large real world population, and to test the hypothesis that CMR yields higher diagnostic performance than SPECT, using X-ray angiography as the reference standard.
Methods
Patients and study design
The methods used in CE-MARC have been published previously.19 Patients were recruited between March, 2006, and August, 2009, from two hospitals (Pinderfields General Hospital and Leeds Teaching Hospitals NHS Trust, UK). Consecutive patients with suspected angina pectoris were screened and enrolled if they had at least one major cardiovascular risk factor and a cardiologist judged them to have stable angina needing investigation, in accordance with contemporary clinical practice. Exclusion criteria19 were previous coronary artery bypass surgery; crescendo angina or acute coronary syndrome; contraindication to CMR (eg, pacemaker) or adenosine infusion (eg, reversible airways disease, atrioventricular block); pregnancy; inability to lie supine; and a glomerular filtration rate of 30 ml/min per 1·73m2 or less. The estimated prevalence of clinically significant coronary disease was 40–60%.19 Patients were included in the primary outcome analysis if they had complete data from both CMR and x-ray angiography and had had no interim cardiovascular events. The study was done in accordance with the Declaration of Helsinki and approved by the local research ethics committee. Patients provided informed written consent.
Randomisation and masking
Patients were randomly assigned with respect to the order they received CMR and SPECT by a 24 h automated randomisation service with stratified permuted blocks to ensure that the groups were balanced for age (<65 years and ≥65 years) and sex. The SPECT, CMR, and x-ray angiogram results were analysed in accordance with international criteria,20–22 by masked, paired readers with at least 10 years of experience of using their modalities. After x-ray angiography, the SPECT result could be made available on request to enable a decision about revascularisation (to mask the treating clinician to the result was deemed unethical); however, CMR results were kept masked.
Procedures
By protocol all patients were scheduled for x-ray coronary angiography. Figure 1 shows the CMR protocol. CMR was done at Leeds General Infirmary with 1·5 Tesla Philips Intera CV scanner (Philips Healthcare, Best, Netherlands). Panel 1 shows the CMR method, with the criteria for positive CMR19 shown in panel 2.
Figure 1.
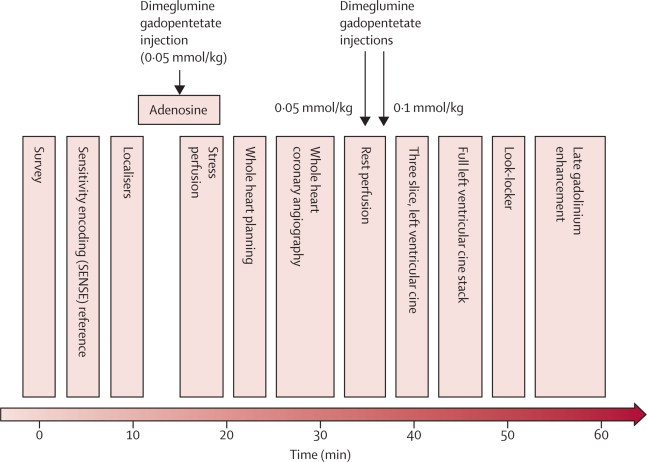
Cardiovascular magnetic resonance protocol
After a low-resolution survey scan and localisers to define the cardiac long and short axes, intravenous adenosine was administered for 4 min at 140 μg/kg per min, during which first-pass stress perfusion imaging was undertaken with 0·05 mmol/kg dimeglumine gadopentetate. 3D whole heart MR coronary angiography was done after the low resolution coronary survey and free-breathing four chamber cine (used to assess slice coverage and diastolic coronary rest period, respectively). Rest perfusion imaging was undertaken a minimum of 15 min after stress perfusion, with a further injection of 0·05 mmol/kg dimeglumine gadopentetate. A final injection of 0·1 mmol/kg dimeglumine gadopentetate was given after this sequence, bringing the overall gadolinium dose to 0·2 mmol/kg. Resting left ventricular function was then assessed, initially for three slices, planned identically to the perfusion slices, and then for the entire left ventricle with contiguous slices. A modified Look-Locker inversion time scout was done before late gadolinium enhancement imaging in short axis, vertical long axis, and horizontal long axis orientations. Times indicated are approximate and sequence blocks are not drawn to scale.
Panel 1. Cardiovascular magnetic resonance imaging parameters.
Balanced steady-state free precession cine imaging
10–12 short axis slices (10 mm thick, no gap) with one slice per breath-hold, 1·6 ms echo time, 3·2 ms repetition time, with a 192 by 192 matrix, 320–400 mm field of view, sensitivity encoding factor 1·7–2·0, and 30–50 phases per cardiac cycle.
Stress and rest perfusion
T1-weighted saturation-recovery, single-shot k-space gradient echo pulse sequence, in three 10-mm short axis slices, 1·0 ms echo time, 2·7 ms repetition time, 15° flip angle, with a 144 by 144 matrix, 320–460 mm field of view (in-plane spatial resolution 2·2–3·2 mm), sensitivity encoding factor 2, and a single saturation pre-pulse per R–R interval shared over three slices.
3D coronary magnetic resonance angiography
Balanced steady-state free precession pulse sequence with T2-weighted, fat saturation pre-pulses, 100–120 slices 0·9 mm thick, free breathing, with a respiratory navigator, 2·3 ms echo time, 4·6 ms repetition time, with a 304 by 304 matrix, 320–460 mm field of view, sensitivity encoding factor 1·7, and duration of acquisition up to 120 ms per R–R interval (determined by the length of the diastolic rest period).
Late gadolinium enhancement
T1-weighted segmented inversion-recovery gradient echo pulse sequence, 10–12 short axis slices with one slice per breath-hold, non-selective 180° pre-pulse, echo time 1·9 ms, repetition time 4·9 ms, flip angle 15°, inversion time adjusted individually according to the Look-Locker scan, with a 240 by 240 matrix, and a 320–460 mm field of view dependent on patient size.
Panel 2. Criteria for a positive CMR.
-
•
Any evidence of regional wall motion abnormality (by visual analysis using the 17-segment model23), each segment scored as 0 (normal), 1 (mild hypokinesia), 2 (severe hypokinesia), 3 (akinesia), or 4 (dyskinesia)
-
•
Hypoperfusion (ischaemia) assessed by visual comparison of stress and rest CMR perfusion scans (16 segments of the 17 segment AHA/ACC model, excluding the apical cap segment) with scores of 0 (normal), 1 (equivocal), 2 (subendocardial ischaemia), or 3 (transmural ischaemia)
-
•
Visual severity (percentage luminal narrowing) of coronary artery stenosis in the coronary MR angiogram (15 coronary segments)
-
•
Any infarct (scar) on late gadolinium-enhancement images (17 segment model) with scores of 0 (none), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (>75%) for each segment.
If any component was positive, the overall CMR result was judged positive; if all components were negative, the CMR overall result was judged negative. CMR=cardiovascular magnetic resonance.
SPECT radionuclide imaging was done at Leeds General Infirmary with a cardiac gamma camera (MEDISO Cardio-C, Budapest, Hungary). Patients underwent a standard 2-day protocol with 99mTc tetrofosmin (Myoview) at a dose of 400 MBq adjusted by weight to a maximum 600 MBq per examination (effective dose 6–9 mSv) as is advised in guidelines.24,25 Stress and rest electrocardiogram gated SPECT images were acquired with an intravenous adenosine protocol identical to that used in CMR (140 μg/kg per min for 4 min) so that the perfusion techniques were directly comparable.19 Patients were imaged supine at 64 projections with 3° intervals, every 40 s over a 180° orbit. At each projection eight ECG gated frames per cardiac cycle were acquired (64 by 64 matrix). Transaxial stress and rest slices of 6 mm thickness (spatial resolution of about 10 mm) were reconstructed with a Butterworth scattered back-projection filter, cut-off frequency 0·4 Nyquist, and an order of 6. Transaxial slices were re-orientated to the cardiac axes for analysis. Evidence of ischaemia was recorded by visual comparison of rest and stress SPECT perfusion scans (17-segment AHA/ACC model; a score of 0 [normal], 1 [equivocal], 2 [moderately reduced], 3 [severely reduced], or 4 [absent] was assigned to each segment). QGS software (version 3.0) was used to calculate end diastolic and end systolic volumes and wall motion scores. As for CMR reporting and according to usual clinical practice, diagnosis was made on the basis of all available SPECT data—ie, rest and stress perfusion, wall motion, and ventricular volumes.
For comparison with CMR, SPECT perfusion summed stress and rest scores (based on a 20-segment model: 0=normal, 1=mildly reduced uptake, 2=moderately reduced uptake, 3=severely reduced uptake, and 4=absent uptake) were calculated with QPS software.19 All x-ray angiograms were done after CMR and SPECT. Clinically significant coronary heart disease was defined as 70% or more stenosis of a first order coronary artery measuring 2 mm or greater in diameter, or left main stem stenosis 50% or more as measured by quantitative coronary angiography with use of QCAPlus software (version 8.11.19), with a post-stenosis diameter used as the reference vessel diameter in cases of ostial disease.
The primary outcome was diagnostic accuracy of the multiparametric CMR protocol to detect clinically significant coronary heart disease defined by x-ray angiography. The main secondary outcome was a comparison of multiparametric CMR (rest and stress perfusion, left ventricular function, coronary magnetic resonance angiography, and late gadolinium enhancement) and SPECT (rest and stress perfusion, left ventricular function) with x-ray angiography as the reference. An additional analysis compared only the equivalent components of the CMR protocol with SPECT.
Statistical analysis
Statistical analyses were done by the Clinical Trials Research Unit, University of Leeds. The sensitivity, specificity, and positive and negative predictive values were calculated with the Wilson score method. Sensitivity and specificity were compared with McNemar's test, and predictive values were compared with the generalised score statistic.26 To compare CMR and SPECT perfusion data for diagnostic performance with receiver operating characteristic analysis, a CMR summed stress score was calculated by adding together all segmental stress perfusion scores. Patients were included in the primary outcome analysis if they had complete data from both CMR and x-ray angiography and had no interim cardiovascular event. Diagnostic performance of CMR and SPECT were compared in patients with assessable results to both tests and x-ray angiography. Statistical analysis was done with SAS software, version 9.2 at a two-sided 5% significance level.19
A CMR result based on the components that are assessable by both modalities (ie, perfusion, ventricular function, and scar, but excluding coronary MR angiogram) was generated. This analysis was done to compare only equivalent data from both modalities and to assess the incremental value of CMR coronary imaging.
Role of the funding source
The sponsors provided financial support for the study but had no role in the study design (other than through its external peer review process), data collection, data analysis, data interpretation, or writing of the report. All authors had access to the primary data and have final responsibility for publication.
Results
Figure 2 shows the trial profile. For the 338 patients excluded after assessment by their cardiologist, the pretest likelihood of coronary heart disease was so low that invasive investigation could not be justified, or invasive angiography was needed so urgently that there could be no delay for non-invasive testing. CMR was not done or completed in 65 (9%) patients, SPECT in 67 (9%), and x-ray angiography in 23 (3%). 95 patients failed to complete one or more tests because of claustrophobia, emergency hospital admission, anxiety, personal or domestic reasons, unrelated illness, death, technical reasons, and eligibility violations. Results were positive in 302 patients for CMR, 239 patients for SPECT, and 282 for angiography. 384 CMR results were negative, 419 for SPECT, and 444 for x-ray angiography. Only SPECT had inconclusive results (26 patients), mainly because of patient movement (n=12) or an attenuation artifact (breast, diaphragmatic, or sub-diaphragmatic). Four patients' results were unavailable for analysis. The table shows baseline characteristics of all patients randomised and of those in the primary analysis; the demographics of all patients randomised, and especially of the primary analysis population, were similar. The overall prevalence of protocol-defined significant coronary heart disease was 39% (table).
Figure 2.
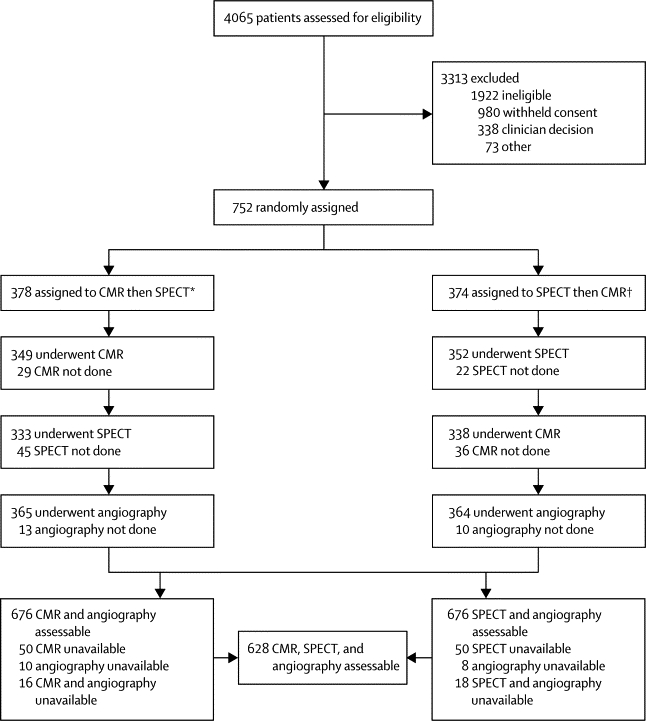
Trial profile
*34 received SPECT before CMR. †56 received CMR before SPECT because of patient and logistic reasons.
Table.
Baseline characteristics of patients
| All randomised patients (N=752) | CMR and x-ray angiography assessable (N=676) | CMR, SPECT, and x-ray angiography assessable (CMR before SPECT; N=316) | CMR, SPECT, and X-ray angiography assessable (SPECT before CMR; N=312) | ||
|---|---|---|---|---|---|
| Age (years) | 60·2 (9·7) | 60·3 (9·5) | 60·8 (9·6) | 59·9 (9·2) | |
| Men | 471 (63%) | 421 (62%) | 193 (61%) | 200 (64%) | |
| Body-mass index (kg/m2) | 29·2 (4·4) | 29·0 (4·3) | 29·1 (4·4) | 28·9 (4·2) | |
| Ethnic origin | |||||
| White | 711 (95%) | 643 (95%) | 295 (93%) | 302 (97%) | |
| Black | 6 (1%) | 5 (1%) | 1 (<1%) | 3 (1%) | |
| Asian | 30 (4%) | 24 (4%) | 17 (5%) | 6 (2%) | |
| Other | 5 (1%) | 4 (1%) | 3 (1%) | 1 (<1%) | |
| Smoking status | |||||
| Never smoked | 257 (34%) | 236 (35%) | 112 (35%) | 112 (36%) | |
| Ex-smoker | 350 (47%) | 315 (47%) | 150 (47%) | 144 (46%) | |
| Current smoker | 145 (19%) | 125 (18%) | 54 (17%) | 56 (18%) | |
| Systolic blood pressure (mm Hg) | 137·9 (20·7) | 138·1 (20·9) | 136·9 (21·5) | 138·6 (20·4) | |
| Diastolic blood pressure (mm Hg) | 79·0 (11·3) | 79·0 (11·3) | 78·5 (11·0) | 79·2 (11·6) | |
| Previous hospital admission for AMI or ACS | 60 (8%) | 54 (8%) | 31 (10%) | 21 (7%) | |
| Previous PCI | 38 (5%) | 37 (5%) | 20 (6%) | 15 (5%) | |
| Hypertension | 394 (52%) | 347 (51%) | 160 (51%) | 154 (49%) | |
| Diabetes mellitus | 96 (13%) | 85 (13%) | 49 (16%) | 34 (11%) | |
| Type 1 | 4/96 (4%) | 4 (5%) | 3 (6%) | 1 (3%) | |
| Type 2 | 92/96 (96%) | 81 (95%) | 46 (94%) | 33 (97%) | |
| Family history of premature heart disease | |||||
| Yes | 430 (57%) | 392 (58%) | 178 (56%) | 187 (60%) | |
| No | 268 (36%) | 237 (35%) | 118 (37%) | 99 (32%) | |
| Unknown | 54 (7%) | 47 (7%) | 20 (6%) | 26 (8%) | |
| Total cholesterol (mmol/L) | 5·2 (1·2) | 5·2 (1·2) | 5·1 (1·2) | 5·2 (1·2) | |
| Simplified Framingham risk score* | 13·7 (3·6; n=692) | 13·6 (3·6; n=622) | 13·7 (3·7; n=285) | 13·5 (3·5; n=291) | |
| Medication | |||||
| Aspirin or clopidogrel | 454 (60%) | 404 (60%) | 189 (60%) | 184 (59%) | |
| Statin | 336 (49%) | 301 (45%) | 151 (48%) | 129 (41%) | |
| ACEi or A2 receptor blockers | 258 (38%) | 229 (34%) | 112 (35%) | 96 (31%) | |
| β-blocker | 235 (34%) | 203 (30%) | 93 (29%) | 93 (30%) | |
| Patients undergoing x-ray angiography† | n=726 | n=676 | n=316 | n=312 | |
| Any significant stenosis | 282 (39%) | 266 (39%) | 125 (40%) | 123 (39%) | |
| Triple-vessel disease | 45 (6%) | 40 (6%) | 21 (7%) | 16 (5%) | |
| Double-vessel disease | 88 (12%) | 83 (12%) | 35 (11%) | 45 (14%) | |
| Single-vessel disease | 149 (21%) | 143 (21%) | 69 (22%) | 62 (20%) | |
| LMS disease | 23 (3%) | 22 (3%) | 12 (4%) | 9 (3%) | |
| LAD disease | 183 (25%) | 169 (25%) | 79 (25%) | 79 (25%) | |
| LCX disease | 133 (18%) | 126 (19%) | 55 (17%) | 65 (21%) | |
| RCA disease | 110 (15%) | 105 (16%) | 51 (16%) | 42 (13%) | |
Data are mean (SD) or n (%) unless otherwise stated.
Percentage risk of an event in the absence of chest pain over 10 years; patients with previous coronary heart disease had no risk score calculated; those older than age 75 years were assumed to be 75 years.27
Numbers of patients undergoing x-ray angiography includes those with completed or partly completed non-invasive test results. AMI=acute myocardial infarction. ACS=acute coronary syndrome. PCI=percutaneous coronary intervention. ACEi=angiotensin converting enzyme inhibitor. A2=angiotensin 2. LMS=left main stem. LAD=left anterior descending. LCx=left circumflex. RCA=right coronary artery.
The median time between CMR or SPECT and x-ray angiography was 21 days (IQR 10–32) and 21 days (12–31), respectively. The median time between CMR and SPECT was 7 days (5–13). Ten patients had 11 serious adverse events, all related to x-ray angiography; eight vascular access site complications (haematoma), one minor neurological event, one ventricular arrhythmia, and one acute event needing percutaneous coronary intervention.
For the primary outcome measure the sensitivity of CMR was 86·5% (95% CI 81·8–90·1), specificity 83·4% (79·5–86·7), positive predictive value 77·2% (72·1–81·6), and negative predictive value 90·5% (87·1–93·0). In the secondary outcome measure population, the sensitivity of SPECT was 66·5% (60·4–72·1), specificity 82·6% (78·5–86·1), positive predictive value 71·4% (65·3–76·9), and negative predictive value 79·1% (74·8–82·8). In this group the comparable values for multiparametric CMR were: sensitivity 86·3% (81·5–90·0), specificity 83·2% (79·1–86·6), positive predictive value 77·0% (71·7–81·5), and negative predictive value 90·3% (86·7–93·0). The differences between the sensitivities and negative predictive values of CMR and SPECT were significantly in favour of CMR (χ2 test, p<0·0001 for both), but the specificities and positive predictive values were not (p=0·916 and p=0·061, respectively). Figure 3 shows example comparisons of the different tests.
Figure 3.
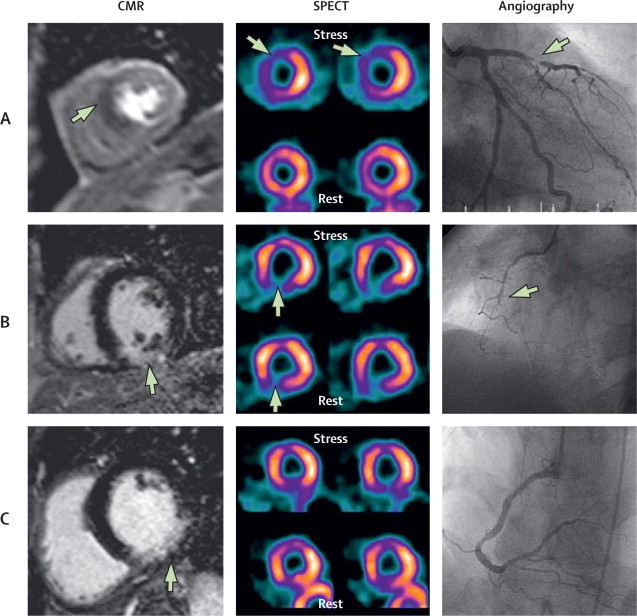
Three examples of CMR, SPECT, and angiographic findings
(A) Stress perfusion CMR shows inducible hypoperfusion (ischaemia) in the septum and anterior wall (arrow shows dark area of hypoperfusion), SPECT is concordant (arrows show lower signal counts during stress), indicating anteroseptal inducible ischaemia, and angiography confirms a stenosis (arrow) in the left anterior descending artery. (B) Late gadolinium-enhanced CMR (arrow shows hyperenhancement in the inferior wall) and SPECT (fixed defect; arrows show comparable inferior defect at rest and stress) are concordant, showing a transmural inferior myocardial infarct with the corresponding right coronary artery chronic total occlusion (arrow) seen at angiography. (C) Late gadolinium-enhanced CMR shows subendocardial inferior infarction (arrow), SPECT was reported as normal (no wall motion abnormality), and the angiogram shows coronary atheroma but no clinically significant stenosis (or occlusion). As per study protocol, CMR in this patient was classified as a false positive, showing the potential limitations of angiography as a reference test for the detection of coronary heart disease. The case also shows that SPECT can miss small subendocardial infarcts. CMR=cardiovascular magnetic resonance. SPECT=single-photon emission computed tomography.
Of the 676 patients who had assessable CMR and x-ray angiography, 598 (88%) had analysable coronary MR angiograms. From the 10 140 (15 for each of the 676 patients) coronary artery segments theoretically available, 5584 (55%) were of analysable quality. When the coronary MR angiogram component was excluded from the overall CMR analysis, CMR sensitivity was 81·6% (76·5–85·8), specificity 85·9% (82·1–88·9), positive predictive value 78·9% (73·7–83·3), and negative predictive value 87·8% (84·2–90·6). The overlap in CIs of these measures with those of the primary outcome analysis population, and the spread and heterogeneity across all four components, suggests that although sensitivity might slightly decrease, overall diagnostic accuracy does not differ if the coronary MR angiogram is excluded.
Compared with SPECT, the differences between the sensitivities, positive predictive values, and negative predictive values of the CMR protocol without coronary MR angiography were significant in favour of CMR (p<0·0001, p=0·01, and p<0·0001, respectively), but the specificities were not (p=0·224).
For the receiver operating characteristic curve analysis, stress CMR (area under the curve [AUC] 0·89, 95% CI 0·86–0·91) significantly out-performed SPECT (0·74, 0·70–0·78; p<0·0001; figure 4A). Stress perfusion CMR was better than SPECT even when the angiographic cutoff value for a clinically significant stenosis was adjusted to 50% or greater for left main stem and 50% or greater for left anterior descending, left circumflex artery, and right coronary artery (AUC for stress CMR 0·84, 95% CI 0·81–0·87 vs AUC for SPECT 0·69, 95% CI 0·65–0·73; p<0·0001; figure 4B).
Figure 4.
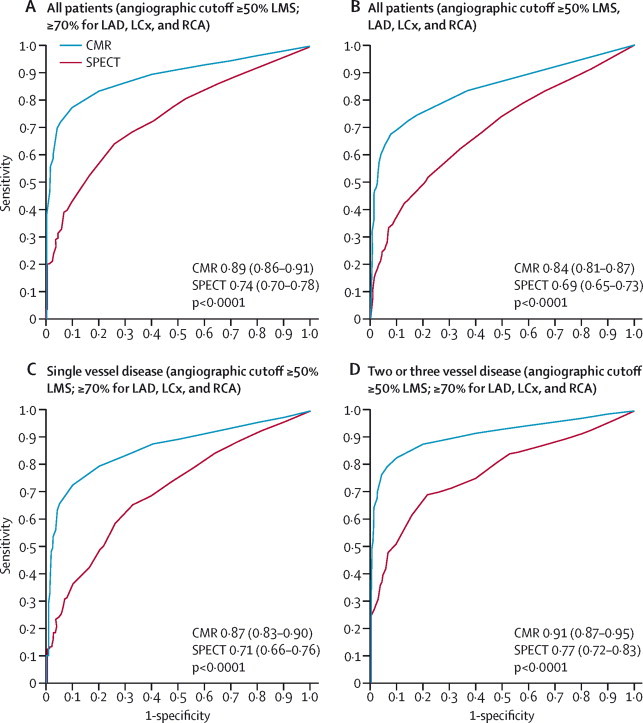
Receiver operating characteristic curves of summed stress scores by population and coronary heart disease definition
Generated using summed stress scores with the CMR stress perfusion component and from SPECT (n=647) for the whole cohort ([A] angiographic cutoff ≥50% LMS; ≥70% for LAD, LCx, and RCA; [B] angiographic cut-off ≥50% for LMS, LAD, LCx, and RCA), patients with single-vessel disease (C), and patients with multivessel (two or three vessel) disease (D). CMR=cardiovascular magnetic resonance. SPECT=single-photon emission computed tomography. AUC=area under the curve. LMS=left main stem. LAD=left anterior descending. LCx=left circumflex. RCA=right coronary artery.
Stress perfusion CMR also performed better than SPECT when single vessel and multivessel coronary artery disease groups were analysed separately (figure 4C, 4D, respectively). For single vessel disease the AUC for stress CMR was 0·87 (0·83–0·90) and for SPECT 0·71 (0·66–0·76; p<0·0001). For multivessel disease, the AUC for stress CMR was 0·91 (0·87–0·95) and for SPECT 0·77 (0·72–0·83; p<0·0001).
Discussion
This trial has shown that in a large population with suspected angina pectoris, CMR is an alternative to SPECT for the detection of clinically significant coronary heart disease, with better sensitivity and negative predictive values. Although CMR is already included in international guidelines for the non-invasive detection of coronary heart disease,28,29 it does not have the large scale diagnostic accuracy and clinical outcome data of SPECT. The results show that CMR offers an accurate assessment of single-vessel and multivessel coronary disease, irrespective of the cutoff used for severity of clinically significant angiographic stenosis (≥50% or 70%). This finding is important because all non-invasive tests have limitations, such that alternative investigative options are needed. For SPECT, these limitations include the exposure of patients to ionising radiation, limited spatial resolution, and attenuation artifacts, whereas for CMR they include claustrophobia, wide abdominal girth, and the presence of some medical implants.
The coronary heart disease prevalence in CE-MARC (39%) is typical of a hospital outpatient population. Only 11% of patients had previous revascularisation or acute coronary syndrome. This trial population differs substantially from that of MR-IMPACT,18 a contrast dose ranging study in which 241 patients were enrolled after x-ray angiography or when scheduled for angiography after a positive SPECT scan. Disease prevalence in the highly selected MR-IMPACT population was 77%; 31% having had coronary angioplasty and 39% myocardial infarction. Furthermore, in CE-MARC all patients were prospectively scheduled to undergo CMR, SPECT, and x-ray angiography at the time of recruitment, minimising selection bias. To avoid confounding between the order of the tests, the order was randomised, and to avoid confounding between observers and the tests, all tests were done and interpreted in accordance with the strict detailed protocols.19 CMR and SPECT stress perfusion used identical adenosine infusion protocols and unlike earlier trials,18 all SPECT studies adopted a unified protocol using 99mTc tetrofosmin (Myoview) and cardiac gating, as advised in guidelines.24,25 CMR and SPECT were reported using international criteria, by two masked independent assessors with at least 10 years experience in their respective modality,20–22 and in a manner inkeeping with usual clinical practice.
Importantly, CE-MARC used a multiparametric CMR protocol, unlike the studies in a previous meta-analysis30 and MR-IMPACT,18 which used only the perfusion CMR components. The additional clinical information provided by multiparametric CMR is important for individual patient management beyond diagnosis. Although SPECT provides information about ischaemia, infarction, and ventricular function, it provides no detail of coronary artery anatomy. However, omitting coronary artery imaging from the multiparametric CMR protocol did not impair overall diagnostic accuracy, and makes the investigation simpler and quicker. Exclusion of the coronary MR angiography component also made the positive predictive value of CMR become statistically superior to that of SPECT, because of a reduction in false-positive CMR results. Although there are no other large-scale CMR trials of similar design (MR IMPACT-2 has not been published), the findings from CE-MARC are inkeeping with those of the recent meta-analysis30 of 26 stress perfusion CMR studies (panel 3). The sensitivity of CMR in CE-MARC was similar to that in the CMR meta-analysis30 (86% vs 89%) and to that of a recent prospective study31 of 136 women (84%). However, direct comparisons should be made with caution (the meta-analysis included many small studies with heterogeneous populations, disease prevalence, and analysis methodologies).
Panel 3. Research in context.
Systematic review
We searched PubMed for original papers in English, with no date restriction, with the terms “cardiovascular magnetic resonance”, “stress perfusion imaging”, “coronary heart disease”, “ischaemic heart disease”, and “single photon emission computed tomography”. There are no large scale diagnostic accuracy studies of a multiparametric cardiovascular magnetic resonance (CMR) protocol for the detection of coronary heart disease. A meta-analysis30 has shown that stress perfusion CMR is highly sensitive but only moderately specific for the detection of coronary heart disease. A small subgroup analysis of the MR-IMPACT trial18 suggests that stress perfusion CMR and single-photon emission computed tomography (SPECT) have much the same diagnostic accuracy.
Interpretation
CE-MARC is the first large-scale trial of a multiparametric CMR protocol for the diagnosis of stable coronary heart disease. CMR had better sensitivity and negative predictive values than SPECT for coronary heart disease diagnosis, with much the same specificity. These findings support the wider adoption of CMR for coronary heart disease diagnosis and its inclusion in evidence-based clinical management guidelines.
The results of CMR compared with SPECT need to be considered in the context of published SPECT data, which are heterogeneous for population, radioisotope tracer, mode of stress, and protocol. Notably, before CE-MARC, SPECT had never been tested prospectively against coronary angiography in such large numbers and in a patient population of this kind. Previous studies of SPECT show a wide range in sensitivity (63–93%) and specificity (10–90%) compared with x-ray angiography.2–4 The sensitivity and specificity of SPECT in CE-MARC fell within the range of these other studies. A UK National Institute for Health and Clinical Excellence technology appraisal2 reported that “considerable uncertainty remains over the true values for sensitivity and specificity of SPECT. In particular, trials that assessed these values were subject to referral bias, in that only SPECT-positive cases were referred for coronary angiography, which was assumed to be the gold standard”. CE-MARC prospectively recruited patients before any imaging procedure and all patients had x-ray angiography, irrespective of clinical intention. Although this design might have increased the number of false negatives for both SPECT and CMR it might be more representative of the real sensitivity of both. The high specificities reported in CE-MARC for both modalities might result from the use of ECG gating during SPECT and an awareness of dark banding artifacts during CMR analysis—a common source of false-positives in CMR perfusion studies. The results of CE-MARC and MR-IMPACT18 are similar (AUC by ROC analysis 0·89 and 0·86 [n=42], respectively). These concordant results using different CMR stress perfusion acquisition protocols show the inherent accuracy of this technique. Although we have not specifically sought to address why CMR has superior accuracy to SPECT, it might relate partly to its higher spatial resolution (2·2–3·2 mm for CMR vs approximately 10 mm in-plane for SPECT).
Our study has some limitations. Most patients were white northern Europeans who had not had previous coronary artery bypass surgery; results might differ in other populations. Also, CE-MARC was a single-centre study in which CMR and SPECT are done in high volumes. Extrapolation to low volume centres should be made with caution. Importantly, a single site and unified pharmacological stress protocol ensured consistency in CMR and SPECT and improved direct comparison.
CE-MARC compared two functional tests (CMR and SPECT) with an anatomical test (angiography), itself an imperfect reference standard. Thus, false-negative results could occur if lesions not causing ischaemia (as assessed by CMR or SPECT) were judged clinically significant on the basis of angiographic stenosis severity. Quantitative coronary angiography, which is considered better than visual estimation, was used, but it is still a crude binary measure that fails to account for diffuse disease, length of diseased segment, serial stenoses, and microvascular disease. Invasive measurement of fractional flow reserve is now the reference standard for the measurement of haemodynamic significance of a coronary artery stenosis and should be used in future studies. Indeed, results from the FAME trial32 showed a wide discrepancy between angiographic stenosis classification and fractional flow reserve measurements.
CE-MARC is the largest, prospective, real world assessment of CMR. The findings of CE-MARC support the wider adoption of CMR for the diagnosis and management of stable coronary heart disease patients, in view of the growing concern of the cancer risk associated with medical-source ionising radiation.
Acknowledgments
Acknowledgments
This study was funded by the British Heart Foundation. We thank for their assistance Gavin Bainbridge and Margaret Saysell (cardiac radiographers); Fiona Richards and Judith Beevers (CE-MARC clinical research nurses); Joanne Copeland, Jayne Fountain, Maria Efthymiou, Gillian Worthy, and Andrew Carter (clinical trials research unit team members); Penelope Thorley (clinical scientist in nuclear medicine); and Peter Tooze (database design and maintenance). Finally, this study would not have been possible without the willing cooperation of the patients in West Yorkshire, UK and the enthusiastic support of the study investigators.
Contributors
JPG planned the study, led the clinical trial, analysed the data, interpreted the results, and wrote the first draft of the report. NM, JFY, and PB collected and analysed the data. JMB planned the study, provided advice on the statistics, analysed the data, and interpreted the results. JN planned the study and analysed the data. CCE did the statistical analyses and interpreted the results. SP planned the study, analysed the data, interpreted results, and co-led the study. JPR and AR planned the study and interpreted the results. CJD and SGB planned the study, analysed the data, and interpreted the results. All authors contributed to the Article's revision, agreed to its submission, and had full access to the original data.
Study investigators
W Baig, P D Batin, K E Berkin, D J Blackman, J M Blaxill, J C Cowan, K M English, A S Hall, M T Kearney, A F Mackintosh, J M McLenachan, C B Pepper, G W Reynolds, R J Sapsford, S Wheatcroft, G J Williams, K K Witte.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.WHO Cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/ (accessed March 14, 2011).
- 2.NICE Myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. National Institute for Health and Clinical Excellence. http://www.nice.org.uk/nicemedia/pdf/TA073guidance.pdf (accessed Nov 18).
- 3.Mowatt G, Vale L, Brazzelli M. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess. 2004;8:1–207. doi: 10.3310/hta8300. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Bairey Merz CN, Pepine CJ, for the WISE investigators Insights from the NHLBI-sponsored women's ischemia syndrome evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 suppl):S4–20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 5.Kim RJ, Wu E, Rafael A. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 6.Kim WY, Danias PG, Stuber M. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 7.Klem I, Heitner JF, Shah DJ. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 8.Kwong RY, Schussheim AE, Rekhraj S. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 9.Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2173–2181. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood JP, Younger JF, Ridgway J, Sivananthan MU, Ball SG, Plein S. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging versus exercise tolerance testing early after acute ST-elevation myocardial infarction. Heart. 2007;93:1363–1368. doi: 10.1136/hrt.2006.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Saadi N, Nagel E, Gross M. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 12.Panting JR, Gatehouse PD, Yang GZ. Echo-planar magnetic resonance myocardial perfusion imaging: parametric map analysis and comparison with thallium SPECT. J Magn Reson Imaging. 2001;13:192–200. doi: 10.1002/1522-2586(200102)13:2<192::aid-jmri1029>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Schwitter J, Nanz D, Kneifel S. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 14.Paetsch I, Jahnke C, Wahl A. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835–842. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 15.Ishida N, Sakuma H, Motoyasu M. Noninfarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229:209–216. doi: 10.1148/radiol.2291021118. [DOI] [PubMed] [Google Scholar]
- 16.Wolff SD, Schwitter J, Coulden R. Myocardial first-pass perfusion magnetic resonance imaging: a multicenter dose-ranging study. Circulation. 2004;110:732–737. doi: 10.1161/01.CIR.0000138106.84335.62. [DOI] [PubMed] [Google Scholar]
- 17.Giang TH, Nanz D, Coulden R. Detection of coronary artery disease by magnetic resonance myocardial perfusion imaging with various contrast medium doses: first European multi-centre experience. Eur Heart J. 2004;25:1657–1665. doi: 10.1016/j.ehj.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Schwitter J, Wacker CM, van Rossum AC. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood JP, Maredia N, Radjenovic A. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials. 2009;10:62. doi: 10.1186/1745-6215-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilkemeier PL, Cooke CD, Grossman GB, McCallister BD, Jr, Ward RP. Standardized reporting of radionuclide myocardial perfusion and function. J Nucl Cardiol. 2009;16:650. doi: 10.1016/j.nuclcard.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Hendel RC, Wackers FJ, Berman DS. American Society of Nuclear Cardiology consensus statement: reporting of radionuclide myocardial perfusion imaging studies. J Nucl Cardiol. 2006;13:e152–e156. doi: 10.1016/j.nuclcard.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Hundley WG, Bluemke D, Bogaert JG. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson. 2009;11:5. doi: 10.1186/1532-429X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerqueira MD, Weissman NJ, Dilsizian V, for the American Heart Association writing group on myocardial segmentation and registration for cardiac imaging Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 24.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS, for the Quality Assurance Committee of the American Society of Nuclear Cardiology Stress protocols and tracers. J Nucl Cardiol. 2006;13:e80–e90. doi: 10.1016/j.nuclcard.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Holly TA, Abbott BG, Al-Mallah M. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–973. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 26.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 27.D'Agostino RB, Sr, Vasan RS, Pencina MJ. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 28.Hundley WG, Bluemke DA, Finn JP. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology oundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendel RC, Patel MR, Kramer CM. CCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Hamon M, Fau G, Née G, Ehtisham J, Morello R, Hamon M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. doi: 10.1186/1532-429X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klem I, Greulich S, Heitner JF. Value of cardiovascular magnetic resonance stress perfusion testing for the detection of coronary artery disease in women. JACC Cardiovasc Imaging. 2008;1:436–445. doi: 10.1016/j.jcmg.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Tonino PA, Fearon WF, De Bruyne B. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]


