Abstract
Inflammatory bowel disease (IBD) is a disease that affects the intestinal tract via an inflammatory process. Patients who suffer from IBD often have diseases that affect multiple other organ systems as well. These are called extraintestinal manifestations and can be just as, if not more debilitating than the intestinal inflammation itself. The skin is one of the most commonly affected organ systems in patients who suffer from IBD. The scientific literature suggests that a disturbance of the equilibrium between host defense and tolerance, and the subsequent over-activity of certain immune pathways are responsible for the cutaneous disorders seen so frequently in IBD patients. The purpose of this review article is to give an overview of the types of skin diseases that are typically seen with IBD and their respective pathogenesis, proposed mechanisms, and treatments. These cutaneous disorders can manifest as metastatic lesions, reactive processes to the intestinal inflammation, complications of IBD itself, or side effects from IBD treatments; these can be associated with IBD via genetic linkage, common autoimmune processes, or other mechanisms that will be discussed in this article. Ultimately, it is important for healthcare providers to understand that skin manifestations should always be checked and evaluated for in patients with IBD. Furthermore, skin disorders can predate gastrointestinal symptoms and thus may serve as important clinical indicators leading physicians to earlier diagnosis of IBD.
Keywords: inflammatory bowel disease, skin disorders, Crohn’s disease, ulcerative colitis
Introduction
Overview
Inflammatory bowel disease (IBD) is an idiopathic and inflammatory disease of the intestinal tract. The two major types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD). As the name implies, UC is limited to the colon and/or rectum (normally continuous lesions in the rectum and colon), and affects only the inner lining (mucosal and submucosal layers) of the gut. In contrast, CD can affect any part of the gut from mouth to anus as non-continuous or skip lesions (a majority of cases start in the terminal ileum), and affect the whole thickness (transmural) of the bowel wall.
Up to 40% of IBD patients may be complicated by extraintestinal manifestations (EIMs) and in some large series of studies, the prevalence of EIMs is higher in CD compared to UC (Vind et al., 2006; Vavricka et al., 2011). In a large cohort study of 950 patients, EIMs were identified in 43% of 580 patients with CD and 31% of 370 patients with UC (Vavricka et al., 2011). Multiple organ systems and almost every organ system may be affected in IBD, including the musculoskeletal system, skin, eyes, hepatobiliary system, lungs, kidneys, immunologic or hematologic system, and cardiovascular system (Williams et al., 2008; Vavricka et al., 2011). The most common EIMs of IBD are peripheral arthritis, aphthous stomatitis, uveitis, and erythema nodosum (EN; Vavricka et al., 2011). Among the organ systems involved, the skin is one of the most commonly affected organ systems. The most common skin or mucocutaneous lesions associated with IBD are EN, pyoderma gangrenosum (PG), and aphthous stomatitis (oral ulceration; Rothfuss et al., 2006; Larsen et al., 2010). Aphthous stomatitis or aphthae are the most common complication of the oral mucosa, while fissure and fistulae are most common complication of the perianal mucosa. EN and PG are immunoreactive in nature as a result of the underlying IBD disease. In addition, skin lesions in IBD patients can also be caused by nutritional deficiencies, such as pellagra and cheilitis. Medication side effects, such as cushingoid features from steroid usage or drug eruptions from immunosuppressant medications have also been noted. Other skin disorders such psoriasis and vitiligo have also been associated with IBD (Timani and Mutasim, 2008).
Mechanisms between IBD and skin disorders
Mucosal T-cells are important in maintaining intestinal homeostasis, defined as the balance between the mucosal epithelium, intestinal microbes, and host immune response (van Wijk and Cheroutre, 2010). Abnormal T-cell response to these microbial antigens can disrupt this equilibrium and is believed to be the mechanism that triggers the chronic inflammation and excessive secretion of cytokines that lead to the development of IBD (Izcue et al., 2009; van Wijk and Cheroutre, 2010; Monteleone et al., 2011). It has been proposed that some EIM’s seen with IBD manifest also as a result of immune dysregulation resulting in a lymphocyte mediated destructive process. Adams and Eksteen (2006) proposed IBD patients with hepatic EIMs could be explained by mucosal T-cells in the gut aberrantly traveling to the liver, becoming exposed to hepatic antigens, and ultimately causing liver damage. This article suggested that a similar mechanism could possibly explain the pathogenesis of other EIM’s (including the skin) in patients with IBD (Adams and Eksteen, 2006).
Studies have implicated several immune pathways in cutaneous disorders of IBD. In addition to the two established effector CD4 T-cells, the Th1 (cell-mediated) and Th2 (humoral), a third novel T-helper cell has been described. Termed Th17 cells for its ability to produce interleukin-17 (IL-17), these cells have been described in various systemic, inflammatory, and autoimmune skin disorders including psoriasis, contact or atopic dermatitis, scleroderma, systemic lupus erythematosus (SLE), Bechet’s disease, and IBD (Asarch et al., 2008). This discovery brings to light new treatment targets for dermatologic disorders in the IBD population.
Classically, CD has been considered to be mediated by Th1 pathways with elevated levels of IFN-Γ and IL-12. On the other hand, UC has been associated with Th2 mediated pathways with elevated levels of IL-5 and IL-13 (van Wijk and Cheroutre, 2010). However, recent evidence from mouse models challenges these viewpoints and suggests that IL-23, induced by Th17 produced cytokines (IL-17 and IL-22, which are present in both CD and UC), is one of the major players in the pathogenesis of IBD (Hue et al., 2006). This IL-23 and Th17 pathway has been proposed as a common pathway of the development of IBD (both CD and UC) and other autoimmune diseases as well (Ahern et al., 2008; van Wijk and Cheroutre, 2010; Abraham and Medzhitov, 2011; Lees et al., 2011). Most recently, high levels of expression of IL-23 was reported in a difficult to treat PG lesion. Treatment with a monoclonal antibody ustekinumab for 14 weeks resulted in marked suppression of IL-23 expression and clinical resolution of the PG lesion (Guenova et al., 2011).
Classification
The cutaneous manifestations of IBD have been classified into the following categories according to pathogenesis (Table 1; Danese et al., 2005; Trost and McDonnell, 2005; Passarini et al., 2007; Mnif et al., 2010; Levine and Burakoff, 2011):
Table 1.
Summary table of cutaneous manifestations of IBD.
| Cutaneous manifestation | Presentation | Incidence |
Pathogenesis or proposed mechanism | |||
|---|---|---|---|---|---|---|
| CD | UC | |||||
| SPECIFIC CUTANEOUS MANIFESTATIONS WITH THE SAME HISTOLOGICAL FEATURES AS THE UNDERLYING IBD | ||||||
| Orofacial: aphthous stomatitis, ulcers | Symptoms: round to oval shaped, can take 2–4 weeks to heal and may frequently scar depending on size | 10% | 4% | CD: non-caseating granuloma with similar mechanism to underlying bowel pathology | ||
| Location: buccal mucosa and lips. | UC: unclear | |||||
| Perianal: fissures, fistulas | Fissures: painless, located posteriorly | 50% (≥1 episode) | Rare | Direct local involvement of skin and mucosa by underlying bowel disease | Fistulas: internal or entero-cutaneous, can destroy the anal sphincter | |
| Metastatic | Symptoms: subcutaneous nodules or non-healing ulcers | Rare | Very rare | Specific granulomatous cutaneous lesions with the same histopathology (non-caseating granulomas with multinucleated giant cells in the dermis) as the intestinal lesions | Location: genital (more common in children) vs. non-genital (more commonly affects lower extremities, abdomen, and trunk) | |
| Affects: adult/female predilection | ||||||
| REACTIVE CUTANEOUS MANIFESTATIONS WITH IMMUNOLOGICAL MECHANISMS RELATED TO THE UNDERLYING IBD | ||||||
| Erythema nodosum | Symptoms: painful, tender, warm nodules with a bruise-like appearance | 4–15% | 3–10% | Panniculitis, perivascular deposits of immunoglobulins, and complement suggesting mechanism related to abnormal immunological response of common antigens between bowel bacteria and skin | Location: typically lower extremities | |
| Affects: female predilection | ||||||
| Pyoderma gangrenosum | Symptoms: non-infectious nodules that develop into deep and painful ulcers. Often debilitating | 1–2% | 5–12% | A type of neutrophilic dermatosis | ||
| Location: lower extremities, peristomal lesions | Altered immune response (cross-reacting autoantibodies to common antigens in the gut and skin), over-expression of pro-inflammatory cytokines (IL-8, IL-16, TNF-α), and neutrophil chemotaxis | |||||
| Affects: female predilection | Pathergy: exaggerated response of skin to local trauma | |||||
| Sweet’s syndrome (SS) | Symptoms: fever, peripheral neutrophilia, tender nodules/papules | Rare | UC > CD | A type of neutrophilic dermatosis | ||
| Location: upper limbs, face, neck. Can be difficult to distinguish from EN if affecting lower extremities | Altered immune response (cross-reacting autoantibodies) | |||||
| Affects: adult/female predilection | ANCA, G-CSF, or other cytokines in activation, maturation, and chemotaxis of neutrophils | |||||
| Bowel-associated dermatosis–arthritis syndrome (BADAS) | Symptoms: erythematous macules developing into painful papules and pustules over 1–2 days. Usually resolve in 1–2 weeks, but can recur every 1–6 weeks. Temporally dependent on IBD activity. Associated with fever, malaise, abdominal pain, arthralgias | Rare | UC > CD | A type of neutrophilic dermatosis | ||
| Location: upper chest, arms, legs | Overgrowth of gut bacteria resulting in production of antibodies to gut bacteria antigen (peptidoglycan) which cross-reacts with skin and joints | |||||
| PDV, PSV, PPV | PDV: pustules in skin folds (axillary, inguinal regions) | Rare | UC > CD | Unclear, but possible related to cross-reacting antigens between the bowel and skin | ||
| PSV: hyperplastic folds of buccal and labial mucosa that progress to shallow ulcers | ||||||
| PPV: variants of PDV and PSV. specific marker of IBD | ||||||
| Affects: adult/Caucasian/male predilection | ||||||
| Leukocytoclastic vasculitis | Symptoms: ranges from palpable purpura to necrotic ulcers. In CD, can occur during remission or exacerbation. In UC, precedes onset of bowel disease. | Rare | UC > CD | Hypersensitivity vasculitis of small vessels due to deposition of immune complexes | ||
| CUTANEOUS DISORDERS OR DERMATOSIS ASSOCIATED WITH IBD | ||||||
| Psoriasis | Symptoms: pruritic and irritated scaly patches. Can be associated with nail changes, arthritis | 11.2% | 5.7% | Genetic (HLA linkage) or Immunologic related mechanisms | ||
| Location: elbows, knees, trunk, but can occur anywhere | ||||||
| Secondary amyloidosis | Symptoms: pruritic rash due to deposits of amyloid in the skin | 0.9% | 0.07% | Type AA Amyloidosis, reactive systemic amyloidosis associated with chronic inflammation | ||
| Vitiligo | Symptoms: irregular white patches of skin | Rare | UC > CD | Related to autoimmune or HLA linkage mechanisms | ||
| Location: face, elbows, knees, hands, feet, genitals | ||||||
| Acquired epidermolysis bullosa | Symptoms: sub-epidermal blisters of the skin and mucous membranes | Rare | UC > CD | Unclear, but related to autoantibodies to type VII collagen in the skin and gut | ||
| SECONDARY CUTANEOUS MANIFESTATIONS EITHER DUE TO COMPLICATIONS OF IBD OR ADVERSE EFFECTS OF IBD TREATMENTS | ||||||
| Zinc deficiency (acrodermatitis enteropathica) | Symptoms: erythematous patches and plaques that progress to crusted vesicles, bullae, or pustules | 40% | No difference compared to control | Reduced mucosa availability for absorption and chronic diarrhea | Location: mouth, anus, limbs, fingers, scalp | |
| Iron deficiency anemia | Symptoms: koilonychias, angular cheilitis, pale skin | 39% | 81% | Malabsorption of iron and chronic intestinal bleeding | ||
| Essential fatty acid deficiency | Symptoms: Xeroderma, dry skin, unspecific eczema | CD > UC | UC < CD | Malabsorption | ||
| Corticosteroid usage | Symptoms: corticosteroid induced acne | Common | Common | Increased sebaceous gland activity | Affects: younger patients | |
| Anti-TNF-αagents | Symptoms: anti-TNF α agent induced psoriasis | Total of 15 cases described | Total of three cases described | Over-expression of IFN-α causing predisposition to psoriasis | ||
Specific cutaneous manifestations or granulomatous cutaneous lesions with the same histological features as the underlying bowel disease.
Reactive cutaneous manifestation of IBD with immunological mechanisms triggered by common antigens shared by gut bacteria and skin.
Cutaneous disorders or dermatosis associated with IBD.
Secondary cutaneous manifestations either due to complications of IBD or adverse effects of IBD treatments.
Cutaneous Manifestations with Same Histological Features as the Underlying IBD
Cutaneous manifestations
Cutaneous or metastatic CD is a rare complication defined as the occurrence of specific granulomatous cutaneous lesions with the same histopathology (non-caseating granulomas with multinucleated giant cells in the dermis surrounded by lymphocytes, plasma cells, and eosinophils) as the intestinal lesions (Georgiou et al., 2006). It occurs more frequently in adult females with established intestinal CD (Georgiou et al., 2006). This disorder presents commonly as subcutaneous nodules or non-healing ulcers in the lower extremities with rare localization to the genital (vulvar or testicular) area; pseudocondyloma mimicking genital warts can also occur (Georgiou et al., 2006; Larsen et al., 2010). Cutaneous CD can be divided into two clinical forms: the genital form (56%) which occurs more in children (characterized by edema, erythema, fissures or ulcers of the labia, scrotum, or penis), and the non-genital form (44%) which most commonly affects the lower extremities (38%), abdomen and trunk (24%), upper extremities (15%), face and lips (11%), and intertriginous areas (8%; Ploysangam et al., 1997). It seems unrelated to the activity of the bowel disease or its response to the IBD treatment (Chalvardjian and Nethercott, 1982; Lebwohl et al., 1984; Lestre et al., 2010).
Various treatment modalities have been tried, such as steroids (topical, intralesional, or systemic), sulfasalazine, metronidazole, azathioprine, methotrexate, hyperbaric oxygen, and anti-TNF-α antibodies (Sarna et al., 2008; Lestre et al., 2010). In cases with severe cutaneous ulcers unresponsive to medical treatment, surgical resection under oral administration of zinc sulfate may be indicated (Georgiou et al., 2006).
Perianal manifestations
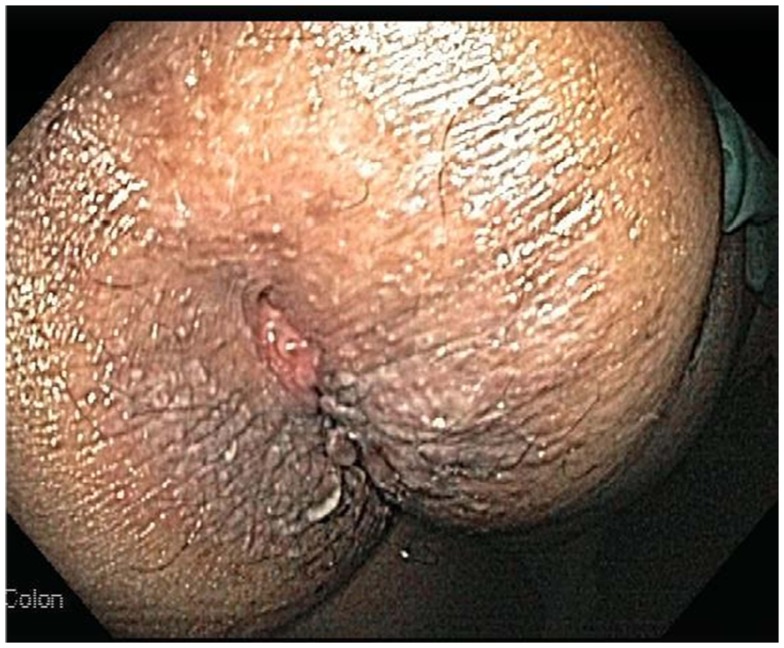
Perianal CD including erythema, abscesses, ulcers, fissures, and fistulas, occur in about 50% of CD patients at some point during their clinical course (Danese et al., 2005). Perianal fissures and fistulas, usually one of the most common skin lesions of IBD, occur mainly in CD (20–60%) and rarely in UC (Roberts and Bunker, 1993; Lebwohl and Lebwohl, 1998). Perianal fissures and fistula are due to direct involvement of skin and mucosa via a similar mechanism as the bowel disease and may antedate the signs and symptoms of bowel disease by several years (Figure 1; Ploysangam et al., 1997; Lebwohl and Lebwohl, 1998). Fissures are usually painless and located posteriorly, whereas fistulas manifest either as a cryptoglandular infection or as a secondary complication of anal fissures. Fistulas can be internal or entero-cutaneous and can undermine ulcers and destroy the anal sphincter (Danese et al., 2005).
Figure 1.
Perianal fissure in a patient with IBD. Note the longitudinal tear pattern in the anoderm.
The recommended treatment of perianal fissures is topical application of glyceryl trinitrate (nitroglycerine) ointment and in some cases this is followed by Botox injections into the affected anal sphincter area (Jonas and Scholefield, 2001). In cases with only limited response to these compounds, calcium channel antagonists (diltiazem) or lateral internal sphincterotomy may be indicated (Fleshner et al., 1995; Singh et al., 2009). For perianal fistulas, combined medical and surgical therapies offer the best chance for success (Lewis and Maron, 2010). Medical treatments include antibiotics (metronidazole, ciprofloxacin), immunosuppressants (azathioprine, 6-MP, cyclosporine, and tacrolimus), and infliximab (Safar and Sands, 2007; Lewis and Maron, 2010). Surgery include fistulotomy for low fistulae (below the dentate line), advance flap or fistula plug for high fistulae (above dentate line), proctectomy with colostomy for severe perianal disease with rectal involvement (Georgiou et al., 2006; Safar and Sands, 2007; Lewis and Maron, 2010).
Orofacial manifestations
Orofacial CD may antedate the typical bowel symptoms by several months to years. Its presentation and severity do not always correlate with the activity of the underlying bowel disease. Orofacial involvement occurs in 5–20% of CD patients and can present as: aphthous stomatitis, pyostomatitis vegetans, angular cheilitis and ulceration, mucosal nodularity (cobblestoning of the buccal mucosa), nodules of gingival and alveolar mucosa, and indurated fissuring of lower lips (Trost and McDonnell, 2005; Georgiou et al., 2006). Biopsy of the lesions in CD patients, although rarely required, can reveal non-caseating granulomas similar to bowel lesions.
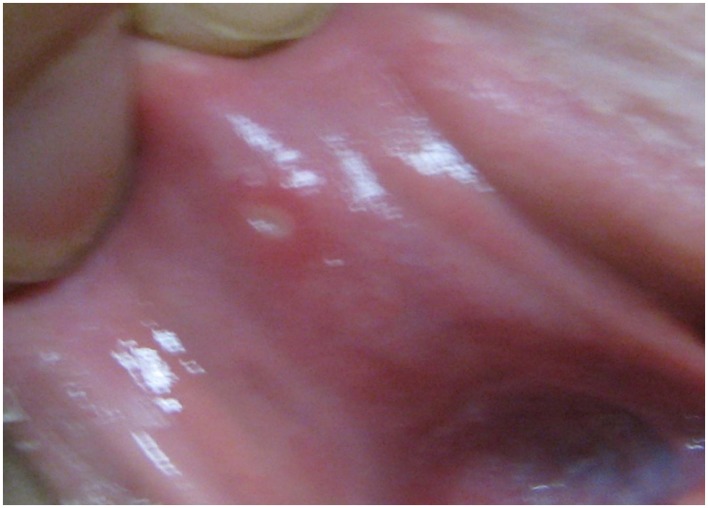
Aphthous stomatitis or ulcers, typically seen in the buccal mucosa and lips, consist of oral ulcers and are more common in CD (10%) than UC (4%; Vavricka et al., 2011). Minor aphthous ulcers are small, shallow, round to oval shaped, have a grayish base, and can be painful, but heal within 2 weeks without scarring (Figure 2). On the other hand, major recurrent ulcers are larger, can last for 6 weeks, and frequently scar (Ship, 1996; Timani and Mutasim, 2008). A study showed that a majority of patients with multiple aphthous ulcers had underlying IBD (Letsinger et al., 2005). The pathogenesis of aphthous stomatitis and UC is still unclear. Studies have not been successful in proving that these ulcers are secondary to vitamin deficiencies as the lesions do not typically improve with vitamin therapy (Basu and Asquith, 1980). Aphthous stomatitis occurs more often in active as opposed to inactive IBD disease: 17.1 vs. 8.6% in CD, 4.1 vs. 3% in UC, but the latter did not reach clinical significance (Vavricka et al., 2011). Other studies suggest that recurrent aphthous stomatitis and symptom onset often parallel UC disease activity (Timani and Mutasim, 2008). Treatment of aphthous stomatitis is symptomatic and consists of topical anesthetics such as viscous xylocaine, steroid elixirs, or topical steroids. Systemic steroids are used for severe or refractory cases (Basu and Asquith, 1980; Trost and McDonnell, 2005; Timani and Mutasim, 2008; Levine and Burakoff, 2011). For secondary bacterial superinfections, topical antibiotics can be used although they are rarely required (Levine and Burakoff, 2011).
Figure 2.
Aphthous stomatitis of the oral mucosa. Note the erythematous ring around the yellow to gray colored ulceration in this minor aphthous stomatitis.
For orofacial CD, the treatment should be directed at the local lesion and underlying systemic disease. For more severe or refractory orofacial CD, treatment should include systemic steroids, immunomodulators, and anti-TNF-α therapy (Quezada et al., 2009).
Reactive Cutaneous Manifestations of IBD
Erythema nodosum
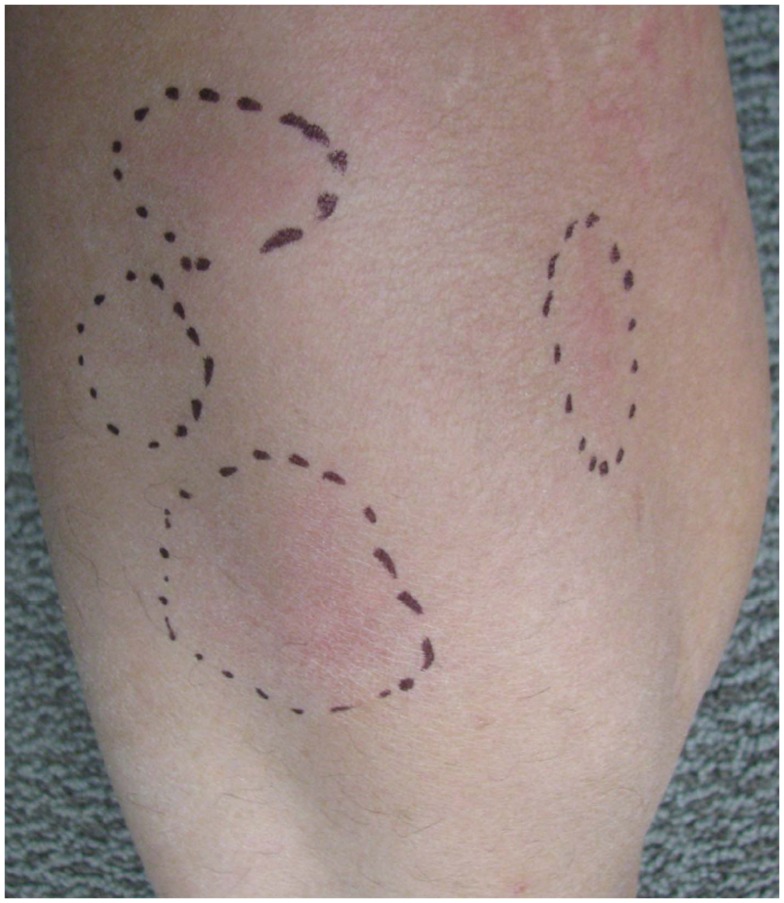
Erythema nodosum, the most common cutaneous manifestation of IBD, affects about 3–10% of UC and 4–15% of CD patients (Lebwohl and Lebwohl, 1998; Georgiou et al., 2006; Timani and Mutasim, 2008; Vavricka et al., 2011). Among patients with IBD, EN has been found to affect 1.9% of females compared to 0.7% of males (Turkcapar et al., 2006). A prospective study of 50 patients with EN found that only 4% were eventually diagnosed with IBD showing that EN rarely precedes the initial diagnosis of IBD (Mert et al., 2004; Trost and McDonnell, 2005; Weinstein et al., 2005). EN is often associated with systemic symptoms of fevers, chills, arthralgias, or arthritis. Arthralgias and arthritis are rare in children, but in adults they can antedate the occurrence of EN lesions by weeks to months (Lebwohl and Lebwohl, 1998; Berkowitz and Lebwohl, 2000). Typical EN lesions present as painful, tender, warm nodules (1–5 cm in diameter), and raised bluish-red subcutaneous lesions or plaques located on the extensor surfaces of extremities, predominantly on the anterior surface of the lower extremities (Figure 3; Mir-Madjlessi et al., 1985; Evans and Pardi, 2007; Timani and Mutasim, 2008; Vavricka et al., 2011). These skin nodules are non-ulcerating, palpable, and can resemble a bruise on the skin, but are not always easily visible (Timani and Mutasim, 2008; Levine and Burakoff, 2011). EN can occur in multiple locations simultaneously and recurrence rate is approximately 20% (Freeman, 2005).
Figure 3.
Erythema nodosum manifested as erythematous nodules of the skin. These lesions are often painful and assume a bruise-like appearance.
Biopsy is generally not necessary as the diagnosis of EN may be confirmed based on the clinical presentation (Agrawal et al., 2007). The histology of EN shows lympho-histocytic infiltrate of lower dermis and focal panniculitis (Danese et al., 2005; Larsen et al., 2010). Panniculitis is inflammation of the subcutaneous fat and thus EN can occur wherever subcutaneous fat is present. The most common site is the pretibial or shin area, but the knees, ankles, arms, or trunk can also be affected (Levine and Burakoff, 2011). Direct immunofluorescence of EN lesions reveal perivascular deposits of immunoglobulins and complement, suggesting that the pathogenesis of reactive cutaneous manifestation of IBD is due to the abnormal immunological response of common antigens between bowel bacteria and skin (Veloso, 1990; Fava and Danese, 2011; Khor et al., 2011). EN lesions have been noted to be correlated with the underlying disease activity and worsened with colitis flares (Timani and Mutasim, 2008). Typically, the time between UC diagnosis and appearance of EN is about 5 years (Mir-Madjlessi et al., 1985). EN in CD patients is mostly associated with colonic involvement (Georgiou et al., 2006).
Since EN lesions mirror bowel disease activity and flare up, treatments targeting underlying IBD disease and flare up always leads to the remission of the cutaneous lesion (Orchard, 2003). However, in situations where lesions occur during the quiescent phase, low doses of oral steroids can lead to rapid remission of the cutaneous lesions in most cases (Georgiou et al., 2006). Other effective alternatives include NSAIDs, potassium iodide, colchicine, dapsone, and immunosuppressants, such as cyclosporine, thalidomide, etc. In recalcitrant cases, infliximab has been used with successful results (Kaufman et al., 2005). In general, EN patients have a shorter time to remission (approximately 5 weeks) than do PG patients (Tromm et al., 2001; Timani and Mutasim, 2008).
Pyoderma gangrenosum
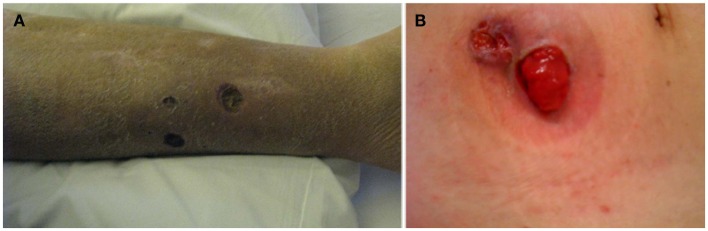
After EN (3–8%), PG represents the second most common cutaneous manifestation of IBD (1–3%), but PG is also the most severe and debilitating (Danese et al., 2005; Georgiou et al., 2006). PG is more common in UC (5–12%) than CD (1–2%) and like EN, some studies have shown a female predilection (Bernstein et al., 2001; Trost and McDonnell, 2005). PG was first described in 1930 by Brunsting as necrotic ulcers containing purulent material (usually sterile on culture) with expanding borders of erythema (Newell and Malkinson, 1982). The non-infectious pustules and nodules eventually expand outward and develop into painful deep ulcers with undermined wound edges (Callen, 1998; Farhi and Wallach, 2008). The ulcer has sharply circumscribed and demarcated borders with a necrotic yellowish base. The ulcers can be single or multiple, unilateral or bilateral, and can range in size from several centimeters to the surface of an entire limb. Other than the classical ulcerative form, PG may also occur in three other forms: the pustular form (multiple painful pustules with halo), bullous form (bullae which rapidly transform into painful erosions and necrotic ulcers), and vegetans form (well-demarcated shallow ulcer, which then gradually transform into an exophytic lesion; Georgiou et al., 2006). PG usually occurs on the extensor surface of the legs, but can appear anywhere on the skin, most noticeably on the abdominal wall adjacent to a postsurgical stoma (Lebwohl and Lebwohl, 1998). Peristomal PG lesions occur from 2 weeks to 3 years after ostomy creation and they typically rapidly evolve from small, erythematous pustules to deep ulcers within hours to days (Cairns et al., 1994). Pathergy, the proposed mechanism behind this occurrence, is characterized by an exaggerated physiological response of skin lesions that develops secondary to local trauma and has been reported in approximately 30% of cases of PG (Callen, 1998; Blitz and Rudikoff, 2001; Ardizzone et al., 2008). The type of trauma can range from postsurgical wounds to minor injuries as non-traumatic as needle sticks from venipuncture (Levine and Burakoff, 2011). This is the reason that PG is worsened with debridement and occurs more often on the leg, around stomas, or around skin biopsy sites (Figure 4). In CD patients, PG is often associated with colonic involvement and is usually diagnosed approximately 10 years after the initial diagnosis of UC (Mir-Madjlessi et al., 1985). Compared to EN, PG often takes a more prolonged course and approximately 35% of patients will experience relapse of PG (Rothfuss et al., 2006). Diagnosis of PG is usually clinical, but skin biopsy may be necessary for confirmation. PG is classified as a type of neutrophilic dermatosis in which the inflammatory infiltrate seen on microscopic examination shows dense dermal neutrophilic infiltrates without any evidence of infection, which was described by some as sterile abscesses (Nischal and Khopkar, 2007; Timani and Mutasim, 2008; Cohen, 2009; Larsen et al., 2010).
Figure 4.
Pyoderma gangrenosum can start as small pustules that as they progress form larger and deeper ulcers. Classically, the borders of the lesions are mucopurulent and purple. Example of pyoderma gangrenosum lesion involving the leg (A) and parastoma (B).
The pathogenesis of PG in IBD is proposed to be an abnormal immune response with cross-reacting autoantibodies directed at common antigens in the bowel and skin (Crowson et al., 2003; Feliciani et al., 2009). It is classified as one of the neutrophilic dermatosis. Other factors such as neutrophil dysfunction, abnormal T-cell response, and over-expression of pro-inflammatory cytokines such as IL-8, IL-16, IL-17, and TNF-α have also been proposed as mechanisms in the pathogenesis of PG (Feliciani et al., 2009; Marzano et al., 2010). A case report has implicated the IL-23 and TH17 axis as being responsible for a PG lesion that subsequently resolved with a monoclonal antibody targeted at inhibiting IL-23 expression (Guenova et al., 2011).
While EN usually correlates with IBD activity, PG correlation with IBD activity is controversial as there are much fewer PG cases. In a study of 14 patients with UC, there was no temporal relationship between the onset of bowel flares and the course of PG lesions (Thornton et al., 1980). In a study of 34 IBD patients, PG was diagnosed in 50% of UC and 75% of CD when underlying bowel disease was active (Levitt et al., 1991). In a study of 986 patients hospitalized for IBD, six patients (0.6%) had PG (Menachem and Gotsman, 2004). Of these six patients, PG appeared 6.5 years on average after the diagnosis of IBD. The majority of patients had active colitis at the onset of PG and half of these patients experienced other associated EIMs, including sacroiliitis, peripheral arthritis, and EN. However, PG does not always respond to treatment of underlying bowel disease and response to bowel resection is unpredictable (Levitt et al., 1991; Menachem and Gotsman, 2004). Further studies need to be done to investigate the relationship between the onset of PG and the underlying IBD activity.
When compared to EN, PG lesions tend to be more severe and resistant to therapy thus necessitating more aggressive therapy. Furthermore, PG patients appear to benefit less than EN patients from colectomy (Goudet et al., 2001). In fact, about 30% of patients with PG do not experience improvement of their lesions with treatment of the underlying IBD (Mir-Madjlessi et al., 1985). In general, treatment of PG includes a combination of wound care, topical medications, antibiotics for secondary infections, and treatment targeted at the underlying colitis (Danese et al., 2005). Although no individual therapy is universally effective, treatment of PG usually involves a regimen of systemic, cutaneous, and/or intralesional medications (Timani and Mutasim, 2008; Cohen, 2009).
Local wound care includes sterile saline lavages, topical antibacterial creams, and hydrocolloid dressings. Topical treatments, such as highly potent steroids with occlusive dressings, nicotine, benzoyl peroxide, sodium cromoglycate, hydrogen peroxide, 5-aminosalicylic acid, and tacrolimus have shown varying success rates (Trost and McDonnell, 2005; Georgiou et al., 2006; Callen and Jackson, 2007). Although intralesional steroid injections can be effective in small or localized disease, the mainstay of treatment remains systemic steroids especially for multiple lesions or generalized form of PG (Georgiou et al., 2006; Timani and Mutasim, 2008). Systemic steroid therapy typically compromises of high dose of oral prednisone around 40–120 mg/day for the average adult initially until lesional clearance, followed by a low maintenance dose that is used to maintain remission (Crowson et al., 2003). However, many alternatives exist. Assuming no concurrent infection, mainstay therapy includes systemic corticosteroids at dosages from 0.5 to 2 mg/kg/day and cyclosporine at initial dosages of 2–5 mg/kg/day (Wollina, 2002; Timani and Mutasim, 2008). Keeping the goal trough serum levels of cyclosporine between 150 and 350 ng/ml has been shown to have good outcomes on PG healing (Curley et al., 1985; Matis et al., 1992; Cohen, 2009; Turner et al., 2010). Other agents including minocycline, sulfasalazine, azathioprine, dapsone, potassium iodide, intravenous immunoglobulin, intravenous steroids, mycophenolate mofetil, cyclophosphamide, methotrexate, plasmapheresis, and hyperbaric oxygen treatment have been employed with various success rates (Wasserteil et al., 1992; Georgiou et al., 2006; Tutrone et al., 2007; Timani and Mutasim, 2008; Cohen, 2009). Anti-TNF-α agents including etanercept, infliximab, and adalimumab have also been reported to treat PG lesions effectively and are used in refractory cases of PG (McGowan et al., 2004; Brooklyn et al., 2006; Alkhouri et al., 2009).
When treating peristomal PG lesions, about two-thirds (12/17) of patients respond to systemic high dose steroids and local wound care, while the rest require additional therapy or long-term medical management. A study showed that no patients were successfully treated with stomal revision (Cairns et al., 1994). To avoid pathergy, unnecessary surgical interventions should be avoided. However, surgery can be considered if medical therapies are not successful. Proper timing of the surgery to avoid active phase of the disease and appropriate administration of immunosuppressants are essential for optimal long-term wound stabilization or remission (Wollina, 2002; Trost and McDonnell, 2005; Wittekindt et al., 2007). For patients with extensive colitis and severe refractory PG lesions unresponsive to medical therapy, proctocolectomy may be considered, but PG has been reported to relapse years after proctocolectomy (Read, 1985; Levitt et al., 1991).
Pyodermatitis vegetans, pyostomatitis vegetans, pyodermatitis–pyostomatitis vegetans
Pyodermatitis vegetans (PDV), a rare skin manifestation of IBD and often regarded as one of clinical forms of PG, has histopathology similar to pyostomatitis vegetans (discussed below) but with treatment similar to PG (Georgiou et al., 2006). PDV occurs mainly in skin folds such as axillary or inguinal area, but can also be present on the trunk or extremities. These lesions are characterized by pustules that quickly rupture, forming erosions with hemorrhagic ground, developing large raised (vegetating) well-demarcated plaques with surrounding pustules (Georgiou et al., 2006; Canpolat et al., 2011).
Pyostomatitis vegetans (PSV), a rare disorder of the oral mucosa that is considered as the oral equivalent of PDV on cutis, is relatively specific for IBD, particularly UC (Storwick et al., 1994; Timani and Mutasim, 2008; Femiano et al., 2009). In contrast to EN and PG which have been shown to have a female predilection, there is a male predominance in PSV with a ratio ranging from 2:1 to 3:1 and the majority of cases occur in Caucasians (Femiano et al., 2009; Ficarra et al., 2010). The pathogenesis of PSV is not known yet, although abnormal immunological responses and/or microbial factors are suggested (Kethu, 2006; Femiano et al., 2009; Ficarra et al., 2010). Lesions are erythematous, thickened, and hyperplastic folds of the buccal and labial mucosa with multiple gray to yellow pustules, which progress to erosions forming shallow, folded, and fissured “snail tract” ulcers, due to degeneration, ulceration, and suppuration of vegetating pustules (Femiano et al., 2009). They often manifest before the diagnosis of IBD (Hansen et al., 1983; Femiano et al., 2009). Lesions also occur at labial attached gingiva, soft and hard palate, oral vestibule, and tonsillar regions. They usually spare the tongue and floor of the mouth (Femiano et al., 2009). Symptoms of burning and pain may be present (Ficarra et al., 2010). Other than oral cavity, mucosal membranes at the vaginal, nasal, and rarely periocular areas can also be affected (Femiano et al., 2009). Peripheral eosinophilia has been found in most cases reported and maybe a valuable aid for the diagnosis of PV (Femiano et al., 2009). Histopathologically, PSV is characterized by epithelial acanthosis and superficial ulceration with intraepithelial and/or subepithelial micro abscesses containing numerous eosinophils and neutrophils with underlying connective tissues containing dense lymphocytic and plasma cell infiltrate (Femiano et al., 2009; Ficarra et al., 2010).
Pyodermatitis vegetans was first reported by Hallopeau (1898) when he described two patients with unusual pustular dermatosis and oral lesions for which he named pyodermite vegetans (Hallopeau, 1898). McCarthy (1949) proposed the term “pyostomatitis vegetans” after he observed similar lesions isolated in the oral cavity (McCarthy, 1949; Femiano et al. 2009). Recently these two entities are considered to be variants of the same disease termed pyodermatitis–pyostomatitis vegetans (PPV; Ficarra et al., 2010). PDV and PSV (or together as a group PPV) are rare cutaneous manifestation of IBD (Storwick et al., 1994; Femiano et al., 2009). In general, bowel disease antedate oral involvement by several years (Leibovitch et al., 2005). The rash of PPV has been reported to correlate with underlying bowel disease activity (Ayangco et al., 2002; Kitayama et al., 2010). The pathogenesis of PSV, PDV, or PPV is not clear, but has been hypothesized to be due to aberrant immune responses in IBD to cross-reacting antigens in the skin and bowel resulting in mucocutaneous manifestations (Femiano et al., 2009).
Employing immunosuppressants and systemic steroids to treat PSV has variable efficacy for treating symptoms and maintaining remission (Timani and Mutasim, 2008). Local therapies including hydrogen peroxide, topical steroids, and antibacterial mouthwashes are usually not effective in controlling disease activity. A case report demonstrated successful PSV remission with topical fluocinonide gel, but the patient relapsed and ultimately required total colectomy to achieve and maintain remission (Calobrisi et al., 1995; Femiano et al., 2009). In fact, subtotal colectomy in a patient with UC resulted in the development of PPV and relapse of bowel disease 5 years after surgery (Kitayama et al., 2010).
Neutrophilic dermatoses
Neutrophilic dermatoses include Sweet’s syndrome (SS) and bowel-associated dermatosis–arthritis syndrome (BADAS). SS, first described by R. D. Sweet and named after him, is characterized by the abrupt onset of tender and red to purple inflammatory nodules or papules that coalesce to form plaques, affecting the upper limbs, face, or neck. In addition to painful and edematous nodules and plaques, vesicles, bullae, or pustules may also be present (Georgiou et al., 2006; Nischal and Khopkar, 2007). Patients with colonic disease and females between the ages of 30 and 50 have increased risk for developing SS (Ardizzone et al., 2008; Timani and Mutasim, 2008). SS is also known as acute neutrophilic dermatosis as it is typically accompanied by fever and peripheral neutrophilia with >70% neutrophils while cutaneous lesions usually show dense aseptic neutrophilic infiltration (Georgiou et al., 2006). These lesions are tender and non-pruritic in nature, but can be difficult to distinguish from EN when they affect the lower extremities (Guhl and Garcia-Diez, 2008). Skin biopsy, which shows neutrophilic infiltrates in the reticular dermis, can be helpful in reaching a diagnosis when there is doubt (Kemmett and Hunter, 1990; Georgiou et al., 2006; Timani and Mutasim, 2008; Cohen, 2009). Patients may also experience fatigue, headache, and other non-specific constitutional symptoms. SS can affect extracutaneous organ systems including, but are not limited to the musculoskeletal, ocular, hepatic, pulmonary, and renal systems (Cohen et al., 1988).
Although the pathogenesis is unclear, SS usually develops as a reactive response to some type of underlying systemic disease, such as infection, malignancy, medications, or IBD (Vij et al., 2010). In fact, studies have shown that patients with SS have some type of underlying systemic disease in 50% of cases and an underlying malignancy in 20% of cases (Kemmett and Hunter, 1990; Souissi et al., 2007). UC and CD are the most common systemic diseases associated with SS (Timani and Mutasim, 2008). SS is associated with active bowel disease (67–80%; Georgiou et al., 2006; Ardizzone et al., 2008). The onset of symptoms from SS usually occur after the initial diagnosis of IBD, but may antedate the onset of IBD (21%) and has also been reported to occur 3 months after proctocolectomy in patients with UC (Darvay, 1996; Ardizzone et al., 2008). Similar to other reactive manifestations of IBD, the pathogenesis of SS has been hypothesized to be related to an altered immune response to common antigens in the skin and gut bacteria (Ali and Duerksen, 2008). Furthermore, ANCA and G-CSF or other cytokines have been proposed to play a pathogenetic role in activation, maturation, and chemotaxis of neutrophils in SS (Diaz-Peromingo et al., 2001; Cohen and Kurzrock, 2003).
First line treatment of SS involves systemic steroids and studies have shown marked symptom improvement with a 6 week steroid course (Cohen et al., 1988; Souissi et al., 2007). When dealing with localized disease, topical, or intralesional steroids are sometimes used with good effects (Timani and Mutasim, 2008). However, recurrence of SS is relatively common with as many as 1/3 of patients re-experiencing symptoms despite treatment (Kemmett and Hunter, 1990). Skin lesions that have not been treated have been documented to heal on their own, but can leave behind scars (Kemmett and Hunter, 1990; Timani and Mutasim, 2008). Other first line options for treatment include colchicine and potassium iodide. Other agents such as indomethacin and clofazimine can be used if first line agents are ineffective or cannot be tolerated. (Cohen and Kurzrock, 2002; Cohen, 2009). Treatments with immunosuppressants and anti-TNF-α have been reported to be successful in SS (Ali and Duerksen, 2008; Cohen, 2009; Larsen et al., 2010).
The BADAS, previously named as bowel bypass syndrome as it was originally reported to occur in up to 20% of patients after laparoscopic jejuno-ileal bypass surgery for obesity, can occur as a complication after various intestinal surgeries, in patients with diverticulitis or appendicitis, and in patients with IBD (Georgiou et al., 2006; Ashok and Kiely, 2007; Patton et al., 2009; Tu et al., 2011). The clinical characteristics of BADAS syndrome are recurrent episodes of fever, malaise, abdominal pain, arthritis, and polyarthralgias of the upper extremities involving the asymmetric large joints and interphalangeal joints of the fingers with accompanying tenosynovitis, and by the appearance of characteristic cutaneous lesions (Ashok and Kiely, 2007). These cutaneous lesions consist of erythematous macules (3–10 mm in diameter), developing into edematous and painful papules with central aseptic vesicles or pustules (2–4 mm in diameter) over the subsequent 1–2 days, which are located on the upper chest and arms (usually on the deltoid muscle region) and EN-like lesions in the legs. These maculopapular rashes occur in crops, resolve within 1–2 weeks without leaving scars and can recur every 1–6 weeks. These cutaneous lesions are temporally dependent on IBD activity (Ashok and Kiely, 2007; Patton et al., 2009).
The histology of BADAS is characterized by perivascular neutrophilic, mononuclear, and eosinophilic (depending on the stage of the lesions) infiltrate with dermal edema, intraepidermal pustules, minimal alterations of the walls of capillaries and venules without the features of leukocytoclastic vasculitis or fibrinoid necrosis (Georgiou et al., 2006; Nischal and Khopkar, 2007). Similar to PG and SS, BADAS has been classified in the spectrum of aseptic neutrophilic dermatoses with histopathology showing dense neutrophil infiltration and no destruction of vessel walls (Nischal and Khopkar, 2007; Patton et al., 2009). By immunofluorescence staining, the deposition of immunoglobulins and complement has frequently been observed at the dermo-epidermal junction. The pathogenesis of BADAS is hypothesized to be due to immune complex-mediated vessel damage, followed by increased migration and accumulation of neutrophils in the perivascular area (Jorizzo et al., 1984). The circulating immune complexes are believed to form due to an immunological response against antigenic bacterial peptidoglycans in the gut, with subsequent deposition in the skin and joints (Jorizzo et al., 1984; Nischal and Khopkar, 2007; Patton et al., 2009). It is theorized that overgrowth of bacteria, such as seen in active IBD, diverticulitis, appendicitis, blind loop after bypass surgery, or in the inflamed bowel segment after surgery, result in inflammation, and the formation of abnormal immunological responses to bacterial antigens especially peptidoglycans (Georgiou et al., 2006; Patton et al., 2009). Similar pathophysiology is also thought to involve the cutaneous manifestations of EN, PG, and SS (Georgiou et al., 2006; Nischal and Khopkar, 2007).
The management of the BADAS includes the treatment of the underlying bowel disorder and the administration of systemic steroids and antibiotics for inhibition of bacterial overgrowth (tetracycline, metronidazole, ciprofloxacin), although the response to these antibiotics are inconsistent (Georgiou et al., 2006; Patton et al., 2009). NSAIDs may be used to alleviate arthralgias or arthritis, but the potential toxicity of NSAIDs on IBD should make the decision to use NSAIDs based on weighing the risk-benefit ratio for each individual patient (Singh et al., 2009). Other drugs such as colchicine, dapsone, and sulfasalazine have been tried with variable successful rates (Georgiou et al., 2006).
Leukocytoclastic vasculitis
Leukocytoclastic vasculitis, one of the rare cutaneous manifestation of IBD, is characterized histopathologically by neutrophil infiltration and nuclear debris of inflamed postcapillary venules with endothelial enlargement and fibrinoid necrosis (Akbulut et al., 2008). The skin presentation ranges from palpable purpura (mediated by deposition of immune complexes in postcapillary venules) on the lower extremities and ankles to necrotic ulcers (Tsiamoulos et al., 2008). Similar to other reactive cutaneous manifestations of IBD, the pathogenesis of leukocytoclastic vasculitis with histology showing hypersensitivity vasculitis of small vessels is proposed to be circulating immune complexes (Mackel and Jordon, 1982; Iannone et al., 2003; Akbulut et al., 2008; Tsiamoulos et al., 2008). In CD, leukocytoclastic vasculitis occurs not only during the onset of bowel disease, but also during periods of exacerbation. On the other hand, in UC, leukocytoclastic vasculitis most commonly precede the onset of bowel disease with a lagging period ranging from 1 month to 2 years (Iannone et al., 2003; Akbulut et al., 2008; Tsiamoulos et al., 2008). In most cases of leukocytoclastic vasculitis, treating the underlying IBD results in resolution of the lesions (Akbulut et al., 2008; Tsiamoulos et al., 2008).
Cutaneous Disorders or Dermatosis Associated with IBD
These disorders include hidradenitis suppurativa, phlebitis, erythema multiforme, urticaria, lichen planus, secondary amyloidosis, and various autoimmune skin disorders, such as acquired epidermolysis bullosa (blistering lesions of the knees, elbows, hands, and feet), bullous pemphigoid, linear IgA bullous dermatosis, vitiligo, and psoriasis (Georgiou et al., 2006; Levine and Burakoff, 2011). Rarely has IBD been reported to be associated with skin neoplasm such as Bowen’s disease and squamous cell carcinoma, which require surgical excision (Georgiou et al., 2006).
Acquired epidermolysis bullosa or epidermolysis bullosa acquisita (EBA) is characterized by autoantibodies to type VII collagen (detected in both the skin and colon of normal individuals), which was also detected in 13/19 CD and 4/31 UC patients (Chen et al., 2002; Hoffmann and Kruis, 2004). In fact, EBA has been reported to be most frequently (25%) associated with CD (Hoffmann and Kruis, 2004). This finding suggests that chronic inflammation in the intestine predisposed IBD patients to develop autoantibodies against type VII collagen in the gut, which in turn attacks the skin at the dermal–epidermal junction resulting in the blistering skin lesions seen in EBA (Hundorfean et al., 2010). Future research is needed to provide more insight in this hypothesis. Compared to an incidence of 0.3% of vitiligo in the normal population, the incidence of vitiligo is higher in CD (0.5%) and UC (1.1%; Pashankar et al., 1999). The pathogenesis of vitiligo in IBD has been proposed to be autoimmune or genetic HLA linkage in nature (Pashankar et al., 1999; Quan et al., 2010). Secondary amyloidosis is rare in IBD and occurs as a systemic response to chronic inflammation. The systemic amyloidosis in IBD is type AA amyloidosis and is more common in CD (0.9%) than UC (0.07%) most likely as a result of a higher degree of inflammation in CD (Sattianayagam et al., 2009).
The most frequently associated cutaneous disease is psoriasis which occurs in 7–11% of the IBD population compared to 1–2% of general population (Danese et al., 2005). In one study, psoriasis was found to be more prevalent in CD (11.2%) than UC (5.7%; Yates et al., 1982; Vavricka et al., 2011). The association of IBD with psoriasis is believed to be both genetically and immunologically related (Georgiou et al., 2006). In a recent study of 146 patients with both IBD and psoriasis compared to a control of 146 patients with only psoriasis, it was found that patients with both IBD and psoriasis had significantly higher rates of autoimmune thyroiditis (6.8 vs.2.1%), hepatitis (6.2 vs. 0.7%), and diabetes (26.7 vs. 11.0%; Binus et al., 2011). Individuals with both IBD and psoriasis also had significantly higher CRPs, ESRs, and sero-negative arthritis (41.1%) compared to individuals with only psoriasis. Certain gene loci on chromosomes 3, 4, 6, and 16 are associated with both CD and psoriasis (Georgiou et al., 2006). A recent study showed that a striking overlap of loci between diseases of CD, UC, psoriasis, ankylosing spondylitis, and primary sclerosing cholangitis (Cho and Brant, 2011). These studies suggest that genetic predispositions or HLA linkages for certain specific immune response patterns and common inflammatory pathways may play an important role in the association of these diseases (Georgiou et al., 2006; Binus et al., 2011).
Secondary Cutaneous Manifestations Due to Complications of IBD or Its Treatments
Manifestations secondary to malnutrition and malabsorption
Skin manifestations of IBD secondary to malnutrition and/or malabsorption include the acquired form of acrodermatitis enteropathica (zinc deficiency), pellagra (niacin deficiency), scurvy (vitamin C deficiency), purpura (vitamin C and K deficiency), stomatitis/glossitis/angular cheilitis (vitamin B deficiency), xeroderma, or dry skin and unspecified eczema (essential fatty acid deficiency), hair and nail abnormalities (malabsorption of amino acid and protein; Trost and McDonnell, 2005; Georgiou et al., 2006; O’Sullivan and O’Morain, 2006). The acquired form of acrodermatitis enteropathica, due to zinc deficiency, is the most common nutritional-deficient cutaneous manifestation of IBD and more common in CD than UC (Dronfield et al., 1977; McClain et al., 1980; Danese et al., 2005). The skin lesions present as psoriasis involving an erythematous patch and plaque, which may evolve into crusted vesicles, bullae, or pustules. The lesions occur frequently around the mouth, anus, limbs, fingers, and scalp (Danese et al., 2005; von Felbert and Hunziker, 2010). Adequate supplementation of the deficient nutrients or vitamins is the treatment of choice. For example, acrodermatitis enteropathica is treated with zinc sulfate 220 mg/day (Danese et al., 2005; Georgiou et al., 2006).
Secondary cutaneous manifestations due to adverse effects of IBD treatment
Skin manifestations from adverse effects can result in a broad range of secondary cutaneous manifestations, such as drug eruptions, urticaria, angioedema, hair loss, lichen planus, erythema multiforme, bullous dermatosis/reaction, Stevens–Johnson syndrome, acne, psoriasis and so on (Georgiou et al., 2006; Fiorino et al., 2009). The following are examples of drugs used in IBD treatments that can cause secondary cutaneous complications: 5-ASA, metronidazole, ciprofloxacin, azathioprine, 6-MP, methotrexate, cyclosporine, steroids, anti-TNF-α agents, etc. (Georgiou et al., 2006; Passarini et al., 2007).
Anti-TNF-α agents have been used successfully in the treatment of IBD and psoriasis, but when treating IBD, anti-TNF-α agents such as infliximab and adalimumab can paradoxically induce psoriasis lesions after the third or fourth infusion (Passarini et al., 2007; Fiorino et al., 2009). The psoriasis lesions regressed after withdrawing the anti-TNF-α agents in 16 out of 17 patients (Fiorino et al., 2009). Although there have only been 18 reported cases of anti-TNF α induced psoriasis in the IBD population, but clinicians should still be aware of this potential side effect (Fiorino et al., 2009). It has been suggested that inhibition of TNF-α induces over-expression of cutaneous IFN-α which then causes predisposition to psoriasis (Iborra et al., 2011). The treatment of psoriasis induced by anti-TNF-α agents is cessation of the offending agents and topical steroid therapy (Iborra et al., 2011).
Conclusion
The most common cutaneous manifestations of IBD are EN and PG (Levine and Burakoff, 2011). All IBD patients should be closely examined for skin manifestations as some skin lesions may be related to IBD activity. Manifestation of one EIM increases the risk of developing additional EIMs, and all EIMs affect both morbidity and mortality in IBD (Monsen et al., 1990; Das, 1999; Vavricka et al. 2011). Similar to other EIMs of IBD, skin lesions may postdate, occur with, or antedate the onset of the underlying disease process (Apgar, 1991). Since dermatological manifestations can precede bowel disease or bowel exacerbation, idiopathic skin lesions such as EN, PG, recurrent multiple aphthous stomatitis, or PPV should alert clinicians to evaluate the possibility of underlying IBD so that early diagnosis and intervention with the appropriate therapy can be initiated (Levine and Burakoff, 2011).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Cedars-Sinai IBD Center for helpful discussions. We thank Cindy Ting for critical reading of the manuscript.
References
- Abraham C., Medzhitov R. (2011). Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 140, 1729–1737 10.1053/j.gastro.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. H., Eksteen B. (2006). Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 6, 244–251 10.1038/nri1784 [DOI] [PubMed] [Google Scholar]
- Agrawal D., Rukkannagari S., Kethu S. (2007). Pathogenesis and clinical approach to extraintestinal manifestations of inflammatory bowel disease. Minerva Gastroenterol. Dietol. 53, 233–248 [PubMed] [Google Scholar]
- Ahern P. P., Izcue A., Maloy K. J., Powrie F. (2008). The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 226, 147–159 10.1111/j.1600-065X.2008.00705.x [DOI] [PubMed] [Google Scholar]
- Akbulut S., Ozaslan E., Topal F., Albayrak L., Kayhan B., Efe C. (2008). Ulcerative colitis presenting as leukocytoclastic vasculitis of skin. World J. Gastroenterol. 14, 2448–2450 10.3748/wjg.14.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Duerksen D. R. (2008). Ulcerative colitis and Sweet’s syndrome: a case report and review of the literature. Can. J. Gastroenterol. 22, 296–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N., Hupertz V., Mahajan L. (2009). Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn’s disease. Inflamm. Bowel Dis. 15, 803–806 10.1002/ibd.20748 [DOI] [PubMed] [Google Scholar]
- Apgar J. T. (1991). Newer aspects of inflammatory bowel disease and its cutaneous manifestations: a selective review. Semin. Dermatol. 10, 138–147 [PubMed] [Google Scholar]
- Ardizzone S., Puttini P. S., Cassinotti A., Porro G. B. (2008). Extraintestinal manifestations of inflammatory bowel disease. Dig. Liver Dis. 40(Suppl. 2), S253–S259 10.1016/S1590-8658(08)60534-4 [DOI] [PubMed] [Google Scholar]
- Asarch A., Barak O., Loo D. S., Gottlieb A. B. (2008). Th17 cells: a new therapeutic target in inflammatory dermatoses. J. Dermatolog. Treat. 19, 318–326 10.1080/09546630802206686 [DOI] [PubMed] [Google Scholar]
- Ashok D., Kiely P. (2007). Bowel associated dermatosis – arthritis syndrome: a case report. J. Med. Case Reports 1, 81. 10.1186/1752-1947-1-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayangco L., Rogers R. S., III., Sheridan P. J. (2002). Pyostomatitis vegetans as an early sign of reactivation of Crohn’s disease: a case report. J. Periodontol. 73, 1512–1516 10.1902/jop.2002.73.12.1512 [DOI] [PubMed] [Google Scholar]
- Basu M. K., Asquith P. (1980). Oral manifestations of inflammatory bowel disease. Clin. Gastroenterol. 9, 307–321 [PubMed] [Google Scholar]
- Berkowitz E. Z., Lebwohl M. (2000). Cutaneous manifestations of inflammatory bowel disease. J. Eur. Acad. Dermatol. Venereol. 14, 349–350 10.1046/j.1468-3083.2000.00137.x [DOI] [PubMed] [Google Scholar]
- Bernstein C. N., Blanchard J. F., Rawsthorne P., Yu N. (2001). The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am. J. Gastroenterol. 96, 1116–1122 10.1111/j.1572-0241.2001.03756.x [DOI] [PubMed] [Google Scholar]
- Binus A. M., Han J., Qamar A. A., Mody E. A., Holt E. W., Qureshi A. A. (2011). Associated comorbidities in psoriasis and inflammatory bowel disease. J. Eur. Acad. Dermatol. Venereol. 10.1111/j.1468-3083.2011.04153.x [DOI] [PubMed] [Google Scholar]
- Blitz N. M., Rudikoff D. (2001). Pyoderma gangrenosum. Mt. Sinai J. Med. 68, 287–297 [PubMed] [Google Scholar]
- Brooklyn T. N., Dunnill M. G., Shetty A., Bowden J. J., Williams J. D., Griffiths C. E., Forbes A., Greenwood R., Probert C. S. (2006). Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 55, 505–509 10.1136/gut.2005.074815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns B. A., Herbst C. A., Sartor B. R., Briggaman R. A., Koruda M. J. (1994). Peristomal pyoderma gangrenosum and inflammatory bowel disease. Arch. Surg. 129, 769–772 10.1001/archsurg.1994.01420310101019 [DOI] [PubMed] [Google Scholar]
- Callen J. P. (1998). Pyoderma gangrenosum. Lancet 351, 581–585 10.1016/S0140-6736(97)10187-8 [DOI] [PubMed] [Google Scholar]
- Callen J. P., Jackson J. M. (2007). Pyoderma gangrenosum: an update. Rheum. Dis. Clin. North Am. 33, 787–802, vi. 10.1016/j.rdc.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Calobrisi S. D., Mutasim D. F., Mcdonald J. S. (1995). Pyostomatitis vegetans associated with ulcerative colitis. Temporary clearance with fluocinonide gel and complete remission after colectomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 79, 452–454 10.1016/S1079-2104(05)80126-X [DOI] [PubMed] [Google Scholar]
- Canpolat F., Cemil B. C., Yilmazer D., Yesilli O., Eskioglu F. (2011). Pyoderma vegetans associated with ulcerative colitis: a case with good response to steroids. Case Rep. Dermatol. 3, 80–84 10.1159/000327221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvardjian A., Nethercott J. R. (1982). Cutaneous granulomatous vasculitis associated with Crohn’s disease. Cutis 30, 645–655 [PubMed] [Google Scholar]
- Chen M., O’toole E. A., Sanghavi J., Mahmud N., Kelleher D., Weir D., Fairley J. A., Woodley D. T. (2002). The epidermolysis bullosa acquisita antigen (type VII collagen) is present in human colon and patients with Crohn’s disease have autoantibodies to type VII collagen. J. Invest. Dermatol. 118, 1059–1064 10.1046/j.1523-1747.2002.01754.x [DOI] [PubMed] [Google Scholar]
- Cho J. H., Brant S. R. (2011). Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 140, 1704–1712 10.1053/j.gastro.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. R. (2009). Neutrophilic dermatoses: a review of current treatment options. Am. J. Clin. Dermatol. 10, 301–312 10.2165/11310730-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Cohen P. R., Kurzrock R. (2002). Sweet’s syndrome: a review of current treatment options. Am. J. Clin. Dermatol. 3, 117–131 10.2165/00128071-200203020-00005 [DOI] [PubMed] [Google Scholar]
- Cohen P. R., Kurzrock R. (2003). Sweet’s syndrome revisited: a review of disease concepts. Int. J. Dermatol. 42, 761–778 10.1046/j.1365-4362.2003.01891.x [DOI] [PubMed] [Google Scholar]
- Cohen P. R., Talpaz M., Kurzrock R. (1988). Malignancy-associated Sweet’s syndrome: review of the world literature. J. Clin. Oncol. 6, 1887–1897 [DOI] [PubMed] [Google Scholar]
- Crowson A. N., Mihm M. C., Jr., Magro C. (2003). Pyoderma gangrenosum: a review. J. Cutan. Pathol. 30, 97–107 10.1034/j.1600-0560.2003.00024.x [DOI] [PubMed] [Google Scholar]
- Curley R. K., Macfarlane A. W., Vickers C. F. (1985). Pyoderma gangrenosum treated with Cyclosporine A. Br. J. Dermatol. 113, 601–604 10.1111/j.1365-2133.1985.tb02385.x [DOI] [PubMed] [Google Scholar]
- Danese S., Semeraro S., Papa A., Roberto I., Scaldaferri F., Fedeli G., Gasbarrini G., Gasbarrini A. (2005). Extraintestinal manifestations in inflammatory bowel disease. World J. Gastroenterol. 11, 7227–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvay A. (1996). Sweet’s syndrome preceding inflammatory bowel disease. Clin. Exp. Dermatol. 21, 175. 10.1046/j.1365-2230.1996.d01-207.x [DOI] [PubMed] [Google Scholar]
- Das K. M. (1999). Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig. Dis. Sci. 44, 1–13 10.1023/A:1026629528233 [DOI] [PubMed] [Google Scholar]
- Diaz-Peromingo J. A., Garcia-Suarez F., Sanchez-Leira J., Saborido-Frojan J. (2001). Sweet’s syndrome in a patient with acute ulcerative colitis: presentation of a case and review of the literature. Yale J. Biol. Med. 74, 165–168 [PMC free article] [PubMed] [Google Scholar]
- Dronfield M. W., Malone J. D., Langman M. J. (1977). Zinc in ulcerative colitis: a therapeutic trial and report on plasma levels. Gut 18, 33–36 10.1136/gut.18.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. E., Pardi D. S. (2007). Extraintestinal manifestations of inflammatory bowel disease: focus on the musculoskeletal, dermatologic, and ocular manifestations. MedGenMed 9, 55. [PMC free article] [PubMed] [Google Scholar]
- Farhi D., Wallach D. (2008). The neutrophilic dermatoses. Dermatol. Nurs. 20, 274–282 [PubMed] [Google Scholar]
- Fava F., Danese S. (2011). Intestinal microbiota in inflammatory bowel disease: friend of foe? World J. Gastroenterol. 17, 557–566 10.3748/wjg.v17.i5.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciani C., De Simone C., Amerio P. (2009). Dermatological signs during inflammatory bowel diseases. Eur. Rev. Med. Pharmacol. Sci. 13(Suppl. 1), 15–21 [PubMed] [Google Scholar]
- Femiano F., Lanza A., Buonaiuto C., Perillo L., Dell’ermo A., Cirillo N. (2009). Pyostomatitis vegetans: a review of the literature. Med. Oral. Patol. Oral. Cir. Bucal. 14, E114–E117 [PubMed] [Google Scholar]
- Ficarra G., Baroni G., Massi D. (2010). Pyostomatitis vegetans: cellular immune profile and expression of IL-6, IL-8 and TNF-alpha. Head Neck Pathol. 4, 1–9 10.1007/s12105-009-0149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino G., Allez M., Malesci A., Danese S. (2009). Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 29, 921–927 10.1111/j.1365-2036.2009.03955.x [DOI] [PubMed] [Google Scholar]
- Fleshner P. R., Michelassi F., Rubin M., Hanauer S. B., Plevy S. E., Targan S. R. (1995). Morbidity of subtotal colectomy in patients with severe ulcerative colitis unresponsive to cyclosporin. Dis. Colon Rectum 38, 1241–1245 10.1007/BF02049164 [DOI] [PubMed] [Google Scholar]
- Freeman H. J. (2005). Erythema nodosum and pyoderma gangrenosum in 50 patients with Crohn’s disease. Can. J. Gastroenterol. 19, 603–606 [DOI] [PubMed] [Google Scholar]
- Georgiou G., Pasmatzi E., Monastirli A., Tsambaos D. (2006). Cutaneous manifestations of inflammatory bowel disease. Hosp. Chron. 1, 158–168 [Google Scholar]
- Goudet P., Dozois R. R., Kelly K. A., Ilstrup D. M., Phillips S. F. (2001). Characteristics and evolution of extraintestinal manifestations associated with ulcerative colitis after proctocolectomy. Dig. Surg. 18, 51–55 10.1159/000050097 [DOI] [PubMed] [Google Scholar]
- Guenova E., Teske A., Fehrenbacher B., Hoerber S., Adamczyk A., Schaller M., Hoetzenecker W., Biedermann T. (2011). Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch. Dermatol. 147, 1203–1205 10.1001/archdermatol.2011.27 [DOI] [PubMed] [Google Scholar]
- Guhl G., Garcia-Diez A. (2008). Subcutaneous sweet syndrome. Dermatol. Clin. 26, 541–551, viii–ix. 10.1016/j.det.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Hallopeau H. (1898). “Pyodermitevegetante” ihre Beziehungen zur dermatitis herpetiformis und dem pemphigus vegetans. Arch. Dermatol. Syphilol. 43, 289–306 10.1007/BF01986902 [DOI] [Google Scholar]
- Hansen L. S., Silverman S., Jr., Daniels T. E. (1983). The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg. Oral Med. Oral Pathol. 55, 363–373 10.1016/0030-4220(83)90191-3 [DOI] [PubMed] [Google Scholar]
- Hoffmann R. M., Kruis W. (2004). Rare extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 10, 140–147 10.1097/00054725-200403000-00013 [DOI] [PubMed] [Google Scholar]
- Hue S., Ahern P., Buonocore S., Kullberg M. C., Cua D. J., Mckenzie B. S., Powrie F., Maloy K. J. (2006). Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 10.1084/jem.20061099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundorfean G., Neurath M. F., Sitaru C. (2010). Autoimmunity against type VII collagen in inflammatory bowel disease. J. Cell. Mol. Med. 14, 2393–2403 10.1111/j.1582-4934.2009.00959.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone F., Scioscia C., Musio A., Piscitelli D., Lapadula G. (2003). Leukocytoclastic vasculitis as onset symptom of ulcerative colitis. Ann. Rheum. Dis. 62, 785–786 10.1136/ard.62.8.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra M., Beltran B., Bastida G., Aguas M., Nos P. (2011). Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a paradoxical side effect. J. Crohns Colitis 5, 157–161 10.1016/j.crohns.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Izcue A., Coombes J. L., Powrie F. (2009). Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 10.1146/annurev.immunol.021908.132657 [DOI] [PubMed] [Google Scholar]
- Jonas M., Scholefield J. H. (2001). Anal fissure. Gastroenterol. Clin. North Am. 30, 167–181 10.1016/S0889-8553(05)70172-2 [DOI] [PubMed] [Google Scholar]
- Jorizzo J. L., Schmalstieg F. C., Dinehart S. M., Daniels J. C., Cavallo T., Apisarnthanarax P., Rudloff H. B., Gonzalez E. B. (1984). Bowel-associated dermatosis-arthritis syndrome. Immune complex-mediated vessel damage and increased neutrophil migration. Arch. Intern. Med. 144, 738–740 10.1001/archinte.1984.00350160088016 [DOI] [PubMed] [Google Scholar]
- Kaufman I., Caspi D., Yeshurun D., Dotan I., Yaron M., Elkayam O. (2005). The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol. Int. 25, 406–410 10.1007/s00296-004-0467-8 [DOI] [PubMed] [Google Scholar]
- Kemmett D., Hunter J. A. (1990). Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J. Am. Acad. Dermatol. 23, 503–507 10.1016/0190-9622(90)70250-L [DOI] [PubMed] [Google Scholar]
- Kethu S. R. (2006). Extraintestinal manifestations of inflammatory bowel diseases. J. Clin. Gastroenterol. 40, 467–475 10.1097/00004836-200607000-00003 [DOI] [PubMed] [Google Scholar]
- Khor B., Gardet A., Xavier R. J. (2011). Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama A., Misago N., Okawa T., Iwakiri R., Narisawa Y. (2010). Pyodermatitis-pyostomatitis vegetans after subtotal colectomy for ulcerative colitis. J. Dermatol. 37, 714–717 10.1111/j.1346-8138.2010.00961.x [DOI] [PubMed] [Google Scholar]
- Larsen S., Bendtzen K., Nielsen O. H. (2010). Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann. Med. 42, 97–114 10.3109/07853890903559724 [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Fleischmajer R., Janowitz H., Present D., Prioleau P. G. (1984). Metastatic Crohn’s disease. J. Am. Acad. Dermatol. 10, 33–38 10.1016/S0190-9622(84)80038-9 [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Lebwohl O. (1998). Cutaneous manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 4, 142–148 10.1002/ibd.3780040209 [DOI] [PubMed] [Google Scholar]
- Lees C. W., Barrett J. C., Parkes M., Satsangi J. (2011). New IBD genetics: common pathways with other diseases. Gut 60, 1739–1753 10.1136/gut.2009.199679 [DOI] [PubMed] [Google Scholar]
- Leibovitch I., Ooi C., Huilgol S. C., Reid C., James C. L., Selva D. (2005). Pyodermatitis-pyostomatitis vegetans of the eyelids case report and review of the literature. Ophthalmology 112, 1809–1813 10.1016/j.ophtha.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Lestre S., Ramos J., Joao A., Serrao V. (2010). Cutaneous Crohn’s disease presenting as genital warts: successful treatment with adalimumab. Eur. J. Dermatol. 20, 504–505 [DOI] [PubMed] [Google Scholar]
- Letsinger J. A., Mccarty M. A., Jorizzo J. L. (2005). Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J. Am. Acad. Dermatol. 52, 500–508 10.1016/j.jaad.2004.10.863 [DOI] [PubMed] [Google Scholar]
- Levine J. S., Burakoff R. (2011). Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. (N Y) 7, 235–241 [PMC free article] [PubMed] [Google Scholar]
- Levitt M. D., Ritchie J. K., Lennard-Jones J. E., Phillips R. K. (1991). Pyoderma gangrenosum in inflammatory bowel disease. Br. J. Surg. 78, 676–678 10.1002/bjs.1800780613 [DOI] [PubMed] [Google Scholar]
- Lewis R. T., Maron D. J. (2010). Efficacy and complications of surgery for Crohn’s disease. Gastroenterol. Hepatol. (N Y) 6, 587–596 [PMC free article] [PubMed] [Google Scholar]
- Mackel S. E., Jordon R. E. (1982). Leukocytoclastic vasculitis. A cutaneous expression of immune complex disease. Arch. Dermatol. 118, 296–301 10.1001/archderm.1982.01650170010012 [DOI] [PubMed] [Google Scholar]
- Marzano A. V., Cugno M., Trevisan V., Fanoni D., Venegoni L., Berti E., Crosti C. (2010). Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin. Exp. Immunol. 162, 100–107 10.1111/j.1365-2249.2010.04201.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis W. L., Ellis C. N., Griffiths C. E., Lazarus G. S. (1992). Treatment of pyoderma gangrenosum with cyclosporine. Arch. Dermatol. 128, 1060–1064 10.1001/archderm.128.8.1060 [DOI] [PubMed] [Google Scholar]
- McCarthy F. P. (1949). Pyostomatitis vegetans; Report of three cases. Arch. Derm. Syphilol. 60, 750–764 10.1001/archderm.1949.01530050112010 [DOI] [PubMed] [Google Scholar]
- McClain C., Soutor C., Zieve L. (1980). Zinc deficiency: a complication of Crohn’s disease. Gastroenterology 78, 272–279 [PubMed] [Google Scholar]
- McGowan J. W. T., Johnson C. A., Lynn A. (2004). Treatment of pyoderma gangrenosum with etanercept. J. Drugs Dermatol. 3, 441–444 [PubMed] [Google Scholar]
- Menachem Y., Gotsman I. (2004). Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Isr. Med. Assoc. J. 6, 88–90 [PubMed] [Google Scholar]
- Mert A., Ozaras R., Tabak F., Pekmezci S., Demirkesen C., Ozturk R. (2004). Erythema nodosum: an experience of 10 years. Scand. J. Infect. Dis. 36, 424–427 10.1080/00365540410027184 [DOI] [PubMed] [Google Scholar]
- Mir-Madjlessi S. H., Taylor J. S., Farmer R. G. (1985). Clinical course and evolution of erythema nodosum and pyoderma gangrenosum in chronic ulcerative colitis: a study of 42 patients. Am. J. Gastroenterol. 80, 615–620 [PubMed] [Google Scholar]
- Mnif L., Amouri A., Tahri N. (2010). Cutaneous manifestations of inflammatory bowel disease. Tunis. Med. 88, 420–423 [PubMed] [Google Scholar]
- Monsen U., Sorstad J., Hellers G., Johansson C. (1990). Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am. J. Gastroenterol. 85, 711–716 [PubMed] [Google Scholar]
- Monteleone G., Pallone F., Macdonald T. T. (2011). Emerging immunological targets in inflammatory bowel disease. Curr. Opin. Pharmacol. 11, 640–645 10.1016/j.coph.2011.09.013 [DOI] [PubMed] [Google Scholar]
- Newell L. M., Malkinson F. D. (1982). “Pyoderma (ecthyma) gangrenosum” by Brunsting, Goeckerman, and O’Leary, October 1930. Commentary: pyoderma gangrenosum. Arch. Dermatol. 118, 743–773 10.1001/archderm.1982.01650220073008 [DOI] [PubMed] [Google Scholar]
- Nischal K. C., Khopkar U. (2007). An approach to the diagnosis of neutrophilic dermatoses: a histopathological perspective. Indian J. Dermatol. Venereol. Leprol. 73, 222–230 10.4103/0378-6323.33634 [DOI] [PubMed] [Google Scholar]
- Orchard T. (2003). Extraintestinal complications of inflammatory bowel disease. Curr. Gastroenterol. Rep. 5, 512–517 10.1007/s11894-003-0042-6 [DOI] [PubMed] [Google Scholar]
- O’Sullivan M., O’Morain C. (2006). Nutrition in inflammatory bowel disease. Best Pract. Res. Clin. Gastroenterol. 20, 561–573 10.1016/j.bpg.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Pashankar D., Prendiville J., Israel D. M. (1999). Vitiligo and Crohn’s disease in children. J. Pediatr. Gastroenterol. Nutr. 28, 227–229 10.1097/00005176-199902000-00029 [DOI] [PubMed] [Google Scholar]
- Passarini B., Infusino S. D., Barbieri E., Varotti E., Gionchetti P., Rizzello F., Morselli C., Tambasco R., Campieri M. (2007). Cutaneous manifestations in inflammatory bowel diseases: eight cases of psoriasis induced by anti-tumor-necrosis-factor antibody therapy. Dermatology (Basel) 215, 295–300 10.1159/000107622 [DOI] [PubMed] [Google Scholar]
- Patton T., Jukic D., Juhas E. (2009). Atypical histopathology in bowel-associated dermatosis-arthritis syndrome: a case report. Dermatol. Online J. 15, 3. [PubMed] [Google Scholar]
- Ploysangam T., Heubi J. E., Eisen D., Balistreri W. F., Lucky A. W. (1997). Cutaneous Crohn’s disease in children. J. Am. Acad. Dermatol. 36, 697–704 10.1016/S0190-9622(97)80320-9 [DOI] [PubMed] [Google Scholar]
- Quan C., Ren Y. Q., Xiang L. H., Sun L. D., Xu A. E., Gao X. H., Chen H. D., Pu X. M., Wu R. N., Liang C. Z., Li J. B., Gao T. W., Zhang J. Z., Wang X. L., Wang J., Yang R. Y., Liang L., Yu J. B., Zuo X. B., Zhang S. Q., Zhang S. M., Chen G., Zheng X. D., Li P., Zhu J., Li Y. W., Wei X. D., Hong W. S., Ye Y., Zhang Y., Wu W. S., Cheng H., Dong P. L., Hu D. Y., Li Y., Li M., Zhang X., Tang H. Y., Tang X. F., Xu S. X., He S. M., Lv Y. M., Shen M., Jiang H. Q., Wang Y., Li K., Kang X. J., Liu Y. Q., Sun L., Liu Z. F., Xie S. Q., Zhu C. Y., Xu Q., Gao J. P., Hu W. L., Ni C., Pan T. M., Yao S., He C. F., Liu Y. S., Yu Z. Y., Yin X. Y., Zhang F. Y., Yang S., Zhou Y., Zhang X. J. (2010). Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat. Genet. 42, 614–618 10.1038/ng.603 [DOI] [PubMed] [Google Scholar]
- Quezada S., Turner P. L., Alexiev B., Daly B., Cross R. (2009). Severe refractory orofacial Crohn’s disease: report of a case. Dig. Dis. Sci. 54, 2290–2295 10.1007/s10620-008-0588-0 [DOI] [PubMed] [Google Scholar]
- Read A. E. (1985). Pyoderma gangrenosum. Q. J. Med. 55, 99–101 [PubMed] [Google Scholar]
- Roberts N., Bunker C. (1993). The gut and the skin. Br. J. Hosp. Med. 50, 31–39 [PubMed] [Google Scholar]
- Rothfuss K. S., Stange E. F., Herrlinger K. R. (2006). Extraintestinal manifestations and complications in inflammatory bowel diseases. World J. Gastroenterol. 12, 4819–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar B., Sands D. (2007). Perianal Crohn’s disease. Clin. Colon Rectal Surg. 20, 282–293 10.1055/s-2007-991027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna J., Sharma A., Marfatia Y. S. (2008). Bilateral nonhealing ulcers in groin: an interesting case of Metastatic Crohn’s disease. Indian J. Sex Transm. Dis. 29, 98–100 10.4103/0253-7184.48735 [DOI] [Google Scholar]
- Sattianayagam P. T., Hawkins P. N., Gillmore J. D. (2009). Systemic amyloidosis and the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 6, 608–617 10.1038/nrgastro.2009.147 [DOI] [PubMed] [Google Scholar]
- Ship J. A. (1996). Recurrent aphthous stomatitis. An update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 81, 141–147 10.1016/S1079-2104(96)80403-3 [DOI] [PubMed] [Google Scholar]
- Singh S., Graff L. A., Bernstein C. N. (2009). Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am. J. Gastroenterol. 104, 1298–1313; quiz 1314. 10.1038/ajg.2009.15 [DOI] [PubMed] [Google Scholar]
- Souissi A., Benmously R., Fenniche S., Zarrouk M., Marrek H., Debbiche A., Ayed M. B., Mokhtar I. (2007). Sweet’s syndrome: a propos of 8 cases. Tunis. Med. 85, 49–53 [PubMed] [Google Scholar]
- Storwick G. S., Prihoda M. B., Fulton R. J., Wood W. S. (1994). Pyodermatitis-pyostomatitis vegetans: a specific marker for inflammatory bowel disease. J. Am. Acad. Dermatol. 31, 336–341 10.1016/S0190-9622(94)70167-9 [DOI] [PubMed] [Google Scholar]
- Thornton J. R., Teague R. H., Low-Beer T. S., Read A. E. (1980). Pyoderma gangrenosum and ulcerative colitis. Gut 21, 247–248 10.1136/gut.21.3.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timani S., Mutasim D. F. (2008). Skin manifestations of inflammatory bowel disease. Clin. Dermatol. 26, 265–273 10.1016/j.clindermatol.2007.10.018 [DOI] [PubMed] [Google Scholar]
- Tromm A., May D., Almus E., Voigt E., Greving I., Schwegler U., Griga T. (2001). Cutaneous manifestations in inflammatory bowel disease. Z. Gastroenterol. 39, 137–144 10.1055/s-2001-11153 [DOI] [PubMed] [Google Scholar]
- Trost L. B., McDonnell J. K. (2005). Important cutaneous manifestations of inflammatory bowel disease. Postgrad. Med. J. 81, 580–585 10.1136/pgmj.2004.031633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiamoulos Z., Karamanolis G., Polymeros D., Triantafyllou K., Oikonomopoulos T. (2008). Leukocytoclastic vasculitis as an onset symptom of Crohn’s disease. Case Rep. Gastroenterol. 2, 410–414 10.1159/000161562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J., Chan J. J., Yu L. L. (2011). Bowel bypass syndrome/bowel-associated dermatosis arthritis syndrome post laparoscopic gastric bypass surgery. Australas. J. Dermatol. 52, e5–e7 10.1111/j.1440-0960.2010.00632.x [DOI] [PubMed] [Google Scholar]
- Turkcapar N., Toruner M., Soykan I., Aydintug O. T., Cetinkaya H., Duzgun N., Ozden A., Duman M. (2006). The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol. Int. 26, 663–668 10.1007/s00296-005-0084-1 [DOI] [PubMed] [Google Scholar]
- Turner R. B., Emer J. J., Weill M., Winterfield L., Friedman S., Qureshi A. A. (2010). Rapid resolution of pyoderma gangrenosum after treatment with intravenous cyclosporine. J. Am. Acad. Dermatol. 63, e72–e74 10.1016/j.jaad.2009.11.683 [DOI] [PubMed] [Google Scholar]
- Tutrone W. D., Green K., Weinberg J. M., Caglar S., Clarke D. (2007). Pyoderma gangrenosum: dermatologic application of hyperbaric oxygen therapy. J. Drugs Dermatol. 6, 1214–1219 [PubMed] [Google Scholar]
- van Wijk F., Cheroutre H. (2010). Mucosal T cells in gut homeostasis and inflammation. Expert Rev. Clin. Immunol. 6, 559–566 10.1586/eci.10.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavricka S. R., Brun L., Ballabeni P., Pittet V., Prinz Vavricka B. M., Zeitz J., Rogler G., Schoepfer A. M. (2011). Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 106, 110–119 10.1038/ajg.2010.343 [DOI] [PubMed] [Google Scholar]
- Veloso T. (1990). Complement deposits in inflammatory bowel disease. Gastroenterology 99, 1541–1542 [DOI] [PubMed] [Google Scholar]
- Vij A., Modi G. M., Suwattee P., Cockerell C. J., Hsu S. (2010). Chronic, recurrent neutrophilic dermatosis: a case report. Dermatol. Online J. 16, 1. [PubMed] [Google Scholar]
- Vind I., Riis L., Jess T., Knudsen E., Pedersen N., Elkjaer M., Bak Andersen I., Wewer V., Norregaard P., Moesgaard F., Bendtsen F., Munkholm P. (2006). Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn’s colitis database. Am. J. Gastroenterol. 101, 1274–1282 10.1111/j.1572-0241.2006.00552.x [DOI] [PubMed] [Google Scholar]
- von Felbert V., Hunziker T. (2010). Acrodermatitis enteropathica-like skin lesions due to Crohn’s disease-associated zinc deficiency. Hautarzt 61, 927–929 10.1007/s00105-010-2060-2 [DOI] [PubMed] [Google Scholar]
- Wasserteil V., Bruce S., Sessoms S. L., Guntupalli K. K. (1992). Pyoderma gangrenosum treated with hyperbaric oxygen therapy. Int. J. Dermatol. 31, 594–596 10.1111/j.1365-4362.1992.tb02728.x [DOI] [PubMed] [Google Scholar]
- Weinstein M., Turner D., Avitzur Y. (2005). Erythema nodosum as a presentation of inflammatory bowel disease. CMAJ 173, 145–146 10.1503/cmaj.050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., Walker D., Orchard T. R. (2008). Extraintestinal manifestations of inflammatory bowel disease. Curr. Gastroenterol. Rep. 10, 597–605 10.1007/s11894-008-0108-6 [DOI] [PubMed] [Google Scholar]
- Wittekindt C., Luers J. C., Klussmann J. P., Huttenbrink K. B. (2007). Pyoderma gangrenosum in the head and neck. Arch. Otolaryngol. Head Neck Surg. 133, 83–85 10.1001/archotol.133.1.83 [DOI] [PubMed] [Google Scholar]
- Wollina U. (2002). Clinical management of pyoderma gangrenosum. Am. J. Clin. Dermatol. 3, 149–158 10.2165/00128071-200203030-00002 [DOI] [PubMed] [Google Scholar]
- Yates V. M., Watkinson G., Kelman A. (1982). Further evidence for an association between psoriasis, Crohn’s disease and ulcerative colitis. Br. J. Dermatol. 106, 323–330 10.1111/j.1365-2133.1982.tb01731.x [DOI] [PubMed] [Google Scholar]