Abstract
Objective To assess the cost effectiveness of the Find and Treat service for diagnosing and managing hard to reach individuals with active tuberculosis.
Design Economic evaluation using a discrete, multiple age cohort, compartmental model of treated and untreated cases of active tuberculosis.
Setting London, United Kingdom.
Population Hard to reach individuals with active pulmonary tuberculosis screened or managed by the Find and Treat service (48 mobile screening unit cases, 188 cases referred for case management support, and 180 cases referred for loss to follow-up), and 252 passively presenting controls from London’s enhanced tuberculosis surveillance system.
Main outcome measures Incremental costs, quality adjusted life years (QALYs), and cost effectiveness ratios for the Find and Treat service.
Results The model estimated that, on average, the Find and Treat service identifies 16 and manages 123 active cases of tuberculosis each year in hard to reach groups in London. The service has a net cost of £1.4 million/year and, under conservative assumptions, gains 220 QALYs. The incremental cost effectiveness ratio was £6400-£10 000/QALY gained (about €7300-€11 000 or $10 000-$16 000 in September 2011). The two Find and Treat components were also cost effective, even in unfavourable scenarios (mobile screening unit (for undiagnosed cases), £18 000-£26 000/QALY gained; case management support team, £4100-£6800/QALY gained).
Conclusions Both the screening and case management components of the Find and Treat service are likely to be cost effective in London. The cost effectiveness of the mobile screening unit in particular could be even greater than estimated, in view of the secondary effects of infection transmission and development of antibiotic resistance.
Introduction
The incidence of tuberculosis in the UK has increased consistently over the past two decades.1 2 More than 9000 cases are reported every year, with 38% of cases occurring in London.2 This increase has been associated with a change in the epidemiology of the disease: although tuberculosis affected the general population in the past, most cases now occur in high risk groups. In particular, tuberculosis is widely recognised to be associated with social risk factors, including homelessness, problematic drug use, and imprisonment.3 Previous work suggests that the outcome of care in these population groups is probably suboptimal.3 4 In a recent study, the estimated prevalence of tuberculosis in hard to reach groups in London was 788 per 100 000 in homeless people, 354 per 100 000 in people with problematic drug use, and 208 per 100 000 in prisoners. By comparison, the overall prevalence of tuberculosis in London was 27 per 100 000 people.3 Although only 17% of tuberculosis cases in London are in hard to reach people, they account for nearly 38% of non-treatment adherent cases, 44% of cases lost to follow-up, and 30% of all highly infectious cases.3 Tuberculosis control therefore needs targeted interventions to address transmission in these groups.
In April 2005, the English Department of Health provided funding to set up a mobile radiography unit to actively screen for tuberculosis disease in London’s vulnerable populations, in view of their low rate of presentation for passive care. The service visits locations where high risk groups can be found, including drug treatment services and hostels or day centres for homeless and impoverished people. All individuals are screened on a voluntary basis regardless of symptom status.
Since September 2007, the mobile screening unit has been encompassed by the “Find and Treat” service. This service aims not only to screen and find active cases, but also to raise awareness, undertake case holding, and provide support for treatment completion for the same hard to reach groups. The unit has visited 210 locations across most London primary care trusts, with a median of 52 individuals screened at each location per year. The service uses links with drug and alcohol support services, hostels, and street outreach and criminal justice services to find cases and to maintain contact with patients during treatment to ensure completion. The availability of staff members to accompany patients to appointments and for home visits reduces the risk of cases being lost to follow-up. Awareness raising events take place in venues across London such as hostels, which are particularly supported by peer workers—individuals from high risk groups who have completed treatment for tuberculosis. The service not only oversees cases referred by the mobile screening unit, but also those referred by tuberculosis clinics across London, who are non-adherent to treatment or lost to follow-up care before treatment completion.
Mobile screening services have also been successfully trialled in the Netherlands and Zimbabwe,5 6where they seem to have potentially rapid effects on tuberculosis transmission and disease.5 The UK secretary of state for health has recently suggested that initiatives like the Find and Treat service could be implemented in cities such as Birmingham, where the prevalence of tuberculosis is rising.7
We aimed to evaluate the cost effectiveness of the Find and Treat service from September 2007 to July 2010 in London, using a decision analytical model set up with patient level data. As the service reaches the end of its pilot phase, the economic assessment of these interventions will help to inform key policy decisions on tuberculosis control in London and elsewhere.
Methods
Decision problem
We evaluated the cost effectiveness of the Find and Treat service by considering costs and outcomes of three groups: patients identified by the mobile screening unit with active pulmonary tuberculosis on chest radiograph examination and further diagnostic tests, patients referred to the service for enhanced case management support (that is, to ensure they complete treatment successfully), and patients referred to the service because they had been lost to follow-up. As controls in our study, we used the current method of passive case finding combined with ad hoc outreach in some primary care trusts, because no other interventions targeting hard to reach cases of tuberculosis have been trialled in London. We compared the following options: having no Find and Treat service, having only one part of the service (the mobile screening unit or the case management component), and having both parts of the service. We undertook the evaluation from the perspective of the healthcare taxpayer perspective.
Data sources
We used the Find and Treat database to obtain information on individuals with active pulmonary tuberculosis screened or managed by the service with record dates between September 2007 and September 2010. Records were not available before September 2007. Although the service used to screen a large number of prisoners, it has mostly stopped since the introduction of radiograph machines in prisons for active case finding in new inmates.
Cases detected by the mobile screening unit and engaged with the Find and Treat service were compared with passively detected control cases with active pulmonary tuberculosis (that is, individuals who presented to London tuberculosis services of their own accord without screening and referral to the Find and Treat service). We excluded patients with non-pulmonary tuberculosis since they would not be detected by chest radiography. A passively presenting group was the most appropriate control, because they received the care services that would be available in London in the absence of Find and Treat. We selected controls from passively detected cases in London that were notified to the Health Protection Agency’s enhanced tuberculosis surveillance system2 between 1 January 2009 (when the system began recording risk factor information) and 9 August 2010. We chose controls that were age matched with actively detected cases (within five year age categories) and that displayed one or more risk factors (a history of homelessness or imprisonment, drug or alcohol abuse, or mental health problems).
We obtained risk factor and clinical information for Find and Treat cases and passively presenting controls from the enhanced tuberculosis surveillance system. Case information was supplemented with data from the Find and Treat database. For each Find and Treat patient, we obtained the date of first screen (for those referred by the mobile screening unit), treatment outcome, and date of outcome, by matching patients to records in the enhanced tuberculosis surveillance system. For patients with symptoms on screening, we calculated diagnostic delays as the time from reported symptom onset to the start of treatment. We obtained dates of symptom onset and treatment by approaching individual clinics when they were not recorded in the databases. If the date of symptom onset was not available, we used the date when the patient sought clinical help. We calculated times to the final outcome as the difference between the date when the service engaged the patient and the date of treatment or episode completion.
The study included 75 mobile screening unit cases, 231 cases referred for case management support, 263 cases referred following loss to follow-up, and 315 passively presented control cases. We excluded cases of extrapulmonary tuberculosis, latent tuberculosis, and suspected tuberculosis; cases merely receiving prophylaxis (and hence unlikely to have active tuberculosis); cases for which the diagnostic delay could not be calculated; and cases younger than 16 years. After these exclusions, the study had 48 mobile screening unit cases, 188 cases referred for case management support, 180 cases referred for loss to follow-up, and 252 passively presenting control cases.
Compartmental model structure
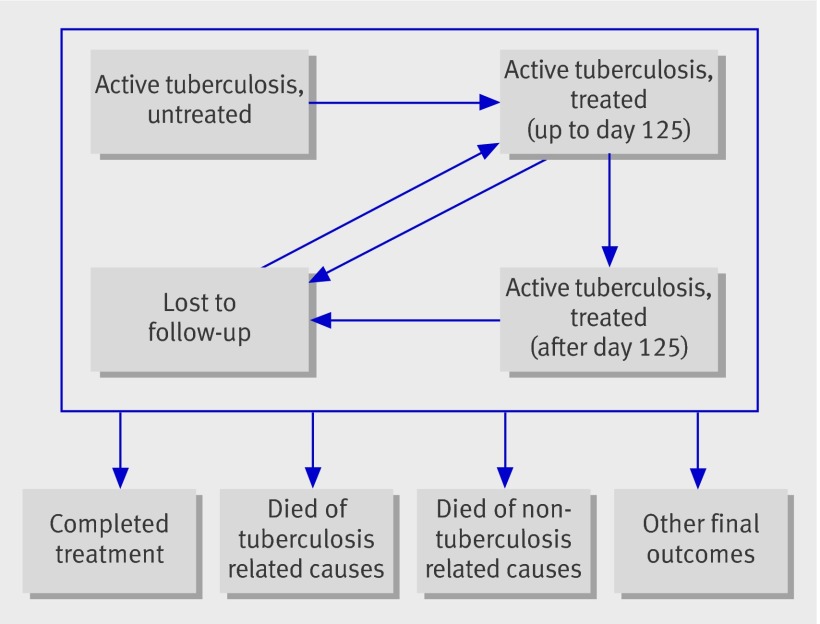
We used a discrete, multiple age cohort, compartmental model8 to model a population of individuals with active tuberculosis. Individuals in the model can occupy one of four health states: active untreated tuberculosis, active treated tuberculosis with up to 125 days of continuous treatment, active treated tuberculosis with more than 125 days of continuous treatment, and lost to follow-up. We split patients undergoing treatment by duration of treatment (with a 125 day cut off), which provided a better fit to data (web appendix). In our model, patients moved between these health states as they started treatment, completed treatment, were lost to follow-up, and re-engaged with treatment (fig ).
Health states in compartmental model for active tuberculosis cases, managed by the Find and Treat service
Every year, cases also have a probability of entering four final outcomes (from which they do not leave): completion of treatment, death due to tuberculosis related causes, death due to other causes, and other final outcomes that the Find and Treat service is not expected to change (such as patients being transferred out of London or stopping treatment for clinical reasons). To estimate the rate of death from causes not related to tuberculosis, we used 2009 all cause mortality rates from the Office for National Statistics. The model followed patients for the remainder of their lives.
The model included actual cases of active pulmonary tuberculosis that had been recorded as screened or managed by the Find and Treat service between September 2007 and September 2010. Since the recruitment period covered three years, we estimated that, on average, the service identified 16 and managed 123 individuals with active tuberculosis each year. Patients entered the model at the age when they were recorded as being detected by the mobile screening unit or referred to the service for case management.
For each case category (that is, cases screened, referred because of complex management issues, or referred after loss to follow-up), we compared the outcomes of a model cohort managed by the Find and Treat service with the outcomes of an equivalent cohort not managed by the service (based on passively detected controls). We then estimated the rates of transition between health states (table 1). We assumed that Find and Treat cases without confirmed active tuberculosis had no additional health benefit or detriment as a result of encountering the service.
Table 1.
Yearly transition rates (%) in model for cases detected or managed by Find and Treat service and for passively treated control cases
| Find and Treat cases | Control cases | |
|---|---|---|
| Previously untreated cases referred for treatment after screening by Find and Treat service | ||
| Completed treatment (if in first year of treatment) | 54.6 | 46.2 |
| Completed treatment (if in subsequent year of treatment) | 67.1 | 56.8 |
| Lost to follow-up | 2.1 | 17.2 |
| Died from tuberculosis related causes | 0 | 0 |
| Had final outcome other than treatment completion, tuberculosis related death, or loss to follow-up | 10.1 | 8.5 |
| Re-engaged with treatment after loss to follow-up | 51.0 | 51.0 |
| Cases referred to Find and Treat service for case management support because of complex issues | ||
| Completed treatment | 61.2 | 51.7 |
| Died from tuberculosis related causes | 3.3 | 3.3 |
| Lost to follow-up | 2.6 | 34.7 |
| Had final outcome other than treatment completion, tuberculosis related death, or loss to follow-up | 11.3 | 9.6 |
| Re-engaged with treatment after loss to follow-up | 51.0 | 51.0 |
| Cases under treatment referred to Find and Treat service because of loss to follow-up | ||
| Re-engaged with treatment (of the 51% who do eventually re-engage) | 81.7 | 0 |
| Lost again to follow-up (if on treatment) | 34.7 | 34.7 |
| Completed treatment (if on treatment) | 41.0 | 40.8 |
| Died from tuberculosis related causes | 2.2 | 2.6 |
| Had final outcome other than treatment completion, tuberculosis related death, or loss to follow-up | 7.6 | 7.6 |
| Untreated cases* | ||
| Died from tuberculosis related causes | 22.9 | 22.9 |
| Recovered | 21.0 | 21.0 |
| Categories specific to Find and Treat cases | ||
| Cases with asymptomatic active tuberculosis (as % of mobile screening unit cases) | 35.4 | – |
| Extremely hard to reach cases (diagnostic delay >131 days) (as % of mobile screening unit cases)† | 22.9 | – |
| Cases that are found and re-engage with treatment (as % of active tuberculosis cases referred to Find and Treat service for loss to follow-up) | 51.7 | – |
| Multidrug resistant or extensively drug resistant active tuberculosis cases‡ | ||
| Mobile screening unit cases | 0.5 | – |
| Other Find and Treat cases | 5.3 | – |
Rates estimated by matching Find and Treat records to enhanced tuberculosis surveillance data, unless stated otherwise.
*Rates estimated by analysis of cases from south Indian study.28
†Includes cases that would not be diagnosed without mobile screening unit.
‡Rates estimated by analysis of Find and Treat records.
Costs and quality of life weights
Untreated cases of active tuberculosis were assumed to have a utility score of 0.68 (standard error 0.5), based on the mean EQ-5D score given to cases diagnosed with tuberculosis in London9 (individual level data obtained from M Kruijshaar, personal communication). The EQ-5D is a standardised instrument used to measure quality of life and its use is preferred by the National Institute for Health and Clinical Excellence (NICE). This utility score of 0.68 was also reported in a Canadian study using an alternative instrument to measure quality of life, the SF-6D.10 For the patients in London, EQ-5D scores improved to 0.81 (standard error 0.04) after 2 months of treatment. Hence we assumed that in the first year of treatment, patients would have an intermediate utility score of 0.79, before reaching a score of 0.81 in subsequent years.9 We assumed cases without active tuberculosis to have the same utility score as population norms.11 Table 2 summarises the economic variables used in the model.
Table 2.
Economic variables in model
| Cost/utility score | Source | |
|---|---|---|
| Find and Treat service | ||
| Mobile screening unit | £530 024 | Find and Treat budget* |
| Case management team | £512 825 | Find and Treat budget† |
| Treatment (per case) | ||
| Non-multidrug resistant tuberculosis | £5522 | NICE report16 |
| Multidrug resistant tuberculosis | £31 329 | NICE report16 |
| Tuberculosis sputum test | £7.12 | National Health Service reference costs15 |
| Quality of life weights | ||
| Active tuberculosis, untreated | 0.68 | EQ-5D study11 |
| Active tuberculosis, first year of treatment | 0.79 | EQ-5D study11 |
| Active tuberculosis, second and subsequent year of treatment | 0.81 | EQ-5D study11 |
*Breakdown of costs: staff costs for registrar, nurse, administrator, driver, and radiographer (£345 624); training and development (£20 000); travel and subsistence (£4800); contracted administration (£15 000); maintenance and cleaning (£25 000); insurance and fuel (£18 000); radiography maintenance (£50 000); and office and management (£51 600).
†Breakdown of costs: staff costs for nurse, social worker, outreach worker, clinical lead and contracted nurse (£361 225); training and development (£30 000); travel and subsistence (£3200); contracted administration (£15 000); and office and management (£103 400).
We obtained costs for staff salaries, training and development, travel and subsistence, administration, maintenance, cleaning, insurance, fuel, office management, and radiography equipment maintenance in 2009-10 from the Find and Treat records. We increased staff costs to take into account qualification and capital overhead costs, by mapping job descriptions of Find and Treat staff to their nearest equivalents (in terms of role and salary) from standard costing sources.12 We did not include non-capital overhead costs, since these were already part of the Find and Treat annual budget.
Chest radiography costs were already included in the overall Find and Treat budget. Patients referred for diagnostic testing (regardless of whether they were subsequently found to have active tuberculosis) were assumed to incur the cost of a laboratory culture test, estimated from the tariff cost for microbiological pathology services.13 We estimated the cost of treating a case of tuberculosis from NICE guidelines.14 Also, increased treatment costs were associated with multidrug resistant patients.14 Based on Find and Treat data, 0.5% of mobile screening unit patients and 5.3% of other Find and Treat patients had multidrug or extensively drug resistant infection (table 1); the remaining patients’ infections were not resistant or were resistant to only one drug. We inflated costs to 2009-10 prices using the hospital and community health services pay and prices index.12
We then added up the costs incurred and quality adjusted life years (QALYs) associated with each health state (fig), with and without the Find and Treat service, with future costs and benefits discounted at a rate of 3.5% per year as recommended by NICE.15
To determine the cost effectiveness of the two components of the Find and Treat service, we allocated costs between the mobile screening unit and the case management team according to the estimates given in the Find and Treat budget (including qualification and capital overhead costs). However, we transferred an additional amount from the case management service budget to the mobile screening unit budget to represent enhanced follow-up of screened cases, based on the proportion of cases followed up who were identified by the mobile screening unit.
Sensitivity analysis
To test the robustness of the results, we made several assumptions (individually and in combination) that were less favourable to Find and Treat.
Increased costs for mobile screening unit
Instead of using standard UK National Health Service capital overheads for the cost of the unit, we assumed that a new mobile unit would need to be purchased, and that it would last five years before being decommissioned. We assumed the cost of a new unit to be £600 000 (excluding maintenance costs, which were already incorporated in the unit’s running budget). We added this amount to the costs of the first year of the service, with discounted costs and outcomes totalled over five years.
Increased cost of tuberculosis treatment
Instead of using costs for tuberculosis treatment from a NICE report, we used higher figures from a costing study,16 and inflated them to 2009-10 prices using the pay and prices index for hospital and community health services. This gave costs of £8300 and £75 000 for treatment of drug sensitive and multidrug resistant tuberculosis, respectively.12
Improved quality of life for untreated tuberculosis case, and poor quality of life for tuberculosis cases on treatment
We raised the QALY weight for untreated tuberculosis cases to the upper 95% limit of the sampling distribution of its mean (utility score 0.76). We also lowered the QALY weight for treated tuberculosis cases to 0.76 (so that putting patients on treatment is not assumed to provide a better health related quality of life until treatment is completed).
Asymptomatic cases detected by mobile screening unit do not always progress to symptomatic disease
We assumed that only 50% of asymptomatic cases with a positive result from the mobile screening unit would progress to symptomatic disease.
Cases referred to Find and Treat service for enhanced case management have a reduced loss to follow-up rate in the absence of the service
We assumed that Find and Treat cases would be lost to follow-up at the same rate as enhanced tuberculosis surveillance controls (17.2% per year) in the absence of the service, rather than at the higher rate we estimated for this extremely hard to reach group (34.7% per year).
Cases referred to Find and Treat service for loss to follow-up could still passively re-engage with treatment
We assumed that even without Find and Treat involvement, these cases could still passively re-engage with treatment at the same rate as enhanced tuberculosis surveillance controls (51% per year).
Results
Transition rates of cases managed and not managed by Find and Treat
The web appendix provides estimated values and full details of the estimation procedure used to compare the transition rates between health states in the two cohorts.
Previously untreated cases referred for treatment after detection by mobile screening unit
We estimated that the 22.9% of patients detected by the mobile screening unit with the longest delays between symptom onset and treatment presentation were unlikely to present for treatment without the activities of the Find and Treat service. Furthermore, 35.4% of mobile screening unit patients were asymptomatic on detection, and hence would not have presented for treatment without the unit. We assumed that asymptomatic patients would progress rapidly to symptomatic disease,17 and that in the absence of the Find and Treat service, they would behave similarly to symptomatic patients (that is, some would present for treatment, but 22.9% would not). Once on treatment, mobile screening unit cases managed by the Find and Treat service had a much lower risk of loss to follow-up than passively presenting controls (loss to follow-up probability after one year: 2.1% for cases, 17.2% for controls).
Cases referred to Find and Treat service for case management support
We found that cases referred to Find and Treat because of complex case management issues had higher rates of completing treatment (61.2% after one year) and lower rates of loss to follow-up (3.3% after one year) than controls.
Cases under treatment referred to Find and Treat service because of loss to follow-up
We estimated that 51.7% of cases of active pulmonary tuberculosis referred to the Find and Treat service because of loss to follow-up would subsequently return to the service. We estimated cases become lost to follow-up after re-engagement at an annual rate of 34.7%. Once this happens, we estimated that the annual probability of re-engagement if cases were again lost to follow-up was 81.7%; we assumed the remaining cases to be permanently lost. Since these lost cases have little hope of re-engaging with treatment after referral, we assumed that, in the absence of the Find and Treat service, these cases would not be re-engaged.
Cost effectiveness of Find and Treat service
Table 3 shows the costs incurred and QALYs accrued by the cohort of active tuberculosis cases described in this report, for scenarios with and without the Find and Treat service. We estimated that every year the service has a net cost of £1.4 million and gains 220 QALYs. Hence the incremental cost effectiveness of the Find and Treat service was estimated to be £6400/QALY gained. The web appendix shows how the service affects the health state of cases over time.
Table 3.
Costs incurred and QALYs accrued by active tuberculosis cases with and without Find and Treat service
| With Find and Treat | Without Find and Treat | Difference | |
|---|---|---|---|
| Costs incurred | |||
| Find and Treat service | £1 000 000 | £0 | £1 000 000 |
| Diagnostic tests | £730 | £330 | £400 |
| Treatment | £690 000 | £310 000 | £400 000 |
| Total | £1 700 000 | £310 000 | £1 400 000 |
| QALYs accrued | |||
| Mobile screening unit for hard to reach patients | 91 | 87 | 4 |
| Mobile screening unit for extremely hard to reach patients | 48 | 29 | 19 |
| Mobile screening unit for asymptomatic cases | 78 | 65 | 13 |
| Referrals because of complex case management issues | 580 | 520 | 60 |
| Referrals because of loss to follow-up | 350 | 220 | 130 |
| Total | 1100 | 920 | 220 |
| Incremental cost effectiveness ratio | – | – | £6400 |
Data are rounded to 2 significant figures.
We also found both components of the service to be cost effective at the same threshold. The mobile screening unit had an incremental ratio of £18 000/QALY gained, whereas the case management component had an incremental ratio of £4100/QALY gained. The ratio increased slightly when we used assumptions that were more unfavourable to the Find and Treat service, but not enough to change the overall conclusion (table 4). In the most unfavourable (and highly unlikely) scenario, which combined all the unfavourable assumptions, the mobile screening unit and case management components had incremental ratios of £26 000/QALY gained and £6800/QALY gained, respectively.
Table 4.
Cost effectiveness comparison of Find and Treat components under unfavourable scenarios
| Scenario | Incremental cost effectiveness ratio | ||
|---|---|---|---|
| Find and Treat service (components combined) | Mobile screening unit | Case management | |
| Base case | £6400 | £18 000 | £4100 |
| Increased mobile screening unit costs | £6700 | £20 000 | £4100 |
| Increased treatment costs | £7600 | £18 000 | £5600 |
| Improved quality of life for untreated tuberculosis and poor quality of life for treated tuberculosis | £6500 | £19 000 | £4200 |
| Asymptomatic mobile screening unit cases do not always progress to symptomatic disease | £6500 | £22 000 | £4100 |
| Cases referred to Find and Treat service for enhanced case management have lower rate of loss to follow-up than those not referred | £7100 | £18 000 | £4600 |
| Cases referred to Find and Treat service for loss to follow-up could passively re-engage with treatment | £7500 | £18 000 | £4700 |
| Most unfavourable scenario to Find and Treat service* | £10 000 | £26 000 | £6800 |
Data rounded to 2 significant figures. *All scenarios combined, apart from base case.
Discussion
Principal findings, strengths, and limitations of study
The Find and Treat service appears to be cost effective, based on a threshold of £20 000-£30 000/QALY gained, used by NICE.15 Although analyses of each Find and Treat component should be interpreted with caution because of overlapping costs between the two services, they both seem to be cost effective separately. We obtained similar results when we made assumptions that were more unfavourable to the Find and Treat service. In the most unfavourable case, in which data were interpreted unfavourably in five instances to create a very highly unlikely scenario, the mobile screening unit had an incremental cost effectiveness ratio of about £26 000 (table 4). NICE would often still consider this ratio to be acceptable,15 particularly since during model parameterisation, whenever data were not available, we made conservative assumptions to ensure that the benefit of Find and Treat was not overestimated.
One limitation of our analysis is the absence of a trial randomising tuberculosis cases to be either managed or not managed by the Find and Treat service. The absence of randomisation meant that we could not be sure of the outcomes of Find and Treat managed cases if the service did not exist. Consequently, we based our model of such outcomes on controls with active tuberculosis in London’s enhanced tuberculosis surveillance system who presented for care. Although we chose controls to have at least one risk factor to represent the potential target population for the Find and Treat service, the service also manages extremely hard to reach individuals, who are often already lost to follow-up at the time of referral or who would never present for care without the mobile screening unit. Hence the comparison of cases with retrospective controls probably underestimates the incremental benefit of the service, although we cannot be certain without a randomised study.
Furthermore, the methods used for the modelling do not fully capture the benefits of the Find and Treat service. For example, we did not incorporate secondary transmission into the economic evaluation, even though the mobile screening unit in particular probably averts several secondary cases by finding highly infectious individuals. For example, in a study of a large ongoing epidemic of isoniazid resistant tuberculosis in London in 2004,18 researchers found 87 cases with traceable contacts, who had 525 contacts between them. Of these contacts, 355 completed screening and 11% of these screened contacts became cases.
Another simplification was that we did not measure the effect of the Find and Treat service on reducing the likelihood of patients developing and transmitting acquired drug resistance (as a result of poor treatment adherence). Drug resistance increases the duration and costs of treatment, as well as the risk of severe disease, thus prevention could be an important benefit of the service. Full capture of these benefits would need a dynamic transmission model. However, since both Find and Treat components already seem cost effective without incorporating dynamic effects, the use of a static model appears to be justified.
Comparisons with other studies and implications
There is wide international consensus that the provision of untargeted chest radiograph examination to the whole population for tuberculosis detection is largely ineffective.17 19 There is also agreement that prompt diagnosis and adequate treatment of active cases is the most crucial element of tuberculosis control. Evaluations of interventions to actively find cases, such as radiological screening of high risk groups, suggest that not only can the screening of high risk groups detect cases more effectively than door to door symptom based screening,5 but also that transmission can be interrupted when the intervention is appropriately targeted.6 The accompanying editorial to the trial by Corbett and colleagues, while supporting active case finding in high prevalence groups, argued that any attempt to scale up such interventions in the community should be accompanied by systems that ensure treatment completion and an assessment of cost effectiveness.20
London has seen a resurgence of tuberculosis on a scale not seen in any other western European capital in the past two decades.21 22 23 The incidence of tuberculosis in the London borough of Brent is comparable to that in Karonga District in Malawi.2 24 It is therefore appropriate that any intervention that provides a cost effective means to identify cases promptly and ensure that they complete treatment is an essential component of the tuberculosis control programme. This study shows that the Find and Treat service provides an intervention that detects hard to reach cases of symptomatic and asymptomatic tuberculosis, and ensures that they receive treatment. The cost effectiveness compares favourably with other interventions currently funded to control tuberculosis in the UK.14 For example, the dual strategy of tuberculin skin testing and interferon gamma release assay testing has an incremental cost effectiveness ratio of £29 955/QALY gained, and universal BCG vaccination of school children has a ratio of £56 000/QALY gained (assuming 0.05% infection prevalence and 10 years of vaccine protection).
The true value of the Find and Treat service to public health will only fully be realised when a cost effective rapid point of care diagnostic tool becomes available. Although there are promising reports of rapid molecular tests endorsed by the World Health Organization,25 26 there is currently no test to accurately detect true latent infection, and molecular assays would benefit from further field evaluation in this setting.
In conclusion, this study shows that the London Find and Treat service is a cost effective intervention. Indeed, both the screening and case management support components of the intervention are likely to be cost effective in London. Although the Find and Treat service alone will probably not reverse the rise in tuberculosis in London, it will probably be helpful to the individuals who have the greatest evidence of ongoing transmission.27 Further studies should assess using point of care testing within community outreach settings such as the mobile screening unit, as well as the role of community based delivery of treatment. Additionally, a randomised trial comparing patients managed and not managed by the Find and Treat service would enable estimates of the service’s benefits to be made with greater certainty.
What is already known on this topic
Tuberculosis in hard to reach groups such as homeless people, prisoners, and problem drug users accounts for 17% of cases in London, 38% of non-treatment adherent cases, 44% of cases lost to follow-up, and 30% of all highly infectious cases
Since September 2007, the tuberculosis Find and Treat service has been funded by the English Department of Health for active case finding, case holding, and treatment completion support for hard to reach groups in London
What this study adds
The incremental cost effectiveness of the Find and Treat service is estimated to be £6400-£10 000/QALY gained
Both the mobile screening unit and the case management component seem to be cost effective, using a threshold of £20 000-£30 000/QALY gained
Contributors: MJ and HRS designed the economic model and retrospective cohort study, respectively, with input from IA and PJW. The Find and Treat evaluation team (Ibrahim Abubakar, Robert Aldridge, Charlotte Anderson, Mark Jit, Michelle Kruijshaar, Laura Pimpin, Helen Stagg, Surinder Tamne, and Peter White) collected the patient data, which were analysed by MJ, HRS, and RWA. MJ drafted the manuscript with input from all the other authors. All authors approved the final version to be published. MJ is the guarantor.
Funding: This work was funded by a grant from the English Department of Health (policy research programme grant reference number 0150305). PJW was partly funded by centre funding from the Medical Research Council. IA and HS are partly funded by the National Institute for Health Research. The authors’ work was independent of the funders, who had no role in the study design, analysis of data, writing of the manuscript, or decision to submit for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: this work was funded by the English Department of Health, Medical Research Council, and National Institute for Health Research; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data for sharing: Full numerical results of the scenarios described here are available from the corresponding author on request.
Cite this as: BMJ 2011;343:d5376
Web Extra. Extra material supplied by the author
Web appendix: Detailed justification for state transition parameters in model
References
- 1.Crofts JP, Gelb D, Andrews N, Delpech V, Watson JM, Abubakar I. Investigating tuberculosis trends in England. Public Health 2008;122:1302-10. [DOI] [PubMed] [Google Scholar]
- 2.Health Protection Agency. Tuberculosis in the UK: annual report on tuberculosis surveillance in the UK. HPA, 2010.
- 3.Story A, Murad S, Roberts W, Verheyen M, Hayward AC. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax 2007;62:667-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson C, Story A, Brown T, Drobniewski F, Abubakar I. Tuberculosis in UK prisoners: a challenge for control. J Epidemiol Community Health 2010;64:373-6. [DOI] [PubMed] [Google Scholar]
- 5.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ,et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010;376:1244-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vries G, van Hest RA, Richardus JH. Impact of mobile radiographic screening on tuberculosis among drug users and homeless persons. Am J Respir Crit Care Med 2007;176:201-7. [DOI] [PubMed] [Google Scholar]
- 7.House of Commons. Hansard (debate): 7 Dec 2010, column 155. 2010. www.publications.parliament.uk/pa/cm201011/cmhansrd/cm101207/debtext/101207-0001.htm.
- 8.Dewilde S, Anderson R. The cost-effectiveness of screening programs using single and multiple birth cohort simulations: a comparison using a model of cervical cancer. Med Decis Making 2004;24:486-92. [DOI] [PubMed] [Google Scholar]
- 9.Kruijshaar ME, Lipman M, Essink-Bot ML, Lozewicz S, Creer D, Dart S,et al. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis 2010;14:296-302. [PubMed] [Google Scholar]
- 10.Guo N, Marra CA, Marra F, Moadebi S, Elwood RK, Fitzgerald JM. Health state utilities in latent and active tuberculosis. Value Health 2008;11:1154-61. [DOI] [PubMed] [Google Scholar]
- 11.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis L. Unit costs of health and social care 2010. Personal Social Services Research Unit, University of Kent, 2010.
- 13.Department of Health. NHS reference costs 2008-2009. 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591.
- 14.National Collaborating Centre for Chronic Conditions. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. NICE clinical guidelines no 33. Royal College of Physicians, 2006. [PubMed]
- 15.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. NICE, 2008. [PubMed]
- 16.White VL, Moore-Gillon J. Resource implications of patients with multidrug resistant tuberculosis. Thorax 2000;55:962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toman K. How does pulmonary tuberculosis develop and how can it be detected at an early stage? In Toman K. Toman’s Tuberculosis. Case detection, treatment, and monitoring—questions and answers. 2nd ed. World Health Organization, 2004:66-71.
- 18.Neely F, Maguire H, Le Brun F, Davies A, Gelb D, Yates S. High rate of transmission among contacts in large London outbreak of isoniazid mono-resistant tuberculosis. J Public Health (Oxf) 2010;32:44-51. [DOI] [PubMed] [Google Scholar]
- 19.Mangura BT, Reichman LB. Periodic chest radiography: unnecessary, expensive, but still pervasive. Lancet 1999;353:319-20. [DOI] [PubMed] [Google Scholar]
- 20.Getahun H, Raviglione M. Active case-finding for TB in the community: time to act. Lancet 2010;376:1205-6. [DOI] [PubMed] [Google Scholar]
- 21.Quabeck L, Haas W. Comparative analysis of tuberculosis epidemiology in capitals and countries in the West/EU-EuroTB region. 2009. www.depol.org/js/ckfinder/userfiles/files/Complete-Programme-IUATLD09.pdf.
- 22.Anderson SR, Maguire H, Carless J. Tuberculosis in London: a decade and a half of no decline [corrected]. Thorax 2007;62:162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zumla A. The white plague returns to London—with a vengeance. Lancet 2010;377:10-1. [DOI] [PubMed] [Google Scholar]
- 24.Crampin AC, Glynn JR, Fine PE. What has Karonga taught us? Tuberculosis studied over three decades. Int J Tuberc Lung Dis 2009;13:153-64. [PMC free article] [PubMed] [Google Scholar]
- 25.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F,et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO endorses new rapid tuberculosis test [press release]. 2010. www.who.int/mediacentre/news/releases/2010/tb_test_20101208/en/index.html.
- 27.Love J, Sonnenberg P, Glynn JR, Gibson A, Gopaul K, Fang Z,et al. Molecular epidemiology of tuberculosis in England, 1998. Int J Tuberc Lung Dis 2009;13:201-7. [PubMed] [Google Scholar]
- 28.Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ 1974;51:473-88. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Detailed justification for state transition parameters in model