Abstract
Objective
Cognitive deficits in schizophrenia are associated with altered activity of the dorsolateral prefrontal cortex, which has been attributed to lower expression of the 67 kDa isoform of glutamic acid decarboxylase (GAD67), the major γ-aminobutyric acid (GABA)-synthesizing enzyme. However, little is know n about the relationship of prefrontal GAD67 m RNA levels and illness severity, translation of the transcript into protein, and protein levels in axon terminals, the key site of GABA production and function.
Method
Quantitative polymerase chain reaction was used to measure GAD67 m RNA levels in postmortem specimens of dorsolateral prefrontal cortex from subjects with schizophrenia and matched comparison subjects with no know n history of psychiatric or neurological disorders (N=42 pairs). In a subset of this cohort in which potential confounds of protein measures were controlled (N=19 pairs), Western blotting was used to quantify tissue levels of GAD67 protein in tissue. In five of these pairs, multilabel confocalimm unofluorescence was used to quantify GAD67 protein levels in the axon terminals of parvalbumin-containing GABA neurons, which are know n to have low levels of GAD67 m RNA in schizophrenia.
Results
GAD67 m RNA levels were significantly lower in schizophrenia subjects (by 15%), but transcript levels were not associated with predictors or measures of illness severity or chronicity. In schizophrenia subjects, GAD67 protein levels were significantly lower in total gray matter (by 10%) and in parvalbumin axon terminals (by 49%).
Conclusions
The findings that lower GAD67 m RNA expression is com m on in schizophrenia, that it is not a consequence of having the illness, and that it leads to less translation of the protein, especially in the axon terminals of parvalbumin-containing neurons, support the hypothesis that lower GABA synthesis in parvalbumin neurons contributes to dorsolateral prefrontal cortex dysfunction and impaired cognition in schizophrenia.
Cognitive impairments are a core feature of schizophrenia and are the best predictor of functional outcome (1). The cognitive deficits span multiple domains (2), suggesting that they reflect an overarching alteration in cognitive control, the ability to adjust thoughts or behaviors in order to achieve goals (3). Cognitive control depends on the coordinated activity of a number of brain regions, including the dorsolateral prefrontal cortex, which plays an integral role in the maintenance and selection of rules that govern behavioral responses (4). Individuals with schizophrenia exhibit altered activation of the dorsolateral prefrontal cortex (5), and dorsolateral prefrontal cortex dysfunction is correlated with cognitive impairment (6).
Frontal lobe gamma band (30–80 Hz) oscillations, which appear to underlie higher-order cognitive processes (7), are positively associated with cognitive control processes in healthy comparison subjects, but schizophrenia patients fail to exhibit the normal task-related increase in gamma band power (8, 9). Gamma oscillations are known to depend on the firing of γ-aminobutyric acid (GABA) neurons (10), and thus alterations in GABA neurotransmission in the dorsolateral prefrontal cortex may contribute to impaired cognition in schizophrenia (11).
GABA signaling is regulated by levels of its synthesizing enzyme, glutamic acid decarboxylase (GAD). The 67-kDa isoform (GAD67) is responsible for the majority of GABA synthesis in the cortex (12), whereas the 65 kDa isoform (GAD65) contributes to GABA synthesis principally during conditions of high synaptic demand (13). Consistent with this interpretation, reducing GAD67 expression in mice results in lower GAD activity and GABA content (14), but reducing GAD65 expression does not significantly alter cortical GABA levels (15). Lower GAD67 mRNA levels in the dorsolateral prefrontal cortex have been consistently reported in schizophrenia (16). However, the relationship between GAD67 mRNA expression and the clinical features of the illness has not been examined in a large sample. Furthermore, since GAD67 expression is activity dependent (17), lower expression might just index the less stimulating social, occupational, and intellectual environment associated with illness chronicity. Accordingly, we quantified GAD67 mRNA levels in postmortem specimens of dorsolateral prefrontal cortex from 42 subjects with schizophrenia and 42 matched comparison subjects and examined their relationship to predictors of illness severity, measures of functional outcome, and duration of illness.
Although lower GAD67 mRNA levels in the dorsolateral prefrontal cortex are consistently found in schizophrenia, how these findings are related to levels of the cognate protein remains unclear despite a single study reporting lower mean levels of both GAD67 mRNA and protein in schizophrenia (18). This question is of particular importance given that mRNA and protein levels are not necessarily correlated, since a number of factors regulate transcription and translation (19). For example, pharmacological manipulation of GABA levels is associated with changes in GAD67 protein but not mRNA (20). Furthermore, measures of protein in postmortem human tissue are subject to confounds that do not influence transcript measures (21). Thus, we employed a quantitative Western blotting strategy that minimized sources of variability to test the hypothesis that cortical GAD67 protein levels are lower in schizophrenia.
Understanding the functional significance of altered GAD67 protein levels requires knowledge of the affected cell class. In schizophrenia, GAD67 mRNA levels are markedly lower in only some 25%–35% of interneurons (22, 23), including the parvalbumin-containing GABA neurons (24) that are crucial to the generation of gamma oscillations (25). Thus, if lower GAD67 mRNA and protein levels in parvalbumin neurons underlie the impaired gamma band oscillations and cognitive control deficits in schizophrenia (10), then lower GAD67 protein levels should be particularly apparent in parvalbumin cells at the principal site of GABA production and function, the axon terminal. Accordingly, we used quantitative fluorescence imaging (26) in a proof-of-concept test of the idea that GAD67 protein levels are much lower in parvalbumin axon terminals than in total cortical gray matter.
Method
Human Subjects
Brain specimens (N=84) were obtained during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh) after consent was obtained from the next of kin. Consensus DSM-IV diagnoses for each subject were made by a committee of experienced clinicians on the basis of medical records and the results of structured interviews conducted with family members of the deceased (27). To reduce biological variance between groups and to control for experimental variation, each schizophrenia subject was matched with one comparison subject for sex and as closely as possible for age and postmortem interval, and tissue samples from members of a pair were always processed together. Comparison subjects had no known history of neurological or psychiatric illness. Tissue was collected from Brodmann’s area 9, and samples were prepared as described in the supplemental Methods section of the data supplement that accompanies the online edition of this article. All procedures were approved by the Committee for the Oversight of Research Involving the Dead and the Institutional Review Board for Biomedical Research at the University of Pittsburgh.
For quantification of GAD67 mRNA levels in schizophrenia, tissue from all 42 subject pairs was utilized. For every subject, the RNA integrity number (RIN) was >7, which is associated with excellent RNA quality (28). Mean age, postmortem interval, brain pH, RIN, and tissue storage time were not significantly different between the two subject groups (Table 1; for individual subject characteristics, see Table S1 in the online data supplement). For measures of total GAD67 protein, only the subject pairs in which both members had postmortem intervals less than 20 hours (N=19; see Results for rationale) were used (Table 1). Of these 19 pairs, five were selected for the parvalbumin axon terminal study (see Results for rationale) (Table 1).
TABLE 1.
Characteristics of the Comparison and Schizophrenia Groups for Each Analysis of Dorsolateral Prefrontal Cortex Specimensa
| Characteristic | Study and Group
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantitative PCR
|
Western Blotting
|
Immunofluorescence
|
||||||||||
| Comparison | Schizophrenia | Comparison | Schizophrenia | Comparison | Schizophrenia | |||||||
| Group (N=42) | Group (N=42) | Group (N=19) | Group (N=19) | Group (N=5) | Group (N=5) | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
|
| ||||||||||||
| Male | 31 | 73.8 | 31 | 73.8 | 14 | 73.7 | 14 | 73.7 | 5 | 100.0 | 5 | 100.0 |
| Race | ||||||||||||
| White | 34 | 81.0 | 29 | 69.0 | 14 | 73.7 | 14 | 73.7 | 5 | 100.0 | 5 | 100.0 |
| Black | 8 | 19.0 | 13 | 31.0 | 5 | 26.3 | 5 | 26.3 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||||||||
| Age at death (years) | 48 | 13 | 47 | 13 | 44 | 13 | 43 | 11 | 45 | 12 | 47 | 9 |
| Postmortem interval (hours) | 17.8 | 5.9 | 18.1 | 8.7 | 12.5 | 4 | 11.4 | 5.4 | 14.3 | 3.6 | 12.3 | 4.7 |
| Brain pH | 6.8 | 0.2 | 6.6 | 0.4 | 6.8 | 0.2 | 6.5 | 0.3 | 6.8 | 0.2 | 6.5 | 0.4 |
| RNA integrity number | 8.3 | 0.6 | 8.2 | 0.7 | 8.5 | 0.6 | 8.2 | 0.6 | 8.6 | 0.4 | 8.1 | 0.6 |
| Freezer storage time (months) | 97 | 43 | 97 | 46 | 95 | 45 | 99 | 44 | 116 | 16 | 110 | 23 |
For each study, there were no significant between-group differences, except for pH in the Western blotting study (t=3.0, df=36, p=0.005).
GAD67 mRNA Levels
GAD67 mRNA levels were assessed by quantitative polymerase chain reaction (qPCR) using the comparative threshold cycle measurement method (27). Four replicate measures were performed for each subject, with a standard threshold for each transcript consistently applied across all subjects. Based on their stable level of expression between schizophrenia and comparison subjects (27), three reference mRNAs (β-actin, cyclophilin A, and glyceraldehyde-3-phosphate dehydrogenase) were used to normalize GAD67 mRNA levels.
Total GAD67 Protein Levels
Western blotting was performed as described in the supplemental Method section in the online data supplement. Bands were measured in arbitrary optical density units by a single investigator (A.A.C.) using the Odyssey Infrared Imaging System software program (LI-COR Biosciences, Lincoln, Neb.). Red (680 nm) and green (800 nm) channels were quantified separately. Optical density was measured by drawing a rectangle around each band. The size of the rectangle was adjusted to include the entire band without including signal from neighboring bands. Each band was measured three times with a new rectangle. Background optical density was measured according to LI-COR specifications.
In an effort to reduce, and account for, the within- and between-subject measurement variance, replicate measures for each subject pair were made both within and across gels. Samples from members of a pair were loaded in adjacent lanes of the same gel. Each pair of samples was loaded four times on a gel, and four replicate gels were run per pair and processed simultaneously (i.e., 16 replicate measures). Of the resulting 608 data points, 28 were dropped before analysis because of obvious gel artifacts, with no more than two of the 16 measures per subject excluded.
GAD67 Protein Levels in Parvalbumin Puncta
Fluorescence immunohistochemistry was used to label two tissue sections per subject with antibodies against GAD65, GAD67, and parvalbumin (see the supplemental Method section in the online data supplement). Using a previously published method for systematic sampling (29), a region within Brodmann’s area 9 where the tissue section was cut perpendicular to the pial surface was identified. Sampling sites were then placed in this region in a systematic random fashion within layers 3–4, defined as 30%–60% of the pia-to-white matter distance (30). The GAD67 mRNA deficit in parvalbumin neurons in schizophrenia is most pronounced in these layers (24). Image stacks (N=180) were collected from the five subject pairs on a confocal microscope as described in the supplemental Method section in the online data supplement.
Because the density of GAD65-immunoreactive axon terminals is not altered in schizophrenia (31), GAD65 immunoreactivity was used to identify immunoreactive puncta (defined as labeled structures 0.06–0.70 μm3 in size) that represent putative axon terminals. Postprocessing and data segmentation were performed using a method that allows for the quantification of fluorescently labeled puncta, the co-localization of different labels in the same puncta, and the quantification of fluorescence intensity in these same structures (26). In this method, a binary channel (mask) is made of the segmented pixels and used to assess fluorescence intensity. Each mask is composed of multiple mask objects (immunoreactive puncta). Mask operations between channels were used to determine GAD65-immunoreactive puncta that were also labeled for parvalbumin and GAD67.
Statistical Analysis
Two analysis of covariance (ANCOVA) models were performed to test the effect of diagnosis on GAD67 mRNA level. The first model included mRNA level as the dependent variable, diagnostic group as the main effect, subject pair as a blocking factor, and storage time, brain pH, and RIN as covariates. Subject pairing may be considered an attempt to balance diagnostic groups for sex, age, and postmortem interval and to account for the parallel processing of tissue samples from a pair and thus not a true statistical paired design. Therefore, we also used a second model without subject pair as a blocking factor that included sex, age, postmortem interval, storage time, brain pH, and RIN as covariates.
A similar approach was used for total protein measures of GAD67, with appropriate adjustments made for covariates. However, to account for the across-gel measurement variability inherent in Western blotting, samples from a single subject pair comprised all lanes of a given gel, and thus only a paired ANCOVA model was used. The use of a paired model is supported by the similar results obtained with both paired and unpaired ANCOVA analyses of GAD67 mRNA levels.
Paired t tests were used to assess whether the GAD67 protein levels in parvalbumin puncta differed between comparison and schizophrenia subjects.
The reported p values for comparisons of GAD67 levels are one-tailed because mRNA levels have been shown to be lower in schizophrenia (16). All other p values are two-tailed.
Results
GAD67 mRNA Expression in Schizophrenia
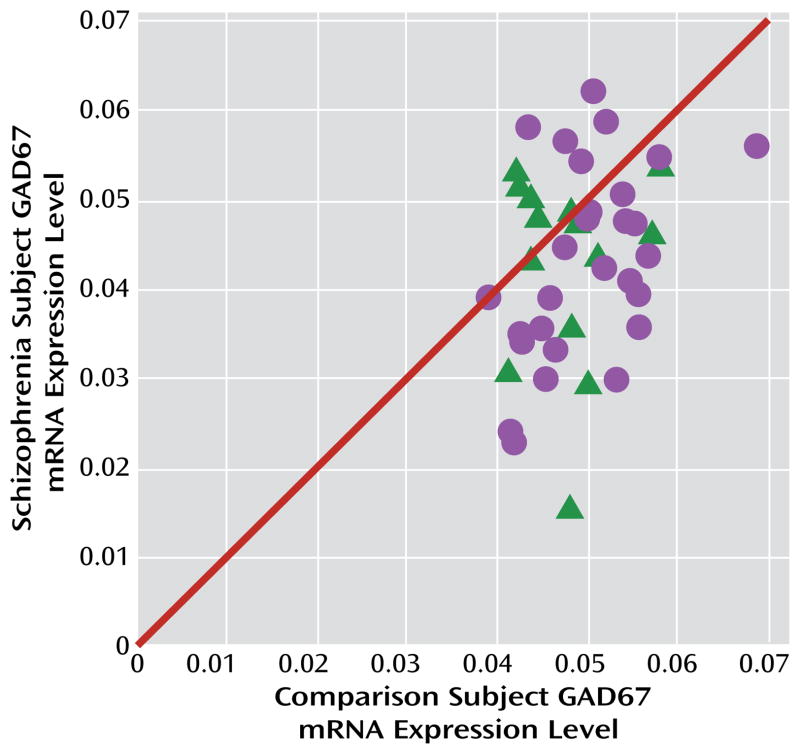
Mean GAD67 mRNA levels were 14.5% lower in the 42 subjects with schizophrenia relative to matched comparison subjects, and the difference was statistically significant (paired: F=7.3, df=1, 38, p=0.005; unpaired: F=6.5, df=1, 76, p=0.006) (Figure 1). None of the covariates were significant in either of the models except for RIN (only in the paired model; F=6.4, df=1, 38, p=0.016). In 23 of these pairs, lower GAD67 mRNA levels had previously been reported by in situ hybridization (using a riboprobe designed against a different portion of the mRNA than the primer set employed here) (32). The pairwise differences in GAD67 mRNA expression as measured by in situ hybridization and by qPCR in the present study were strongly positively correlated (r=0.68, p<0.001). The new set of 19 subject pairs also showed a 12.3% lower level of GAD67 mRNA in schizophrenia (paired: F=4.1, df=1, 15, p=0.031; unpaired: F=6.0, df=1, 30, p=0.010).
FIGURE 1. GAD67 mRNA Levels in the Dorsolateral Prefrontal Cortex in Schizophrenia and Comparison Subjectsa.
a The scatterplot indicates GAD67 mRNA levels for each matched pair of comparison subject and subject with schizophrenia (circles) or schizoaffective disorder (triangles). Pairs below the diagonal unity line have lower GAD67 mRNA levels in the schizophrenia or schizoaffective disorder subject relative to the comparison subject. Levels of GAD67 mRNA were 14.5% lower in the schizophrenia subjects (F=7.3, df=1, 38, p=0.005).
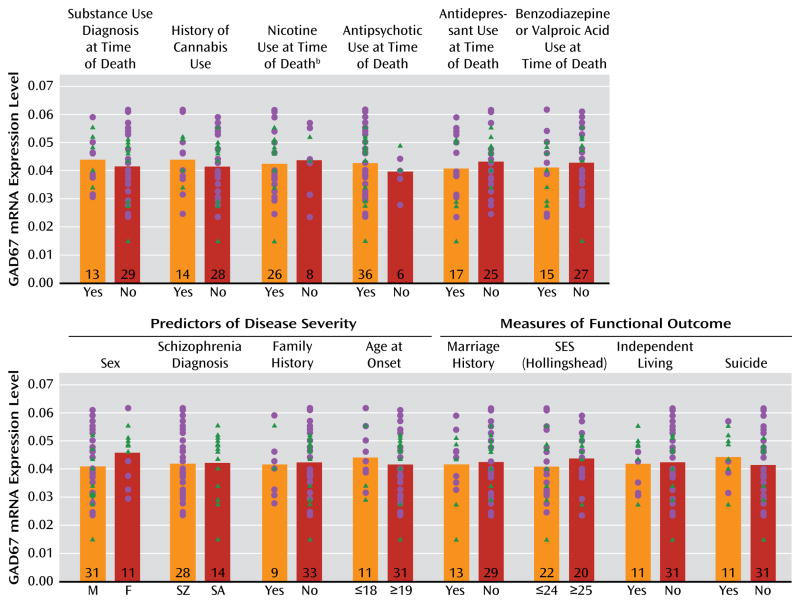
GAD67 mRNA levels in the schizophrenia subjects did not differ as a function of diagnosis of substance abuse or dependence current at the time of death; use of antidepressants, benzodiazepines, sodium valproate, antipsychotics, or nicotine at the time of death; or history of cannabis use (Figure 2). Additionally, regression models were built to look for significant interaction terms between the variables (with the exception of nicotine use at time of death, because data were not available for all subject pairs), and a sequence of models with increasing interaction complexity was fit. A model with main effects and all two-way interactions revealed no significant effects.
FIGURE 2. Effect of Substance Abuse, Psychotropic Medication Use, Predictors of Illness Severity, and Measures of Functional Outcome on GAD67 mRNA Levels in the Dorsolateral Prefrontal Cortex in Schizophrenia Subjectsa.
aGAD67 mRNA levels in subjects with schizophrenia (circles) or schizoaffective disorder (triangles) did not differ significantly as a function of the presence or absence of substance abuse or dependence at time of death; history of cannabis use; nicotine use at the time of death; or use of antipsychotics, antidepressants, or benzodiazepines and/or valproic acid at the time of death. Additionally, GAD67 mRNA levels did not significantly differ based on the presence or absence of predictors of disease severity (sex, schizophrenia versus schizoaffective disorder diagnosis, first-degree relative with schizophrenia, or age at onset of illness) or measures of functional impairment (history of marriage, highest achieved socioeconomic status, independent living, or death by suicide). M=male; F=female; SZ=schizophrenia; SA=schizoaffective disorder; SES=socioeconomic status. Numbers at the bottom of bars indicate number of schizophrenia subjects per group.
b Information was not available for all subjects.
The within-pair percent differences in GAD67 mRNA in schizophrenia relative to comparison subjects were heterogeneous (range=−67% to +34%), and thus we examined several factors that might contribute to the variability. As shown in Figure 2, among schizophrenia subjects, GAD67 mRNA levels did not differ as a function of factors that predict a more severe course of illness (male sex, a diagnosis of schizophrenia rather than schizoaffective disorder, a first-degree relative with schizophrenia, early age at illness onset [<19 years of age]) or measures of illness severity (suicide, no history of marriage, low socioeconomic status as measured by the Hollings head Index of Social Position [33], absence of independent living at the time of death). Additionally, GAD67 mRNA levels were not significantly correlated with age at illness onset, age at the time of death, or age-corrected duration of illness.
GAD67 Protein Levels in Schizophrenia
Determination of quantitative Western blotting experimental parameters
To maximize our ability to account for potential differences in the amount of protein loaded per well, we measured the levels of two commonly used normalizers, β-actin and β-tubulin. Mean values for both proteins did not differ between the schizophrenia and comparison subjects (N=15 pairs), but the coefficient of variation of the within-pair difference was three times greater for β-actin (0.104) than for β-tubulin (0.036). Therefore, we corrected each GAD67 optical density measure by the β-tubulin optical density measure from the same lane. To determine an appropriate protein load, we assessed the linearity and proportionality of the GAD67 and β-tubulin antibodies as a function of total protein loads of 10, 20, 30, and 40 μg (see Figure S1 in the online data supplement). GAD67 and β-tubulin optical density increased in a highly linear (r=0.99, p=0.001; r=0.99, p=0.026, respectively) and proportional fashion as protein load increased from 10 to 40 μg and 20 to 40 μg, respectively. Accordingly, we loaded each lane with 25 μg of total protein.
Total GAD67 protein levels in schizophrenia
Protein degradation begins at death, but the effect of postmortem interval on protein levels is variable across proteins and brain regions (21). Hence, we examined the effect of postmortem interval on GAD67 protein levels in dorsolateral prefrontal cortex from six comparison subjects as well as one monkey (see the supplemental Results section in the online data supplement). Based on these findings, we used only subject pairs with postmortem intervals <20 hours for Western blot studies.
Uncorrected and tubulin-corrected GAD67 protein levels were, respectively, 12.9% (F=5.6, df=1, 17, p=0.015) and 10.1% (F=3.4, df=1, 17, p=0.040) lower in the schizophrenia subjects (Figure 3). In contrast, uncorrected tubulin levels did not differ between schizophrenia subjects and comparison subjects. Tissue storage time was not significant in either the uncorrected or the corrected model.
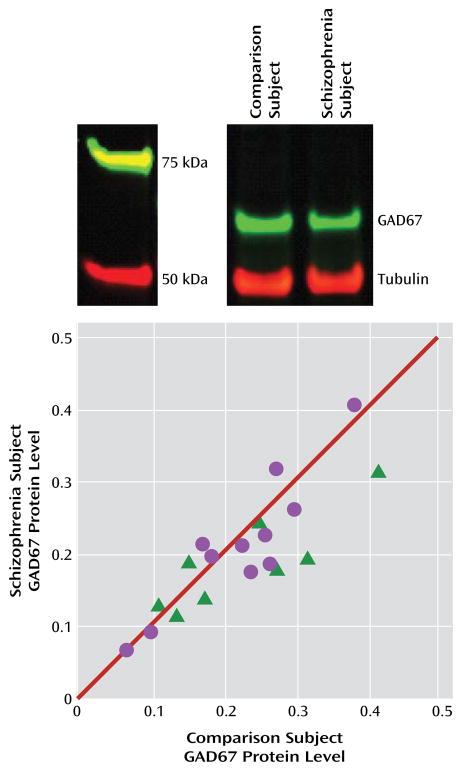
FIGURE 3. GAD67 Protein Levels in the Dorsolateral Prefrontal Cortex in Schizophrenia Subjects Relative to Comparison Subjectsa.
a The image at top shows lanes from a representative blot loaded with tissue from a comparison and schizophrenia subject pair. The plot shows tubulin-normalized GAD67 protein values for each pair of comparison and schizophrenia (circles) or schizoaffective disorder (triangles) subjects. Values below the diagonal unity line indicate lower GAD67 protein levels in the schizophrenia subject relative to the comparison subject. GAD67 protein levels were 10.1% lower in schizophrenia subjects (F=3.4, df=1, 17, p=0.040).
As depicted by the scatter along the unity line in Figure 3, GAD67 protein levels varied substantially across subject pairs. This scatter was more than four times greater than for GAD67 mRNA levels (Figure 1), suggesting that it represented measurement variance. Hence, we performed an additional 16 replicates in four subject pairs. The absolute levels of GAD67 protein differed between the first and second measurements in the same subject, but the percent difference within each subject pair was highly consistent (intraclass correlation coefficient=0.97). Together, these findings indicate that measurement variance contributes substantially to the distribution of GAD67 protein levels along the unity line in Figure 3, but the distance from the unity line for each subject pair likely reflects the biological effect of schizophrenia.
Correlation of Total GAD67 mRNA and Protein Levels
Because of a lack of tissue availability, different comparison subjects were utilized between the total mRNA and protein studies for subject pair 4 (see Table S1 in the online data supplement). In the remaining 18 pairs of subjects, the within-pair percent differences in GAD67 mRNA and protein were positively correlated (r=0.40, p=0.105), supporting the idea that a reduction in GAD67 mRNA expression results in less translation of the cognate protein.
GAD67 Protein Levels in Parvalbumin Puncta
The deficit in GAD67 mRNA is known to be present in a subset of interneurons, including those that express parvalbumin (24). Thus, we conducted a proof-of-concept test of the hypothesis that the deficit in GAD67 protein levels is much larger in the axon terminals of parvalbumin cells than in total cortical gray matter. Five subject pairs with a range of differences in total GAD67 protein levels (−24% to +16%; mean −5%) were selected. We created masks of GAD65-labeled puncta, with each mask object representing a putative axon terminal. In a total of 30,630 GAD65+/parvalbumin+ puncta, we measured GAD67 fluorescence intensity.
A potential confound of fluorescent intensity measures in human cortex is the autofluorescence (~350–700 nm) of lipofuscin, an intracellular lysosomal protein that accumulates with age (34). To eliminate lipofuscin as a confound (see the supplemental Method section and Figure S2 in the online data supplement), we imaged and masked lipofuscin in the 403-nm channel and excluded any GAD67+/GAD65+/parvalbumin+ puncta that overlapped with the 403-nm masks. The effectiveness of this procedure was confirmed by the finding that lipofuscin volume, as expected, was strongly positively correlated with subject age (r=0.87, p=0.002; see Figure S2). The total volume of masked lipofuscin did not significantly differ between comparison and schizophrenia subjects, and lipofuscin volume per subject was not significantly correlated with GAD67 intensity in parvalbumin puncta.
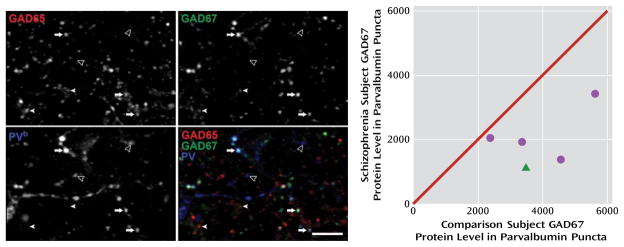
The fluorescence intensity of GAD67 in parvalbumin puncta was 48.8% lower (t=3.9, df=4, p=0.008) in schizophrenia subjects (Figure 4), a difference nearly 10 times greater than the 5% reduction in total GAD67 protein found in these same subject pairs by Western blotting. These data suggest that GAD67 protein deficits are especially pronounced in parvalbumin neurons, consistent with previous findings of cell type-specific deficits in GAD67 mRNA (24).
FIGURE 4. GAD67 Protein Levels in Parvalbumin Puncta in the Dorsolateral Prefrontal Cortex in Comparison and Schizophrenia Subjectsa.
a The image projections (consisting of three z-planes) on the left show representative puncta obtained in the GAD65 (upper left), GAD67 (upper right), and parvalbumin (bottom left) channels; the bottom right image shows a color overlay of all three channels. PV=parvalbumin; arrows indicate GAD65+/GAD67+/parvalbumin+ puncta; filled arrowheads indicate GAD65+/GAD67+/parvalbumin–puncta; open arrowheads indicate GAD65−/GAD67−/parvalbumin+ puncta. Scale bar=10 μm. The scatterplot on the right shows GAD67 protein values in parvalbumin puncta plotted for each pair of comparison and schizophrenia (circles) or schizoaffective disorder (triangle) subjects. Values below the diagonal unity line indicate lower GAD67 protein levels in parvalbumin puncta in the schizophrenia subject relative to the comparison subject. Mean GAD67 protein levels in parvalbumin puncta were 48.8% lower in schizophrenia subjects (t=3.9, df=4, p=0.008).
b For presentation purposes, pixel values were increased equally in order to visualize low-, medium-, and high-intensity puncta in the parvalbumin channel, resulting in the saturation of some pixels.
Discussion
In this study, we documented lower mean GAD67 mRNA levels in the dorsolateral prefrontal cortex of schizophrenia subjects in the largest cohort reported to date. In a subset of this cohort in which potential confounds of protein measures were controlled, total GAD67 protein levels were also similarly lower, consistent with the hypothesis that lower mRNA levels lead to less translated protein. Finally, the GAD67 protein findings were especially pronounced in the axon terminals of parvalbumin neurons, providing evidence of a cell type-specific deficit in GAD67 protein at the site of GABA synthesis and release (Figure 5).
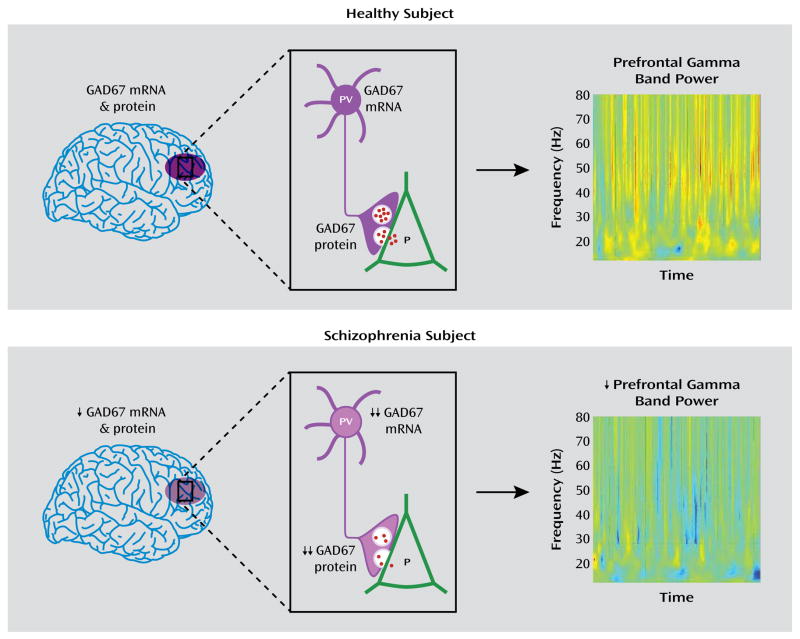
FIGURE 5. Schematic Summary and Predicted Functional Consequences of Lower GAD67 mRNA and Protein Levels in the Dorsolateral Prefrontal Cortex of Subjects With Schizophreniaa.
aIn healthy subjects, normal tissue levels of GAD67 mRNA and protein in the dorsolateral prefrontal cortex (purple oval in top panel, left) are associated with normal levels of GAD67 mRNA and protein in parvalbumin (PV)-containing cell bodies and axon terminals, respectively (top panel, center), and normal amounts of GABA (red dots) release from synaptic vesicles onto pyramidal (P) neurons, providing the inhibitory inputs required for normal prefrontal gamma band power as measured by EEG during cognitive control tasks (top panel, right). In schizophrenia, modest reductions in tissue levels of GAD67 mRNA and protein (bottom panel, left) are associated with pronounced reductions of GAD67 mRNA and protein in PV neurons and axon terminals, respectively (bottom panel, center). The predicted reduction in GABA release from synaptic vesicles leads to the lower gamma band power present over the dorsolateral prefrontal cortex during cognitive control tasks (bottom panel, right). Warmer colors in the heat maps of gamma band power indicate higher power, and cooler colors indicate lower power. Heat maps are from Cho et al. (8). Copyright 2006, Proceedings of the National Academy of Sciences.
As in previous studies of GAD67 mRNA (16), the apparent magnitude of the GAD67 mRNA and protein deficits in this study differed substantially across schizophrenia subjects. This variability does not appear to be due to differences across schizophrenia subjects in potential confounding factors, such as substance abuse or psychotropic medication use at the time of death. The similar levels of GAD67 mRNA in subjects both on and off antipsychotic medications at the time of death are consistent both with previous studies of schizophrenia (11) and with the absence of a deficit in GAD67 mRNA levels in monkeys treated long term with high-dosage haloperidol decanoate (23) or low-dosage oral haloperidol or olanzapine (35).
The variability in GAD67 mRNA levels across schizophrenia subjects also does not appear to be accounted for by factors that predict a more severe form of the illness, including male sex, a diagnosis of “pure” schizophrenia, a family history of schizophrenia, and an earlier age at illness onset. Additionally, factors that are a more direct metric of illness severity and functional outcome (death by suicide, lower socioeconomic status, not living independently, and no history of marriage) were not associated with lower levels of GAD67 mRNA in schizophrenia subjects. These data indicate that the GAD67 mRNA deficit is a conserved feature associated with a diagnosis of schizophrenia that is robust to the influence of multiple other factors.
Since GAD67 expression is activity regulated (17), it is possible that lower GAD67 mRNA levels in schizophrenia are a consequence of reduced cortical activity associated with having a chronic psychiatric illness or severe cognitive impairments. However, the latter explanations seem unlikely since 1) GAD67 mRNA levels did not differ between schizophrenia subjects with or without factors that predict or reflect a more severe illness course, and 2) the within-pair percent differences in GAD67 mRNA and protein levels were not significantly correlated with duration of illness. Furthermore, lower frontal gamma power, predicted to result from less GABA synthesis in parvalbumin neurons (10, 25), is present during both the first episode (9) and the chronic phase (8) of schizophrenia. Taken together, these findings suggest that lower GAD67 mRNA levels are not attributable to substance abuse, psychotropic medication use, other factors related to illness course, or chronic psychiatric illness. Thus, lower GAD67 levels appear to be a common component of, and not a consequence of, the disease process of schizophrenia.
The substantially greater deficit of GAD67 protein in parvalbumin puncta than in total gray matter is convergent with previous findings that the deficit in GAD67 mRNA expression is prominent in parvalbumin neurons (24) and not detectable in the majority of other GABA neurons (22, 23). Lower levels of GAD67 protein in parvalbumin terminals suggest that these terminals synthesize less GABA and therefore are less able to provide the inhibition of pyramidal neurons that is required for gamma oscillations (10, 25). Thus, impaired GABA synthesis in parvalbumin terminals, resulting in lower inhibition, is a plausible mechanism for the reduced prefrontal gamma oscillation power observed during cognitive control tasks in schizophrenia patients (8, 9) (Figure 5).
However, directly demonstrating that lower GAD67 mRNA and protein levels in the dorsolateral prefrontal cortex (or other cortical regions where lower GAD67 mRNA levels have been observed [16]) lead to less cortical GABA in schizophrenia is challenging. Indeed, it is possible that GAD67 expression could be down-regulated in response to reduced GABA metabolism; for example, pharmacological inhibition of GABA transaminase, which metabolizes GABA, is associated with elevated levels of cortical GABA and lower levels of GAD67 protein (12). Perhaps consistent with this interpretation, a magnetic resonance spectroscopy study reported elevated GABA levels in the anterior cingulate and parieto-occipital cortices of patients with chronic schizophrenia (36). However, some studies found no differences, and others reported lower levels of cortical GABA in patients with schizophrenia (37, 38). Interestingly, lower GABA levels in the visual cortex in patients with schizophrenia were correlated with reductions in a behavioral measure of visual inhibition that is known to depend on GABA neurotransmission (38). Similarly, a trend-level negative correlation was found between frontal GABA levels and number of errors on a working memory task in patients with early-stage schizophrenia (39), consistent with the idea that lower GABA synthesis results in cognitive impairments. However, since spectroscopic studies measure total tissue GABA levels, and not GABA levels in synaptic vesicles or the extracellular space, their relevance to GABA synthesis and transmission remains uncertain. The hypothesis that GABA hypofunction results in cognitive impairments in schizophrenia is supported by findings of impaired working memory performance in rats and monkeys after pharmacological blockade of prefrontal GABAA receptors (40, 41).
Together, the findings of this study demonstrate that lower levels of GAD67 mRNA are accompanied by lower levels of GAD67 protein, which is especially pronounced in the axon terminals of parvalbumin GABA neurons, providing a plausible mechanistic basis for impaired cortical gamma oscillations and cognitive control deficits in schizophrenia (Figure 5). Notably, comparisons across the subjects with schizophrenia suggest that a deficit in GABA synthesis is likely to contribute to cognitive dysfunction in schizophrenia and is not likely a consequence of experiencing the illness.
Supplementary Material
Acknowledgments
Supported by a Scottish Rite Schizophrenia Fellowship and an Andrew Mellon Predoctoral Fellowship to Ms. Curley; and by NIH grant MH-085108 to Dr. Fish, grant MH-084016 to Dr. Volk, and grants MH-043784 and MH-084053 to Dr. Lewis.
The authors thank Robert Sweet, Elizabeth Sengupta, Carol Sue Johnston, Siyu Li, and Mary Brady for technical assistance.
Footnotes
Presented in part at the annual meeting of the Society for Neuroscience, Chicago, October 17–21, 2009, and at the annual meeting of the Society for Biological Psychiatry, New Orleans, May 20 –22, 2010.
Dr. Lewis receives investigator initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium, and Pfizer and has served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Merck, Neurogen, and SK Life Science. Dr. Sampson is a statistical consultant to Johnson & Johnson Pharmaceutical Research and Development. All other authors report no financial relationships with commercial interests.
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 7.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 12.Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 13.Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- 14.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 19.Nelson PT, Keller JN. RNA in brain disease: no longer just “the messenger in the middle. J Neuropathol Exp Neurol. 2007;66:461–468. doi: 10.1097/01.jnen.0000240474.27791.f3. [DOI] [PubMed] [Google Scholar]
- 20.Rimvall K, Sheikh SN, Martin DL. Effects of increased gamma-aminobutyric acid levels on GAD67 protein and mRNA levels in rat cerebral cortex. J Neurochem. 1993;60:714–720. doi: 10.1111/j.1471-4159.1993.tb03206.x. [DOI] [PubMed] [Google Scholar]
- 21.Beneyto M, Sibille E, Lewis DA. Human postmortem brain research in mental illness syndromes. In: Charney DS, Nestler E, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2008. pp. 202–214. [Google Scholar]
- 22.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 23.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fish KN, Sweet RA, Deo AJ, Lewis DA. An automated segmentation methodology for quantifying immunoreactive puncta number and fluorescence intensity in tissue sections. Brain Res. 2008;1240:62–72. doi: 10.1016/j.brainres.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imbeaud S, Graudens E, Boulanger V, Barlet X, Zaborski P, Eveno E, Mueller O, Schroeder A, Auffray C. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res. 2005;33:e56. doi: 10.1093/nar/gni054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollingshead AB. Two-Factor Index of Social Position. New Haven, Conn: Yale University; 1965. [Google Scholar]
- 34.Porta EA. Pigments in aging: an overview. Ann NY Acad Sci. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, Hori H, Ueda N, Korogi Y, Nakamura J. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol. 2009;24:639–645. doi: 10.1002/hup.1070. [DOI] [PubMed] [Google Scholar]
- 40.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 41.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.