Abstract
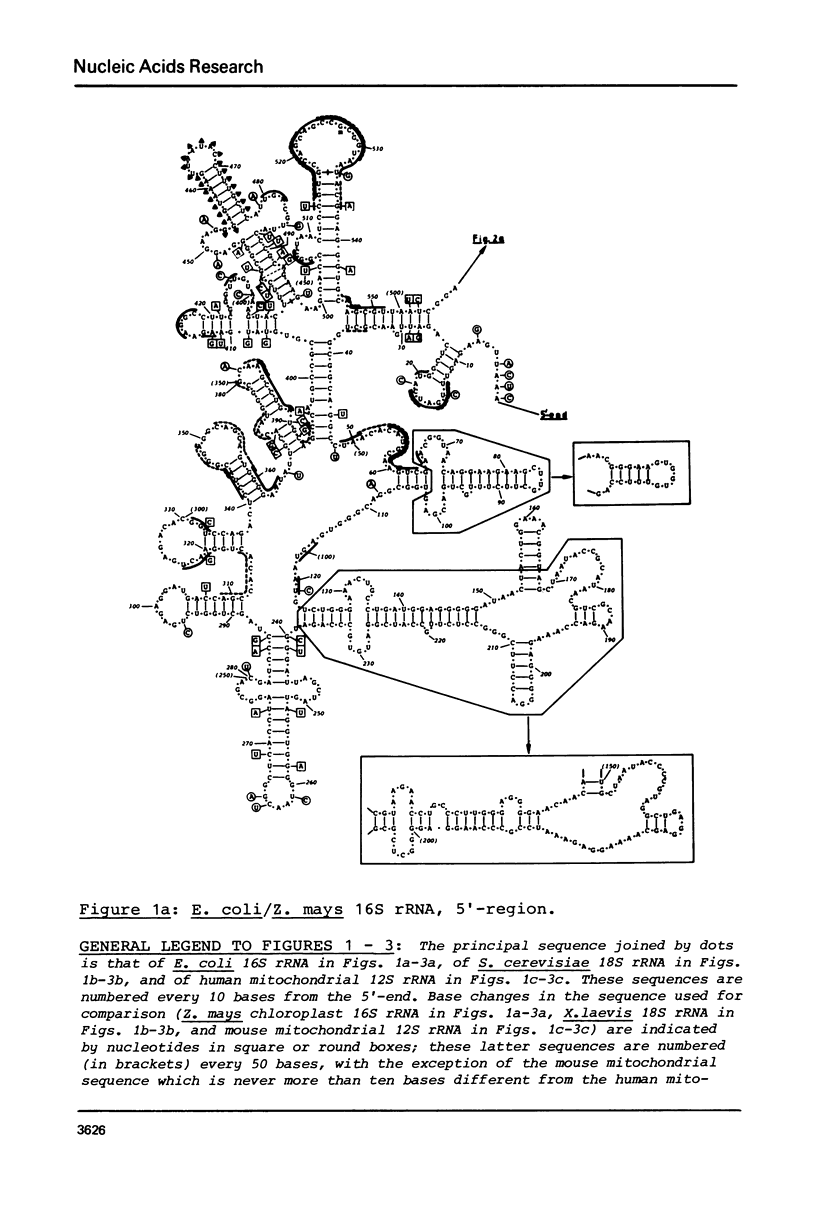
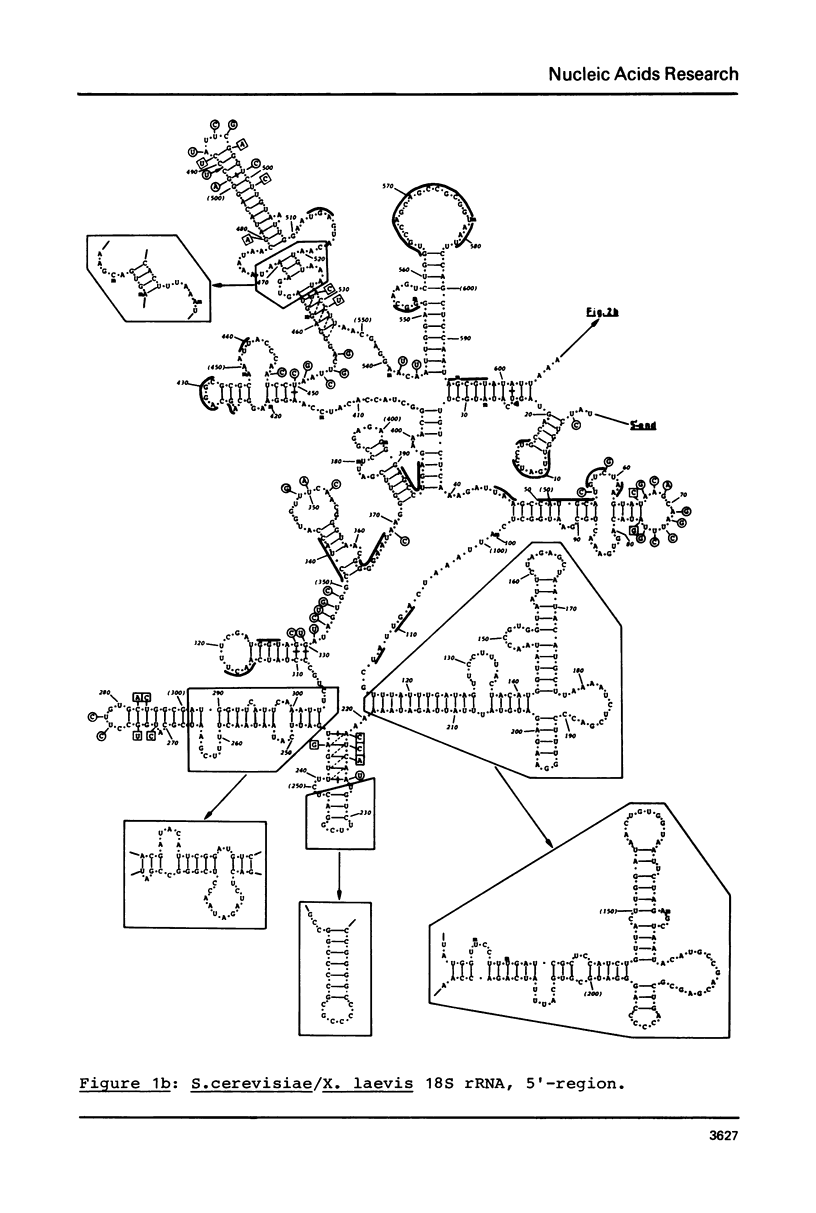
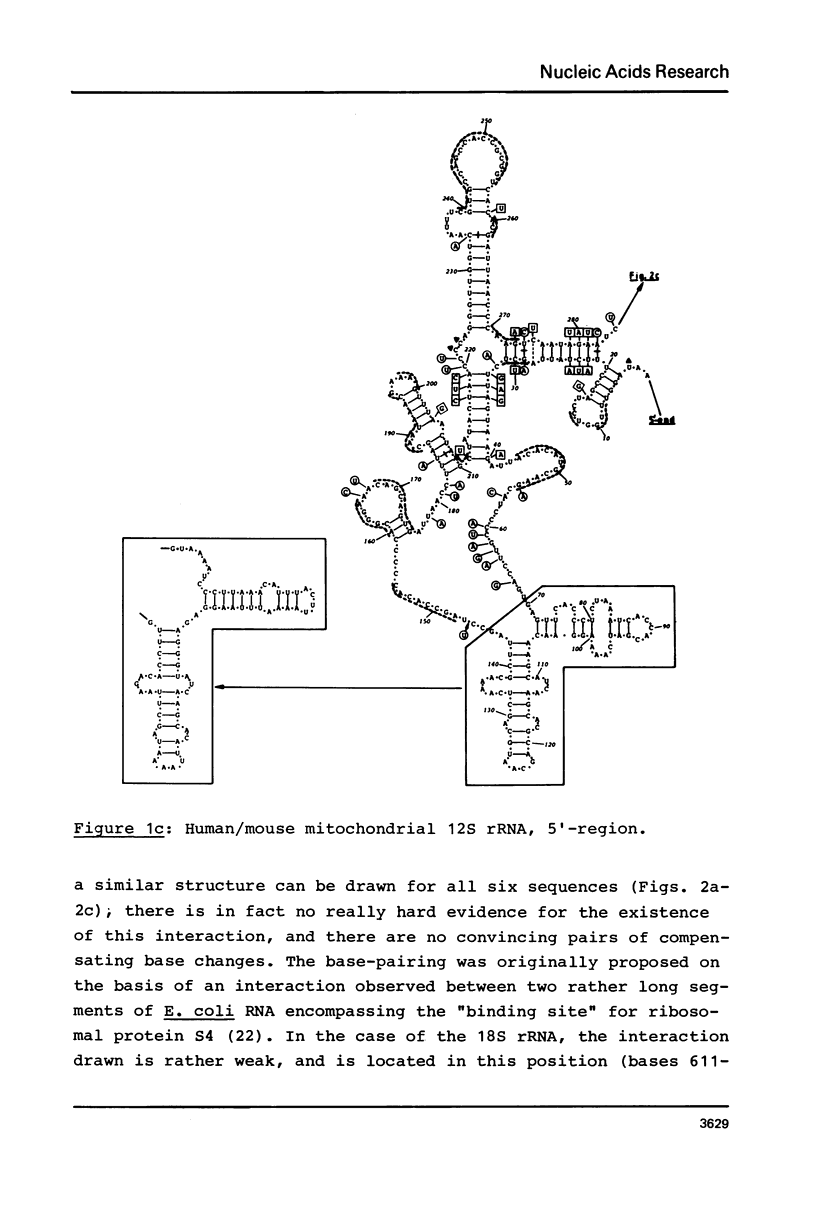
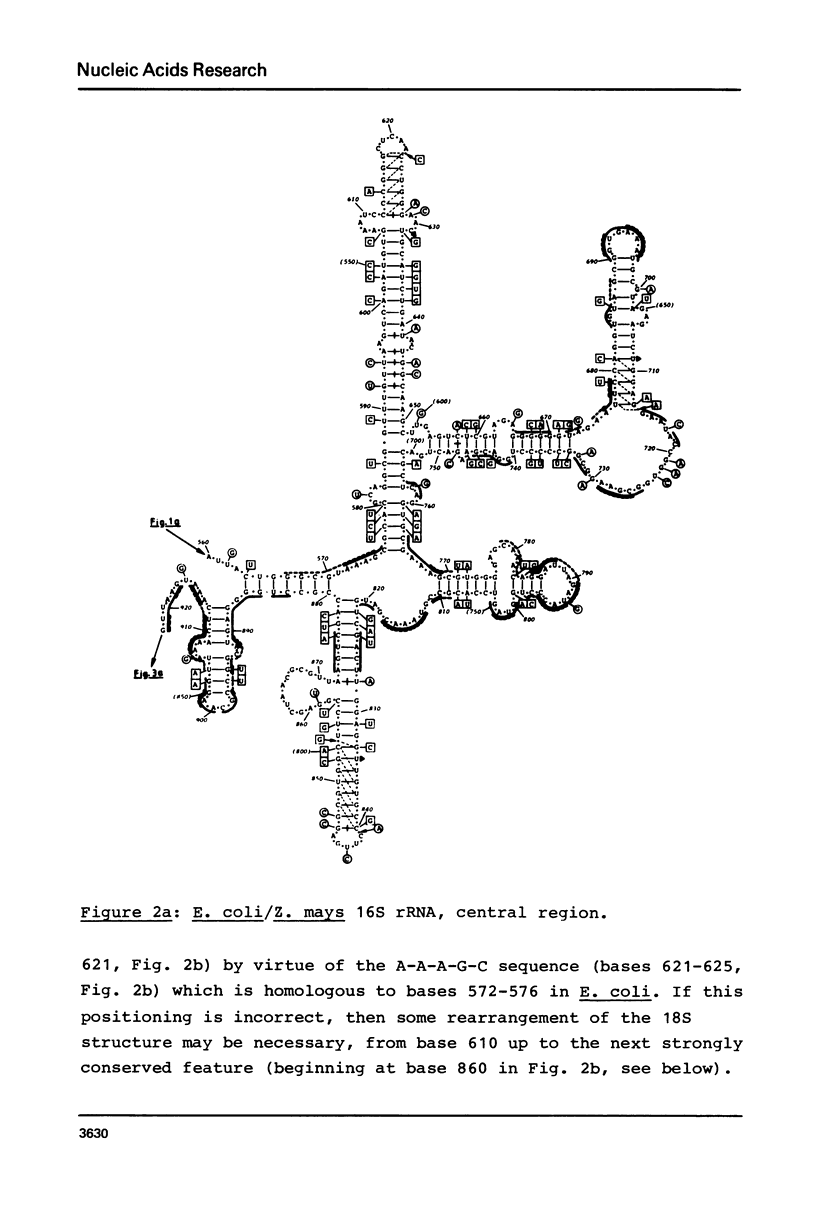
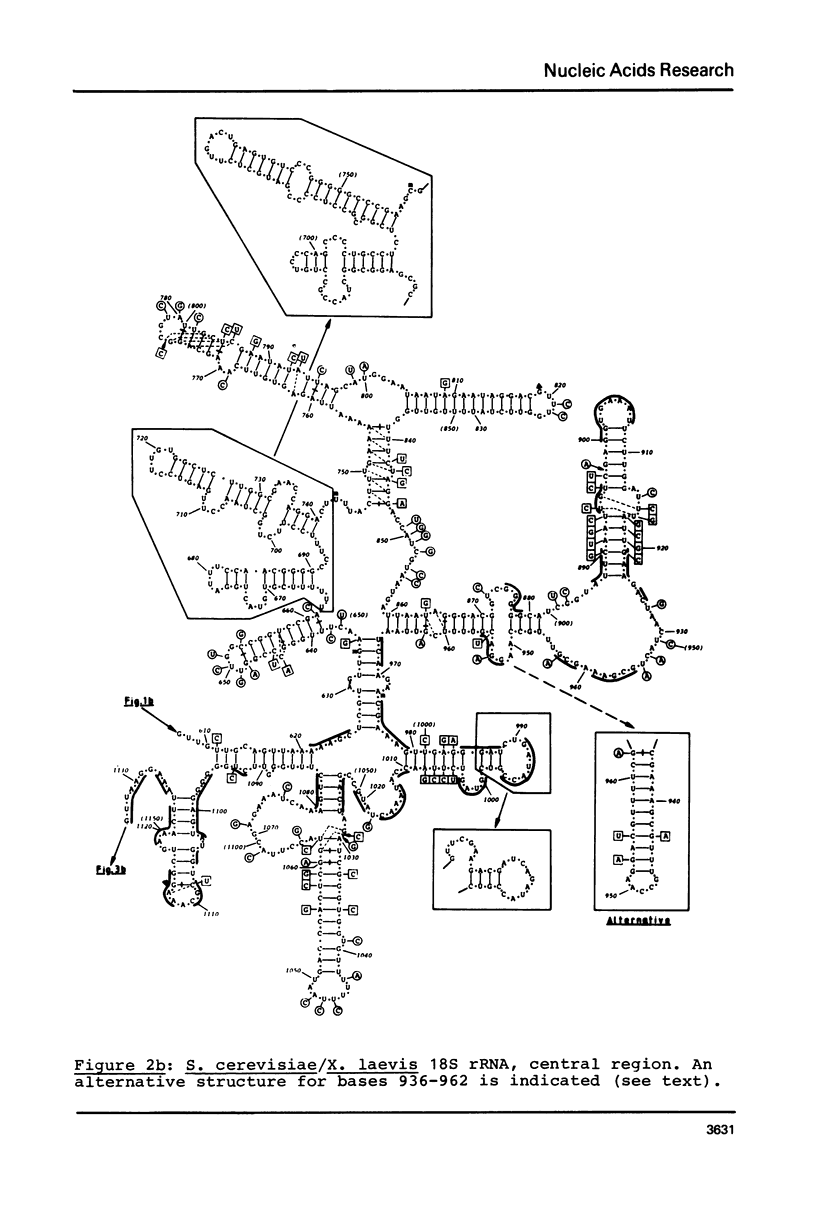
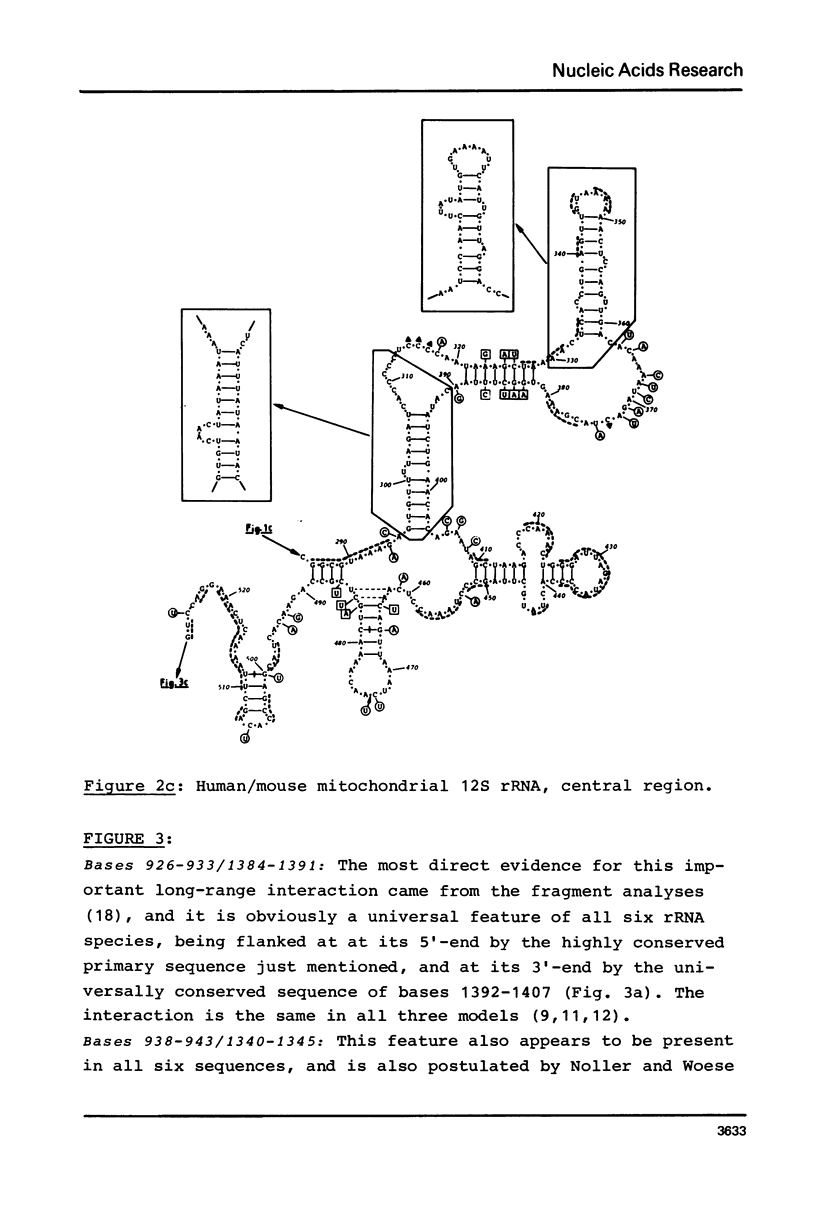
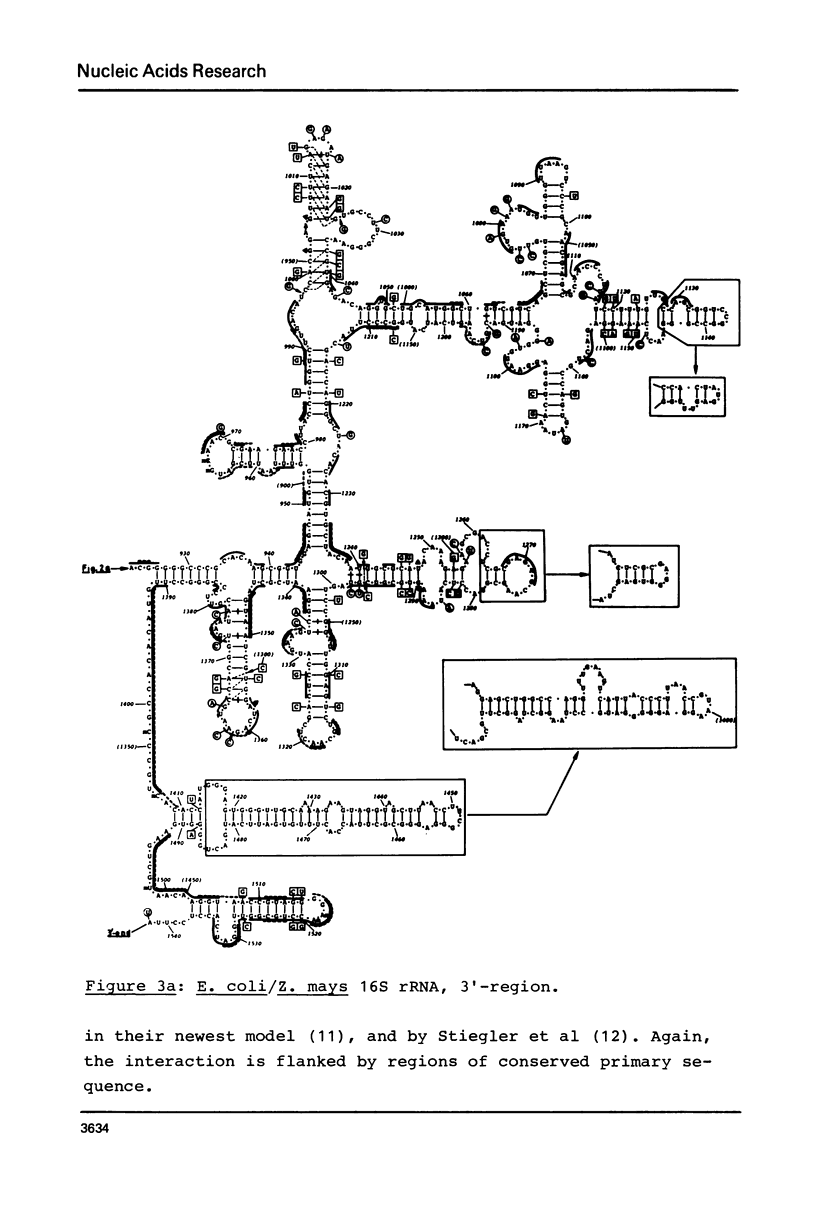
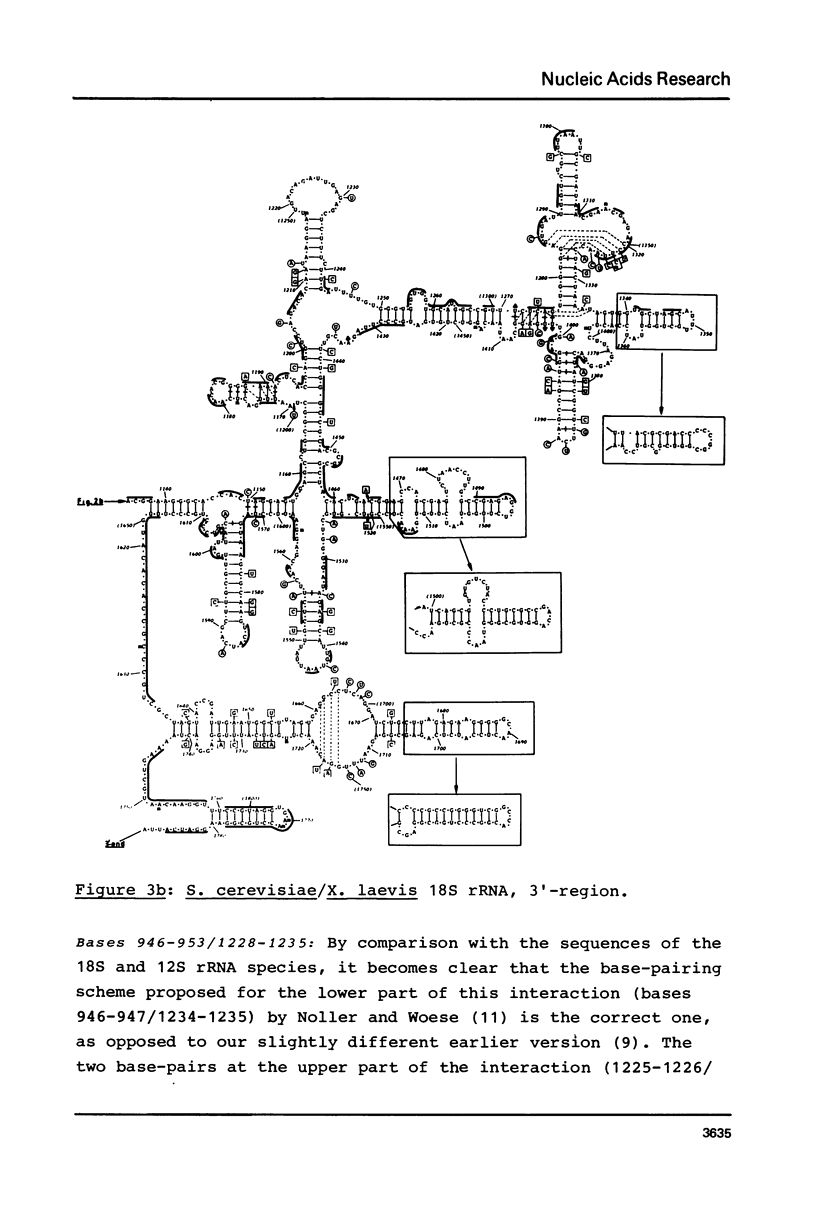
Secondary structure models are presented for three pairs of small subunit ribosomal RNA molecules. These are the 16S rRNA from E. coli cytoplasmic and Z. mays chloroplast ribosomes, the 18S rRNA from S. cerevisiae and X. laevis cytoplasmic ribosomes, and the 12S rRNA from human and mouse mitochondrial ribosomes. Using the experimentally-established secondary structure of the E. coli 16S rRNA as a basis, the models were derived both by searching for primary structural homology between the three classes of sequence (12S, 16S, 18S), and also by searching for compensating base changes in putative helical regions of each pair of sequences. The models support the concept that secondary structure of ribosomal RNA has been extensively conserved throughout evolution, differences in length between the three classes of sequence being accommodated in distinct regions of the molecules.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R. J., Dubin D. T. Methylated regions of hamster mitochondrial ribosomal RNA: structural and functional correlates. Nucleic Acids Res. 1981 Jan 24;9(2):323–337. doi: 10.1093/nar/9.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ungewickell E., Garrett R. A. The topography of the 5' end of 16-S RNA in the presence and absence of ribosomal proteins S4 and S20. Eur J Biochem. 1980 Feb;103(3):439–446. doi: 10.1111/j.1432-1033.1980.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Glotz C., Brimacombe R. An experimentally-derived model for the secondary structure of the 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1980 Jun 11;8(11):2377–2395. doi: 10.1093/nar/8.11.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Woese C. R. Secondary structure of 16S ribosomal RNA. Science. 1981 Apr 24;212(4493):403–411. doi: 10.1126/science.6163215. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Experimental determination of interacting sequences in ribosomal RNA. Nature. 1979 Sep 27;281(5729):271–276. doi: 10.1038/281271a0. [DOI] [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Batchikova N. V., Skriabin K. S., Baev A. A. Opredelenie pervichnoi struktury fragmentov ribosomnogo operona pekarskikh drozhzhei, kodiruiushchikh 18 S rRNK. Dokl Akad Nauk SSSR. 1979;248(3):760–762. [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Zakharyev V. M., Krayev A. S., Skryabin K. G., Bayev A. A. The structure of the yeast ribosomal RNA genes. I. The complete nucleotide sequence of the 18S ribosomal RNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Dec 11;8(23):5779–5794. doi: 10.1093/nar/8.23.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Samols D. R., Hagenbuchle O., Gage L. P. Homology of the 3' terminal sequences of the 18S rRNA of Bombyx mori and the 16S rRNA of Escherchia coli. Nucleic Acids Res. 1979 Nov 10;7(5):1109–1119. doi: 10.1093/nar/7.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structure secondaire et topographie du RNA ribosomique 16S d'Escherichia coli. C R Seances Acad Sci D. 1980 Dec 8;291(12):937–940. [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Singh-Bergmann K. Binding sites for ribosomal proteins S8 and S15 in the 16 S RNA of Escherichia coli. Biochim Biophys Acta. 1979 Jul 26;563(2):422–431. doi: 10.1016/0005-2787(79)90061-3. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Localisation of a series of intra-RNA cross-links in 16S RNA, induced by ultraviolet irradiation of Escherichia coli 30S ribosomal subunits. Nucleic Acids Res. 1980 Jun 11;8(11):2397–2411. doi: 10.1093/nar/8.11.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Max-Planck-Institut für Molekulare Genetik, Abteilung Wittmann, Berlin-Dahlem, GFR. Nucleic Acids Res. 1979;6(5):1775–1790. doi: 10.1093/nar/6.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]



