Abstract
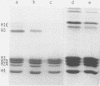
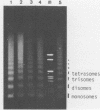
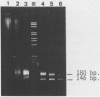
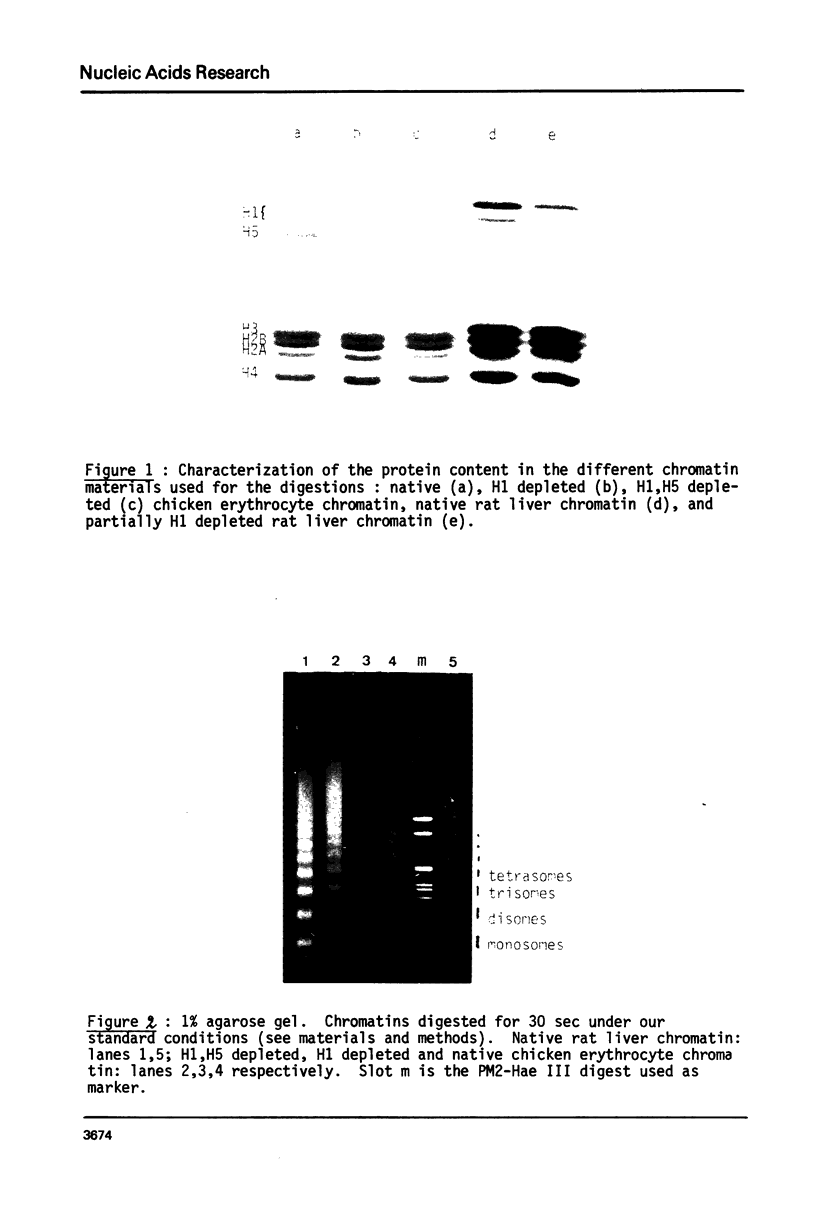
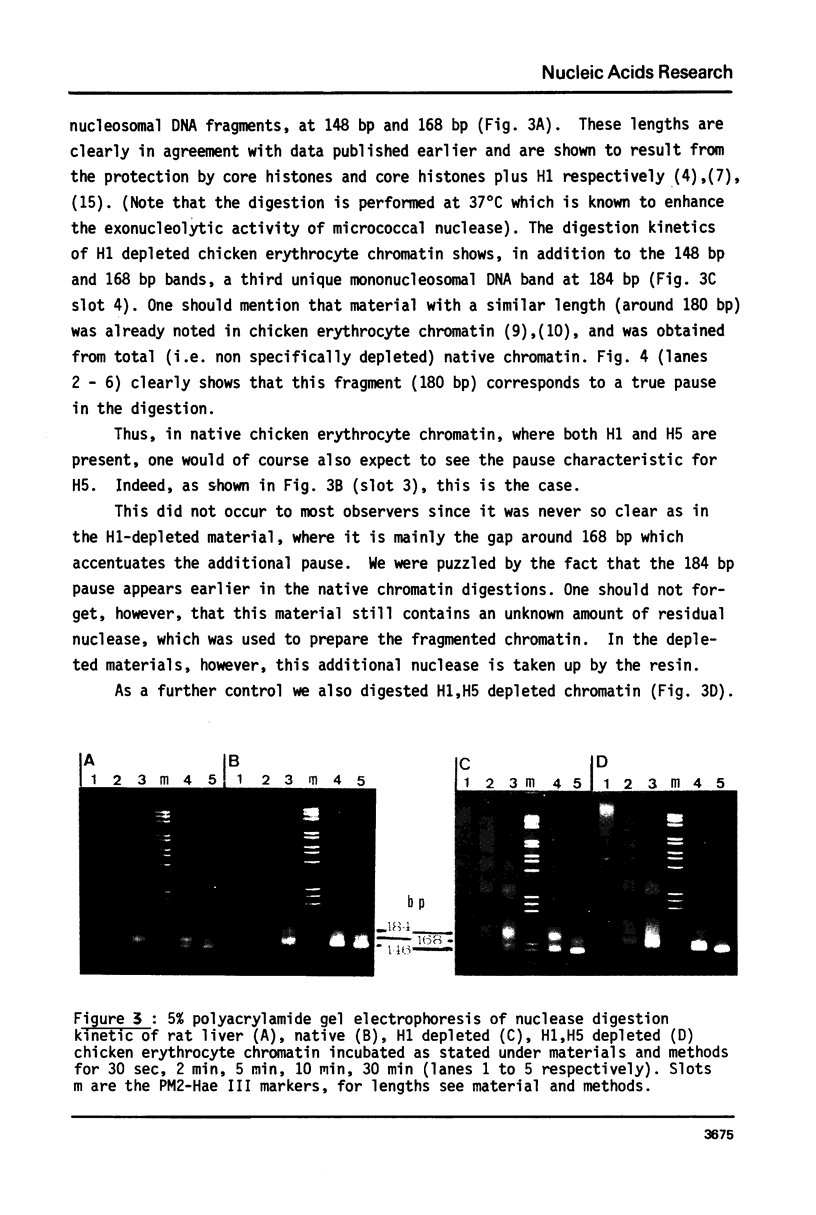
Several authors, including ourselves, have reported the existence of chromatosomes with DNA size larger than 166 bp in bird erythrocyte chromatin. It was tempting to correlate this increased DNA size with the presence of histone H5. In order to substantiate this hypothesis, we performed a micrococcal nuclease digestion kinetic on: chicken erythrocyte chromatin, either native, selectively depleted from H1, or from H1 and H5; and rat liver chromatin, either native or partially H1 depleted. The comparative analysis of the lengths of DNA in the chromatosome size region led to the following conclusions: - denaturing gels clearly reveal a first discrete pause at 178 nucleotides in H1 depleted chicken erythrocyte chromatin as well as in partially H1-depleted rat liver chromatin, before the material accumulates at the next intermediate 166 nucleotide chromatosome pause. - the generation of all discrete chromatosome bands is critically dependent on low ionic strength conditions and low Ca++ concentrations during the digestion, suggesting it may result from the protection of DNA cleavage sites by histone H5 or H1, C or N terminal domains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Bakayeva T. G., Bakayev V. V. Separation of nucleosomes containing histones H1 and H5. Mol Biol Rep. 1978 Oct 16;4(3):185–189. doi: 10.1007/BF00777522. [DOI] [PubMed] [Google Scholar]
- Belyavsky A. V., Bavykin S. G., Goguadze E. G., Mirzabekov A. D. Primary organization of nucleosomes containing all five histones and DNA 175 and 165 base-pairs long. J Mol Biol. 1980 May 25;139(3):519–536. doi: 10.1016/0022-2836(80)90144-8. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Moss T., Bradbury E. M. High-resolution proton-magnetic-resonance studies of chromatin core particles. Eur J Biochem. 1978 Sep 1;89(2):475–482. doi: 10.1111/j.1432-1033.1978.tb12551.x. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasters I., Muyldermans S., Wyns L., Hamers R. Differences in rearrangements of H1 and H5 in chicken erythrocyte chromatin. Biochemistry. 1981 Mar 3;20(5):1104–1110. doi: 10.1021/bi00508a010. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Miki B. L., Neelin J. M. DNA repeat lengths of erythrocyte chromatins differing in content of histones H1 and H5. Nucleic Acids Res. 1980 Feb 11;8(3):529–542. doi: 10.1093/nar/8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S., Lasters I., Wyns L., Hamers R. Preparation and purification of mononucleosome particles containing histone H5. FEBS Lett. 1980 Sep 22;119(1):93–96. doi: 10.1016/0014-5793(80)81005-2. [DOI] [PubMed] [Google Scholar]
- Muyldermans S., Lasters I., Wyns L., Hamers R. Upon the observation of superbeads in chromatin. Nucleic Acids Res. 1980 May 24;8(10):2165–2172. doi: 10.1093/nar/8.10.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S., Lasters I., Wyns L. Histone H1 can be removed selectively from chicken erythrocyte chromatin at near physiological conditions. Nucleic Acids Res. 1980 Feb 25;8(4):731–739. [PMC free article] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Nucleosome-nucleosome interaction in chromatin. FEBS Lett. 1979 Mar 1;99(1):123–128. doi: 10.1016/0014-5793(79)80263-x. [DOI] [PubMed] [Google Scholar]
- Riley D., Weintraub H. Nucleosomal DNA is digested to repeats of 10 bases by exonuclease III. Cell. 1978 Feb;13(2):281–293. doi: 10.1016/0092-8674(78)90197-6. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Haye K. R., Litwack A. H., Phelps B. M. Nucleosome repeat lengths in the definitive erythroid series of the adult chicken. Biochim Biophys Acta. 1980 Feb 29;606(2):316–330. doi: 10.1016/0005-2787(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Weintraub H. The nucleosome repeat length increases during erythropoiesis in the chick. Nucleic Acids Res. 1978 Apr;5(4):1179–1188. doi: 10.1093/nar/5.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischet W. O., Allen J. R., Riedel G., Van Holde K. E. The effects of salt concentration and H-1 depletion on the digestion of calf thymus chromatin by micrococcal nuclease. Nucleic Acids Res. 1979;6(5):1843–1862. doi: 10.1093/nar/6.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]