Drug resistance in acute myeloid leukemia (AML) patients is attributed to high levels of anti-apoptotic Bcl-2 family members. Strasser and colleagues now show that one such family member, Mcl-1, is essential for the sustained survival and growth of AML in mice and humans. Mcl-1 removal leads to death of transformed AML cells and produces lasting remissions in mice, highlighting its importance as a potential therapeutic target in AML treatment.
Keywords: acute myeloid leukemia, apoptosis, Mcl-1, Bcl-xL
Abstract
Acute myeloid leukemia (AML) frequently relapses after initial treatment. Drug resistance in AML has been attributed to high levels of the anti-apoptotic Bcl-2 family members Bcl-xL and Mcl-1. Here we report that removal of Mcl-1, but not loss or pharmacological blockade of Bcl-xL, Bcl-2, or Bcl-w, caused the death of transformed AML and could cure disease in AML-afflicted mice. Enforced expression of selective inhibitors of prosurvival Bcl-2 family members revealed that Mcl-1 is critical for survival of human AML cells. Thus, targeting of Mcl-1 or regulators of its expression may be a useful strategy for the treatment of AML.
Acute myeloid leukemia (AML) is a heterogeneous disease, with treatment outcome after intensive chemotherapy strongly influenced by cytogenetic and molecular characteristics of the cancer. Several recurrent AML oncogenic fusion lesions have been linked to a high risk of relapse after chemotherapy, including chromosomal translocations involving the Mll (mixed-lineage leukemia) gene (MLL-ENL and MLL-AF9, particularly found in infants or individuals that had previously been treated with topoisomerase inhibitors) (Ayton et al. 2004) or Aml1 gene (AML1-ETO9, found in ∼15% of human AML cases) (Yan et al. 2006; Jiao et al. 2009). Mice transplanted with bone marrow-derived hematopoietic stem/progenitor cells that had been retrovirally transduced with the AML1-ETO9a (Yan et al. 2006) oncogene succumb to myeloblastic AML highly reminiscent of the human disease induced by the same oncogenic lesion. Similar transduction/transplantation models for expression of MLL-ENL (Lavau et al. 2000), MLL-AF9 (Somervaille and Cleary 2006), Mixl1 (Glaser et al. 2006), or Hoxa9 (Thorsteinsdottir et al. 2002) oncogenes cause monocytic and myelomonocytic AML in mice, recapitulating the human subtypes of AML often associated with these oncogenes. Like human AML (Estey and Dohner 2006), these murine leukemias often respond only transiently to anti-cancer therapeutics, with relapse of drug refractory disease in a significant proportion (Zuber et al. 2009). Anti-cancer therapy kills many tumor cells (at least in part) through induction of apoptosis, and defects in the programmed cell death machinery are frequently associated with poor response (Kaufmann et al. 1998; Johnstone et al. 2002). Recent studies have identified somatic amplification of the genomic regions containing the anti-apoptotic genes mcl-1 or bcl-x (Beroukhim et al. 2010) or post-translational stabilization of Mcl-1 protein (Schwickart et al. 2010) in diverse human cancers. We used mouse as well as human AMLs to determine which anti-apoptotic Bcl-2 family members are essential for development and sustained growth of this hematological malignancy to gain insight into which of these proteins should be targeted for therapeutic benefit.
Results and Discussion
Deletion of mcl-1 but not bcl-x kills transformed myeloid cells in culture
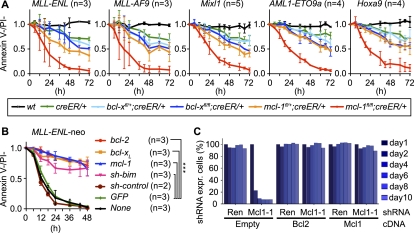
We employed gene targeted mice expressing a tamoxifen-regulated Cre recombinase estrogen receptor fusion protein (Rosa26-Cre-ERT2 mice, hereafter called Cre) (Seibler et al. 2003) to allow controlled inactivation of loxP targeted (floxed, hereafter denoted fl) bcl-x (Wagner et al. 2000) or mcl-1 (Vikstrom et al. 2010) alleles (Supplemental Fig. 1A). We used cells of six different genotypes for analysis: wild-type (wt) to reveal toxic effects of tamoxifen, cre/+ heterozygotes to detect toxicity of the activated Cre recombinase, and cre/+ cells that are either heterozygous (fl/+) or homozygous (fl/fl) for conditional bcl-x or mcl-1 alleles to examine the consequences of loss of the corresponding anti-apoptotic proteins (Bcl-xL and Mcl-1). Bone marrow-derived hematopoietic stem/progenitor cells were infected with retroviruses encoding MLL-ENL, MLL-AF9, AML1-ETO9a, Mixl1, or Hoxa9 plus GFP (from an IRES) as a marker (Supplemental Fig. 1B). Each transduced cell population readily yielded immortalized cell lines exhibiting an immature myeloid profile, with the exception of AML1-ETO9a transduced cells, which exhibited a more differentiated morphology (Supplemental Fig. 1C). Treatment with tamoxifen resulted within 24 h in near complete recombination of both conditional bcl-xfl or mcl-1fl alleles (Supplemental Fig. 1D). Moreover, this treatment caused nearly complete loss of Bcl-xL protein in bcl-xfl/fl;cre/+ cells and almost complete loss of Mcl-1 (and increase of Bim but not tBid or Puma) protein in mcl-1fl/fl;cre/+ cells (Supplemental Fig. 1E). Tamoxifen exerted no toxicity on wild-type cells, but Cre activation reduced the viability of cre/+ cells to ∼70% compared with untreated cultures (Fig. 1A; Supplemental Fig. 1F). Bcl-xfl/+;cre/+ and bcl-xfl/fl;cre/+ cells showed slightly increased death compared with the cre/+ cells, displaying an initial drop in viability to 57%–74% or 45%–57%, respectively (Fig. 1A; Supplemental Fig. 1F). Addition of tamoxifen reduced the viability of mcl-1fl/+;cre/+ cells to 37%–55% and, remarkably, completely killed all mcl-1fl/fl;cre/+ lines (Fig. 1A). These results show that Mcl-1 but not Bcl-xL is essential for sustained survival of transformed myeloid cells in vitro.
Figure 1.
Impact of conditional deletion of bcl-x or mcl-1 in transformed myeloid and AML cells in vitro. (A) Cells of the indicated genotypes, transformed with the oncogenes indicated, were grown for 72 h in medium with or without tamoxifen. Viable (AnnexinV−/PI−) cells were enumerated by flow cytometry. Graphs represent the means ± SEM of the ratio of viable tamoxifen-treated cells versus viable untreated cells (n = 3–5 independently transduced and sorted cell lines for each transforming oncogene and genotype). (B) MLL-ENL/neo transformed mcl-1fl/fl;creER/+ cells were secondarily transduced with retroviruses encoding GFP alone or GFP plus Bcl-2, Bcl-xL, Mcl-1, or shRNA for knockdown of Bim and purified on the basis of GFP expression. Cells were then cultured in the presence or absence of tamoxifen and their survival was measured; data are presented as in A. (***) P < 0.001. (C) RNAi-mediated suppression of Mcl-1 in MLL-AF9 plus N-Ras AML cells. Graphs represent the percentages of cells coexpressing shRNA and the cDNAs indicated over time following doxycycline treatment.
We next examined the mechanism by which these transformed cells are killed upon mcl-1 gene deletion. Overexpression of Bcl-2, Bcl-xL, or Mcl-1 (Supplemental Fig. 1G) prevented this cell death (Fig. 1B). Among the apoptosis initiators tested, Bim was the most highly expressed in these cells, and, remarkably, knockdown of this BH3-only protein by shRNA (Supplemental Fig. 1H) prevented apoptosis of MLL-ENL transformed cells undergoing mcl-1 deletion (Fig. 1B). These results demonstrate that Mcl-1 deletion kills transformed myeloid cells in vitro by activating the “Bcl-2-regulated” (also called “mitochondrial,” “intrinsic,” or “stress”) apoptotic pathway in a Bim-dependent manner (Youle and Strasser 2008).
Murine AML are more sensitive to Mcl-1 deletion than nontransformed stem cells and myeloid progenitors
We next generated AML in vivo by reconstituting lethally irradiated mice with MLL-ENL or MLL-AF9 retrovirus-infected bone marrow-derived hematopoietic stem/progenitor cells. We used the Cre/loxP system to induce loss of Bcl-xL or Mcl-1 (Supplemental Fig. 2A), or tetracycline-regulated RNAi (Zuber et al. 2011a) for knockdown of Mcl-1 (Supplemental Fig. 2B). All recipient mice developed AML within 5 wk after reconstitution (Supplemental Fig. 2C,D). Knockdown of Mcl-1 by RNAi caused apoptosis and rapid depletion of (dsRed+/Venus+) mcl-1 shRNA-expressing AML cells that had been transformed by combined expression of the MLL-AF9 and N-ras oncogenes (Fig. 1C). In agreement with the data described above (Fig. 1B), this death could be inhibited by overexpression of Bcl-2 or Mcl-1 (Fig. 1C).
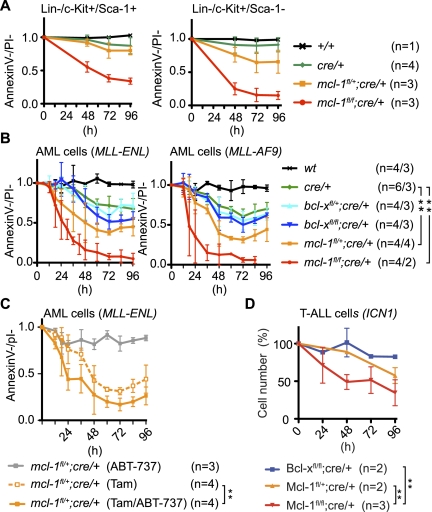
For Mcl-1 inhibition to become a feasible strategy for AML therapy, there needs to be a “therapeutic window” in which leukemic cells are more sensitive than normal hematopoietic stem cells (which require Mcl-1 for survival) (Opferman et al. 2005). Treatment with tamoxifen caused efficient recombination of mcl-1fl alleles in FACS-sorted nontransformed LSK (lineage marker−Sca-1+c-Kit+) stem and myeloid progenitors from mcl-1fl/+;cre/+ or mcl-1fl/fl;cre/+ mice, as demonstrated by flow-cytometric detection of the hCD4 reporter (Supplemental Fig. 3A). Mcl-1 deletion lowered the viability of mcl-1fl/+;cre/+ LSK cells to 80% and that of mcl-1fl/fl;cre/+ LSK cells to 34%, compared with untreated cells (Fig. 2A). Myeloid progenitors were slightly more susceptible to Mcl-1 deletion, showing viability of 91% for cre/+, 66% for mcl-1fl/+;cre/+, and 15% for mcl-1fl/fl;cre/+ cells (Fig. 2A). When MLL-ENL and MLL-AF9 transformed AML cells were treated in vitro with tamoxifen for 48 h, no effect was observed in wild-type cells, whereas the viability of cre/+ cells dropped to ∼65% (Fig. 2B). The bcl-xfl/+;cre/+ and bcl-xfl/fl;cre/+ leukemic cells displayed a reduction in viability that was slightly higher compared with that of cre/+ cells (Fig. 2B). In contrast, the viability of mcl-1fl/+;cre/+ cells dropped to ∼35%, and most mcl-1fl/fl;cre/+ cells were killed within 72 h (Fig. 2B). These results demonstrate that while Mcl-1 is important in both normal stem/myeloid progenitor cells and AML cells, the AML cells were significantly more susceptible to loss of Mcl-1, even when they were cocultured (Supplemental Fig. 3B). Thus, it might be possible to establish a “therapeutic window” for Mcl-1 inhibitors in AML therapy.
Figure 2.
Impact of conditional in vitro deletion of bcl-x or mcl-1 in normal hematopoietic stem and progenitor cells and in vivo derived AML cells. (A) Normal LSK (left panel) and Lin−c-Kit+Sca-1− myeloid progenitors (right panel) were cultured in the presence or absence of tamoxifen and cell survival was determined; data are presented as in Figure 1A. (B) Leukemic cells from mice bearing MLL-ENL-induced (left panel) or MLL-AF9-induced (right panel) AML were cultured in the presence or absence of tamoxifen and cell survival was determined; data are presented as described in Figure 1A. (**) P < 0.01. (C) MLL-ENL transformed AML cells of the indicated genotypes were grown in medium containing tamoxifen, ABT-737 (1 μM), or tamoxifen plus ABT-737 (1 μM). Cell survival was determined, and data are presented as described in Figure 1A. (**) P < 0.01. (D) Leukemic cells from mice bearing ICN1-induced T-ALL of the indicated genotypes were cultured in the presence or absence of tamoxifen. Cell survival was determined, and data are presented as described in Figure 1A. (**) P < 0.01.
Anti-apoptotic Bcl-xL, Bcl-2, and Bcl-w are collectively dispensable for sustained in vitro survival of AML
Although loss of Bcl-xL had only a minor impact, it may have a critical function in AML cell survival that overlaps with Bcl-2 and/or Mcl-1. We therefore treated MLL-ENL transformed AML cells with ABT-737, a BH3 mimetic that potently binds and inactivates Bcl-xL, Bcl-2, and Bcl-w (but not Mcl-1 or A1) (Oltersdorf et al. 2005), but on its own this had only a minor impact on viability (Fig. 2C). Moreover, ABT-737 further decreased the viability of mcl-1fl/+;cre/+ AML cells that had also been treated with tamoxifen to lose one mcl-1 allele to only a limited extent (Fig. 2C). This indicates that, collectively, Bcl-2, Bcl-xL, and Bcl-w have only a minor role in sustaining the survival of AML cells.
Sustained survival of T-ALL is possible in the absence of Mcl-1
To examine whether Mcl-1 is generally essential for the sustained survival and growth of all types of leukemic cells, we generated T-ALL by reconstituting lethally irradiated mice with bcl-xfl/fl;cre/+, mcl-1fl/+;cre/+, or mcl-1fl/fl;cre/+ bone marrow cells that had been retrovirally transduced with an expression vector encoding intracellular NOTCH1 (ICN1), an oncogene implicated in human T-ALL (Supplemental Fig. 4A; Allman et al. 2001). Treatment of GFP+ leukemic T cells maintained on OP9-DL1 feeder cells with tamoxifen led to efficient recombination of the mcl-1fl allele, as evidenced by hCD4 reporter expression (Supplemental Fig. 4B). Although loss of Mcl-1 (but not loss of Bcl-xL) elicited some reduction in T-ALL viability in vitro, a large fraction of T-ALL cells could survive longer term in the absence of Mcl-1 (Fig. 2D). This is in striking contrast to the obliteration of AML cells caused by Mcl-1 loss (Figs. 1A,C, 2B), and thereby demonstrates that the requirement for Mcl-1 for sustained growth and survival of transformed cells is specific to certain types of leukemia.
Deletion of mcl-1 but not bcl-x kills murine AML within the whole animal
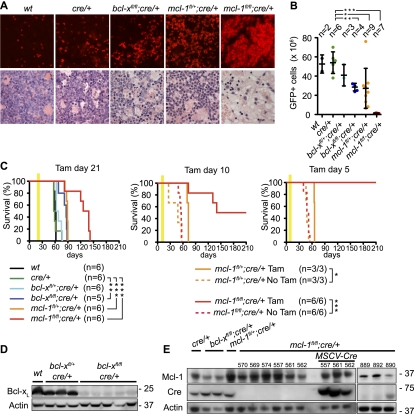
The impact of loss of Mcl-1 or Bcl-xL on AML within the whole animal was assessed by administering AML-burdened mice with tamoxifen. TUNEL staining of the marrow 3 d after tamoxifen treatment revealed significant (albeit moderate) apoptosis of bcl-xfl/fl;cre/+ AML cells, more abundant death of mcl-1fl/+;cre/+ AML cells, and widespread apoptosis of mcl-1fl/fl;creER/+ leukemic blasts (Fig. 3A, top panels; Supplemental Fig. 5A). Accordingly, almost no leukemic blasts were evident in histological examination 5 d after tamoxifen treatment of mice that had previously developed MLL-ENL transformed mcl-1fl/fl;cre/+ AML (Fig. 3A, bottom panels). On the fifth day after starting treatment, we found 24 × 106 to 70 × 106 GFP+ wild-type, cre/+, bcl-xfl/+;cre/+, or bcl-xfl/fl;cre/+ cells and 26 × 106 ± 21 × 106 GFP+ mcl-1fl/+;cre/+ AML cells but almost no (0.2 × 106 ± 0.2 × 106) GFP+ mcl-1fl/fl;cre/+ AML cells (Fig. 3B). All mice treated with tamoxifen 3 wk after transplantation with MLL-ENL transduced bone marrow cells relapsed with AML. In contrast, when treated with tamoxifen at day 10 or day 5 post-transplantation, only half or none, respectively, of the mcl-1fl/fl;cre/+ AML-burdened mice became sick during observation for >1 year. In contrast, their untreated counterparts survived for only 49 ± 5 d (P < 0.001), and untreated or tamoxifen-treated mcl-1fl/+;cre/+ AML-burdened mice lived for 49 ± 3 d or 66 ± 1 d, respectively (Fig. 3C). These findings were confirmed by demonstrating that tetracycline-induced RNAi-mediated knockdown of Mcl-1 in vivo caused clearance of AML cells from the liver and spleen and substantially prolonged survival of such tumor-burdened mice (Supplemental Fig. 5B–D).
Figure 3.
Conditional deletion of mcl-1 causes regression of AML in tumor-burdened mice. (A) Histological examination of bone marrow from mice burdened with MLL-ENL-induced AML of the indicated genotypes. The top panels show apoptosis of cells (detected by TUNEL staining) after 3 d of treatment with tamoxifen. The bottom panels show the presence of leukemic blasts after 5 d of treatment with tamoxifen. (B) Symptomatic (elevated leukocyte counts, thrombocytopenia, and anemia) mice bearing MLL-ENL-induced AML of the indicated genotypes were treated for 5 d with tamoxifen. Shown are the total numbers of GFP+ leukemic cells collected from two femora and two tibiae on the sixth day. (*) P < 0.05; (**) P < 0.01; (***) P < 0.001. (C) Survival of AML-burdened mice that were treated with tamoxifen (treatment window indicated by yellow bar) 21 d (left panel) 10 d (middle panel) or 5 d (right panel) after transplantation with MLL-ENL transformed AML cells of the indicated genotypes. Untreated mice (dotted lines) from the same cohort treated with tamoxifen at 10 d or 5 d all developed disease, thus confirming the presence of AML at that time pointl (*) P < 0.05; (**) P < 0.01; (***) P < 0.001. (D,E) Western blot analysis to detect Bcl-xL (D) or Mcl-1 and CreER proteins (E) in leukemic cells of the indicated genotypes from mice that relapsed with AML after tamoxifen treatment.
All relapsed bcl-xfl/fl;cre/+ and mcl-1fl/+;cre/+ AML from tamoxifen-treated mice had efficiently deleted their floxed alleles, as reflected by the absence of Bcl-xL protein (Fig. 3D) or by Southern blot analysis and hCD4 reporter expression (Supplemental Fig. 6A,B). In contrast, all relapsed mcl-1fl/fl;cre/+ AML arose from variant leukemic cells bearing mutations that debilitate CreERT2-mediated recombination. Most (eight out of nine) of these relapsed mcl-1fl/fl;cre/+ AML had not recombined both mcl-1fl alleles due to mutated CreERT2, as evidenced by the absence of hCD4 reporter (Supplemental Fig. 6B) and Cre-ERT2 protein expression (Fig. 3E). Retroviral reintroduction of CreERT2 and tamoxifen treatment allowed mcl-1fl recombination and consequently rapidly killed such cells in vitro (Supplemental Fig. 6C). Another mcl-1fl/fl;cre/+ AML (#890) contained CreERT2 protein but had recombined only one mcl-1 allele due to a deletion mutation in one loxP site, as reflected by Western blotting (Fig. 3E), hCD4 expression, and DNA sequencing (Supplemental Fig. 6B,D). These results show that Mcl-1 is essential for sustained survival and expansion of AML within the whole animal—only AML cells that had escaped mcl-1 deletion could cause tumor relapse.
Mcl-1 is critical for sustained survival of human AML-derived cell lines and primary human AML
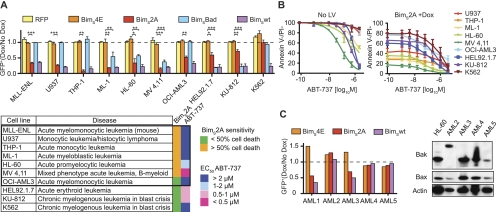
These gene deletion studies in murine AML indicated that pharmacological inhibition of Mcl-1 might be effective for treatment of this malignant disease in humans. However, small organic BH3 mimetic compounds specifically targeting Mcl-1 have yet to be developed. To test the impact of functional inactivation of Mcl-1 in human AML, we employed lentiviral expression of BimS-derived BH3 variant ligands: BimS4E, an inert Bim variant (negative control) (Chen et al. 2005); BimS2A to target Mcl-1 but not Bcl-2, Bcl-xL, Bcl-w, or A1 (Lee et al. 2008); BimSBad to target Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1 or A1 (Merino et al. 2009); and BimSwt to target all prosurvival Bcl-2 proteins (Chen et al. 2005) (Supplemental Fig. 7A). Doxycycline-induced expression of RFP or BimS4E for 72 h in MLL-ENL transformed mouse AML or multiple human AML- and chronic myeloid leukemia (CML)-derived cell lines did not reduce the numbers of lentivirally transduced (GFP+) cells compared with uninduced cultures (Fig. 4A; Supplemental Fig. 7B). In contrast, BimSwt expression greatly reduced the viability of all tested cell lines (Fig. 4A; Supplemental Fig. 7B). Importantly, blockade of Mcl-1 by BimS2A potently killed six of seven AML-derived cell lines but had only minor impact on the human erythroleukemia-derived (HEL92.1.7) and CML-derived (KU-812 and K562) cell lines (Fig. 4A). Interestingly, the few GFP+ MV4,11 cells that remained viable after 3 d of doxycycline treatment exhibited low or no BimS2A expression (Supplemental Fig. 7C), indicating that selection against Mcl-1 blockade had occurred. In contrast, BimSBad expression (Fig. 4A) or ABT-737 treatment (Fig. 4B), both of which block Bcl-2, Bcl-xL, and Bcl-w (Oltersdorf et al. 2005; Merino et al. 2009), did not reduce the viability of most AML lines, except the ML-1, HL-60, and MV4,11 cells (small but significant effect), but they potently killed the human erythroleukemia (HEL92.1.7) and CML (KU-812) cell lines. BimS2A-mediated Mcl-1 blockade in combination with ABT-737 treatment for 40 h led to almost complete killing of all cell lines tested (Fig. 4B), consistent with the notion that cells cannot survive when a broad range of prosurvival Bcl-2 proteins are inactivated.
Figure 4.
Impact of functional inactivation of Mcl-1 by inducible expression of Bim-derived BH3-like ligands that selectively neutralize different prosurvival Bcl-2 family members in human leukemia-derived cell lines and primary human AML cells. (A) Mouse AML cells and human leukemia-derived cell lines (U937, THP-1, ML-1, HL-60, MV4,11, OCI-AML3, HEL92.1.7, KU-812, and K562) were transduced with lentiviral constructs that allow inducible expression of BimS4E (negative control, no binding to Bcl-2-like proteins), BimS2A (binding only Mcl-1), BimSBad (binding Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1 or A1), or BimSwt (binding all Bcl-2 prosurvival proteins) and were either left untreated or treated with doxycycline. Graphs represent the ratio of GFP+ cells (doxycycline-treated compared with untreated) detected by flow cytometry of at least three experiments for each cell line. In the table, cell lines derived from AML are boxed; the heat map summarizes the sensitivity of all cell lines to BimS2A expression or ABT-737 treatment. (B) The cell lines indicated were treated with the indicated doses of ABT-737 alone (left panel) or in combination with induced BimS2A (inhibits Mcl-1) expression (right panel). (C) Primary human AML cells were transduced with BimS4E-encoding (negative control), BimS2A-encoding (inhibits Mcl-1), or BimSwt-encoding (inhibits all prosurvival Bcl-2 family members) lentiviruses and were either left untreated or treated with doxycycline. Shown is a graph representing the ratio of doxycycline-treated compared with untreated GFP+ AML blast cells from one representative experiment for each patient sample. Western blot analysis to detect Bax and Bak protein levels in primary AML cells. Low levels of Bax and Bak were detected in sample #5; low levels of Bax and abnormal size of Bak were detected in sample #2.
We next transduced primary AML cells from patients (Supplemental Fig. 7D) with the aforementioned expression constructs to block Mcl-1 (Bims2A) or all prosurvival Bcl-2 proteins (Bimswt), using Bims4E as a negative control. Interestingly, two primary human AMLs (#1 and #3) showed substantially reduced viability after doxycycline-mediated induction of BimS2A or BimSwt but not after induction of (inactive) BimS4E (Fig. 4C). Some primary human AML, however, did not show a significant drop in viability after doxycycline treatment. Although in some AML cells this may have been due to insufficient induction of Bims2A or Bimswt, we remarkably found mutated Bak (abnormal protein size) and abnormally low Bax levels in AML #2 and very low expression levels of both Bax and Bak in AML #5 (Fig. 4C). It appears likely that the therapeutic treatments that these patients received prior to our analysis selected for outgrowth of AML with such generalized defects in the “Bcl-2-regulated” apoptotic pathway.
Collectively, these results demonstrate that Mcl-1 is critical for the sustained survival and expansion of mouse as well as human AML resulting from a broad range of oncogenic lesions, whereas Bcl-xL, Bcl-w, or Bcl-2, as reported (Zuber et al. 2011b), play minor prosurvival roles in these cancers. As such, these findings encourage the development of pharmacologic inhibitors that target Mcl-1 or regulators of its expression for treatment of this hematological malignancy.
Materials and methods
Animals
Experiments with mice were conducted according to the guidelines of The Walter and Eliza Hall Institute Animal Ethics Committee. bcl-xfl/fl (Wagner et al. 2000), mcl-1fl/fl (Supplemental Fig. 1A; Vikstrom et al. 2010) and the Rosa26-CreERT2 (Seibler et al. 2003) (Taconic Artemis) gene targeted mice (all on a C57BL/6 background) have been described. Mice received one aliquot containing 4 mg of tamoxifen (Sigma, T5648) per day for five consecutive days by oral gavage as described previously (Anastassiadis et al. 2010).
Generation and culture of transformed leukemic cells
MLL-ENL and MLL-AF9 retroviral constructs were obtained from Drs. R. Slany and M. Cleary, the AML1-ETO9a retroviral construct was obtained from Dr. D.-E. Zhang (Addgene plasmid 12433) (Yan et al. 2006), the Hoxa9 retroviral construct was obtained from Dr. C. Largman (Addgene plasmid 8515) (Shen et al. 1997), and the Mixl1 retroviral construct was described previously (Glaser et al. 2006). The Bcl-2, Bcl-xL, and Mcl-1 retroviral expression constructs were provided by Dr. D.C.S. Huang. The CreER-hCD2 retroviral construct was provided by Dr. M. Bussliner (IMP, Vienna, Austria), and the retroviral construct for shRNA-mediated knockdown of Bim has been described (Bouillet et al. 2005). Lentiviral expression constructs were generated by KpnI digestion, and by cloning a synthesized 1638-base-pair (bp) fragment containing GFP-WPRE and AgeI/ClaI digestion and cloning of BimS variants into the TRIPZ vector (Open Biosystems). Viral supernatants were produced by CaCl2 cotransfection of 293T cells with expression constructs and packaging plasmids. Fetal liver (E13.5 [embryonic day 13.5] embryos) or bone marrow cells from 5-FU-treated mice were enriched for immature cells by immunomagnetic depletion of mature lymphoid (CD3+, CD4+, CD8+, and B220+) myeloid (Mac1+ and Gr1+) and erythroid (Ter119+) cells. Viral transduction was performed as described (Metcalf et al. 2007). Transduced cells were cultured in Dulbecco's modified Eagle's medium supplemented with 20% FBS (HyClone), 2 mM L-glutamine, 100 ng/mL murine stem cell factor, and 10 ng/mL IL-3 (Peprotech). Infected cells were either FACS-sorted on the basis of GFP expression for in vitro studies or injected unsorted into sublethally γ-irradiated (5.5 Gy) C57BL/6 mice. Cells retrovirally transduced with the MLL-ENL/neo expression vector were plated in methylcellulose medium M3234 (Stem Cell Technologies) supplemented with 100 ng/mL murine stem cell factor, 10 ng/mL IL-3, and 1 mg/mL G418 (InvivoGen). G418-resistant cells from the third plating were transduced with retroviral constructs encoding Bcl-2 family members or RNA hairpins for knockdown of Bim and were linked with IRES-GFP, and transduced cells were subsequently isolated by cell sorting. Cre-mediated recombination in vitro was induced by treatment with 10−7 M 4-hydroxy tamoxifen (Sigma, H7904) (Glaser et al. 2009). For analysis of protein levels by Western blotting, mcl-1fl/fl;creER/+ cells were treated with tamoxifen in the presence of the pan-caspase inhibitor qVD-OPh (25 μM; MP Biomedicals) to protect them from apoptotic death.
Detailed methodology is described in the Supplemental Material.
Acknowledgments
We thank Drs. L. Hennighausen, J.M. Adams, S. Cory, D.C.S. Huang, M. Cleary, D.-E. Zhang, C. Largman, and M. Busslinger for gifts of mice and retroviral constructs. This work was supported by grants and fellowships from the Deutsche Forschungsgemeinschaft (DFG) (to S.G.), the Lady Tata Memorial Trust (to S.G.), the NHMRC (programs 257500 and 461221; projects 637326 and 1008329; fellowships 356203, 461299, and 575501; and Independent Research Institutes Infrastructure Support Scheme grant 361646), the Leukemia and Lymphoma Society (SCOR grant 7413), the NIH (CA43540 and CA80188), the Victorian Cancer Agency, the Leukemia Foundation of Australia, and the Australian Cancer Research Fund, and by operational infrastructure grants through the Australian Government (IRISS) and the Victorian State Government (OIS).
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.182980.111.
References
- Allman D, Karnell FG, Punt JA, Bakkour S, Xu L, Myung P, Koretzky GA, Pui JC, Aster JC, Pear WS 2001. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. J Exp Med 194: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis K, Glaser S, Kranz A, Berhardt K, Stewart AF 2010. A practical summary of site-specific recombination, conditional mutagenesis, and tamoxifen induction of CreERT2. Methods Enzymol 477: 109–123 [DOI] [PubMed] [Google Scholar]
- Ayton PM, Chen EH, Cleary ML 2004. Binding to nonmethylated CpG DNA is essential for target recognition, transactivation, and myeloid transformation by an MLL oncoprotein. Mol Cell Biol 24: 10470–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel C, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm J, Dobson J, Urashima M, et al. 2010. The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Robati M, Bath ML, Strasser A 2005. Polycystic kidney disease prevented by transgenic RNA interference. Cell Death Differ 12: 831–833 [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS 2005. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403 [DOI] [PubMed] [Google Scholar]
- Estey E, Dohner H 2006. Acute myeloid leukaemia. Lancet 368: 1894–1907 [DOI] [PubMed] [Google Scholar]
- Glaser S, Metcalf D, Wu L, Hart AH, DiRago L, Mifsud S, D'Amico A, Dagger S, Campo C, Chan AC, et al. 2006. Enforced expression of the homeobox gene Mixl1 impairs hematopoietic differentiation and results in acute myeloid leukemia. Proc Natl Acad Sci 103: 16460–16465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser S, Lubitz S, Loveland KL, Ohbo K, Robb L, Schwenk F, Seibler J, Roellig D, Kranz A, Anastassiadis K, et al. 2009. The histone 3 lysine 4 methyltransferase, Mll2, is only required briefly in development and spermatogenesis. Epigenetics Chromatin 2: 5 doi: 10.1186/1756-8935-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao B, Wu CF, Liang Y, Chen HM, Xiong SM, Chen B, Shi JY, Wang YY, Wang JH, Chen Y, et al. 2009. AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia-M2. Leukemia 23: 1598–1604 [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW 2002. Apoptosis: A link between cancer genetics and chemotherapy. Cell 108: 153–164 [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, Reed JC 1998. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood 91: 991–1000 [PubMed] [Google Scholar]
- Lavau C, Luo RT, Du C, Thirman MJ 2000. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci 97: 10984–10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EF, Czabotar PE, van Delft MF, Michalak E, Boyle M, Willis SN, Puthalakath H, Bouillet P, Colman PM, Huang DCS, et al. 2008. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol 180: 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD, et al. 2009. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 186: 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, Glaser S, Mifsud S, Di Rago L, Robb L 2007. The preleukemic state of mice reconstituted with Mixl1-transduced marrow cells. Proc Natl Acad Sci 104: 20013–20018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681 [DOI] [PubMed] [Google Scholar]
- Opferman J, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ 2005. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307: 1101–1104 [DOI] [PubMed] [Google Scholar]
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F, Eastham-Anderson J, et al. 2010. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463: 103–107 [DOI] [PubMed] [Google Scholar]
- Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, Hennek T, Rode A, Heimann C, Faust N, Kauselmann G, et al. 2003. Rapid generation of inducible mouse mutants. Nucleic Acids Res 31: e12 doi: 10.1093/nar/gng012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WF, Montgomery JC, Rozenfeld S, Moskow JJ, Lawrence HJ, Buchberg AM, Largman C 1997. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol 17: 6448–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML 2006. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 10: 257–268 [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Mamo A, Kroon E, Jerome L, Bijl J, Lawrence HJ, Humphries K, Sauvageau G 2002. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood 99: 121–129 [DOI] [PubMed] [Google Scholar]
- Vikstrom I, Carotta S, Luethje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, et al. 2010. Mcl-1 is essential for germinal center formation and B cell memory. Science 330: 1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Claudio E, Rucker EB 3rd, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L 2000. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development 127: 4949–4958 [DOI] [PubMed] [Google Scholar]
- Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, Chen IM, Chen Z, Rowley JD, Willman CL, et al. 2006. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med 12: 945–949 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A 2008. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, McCurrach ME, Yang MM, Dolan ME, Kogan SC, et al. 2009. Mouse models of human AML accurately predict chemotherapy response. Genes Dev 23: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW 2011a. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol 29: 79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, Shi J, Weissmueller S, Fellmann C, Taylor MJ, et al. 2011b. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev 25: 1628–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]