Abstract
Prevention trials of whole foods or simple extracts offer prospects for reducing an expanding global burden of cancer effectively and, in contrast to promising isolated phytochemicals or pharmaceuticals, frugally. We use the term “green chemoprevention” to differentiate a food-centered approach that is sustainable in underserved populations. It can be applied to personalized medicine just as well as can a pharmaceutical approach, but only green chemoprevention can be applied in both rich and poor settings. This MiniReview discusses some of the challenges of conducting food-based trials in developing countries, with particular emphasis on moving the limited number of promising phase II trials forward as placebo-controlled randomized trials, the gold standard for prevention studies. How does one define a placebo for a food? What is the regulatory context of such a food-based product? How can such products be produced and standardized to the benefit of a larger, individual trial and, importantly, the research community at large? What are the challenges and opportunities of conducting such trials in the international setting? Last, how does one make the science practical?
Introduction
Aging and growth of the world population, together with adoption of life-style factors such as smoking, obesogenic diets, and sedentary lifestyles, are escalating the global burden of cancer. It is estimated that 7.6 million cancer deaths occurred in 2008 and that this toll will reach more than 13 million by 2030 (1). Moreover, within the next two decades nearly 70% of cancer deaths will occur in the developing world(2). Cancer and other chronic diseases will bankrupt medical care–delivery systems and cause enormous suffering if their progression cannot be slowed or reversed. It is suggested that a substantial proportion of the worldwide burden of cancer could be prevented through the application of existing knowledge of cancer control and by implementing programs for tobacco control, vaccination, and early detection and treatment, as well as public health campaigns promoting physical activity and consumption of healthier diets (3). Although unhealthy eating is in part to blame for the rise in prevalence of some chronic diseases, dietary approaches can be instrumental in preventing or delaying a variety of cancers. Indeed, combined modification of diet and behavior constitute one of the only available tools for widespread change in many populations in the developing world. Here especially, the practice of frugal medicine becomes essential; interventions need to be effective, safe, tolerable, practical, and inexpensive. As the Western world spins toward personalized medicine and prevention, the economic realities argue that most of the world population at risk for cancer will not have access to new-generation targeted synthetic molecules for treatment or prevention. They will have access, however, to local foodstuffs, and thus an appreciation of the mechanisms of chemopreventive action of Western foods can be translated to indigenous foods and/or guide the introduction of culturally appropriate targeted protective foods. It is in this sense—food-centered approaches that are sustainable in underserved populations—that we use the term “green” chemoprevention. It can be applied to personalized medicine as well as can a pharmaceutical approach, but only green chemoprevention can be applied in poor as well as rich settings. This MiniReview follows on to a group of international papers in the previous issue of the journal on cancer prevention in developing countries or regions (4–8)..
Chemoprotection Trials of Standardized Foods
Translation of research findings from field to bench to bedside must assess the best approaches to preventive interventions. Many of our colleagues have reviewed the body of evidence supporting the chemoprotective effects of isolated phytochemicals (e.g., isothiocyanates from crucifers, organosulfides from garlic and onions, and polyphenols from berries and teas). All these compounds come from foods—vegetables, fruits, herbs, spices, and teas. We and others believe that there is ample evidence to suggest that whole foods themselves may be the most effective way to reduce the risk of a variety of cancers, and that the delivery of complex mixtures of a number of individually bioactive phytochemicals permits the upregulation and/or inhibition of multiple steps in the development of neoplasias (9, 10). A very slim, but growing portfolio comprises the prevention trials that have employed whole foods or simple extracts (juices and extracts of fruits and vegetables) rather than isolated phytochemicals or pharmaceuticals.
There are at least three general approaches that can be used to standardize foods (fruits or vegetables) for such trials. First, an approach can use random selection, where either foods are supplied by the investigator or the participants are told what to eat; then ingestion of the phytochemical(s) of interest is estimated. In a recent trial with tomato products, for example, participants were instructed to consume “a diet rich in tomato products (providing at least 25 mg of lycopene per day)” and were given guidance on how to do so (11). In this approach, phytochemical intake is surmised post facto. A second approach involves growing or purchasing and processing a large, single lot of food specifically for the trial. After composition or phytochemical analysis, the food can be delivered to participants with a controlled phytochemical “loading.” It is therefore possible to carry out a dose-response study, which is impractical with the first approach. Most of the trials highlighted below utilize this second approach, but it is still possible that clinical results could be unique for a particular food lot, or batch. The third approach is specifically and deliberately to grow plants based on their phytochemical content and to characterize them, including screens for human pathogens, measuring microbial content, analyzing heavy metals and pesticides, and verifying phytochemical titer. We have used this approach for our work with broccoli sprouts since environmental effects are minimized by growth in a controlled environment and plant genotype essentially controls phytochemical content (12). A modification of this approach was described in early work by Ip and Lisk (13), who developed selenium-enriched garlic by growing it in selenium-enriched soils resulting in the bioaccumulation of abnormally high selenium levels. They then used this garlic in a rat mammary tumor model and speculated about the utility of such an approach for human chemoprevention.
Most of the work with both berries and broccoli sprouts has been performed with freeze-dried (lyophilized) preparations, which concentrate their phytochemicals or bioactives about 10-fold simply by the removal of water and permit easier handling and dosification, greatly prolong storage life, and enhance product uniformity. Since lyophilization conditions can vary and the process removes other substances (e.g., essential oils) as well as water, lyophilization is not sensu stricto exclusively a “concentration” step since some biologically active molecules may be lost and the composition of the food might be modified more than desired.
In the following subsections, we briefly highlight a few of the food-based interventions that are directed toward cancer protection. These foods have been selected because the interventions conducted with them exemplify the three general approaches indicated previously and because they in fact represent the majority of food-based trials of which we are aware. We have specifically excluded consideration of trials that utilize herbal mixtures or purified phytochemicals, e.g., epigallocatechin-gallate (EGCG; ref. 14), both of which are in many ways more closely aligned with dietary supplement or drug trials and have been reviewed in that context by many others. As such, we do not address the encouraging recent clinical trials of curcumin (15) or the extensive work in progress on polyunsaturated fatty acids (PUFAs). Furthermore, foods can be divided roughly into animal and plant domains, and the interest in chemoprevention focuses almost exclusively on reducing or qualitatively altering meat intake and increasing plant intake (also with qualitative fine tuning). Therefore, we have grouped the foods we consider here as either fruits or vegetables, including teas as a vegetable since they are in essence hot-water leaf infusions. Also, they are not artificially enriched beyond the obvious preferential exclusion of highly lipophilic substances from an aqueous infusion—as is true for broccoli sprout extracts. National Clinical Trial (NCT) numbers in parentheses refer to clinical trial designations on the NIH registry at www.clinicaltrials.gov.
Fruits
Berries
Stoner (16) elegantly described the factors driving the preclinical and early clinical study of berries in chemoprevention as well as considerations that influence the selection of powdered freeze-dried berries as opposed to berry extracts or pure anthocyanins, the presumed bioactive components. Powdered freeze-dried berries are relatively inexpensive (certainly in comparison with purified anthocyanins) and contain numerous molecules with possible chemopreventive activity, potentially contributing to multiple modes of action against carcinogenesis. Counterbalancing these advantages are concerns about standardization of batches of berry powders and potential contamination with insecticides, heavy metals, and microbes. In China, Chen et al. recently conducted a randomized phase II chemoprevention trial of two doses (60 g/d or 30 g/d) of whole strawberries lyophilized at a commercial food lyophilizing facility for reducing the histology and molecular features of esophageal dysplasia (4). This study used a single lot of well-characterized powder prepared from standard commercial varieties, thus addressing some of the aforementioned concerns. Sixty g/day (but not 30 g/day) produced a significant reduction in histological grade of the premalignant lesions. The trial was conducted within the ‘‘esophageal cancer belt,” a high-risk area stretching from northern Iran through the central Asian republics to north-central China, where 90% of cases are squamous cell carcinomas (SCC; ref. 17). Major risk factors for SCC in these areas probably include poor nutritional status, low intake of fruits and vegetables, and drinking beverages at high temperatures (18). The earlier Nutritional Intervention Trial conducted in Linxian, China, well recognized as the highest incidence area for esophageal SCC in the world, had demonstrated that supplementation with vitamins and minerals (50 μg selenium, 30 mg vitamin E, and 15 mg beta-carotene) decreased mortality from all causes, cancer overall, and gastric cancer (19). Non-significant effects on mortality from esophageal cancer were reported. Thus, the population-based evidence for the strawberry-based trial was solid, if not entirely secure. Animal models have highlighted the chemopreventive efficacy of strawberries and other berries against chemical-induced tumorigenesis in the rat esophagus (20, 21). It is important to note that China is becoming a major producer in the world strawberry market, so that unlike raspberries and blackberries, this fruit is available to this population in the marketplace.
The present group previously evaluated freeze-dried black raspberries, blueberries, and strawberries in preclinical in-vitro and in-vivo studies (reviewed in ref. 22). Clinical trials of these berries and their extracts now include oral delivery for prevention of non-small–cell lung cancer (NCT00681512) and familial adenomatous polyposis (NCT00770991); lozenges for treatment of oral SCC (NCT01465776) and head and neck cancer (NCT01469429); and oral gels in a multi-center oral cancer chemoprevention trial (NCT01192204; also see refs. 23–25).
Pomegranate
At least eight separate studies of pomegranate are currently listed on the website www.clinicaltrials.gov. These trials are evaluating the effects of currently marketed pomegranate juice, extract, or pills (capsules containing an extract) on prostate cancer progression or recurrence or biomarkers thereof. We are only aware of published results from a single phase II study in men after treatment for prostate cancer, which showed a significant prolongation of prostate-specific antigen (PSA) doubling time (26).
Tomato
There have now been several small clinical interventions in which tomato products have been prescribed for men with recurring prostate cancer (12) and for women at a high risk for breast cancer (27). Although these trials have been rigorous and well designed, they have not delivered a standardized preparation of tomatoes, but rather have dictated that a minimum quantity of tomato products must be consumed by subjects in “the tomato arm.”
Vegetables
Broccoli sprouts
Some of our early clinical work utilized fresh broccoli sprouts (13, 28–30), and in part, this encouraged us to develop freeze-dried standardized sprout extracts from specifically selected cultivars and seed sources that are grown for us in a prescribed manner. We also utilize a boiling water extraction step, and many of our 24 broccoli sprout studies listed on www.clinicaltrials.gov have added an enzymatic conversion step before lyophilization, as summarized later in this article.
Tea
Both black and green teas are abundant sources of polyphenols and have been studied in the clinic. Polyphenolic extracts have been delivered as pure teas and as concentrates (e.g., Polyphenon E). The preclinical experience with teas for cancer prevention has been summarized recently (31–33). Early trials addressing pharmacokinetic endpoints (34) have guided at least one phase II randomized, [placebo]-controlled trial with encouraging results in patients with high-risk oral premalignant lesions (35). At present, more than 40 clinical cancer-prevention or -treatment trials of tea or an enriched tea concentrate (e.g., Polyphenon E) are listed on www.clinicaltrials.gov.
Garlic
Aged garlic extract or garlic oil has been evaluated for its ability to inhibit the development of precancerous gastric lesions (36) and colorectal adenomas (37). The colorectal trial was small (51 subjects) and demonstrated that taking daily garlic extract for a year had an effect, but there was no effect on precancerous lesions in the gastric study involving 3365 subjects randomized to treatments that included garlic taken twice daily for 7.3 years. Other garlic trials appear to have been completed (NCT00079170; NCT0123591), but we are not aware that results have been published.
Ginger root
Ginger has been shown to downregulate cyclooxygenase (COX) in vitro and to reduce adenomas in rats. A recent small early-phase randomized trial of ginger root extract for effects on eicosanoids found that the extract (versus placebo) significantly decreased the primary endpoint of mean change in prostaglandin E2 levels in biopsies (when normalized to free arachidonic acid) of the colon mucosa of healthy volunteers (38); the extract was tolerable and safe. This trial established a dose (originally based on a phase I trial; ref. 39) and formulation of ginger root extract that the investigators suggested for moving forward into clinical testing in people at a high risk of colorectal cancer.
Clearly the goal of food-based approaches to prevention is enticing, but there are a number of daunting challenges in designing and conducting food-based intervention trials, particularly when they require international collaborations. What are some next steps and key challenges?
Randomized Placebo-controlled Clinical Trials of Food—What Is the Placebo?
The gold standard for assessing the efficacy of new chemopreventive agents, and indeed many medicines, has been the phase III randomized placebo-controlled clinical trial (RCT). RCTs of single-agent tamoxifen, finasteride, or celecoxib (40–42) and of combinations such as α-difluoromethylornithine (DFMO) and sulindac (43) exemplify this approach in the prevention setting. While the demand to use “the best treatment” excludes the use of placebo in the control group where a standard of care has been established, and presents an obstacle to the scientific evaluation of a number of drugs and treatments in general, this demand regrettably is of limited relevance to cancer prevention, where few drugs have been Food and Drug Administration (FDA)-approved. The use of placebo is justified whenever it does not cause serious damage or considerable suffering to the well-informed patient. However, it is unclear whether the standard of best available treatment should be local or international (44). Chen et al. (4) proposed “to test strawberries-alone and combined with other preventive agents in randomized placebo-controlled trials” as the next step in their program of study. But what should the placebo be in such a food-based study?
For chemoprevention trials, placebos have an important role as control interventions to determine specific effects and to reduce bias by enabling blinding (45). In our RCTs of broccoli sprout preparations conducted in China, we have used a variety of strategies to develop appropriate placebos. None have been ideal. Our three main approaches have been as follows: 1) Remove/extract the active component(s), 2) add the active components to an ”inert” vehicle, and 3) unmatched placebo. In our first RCT (46), we imported broccoli seeds and grew three-day-old sprouts in an improvised greenhouse. Plants were plunged into boiling water for 15 minutes to extract glucoraphanin, the stable, water-soluble glucose conjugate precursor to the chemopreventive compound sulforaphane. Extensive quality-control measures established the safety of, and glucoraphanin concentration in, the beverage. The placebo beverage was a sequential fourth hot-water extract of the plant material. At this stage, all of the glucoraphanin as well as other uncharacterized plant components were eliminated. Nonetheless, the placebo beverage still had a vegetable smell and coloration, albeit with a different complex of tastes. The logistics of scaling up this process for larger, longer trials, as well as a changing regulatory landscape, prompted us to develop an alternative approach to formulating a placebo for a trial that is ongoing as we write this MiniReview. With commercial sprouters and a freeze-drying facility with good [food] manufacturing practice (GMP) compliance, we prepared large-scale batches of glucoraphanin-rich and sulforaphane-rich powders from the hot water sprout extracts. These powders were then shipped to China and are reconstituted daily in a beverage composed of commercial bottled water, pineapple juice, and lime juice. This admixture of liquids provides blinding to taste and visual aspects of both the placebo and test beverages. Are participants completely deceived? We think not. In an earlier RCT of the drug oltipraz, one participant sent out an assigned pill to a relative working in the pharmaceutical industry for analysis, which amused us, especially since this was rural China in the mid 1990s—a very different time and place. Blinding may be in the eye of the investigator, but rarely of the study participant. Albeit in a cross-over design trial in which each individual served as his or her own control rather than in an RCT, our third approach used a dilute mango juice beverage during run-in and wash-out phases of the trial, which compared effects of either glucoraphanin- or sulforaphane-rich mango juice beverages containing reconstituted lyophilized powders (47, 48). The mango juice provided no blinding to taste or color of the beverage. The unmatched placebo worked well in this setting, although most participants at the end of the study acknowledged that they liked the placebo better than either of the active intervention formulations.
In addition to blinding, the other key aspect of selecting an appropriate placebo is to facilitate evaluation of the specificity of the effects (adverse or efficacious) of the intervention agent. In regard to food placebos, developing a placebo material that is completely lacking in bioactive materials is exceedingly difficult. Sulforaphane, like the anthocyanins featured in the Chen et al. (4) study, has multiple molecular targets and actions. Since our study endpoints in China typically focus on alteration of carcinogen metabolism and formation of DNA adducts, we screen for possible placebo components devoid of these actions in simple in-vitro bioassays. With more complicated study endpoints, such as histological evaluation of esophageal dysplasia, where multiple targets and actions occur, verification that the placebo is devoid of biological activities becomes more difficult. Active placebos can confound a clinical trial and might give a misleading negative result. Since the default effect of such confounding is to push the outcome towards the null, a bioactive placebo is highly undesirable. Perhaps the recent Chen et al. (4) outcome of apparently no effect with the lower dose of strawberries suggests that dilution may be a solution to consider for a useful placebo. In any case, achieving objective evaluation of specificity of effects together with effective blinding of study participants and investigators in a food-based trial is far more challenging than in a drug-based trial, where masking generally can be readily achieved with opaque capsules and inert fillers.
Standardization of the Test Material
Good science demands a rigorous understanding of the composition of foods utilized in prevention studies. A shifting regulatory landscape also requires a high level of quality control and safety evaluation in the use of food-derived products as medicines. Classification of foods versus dietary supplements versus drugs has a significant impact on the approval processes for use of these materials in clinical trials. The U.S. FDA defines drugs as (emphasis ours) “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals.” The FDA regulates both finished dietary supplement products and dietary ingredients under a set of regulations different than those covering “conventional” foods and drug products (prescription and over-the-counter). Under the Dietary Supplement Health and Education Act of 1994, the manufacturer of dietary supplements or ingredients is responsible for ensuring the safety of these products before they are marketed. These products do not need to be registered with the FDA, unlike drugs, for which Investigational New Drug (IND) applications must be filed and approved before initiating studies.
In the past decade, our intervention test article (hot water extracts of three-day-old broccoli sprouts) has been classified as a food, a dietary supplement, and a drug, by both our Institutional Review boards (IRBs) and the FDA, often not synchronously or with clarity of purpose. Often legal liability considerations rather than scientific considerations have driven these decisions. A uniform regulatory approach, nationally and internationally, will be needed to facilitate investigators, IRBs, and national regulatory bureaus in the evaluation of food-based approaches for disease prevention.
Standardization and validation of the plant source has most notably come to general attention with regard to herbal medicines and dietary supplements. Contamination of herbal extracts with foreign plant material (incorrect or perhaps fraudulent identification of plant species), excessive quantities of trace elements, pesticides, filth (e.g., rodent hairs and insect parts), undeclared synthetic substances, microbes, or antibiotics have all been documented, along with misrepresentation of the sourcing of plants. The issues associated with scientific development of medicines from plants have been summarized in a historical context by Talalay and Talalay (49). Issues surrounding poorly documented trials with “herbal” and “botanical” products (e.g., ginseng, black cohosh, grape seed extract) have added to the confusion and reluctance of some clinical practitioners to embrace food-based trials (50). The development of standardized vegetables and fruits for intervention must be guided by most of the same concerns in place for herbal or botanical remedies and dietary supplements. It is particularly important that issues surrounding content, source, and botanical variety and uniformity of preparation must be considered to ensure that dosing of subjects can be validly compared based on a biologically useful criterion. In most but not all cases, this standardization is based upon measuring the quantity of a particular phytochemical or phytochemicals within the fruit or vegetable.
Epidemiology points to foods (e.g., fruits and vegetables) for prevention, but modern science cannot fully validate their efficacy unless scientists can repeat the outcomes of published interventions. Therefore, reproducible delivery of standardized foods is critical to the clinical testing of foods and food combinations for their chemoprotective potential. In other words, clinical testing of foods and food combinations that can be validated is not likely to happen unless and until these foods are documented and characterized in such a way that others can replicate the dosing regimen and deliver the same amount of the phytochemical(s) of interest within a reasonably similar background (plant) matrix. The use of crops grown for human consumption requires knowledge of the following plant-related factors: 1) Genetics or pedigree, 2) environment or provenance, and 3) contamination (both deliberate and accidental).
Pedigree
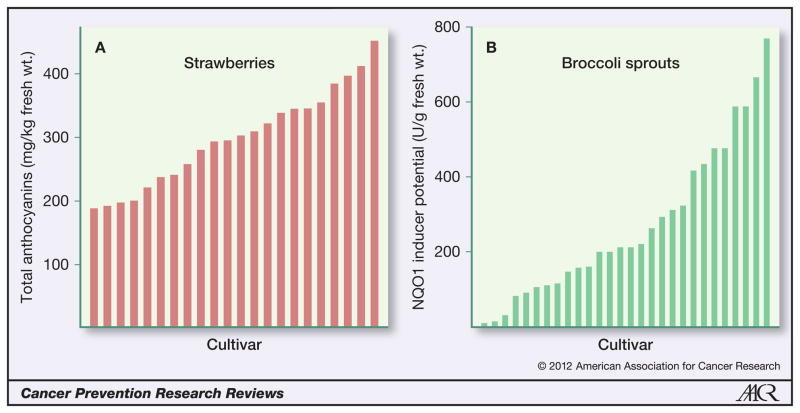
Designation of the genus and species or subspecies is generally sufficient to indicate common plant names such as strawberry or broccoli. The terms “variety” and “cultivar,” however, are genetic-based botanical or horticultural designations that specify a type of rose (e.g., Midas Touch or Crimson Queen) or apple (e.g., Macintosh, Gala, or Golden Delicious). For vegetables like broccoli and fruit like strawberries, the cultivar designation is not generally apparent to the consumer. Growers, packers, and distributors select cultivars for the market based on economics and availability so that the cultivar on a consumer’s plate may vary from one trip to the supermarket or farmers market to the next. Our experience with broccoli and broccoli sprouts is that content of glucoraphanin (which is then converted to biologically active sulforaphane) differs between 10- and 100-fold among cultivars (13, 51) and varies greatly with growing conditions (52) and even with position on the broccoli head (53). Strawberry cultivars have similar quantitative and qualitative variations in polyphenols (54, 55). Therefore, documentation of cultivar and source is essential to replication of a clinical or laboratory result (Fig. 1).
Figure 1.
A, the cultivar and harvest time (and various other factors) dramatically affect levels of total anthocyanins (pictured), cyanidin, ellagic acid, p-coumaric acid, chlorogenic acid, caffeic acid, ascorbic acid, and pelargonidin in 22 cultivars of field-grown strawberries (redrawn from ref. 54). B, similarly, a variety of factors influence the glucosinolate content of broccoli, which translates directly to NAD(P)H quinone:oxidoreductase 1 (NQO1) induction in Hepa1c1c7 cells (pictured) and is exclusively attributed to sulforaphane content. Data are from 28 broccoli cultivars grown to the three-day-old stage in the authors’ lab, as summarized in reference 13.
Environment
The geographic location of crop production and time of year, as well as all of the environmental variables in a growing season, can have a profound effect on the content of trace elements and phytochemicals. Phytochemicals are plant secondary metabolites and are largely defense compounds. Their titer and relative predominance within plant tissues respond acutely to environmental perturbations (including predation). Growth in conventional versus organic agricultural settings has an even more dramatic effect on phytochemical content than on the macro-components (e.g., carbohydrates, proteins, fat; ref. 56).
Contaminants
Contamination of fruits and vegetables can vary greatly and should be screened for, well documented, and eliminated if at all possible. Microbes (plant pathogens and commensals and human pathogens) are the primary potential contaminants. Plants naturally host a large external microbial community, but improper agricultural practices can bring unacceptably high levels of human pathogens (e.g., E. coli 0157:H7) into contact with fruits and vegetables destined for the market. This is a significant problem with both berries and sprouts, as it is with any other commercially available fruit or vegetable, and contamination issues led us to assist the FDA in developing and codifying procedures for safely producing vegetable sprouts (57). Pesticides and environmental toxins are more insidious, but again, proper testing can assure safety. Deliberate contamination, introduction of undeclared substances, or dilution with other (inactive) botanicals is a greater problem in the supplement industry than with whole foods. Nevertheless, within weeks of the introduction of broccoli sprouts as a food in 1997, unscrupulous profiteers were misrepresenting (as broccoli) both sprouts and seeds that laboratory testing proved not to be broccoli or even cruciferous vegetables in some cases (13).
Chen et al. (4) set an example by the excellent documentation of source and phytochemical content of their lyophilized strawberries, as well as by their processing, handling, and dosification conditions. These exemplary methods are equally well detailed in earlier publications from this group on their work with lyophilized black raspberries (16). They have set a high bar for others to follow, and we urge that future potential clinical investigators of whole foods or simple food extracts view their example as a minimal entry requirement. For fruit and vegetable interventions that employ plant foods of poorly documented provenance or pedigree, investigators should deposit voucher specimens that include viable seeds or other means to propagate those plants in appropriate land grant university or private herbaria/hortoria, or with the US Department of Agriculture’s National Germplasm System (58).
Production
One of the most frustrating obstacles that scientifically rigorous whole-food trialists face is the lack of readily accessible large-scale facilities where the plants can be reliably and safely produced and processed under conditions that allow for rigorous investigator supervision and/or participation in the process and standardization of the product for content of specific phytochemicals (at times within the context of rather artificial IND “drug” constraints). We return to the example with which we are most familiar—our trials with broccoli sprouts—in order to demonstrate some of the challenges to conducting trials of this sort, for which we, as a scientific community, must develop a better operational paradigm.
We have prepared highly standardized, freeze-dried boiling water extracts of broccoli sprouts under food-compliant GMP conditions and under the direct personal supervision of scientists at Johns Hopkins Medical School. These preparations are now being tested clinically for oral and topical use. More than 24 clinical studies (over a dozen have INDs) have been undertaken or are being planned for a broad range of clinical investigations: From pharmacokinetics, safety, and tolerance studies to preventive interventions in various disease states, including cancer-risk settings, asthma, chronic obstructive pulmonary disease, skin pathology, and a number of neurodegenerative disorders. Some of these studies are being conducted at Johns Hopkins, and many are now at other institutions in the U.S. and other countries. Hopkins co-investigators (JWF and PT) in these studies supply the broccoli sprout extract (BSE) together with extensive chemistry, composition, and safety data that are used in applications for IRB and IND approvals. This BSE has been administered to volunteers as follows: 1) Redissolved in water, 2) in a food matrix (e.g., mango juice, pineapple/lime juice, or cheese soup), or 3) in gelatin capsules hand-made by a specialty pharmacist. This process includes regular and time-consuming quality and stability testing and record-keeping. We thus share full responsibility for the design and proper conduct of the clinical studies of BSE; we also receive real-time information on the progress and any complications in these studies. As a result, much of the record-keeping, regulatory, and product development functions that would be shouldered by a food or drug company in other scenarios are squarely ours in this scenario—not the most efficient use of the time of research scientists.
We suggest that many of the food-based trials described above have been faced with at least some of the issues of cost, control, responsibility, standardization, reproducibility, and regulation that we have attempted to highlight. Scientific consensus on the efficacy of clinical approaches requires more than a single trial, and satisfactory replication of results requires a better mechanism for dealing with the production of reproducible, standardized material for food-based trials. We have already solicited advice and assistance from colleagues in the chemoprevention community and elsewhere in our efforts to develop a suitable management and manufacturing structure that will permit the continued supply of high-quality, standardized food (extracts or concentrates) for clinical studies to collaborators worldwide. The following attractive ideas have been fielded thus far: 1) The creation of a not-for-profit corporation or a foundation to manage, finance, and take responsibility for this process; 2) direct participation of the NIH; 3) involvement of a commercial operation (e.g., one of the food-processing or freeze-drying companies that has serviced the berry trials and our BSE trials); and 4) involvement of a large university food-science group. Intellectual property, control, and production-priority issues may complicate some or all of these approaches. We present the dilemma with the hope that our colleagues will engage in a dialogue and that a feasible and practical mechanism will emerge to facilitate the development of an investigator-controlled, but simple economical paradigm for providing standardized foods for cancer prevention research trials worldwide.
Frugal medicine demands it, and sound science would greatly benefit from it. Transferring this process to the pharmaceutical industry is not an option. Cancer chemoprevention most certainly has a niche for pharmaceuticals, and perhaps these companies will eventually even have a very large presence at the table. Nonetheless, validated, efficacious dietary approaches would clearly be the most sustainable and rational approaches to prevention. We submit that they are absolutely essential in the international arena of developing regions, and as we move into the era of baby-boomer health-care economics, in developed countries and regions as well. All these barriers to disease prevention with berries, broccoli, and other foods for chemoprevention can be overcome.
International Studies: Opportunities and Challenges
Identification of geographically delineated, high-risk cohorts in economically developing regions, such as those in the esophageal “hotspot” of Linxian, China, or the hepatocellular carcinoma “hotspot” of Qidong, China, offers pragmatic advantages for efficient clinical trial design and conduct in regions where frugal medicine is needed. There are several key prerequisite elements, however, for developing productive international collaborations. First and foremost, the right collaborators, linked by a genuine interest in building friendships and cultural understanding, must be identified. Trans-national investigators should provide the projects with complementary sets of skills and resources that enhance all collaborative roles. Diligence in maintaining regular channels for communicating and meeting are also important. Our Johns Hopkins investigators have collectively spent nearly a decade of cumulative time “on the ground” in Qidong during the course of the development, conduct, and renewal of our collaborative studies of BSE and other chemopreventive agents. Multiple reciprocal visits of Qidong scientists, clinicians, public health leaders, and government leaders to Johns Hopkins have further nurtured the learning experiences and facilitated the timely conduct of the science.
Regulations at home and abroad govern the design and conduct of these collaborative trials. Meshing of regulatory and cultural distinctions between the collaborating countries can be challenging; this has certainly been true for our Sino-U.S. projects, where the barriers have been bilateral. The Federalwide Assurance process, which defines the structure and functions of IRBs, may need to be implemented at the local collaborating institutions. Import of study food materials (including plant seeds or freeze-dried powders), components of placebo beverages, or study supplies and export of biological samples are subject to strict regulation in China and other countries. Export of biospecimens may be forbidden in some countries, necessitating the establishment of local laboratories with the requisite analytical capabilities. Transport of these items can be very expensive, and customs delays are extensive. Cumbersome and protracted processes for securing permissions to open and conduct trials are an unfortunate hallmark of international collaborations. Obtaining and synchronizing regulatory, infrastructure, and implementation factors are always underestimated in terms of scope and time. Passion and commitment to achieve the goal are essential prerequisites, as is patience. When international collaborative research works, the excitement of new relationships, of building capacity, of making a difference, and of doing good science collectively provides enormous satisfaction. Successful translation of such collaborative work on early-phase trials into RCTs promises to bring enormous, affordable benefit to places that have great needs.
Getting from Clinical Trials to Frugal Medicine
Getting greener
Although good science demands the use of standardized foods in research trials, frugal medicine will not be served by these products. Preparation of standardized test articles such as lyophilized berries or broccoli sprouts is expensive in relation to the economics of the developing world. Early collaboration with phytochemical experts is therefore highly desirable and may even lead to exciting and unanticipated new discoveries, as illustrated by our studies of the Moringa tree (Moringa oleifera; refs. 59, 60). Moringa species, which are not crucifers, contain a multiply glycosylated isothiocyanate precursor that is as potent an inducer of the nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) response as is sulforaphane (61) and is as potent an antibiotic against the bacterium responsible for much of the world’s gastric cancer (62). The Moringa tree is of Himalayan origin, grows extensively in the dryland tropics globally, and can thrive where most other edible plants cannot. All plant parts are edible, and various governmental agencies and non-governmental organizations (NGOs) have advocated intensive food use of Moringa as a sustainable intervention to combat starvation in these regions, where food availability is marginal or non-existent and arable land is minimal. Another attractive feature of these plants is that local populations are already consuming them, both as famine foods and in normal local cuisine (63).
The drivers
Neither identifying the problems, nor making tremendous progress towards solving them, will in and of itself lead to a diminution of the scourge of cancer. There will be many stakeholders in the greening of preventive medicine, and the sooner we acknowledge, encourage, and perhaps regulate their actions, the sooner real progress will be made from a population perspective. Whereas large pharmaceutical companies have been the rich uncles for much work on disease treatment, and they have profited mightily from that investment, we expect that they will not be the stakeholders in a chemoprevention paradigm shift. We expect that the drivers of this revolution will be the food producers (farmers and seed companies) and the packers, processers, distributors, and retailers who get food to the public. They stand to profit from the value added at a number of levels, including cultivar differentiation, “healthy food” formulations, introduction of new foods, and re-introduction of “old foods” into new markets. This process can be augmented and directed 1) by enhancing meaningful partnerships between the NIH and the US Department of Agriculture, 2) by approaching the regulation of this inevitable shift with wisdom and based upon scientific principals, 3) by demanding that the new drivers (food and agricultural interests) provide a larger share of the funding for chemoprevention research and for effective public outreach, and 4) by increasing dramatically the level of nutrition education in our health-related graduate schools and in our medical and nursing schools.
The end game
It is certainly plausible that, if ultimately shown to be effective by scientific consensus, Western foods such as those highlighted above could contribute to reducing the prevalence of cancer by becoming more widely consumed. It is not as plausible, nor are we recommending, that these foods would “catch on” in other parts of the world, where they are neither available nor part of the local cuisine. Therefore, we view the trials highlighted above as principally applicable to a preventive strategy in Western countries and as a powerful guide for selecting other foods (vegetable or fruit) based upon the botanical, phytochemical, and mechanistic (e.g., chemopreventive) profiles of culturally acceptable and available foods in other places with populations at risk for cancers. That being said, we and others have witnessed a recent dramatic upturn in the production of both strawberries and broccoli in China (Fig. 2). Whether this is connected with the extremely widespread awareness of the clinical trials discussed above in the applicable regions of China, one can only speculate, but the local peasant farming populations have become keenly aware of the potential value (both medical and financial) of both crops. The “green revolution” in meeting the needs of worldwide food production can now be followed by a “green” evolution into disease prevention.
Figure 2.
Jiang Luo, Qidong, China—December 2011. In the last half-decade, broccoli has become the major late-season crop in eastern Qidong. Although largely produced for export, the vegetable is beginning to penetrate local markets.
Acknowledgments
USPHS (P01 ES0906052, R01 CA39416, R01 CA93780), and the Lewis B. and Dorothy Cullman Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lingwood RJ, Boyle P, Milburn A, Ngoma T, Arbuthnott J, McCaffrey R, et al. The challenge of cancer control in Africa. Nature Rev Cancer. 2008;8:398–403. doi: 10.1038/nrc2372. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. (2011) Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Chen T, Yan F, Qian JM, Guo MZ, Zhang HB, Tang XF, et al. Randomized clinical chemoprevention trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev Res. 2012:5. doi: 10.1158/1940-6207.CAPR-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res. 2012:5. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane MA. Preventing cancer with vaccines: progress in the global control of cancer. Cancer Prev Res. 2012:5. doi: 10.1158/1940-6207.CAPR-11-0533. [DOI] [PubMed] [Google Scholar]
- 7.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res. 2012:5. doi: 10.1158/1940-6207.CAPR-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald DW, Bezak K, Ocheritina O, Riviere C, Wright TC, Jr, Milne GL, et al. The effect of HIV and HPV co-infection on cervical COX-2 expression and systemic prostaglandin E2 levels. Cancer Prev Res. 2012:5. doi: 10.1158/1940-6207.CAPR-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey JW, Kensler TW. Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem Res Toxicol. 2007;20:572–576. doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu RH. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 11.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW-M, Ferketich AK, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–154. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 12.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ip C, Lisk DJ, Stoewsand GS. Mammary cancer prevention by regular garlic and selenium-enriched garlic. Nutr Cancer. 1992;17(3):279–286. doi: 10.1080/01635589209514197. [DOI] [PubMed] [Google Scholar]
- 14.Urusova DV, Shim J-H, Kim DJ, Jung SK, Zykova TA, Carper A, et al. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev Res. 2011;4:1366–77. doi: 10.1158/1940-6207.CAPR-11-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res. 2011;4(3):354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 18.Islami F, Boffetta P, Ren JS, Pedoeim L, Khatib D, Kamangar F. High-temperature beverages and foods and esophageal cancer risk–a systematic review. Int J Cancer. 2009;125:491–524. doi: 10.1002/ijc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–18. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, Morgan C, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–6. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Black raspberries inhibit N-nitrosomethylbenzylamine (NMBA)-induced angiogenesis in rat esophagus parallel to the suppression of COX-2 and iNOS. Carcinogenesis. 2006;27:2301–7. doi: 10.1093/carcin/bgl109. [DOI] [PubMed] [Google Scholar]
- 22.Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York: 2011. p. 313. [Google Scholar]
- 23.Mallery SR, Tong M. Cancer prevention in humans at high-risk for development of cancer: Prevention of oral dysplasia in humans by berry formulations. (Chapter 13) In: Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York: 2011. pp. 247–258. [Google Scholar]
- 24.Knobloch TJ, Casto BC, Agrawal A, Clinton SK, Weghorst CM. Cancer prevention in populations high at-risk for the development of oral cancer: Clinical trials with black raspberries. (Chapter 14) In: Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York: 2011. pp. 259–280. [Google Scholar]
- 25.Stoner GD, Wang L-S, Sardo C, Arnold M, Martin E, Frankel W. Effects of black raspberries on cellular and epigenetic biomarkers of colon cancer development in humans. (Chapter 15) In: Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York: 2011. pp. 281–303. [Google Scholar]
- 26.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin JM, Olivo-Marston S, Vitolins MZ, Bittoni M, Reeves KW, Degraffinreid CR. Effects of tomato- and soy-rich diets on the IGF-I hormonal network: A crossover study of postmenopausal women at high risk for breast cancer. Cancer Prevent Res. 2011;4(5):702–710. doi: 10.1158/1940-6207.CAPR-10-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res. 2009;2(4):353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Disposition of chemoprotective glucosinolates and isothiocyanates of broccoli sprouts. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 30.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 31.Gullett NP, Ruhul Amin ARM, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, et al. Cancer prevention with natural compounds. Sem Oncol. 2010;37(3):258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;2(7):931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 33.Yang CS, Ju J, Lu G, Xiao H, Hao X, Sang S, et al. Cancer prevention by tea and tea polyphenols. Asia Pac J Clin Nutr. 2008;17(S1):245–248. [PMC free article] [PubMed] [Google Scholar]
- 34.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 35.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res. 2009;2:931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You W-C, Brown LM, Zhang L, Li J-Y, Jin M-L, Chang Y-S, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–983. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr. 2006;136:821S–826S. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- 38.Zick SM, Turgeon DK, Vareek SK, Ruffin MT, Litzinger AJ, Wright BD, et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev Res. 2011;4(11):1929–1937. doi: 10.1158/1940-6207.CAPR-11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 41.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 42.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 43.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickens BM, Cook RJ. Challenges of ethical research in resource-poor settings. Int J Gynec Obstet. 2003;80:79–86. doi: 10.1016/s0020-7292(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 45.Linde K, Fässler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Phil Trans R Soc B. 2011;366:1905–12. doi: 10.1098/rstb.2010.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kensler TW, Chen J-G, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthene tetraols in a randomized clinical trial in He Zuo Township, Qidong, PRC. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 47.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: Results of a short term, cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4:384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Muñoz A, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr229. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talalay P, Talalay P. The importance of using scientific principles in the development of medicinal agents from plants. Acad Med. 2001;76(3):238–247. doi: 10.1097/00001888-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Ruhlen R, Sauter E. Plant based therapies to prevent/treat cancer. Expert Rev Clin Pharmacol. 2010;3(1):1–3. doi: 10.1586/ecp.09.46. [DOI] [PubMed] [Google Scholar]
- 51.Farnham MW, Wilson PE, Stephenson KK, Fahey JW. Genetic and environmental effects on glucosinolate content and chemoprotective potency of broccoli. Plant Breeding. 2004;123:60–65. [Google Scholar]
- 52.Pereira FM, Rosa E, Fahey JW, Stephenson KK, Carvalho R, et al. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italic) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agric Food Chem. 2002;50:6239–6244. doi: 10.1021/jf020309x. [DOI] [PubMed] [Google Scholar]
- 53.Fahey JW, Stephenson KK. Cancer chemoprotective effects of cruciferous vegetables. HortScience. 1999;34(7):1159–1163. [Google Scholar]
- 54.Vallverdu-Queralt A, Medina-Remon A, Casals-Ribes I, Lamuela-Raventos RM. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. [Google Scholar]
- 55.Pozo-Insfran DD, Duncan CE, Yu KC, Talcott ST, Chandler CK. Polyphenolics, ascorbic acid, and soluble solids concentrations of strawberry cultivars and selections grown in a winter annual hill production system. J Amer Soc Hort Sci. 2006;131(1):89–96. [Google Scholar]
- 56.Wang SY. Correlation of antioxidants and antioxidant enzymes to oxygen radical scavenging activities in berries. (Chapter 4) In: Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York: 2011. pp. 79–97. [Google Scholar]
- 57.Fahey JW, Ourisson PJ, Degnan FH. Pathogen detection, testing, and control in fresh broccoli sprouts. Nutr J. 2006;5:13. doi: 10.1186/1475-2891-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nesbitt M, McBurney RPH, Broin M, Beentje HJ. Linking biodiversity, food and nutrition: The importance of plant identification and nomenclature. J Food Comp Anal. 2010;23:486–498. [Google Scholar]
- 59.Fahey JW. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees for Life J. 2005;1:5. http://www.tfljournal.org/article.php/200512.01124931586.
- 60.Olson ME, Fahey JW. Moringa oleifera: un árbol multiusos par las zonas tropicales secas. Revista Mexicana de Biodiversidad. 2011;82:1071–1082. [Google Scholar]
- 61.Fahey JW, Dinkova-Kostova AT, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. In: Sies H, Packer L, editors. Methods in Enzymology. Part B. Vol. 382. Elsevier Science; San Diego, CA: 2004. pp. 243–258. Chapter 14. [DOI] [PubMed] [Google Scholar]
- 62.Haristoy X, Fahey JW, Scholtus I, Lozniewski A. Evaluation of antimicrobial effect of several isothiocyanates on Helicobacter pylori. Planta Medica. 2005;71:326–330. doi: 10.1055/s-2005-864098. [DOI] [PubMed] [Google Scholar]
- 63.Thurber M, Fahey JW. Adoption of Moringa oleifera to combat under-nutrition viewed through the lens of the 'Diffusion of Innovations' theory. Ecol Food Nutr. 2009;48:212–225. doi: 10.1080/03670240902794598. [DOI] [PMC free article] [PubMed] [Google Scholar]