Abstract
Eradication of infectious disease is our global health challenge. After encountering intestinal infection with a bacterial pathogen, the host defense program is initiated by local antigen-presenting cells (APCs) that eliminate invading pathogens by phagocytosis and establish localized inflammation by secreting cytokines and chemokines. These pathogen-experienced APCs migrate to the mesenteric lymph nodes, where host immune responses are precisely orchestrated. Initiation and regulation of this defense program appear to be largely dependent on innate immunity which is antigen non-specific and provides a rapid defense against broader targets. On the other hand, many bacterial enteropathogens have evoked abilities to modify the host defense program to their advantage. Therefore, better understanding of the host-pathogen interactions is essential to establish effective eradication strategies for enteric infectious diseases. In this review, we will discuss the current understanding of innate immune regulation of the host defense mechanisms against intestinal infection by bacterial pathogens.
Keywords: innate immunity, bacterial pathogens, host defense, macrophages, enteropathogen, Gram-negative, toll-like receptors, Nod-like receptors, mucosal defense, protective immunity, antimicrobial peptide, secretory IgA, commensals
Introduction
Intestinal bacterial infection is a common health problem and could lead to a major impact on public health and the global economy [1]. Many pathogenic bacteria cause foodborne illness resulting in epidemics. Outbreaks often occur following natural disasters (e.g., recent outbreaks of Vibrio cholera in Haiti) making already dire situations catastrophic [2–4]. Recent advances of our knowledge have led to the idea that intestinal mucosa maintains physiological homeostasis through host-commensal interactions, and that gastrointestinal infections occur through host-pathogen interactions. However, it is largely unknown how the host-pathogen interactions lead to intestinal pathology and systemic diseases.
The gastrointestinal mucosa is under continuous exposure to a milliard of microorganisms that comprise the commensal flora. Mucosal immunity maintains tolerance to commensal flora while inducing effector immunity to pathogens via a fine interplay between innate and adaptive immune responses. In this process, innate immunity is responsible for recognition of microorganisms and initiation of effector and/or regulatory immune responses. In addition, innate immunity triggers mucosal restitution programs following epithelial injury or inflammation. Therefore, innate immunity is crucial for regulation of host-commensal interactions in the maintenance of intestinal homeostasis.
How the host immunity induces effector responses only to harmful pathogens amongst diverse commensal flora in the intestine is an essential question. Upon pathogenic infection, both virulence factors of the pathogen and the host defense mechanisms determine the consequence of intestinal immune responses. Many commensal bacteria that colonize the intestine have diminished virulence [5]. By contrast, most clinically significant bacterial enteropathogens have evolutionarily acquired an ability to evade host immune defenses with individually unique virulence factors [6–8]. The strategies used by these pathogens for immune evasion mainly target innate immunity, highlighting the importance of innate immunity in intestinal defense mechanisms.
On the host side, identification of the pathogens and selective induction of immune responses require utilization of innate immunity, which is initiated by pattern recognition receptors (PRRs) on APCs including macrophages, dendritic cells (DCs), and intestinal epithelial cells (IECs). Recognition of specific pathogen-associated molecular patterns (PAMPs) by PRRs initiates an innate immune response inducing the production of antimicrobial peptides, phagocytic microbial killing, and expression of cytokines, chemokines, and reactive oxygen species. These processes lead to the recruitment of acute inflammatory cells in order to establish localized inflammation. Diverse pathogen patterns are precisely recognized by toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) like receptors (NLRs). Simultaneously, activated APCs initiate adaptive immunity, which has the ability to terminate the infection and inflammation.
Intestinal Innate Immune System Forms Host-Pathogen Interactions
Most enteropathogens invade our body through microfold cells (M-cells) within the follicle-associated epithelium (FAE) that covers intestinal Peyer's patches (PPs). Pathogens can easily attach to the surface of M-cells because the surface has a poorly formed glycocalyx layer. M-cells in turn actively uptake the attached pathogens by transcytosis, a process involving TLR signaling [9]. When pathogens are delivered to PPs, they are phagocytosed by APCs; i.e., macrophages and DCs. Pathogens that have an ability to evade phagocytic elimination by APCs establish an infection and colonize PPs. The initial colonization by the pathogen induces localized inflammation in PPs characterized by recruitment of neutrophils, DCs and monocytes, leading to activation of CD4+ T cells and production of secretory IgA [10,11]. The existence of an M-cell-independent route of infection has also been suggested [12]. The CX3CR1+ subset of DCs is thought to sample luminal contents through a dendrite extended between the epithelial cells, and may initiate the host immune response [13]. This transepithelial dendrite formation is known to increase with oral Salmonella infection, and this process also involves TLR signaling [14]. However, the roles of these DCs in host defense against in vivo pathogenic infection have been controversial [15].
Pathogens that survive primary host responses in PPs or intraepithelial DCs travel to and colonize the mesenteric lymph nodes (MLNs). In this process, pathogens (especially intracellular pathogens) may utilize phagocytes as a carrier to transport them to the MLNs. In particular, TLR5 signaling in DCs seems to play a role in the transfer of Salmonella to the MLNs because TLR5−/− mice show a reduced number of Salmonella-harbored DCs in the MLNs after mucosal infection [16]. Taking the risk of MLN colonization underlines the importance of transferring the pathogen-experienced APCs to the MLNs for the host to establish an organized immune response against bacterial dissemination [17,18]. In fact, mice with surgically removed MLNs demonstrate increased bacterial dissemination and severe immunopathology in peripheral organs [19].
We have discussed that innate immunity is responsible for induction of localized inflammation in intestinal mucosa, PPs, and MLNs after enteric infection with bacterial pathogens. Although it may result in severe enterocolitis and lymphadenitis, regional inflammation is required for prevention of systemic dissemination of the pathogen. TLR4−/− mice are unable to recruit neutrophils in response to chemically induced mucosal injury and have enhanced bacterial translocation to the spleen [20]. Therefore, prevention of systemic dissemination of the infected pathogen is one of the important tasks of the innate immune defense in the face of bacterial infections in the intestine.
Mouse Models of Intestinal Bacterial Infection
One of the obstacles in investigating the role of intestinal defense mechanisms against enteropathogens is that experimental mice are resistant to many pathogens that cause serious diseases in humans. Nevertheless, some mouse models of intestinal bacterial infection have been established (Table 1). Individual models have unique features that are useful to investigate specific aspects of host immune responses as well as in vivo function of the virulence factors of the pathogens.
Table 1.
Well-established mouse models of intestinal bacterial infection.
| Pathogens | Type | Gram staining | Pathology |
|---|---|---|---|
| S. typhimurium | Invasive | Negative | Oral infection leads to systemic dissemination of S. typhimurium demonstrating typhoid-like disease. Infection with S. typhimurium after pretreatment of mice with streptomycin (elimination of commensals) demonstrates intestinal pathology (enterocolitis). |
| Y. enterocolitica | Invasive | Negative | Oral infection demonstrates intestinal inflammation, microabscesses in the PPs and MLNs. Infection eventually disseminates to the spleen and the liver. |
| C. rodentium | Non-invasive | Negative | A model pathogen of EPEC and EHEC in human disease. Oral infection leads to colonization of the large intestine. Induces diarrhea disease with colitis. |
| H. hepatices | Non-invasive | Negative | Natural infection leads to life-long colonization of this pathogen in mice. Inoculation of normal mice with this pathogen does not demonstrate pathology, but influences the course of colitis models. Infection to lymphopenic mice demonstrates severe colitis. |
Salmonella enterica serovar typhimurium infection of mice is a well-established model of intestinal as well as systemic Salmonella infection in humans. Except for infection with typhi, paratyphi, and sendai serovars, natural infection with Salmonella manifests enterocolitis in most human cases [21]. In mice, however, S. typhimurium causes a systemic disease resembling human typhoid fever but neither colonizes the intestine nor manifests as enterocolitis after oral infection [22]. Interestingly, pretreatment of mice with antibiotics (e.g., streptomycin) leads to acute intestinal inflammation in response to S. typhimurium infection, highlighting the importance of commensals in the establishment of intestinal versus systemic pathology by this pathogen [22].
Yersinia enterocolitica orally infects mice without the elimination of commensals and the mouse model of Y. enterocolitica infection reproduces the manifestations of human disease [23]. Once Y. enterocolitica establishes an infection, it forms microcolonies, microabscesses, and granulomatous lesions in PPs and the MLNs, and may disseminate to the liver and spleen. Therefore, together with S. typhimurium, the mouse model of Y. enterocolitica infection is useful to investigate intestinal immune mechanisms and defense against systemic dissemination of invading pathogens.
Citrobacter rodentium is an enteric pathogen that causes natural infection in mice and is considered a good model for human enteropathogenic Escherichia coli (EPEC) and enterohomorrhagic E. coli (EHEC), because it carries similar virulence factors as EPEC and EHEC. In stark contrast to S. typhimurium and Y. enterocolitica, C. rodentium is not an invasive pathogen. C. rodentium colonizes the surface of the cecal lymphoid patches a few hours post oral infection and spreads toward the distal colon within a few days [24]. After attaching to colonocytes, C. rodentium breaks epithelial barrier integrity through tight junction disruption by its virulence factors, which manifests diarrhea with colitis and epithelial hyperplasia [25].
Helicobacter hepaticus naturally infects mice and can induce intestinal pathology and chronic active hepatitis in immune deficient mice [26]. H. hepaticus does not manifest enterocolitis in normal mice, but infection with this pathogen exacerbates colonic inflammation in mouse colitis models [26]. A major component of this colitis is mediated by innate immunity, as evidenced by the severe colitis induced by oral infection of lymphopenic RAG2−/− mice with H. hepaticus [27]. In addition, adoptively transferred regulatory T cells inhibit induction of colitis in H. hepaticus infected RAG2−/− mice. Accordingly, this model is advantageous to study how bacterial signaling influences intestinal inflammation in the context of both innate and adaptive immune responses.
TLRs and NLRs in Intestinal Defense against Bacterial Pathogens
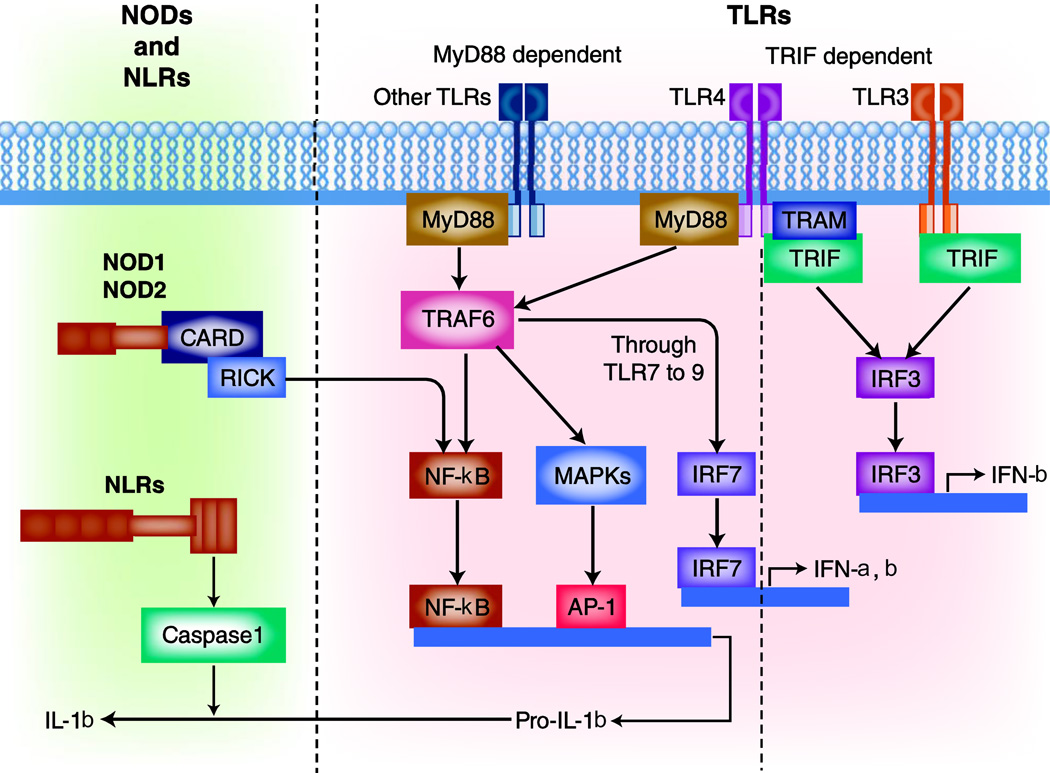
Recognition of PAMPs by specific PRRs triggers innate immunity, which induces a variety of gene expression via distinct intracellular signaling pathways. Figure 1 shows the complex interactions of intracellular signaling pathways under the activation of PRRs. TLRs are expressed on the plasma membrane or endosomes, and NLRs are expressed within the cytosol of most cell types in intestinal mucosa. Each pathogen induces a unique pattern of signaling pathways as different PRRs may simultaneously recognize more than one PAMP on each pathogen. In addition, different cell types induce different responses to the same pathogen. This combination of cellular and molecular diversity increases the host capacity to establish organized and directed immune responses to a variety of pathogens. Expression of most TLRs in IECs and resident APCs in the lamina propria seems to be down-regulated presumably to avoid excessive immune responses to the commensals [28–30]. However, the in vivo functional consequences of these TLRs in individual cell types in the intestine are yet to be fully determined.
Figure 1. Signal transduction pathways of TLR and NLR.
Pathogens are recognized by the host APCs through TLRs, which induce intracellular signaling mainly through two adapter molecules, myeloid differentiation factor 88 (MyD88) or Toll/interleukin-1 receptor domain-containing adapter inducing IFN-β (TRIF). While MyD88 is used by most TLRs, the TRIF pathway can be exclusively induced by TLR4 and TLR3. The MyD88 pathway strongly induces NF-κB activation and pro-inflammatory cytokine secretion associated with pathogen clearance [83]. The TRIF pathway, on the other hand, induces type I IFNs and slower NF-κB activation [84,85]. On the other hand, pathogens can be recognized by NLRs in the cytosol, which assemble a protein complex named inflammasome to activate caspase-1. Activated caspase-1 induces cellular apoptosis and promotes secretion of IL-1β and IL-18.
Most bacterial pathogens that cause intestinal pathology in humans, such as Yersinia, Salmonella, Vibrio or Shigella, are Gram-negative species. TLR4 is highly suspected to be involved in the host defense mechanisms against these pathogens, as lipopolysaccharide (LPS) of the outer membrane of the Gram-negative bacteria is the major ligand for TLR4. In fact, TLR4 deficient mice are highly susceptible to oral and systemic infection with S. typhimurium as well as Y. enterocolitica due to impaired bacterial killing by macrophages and defective cytokine production [31–33]. Invasive enteropathogens that carry flagella can be recognized by TLR5 or NLRC4 at the host cell plasma membrane or cytosol, respectively. Phagocytes infected with S. typhimurium in vitro activate NLRC4, which induces cellular production of IL-1β and IL-18 via caspase-1 activation [34–36]. This process appears to be important for host defense as mice deficient in caspase-1, IL-1β, and IL-18 are individually susceptible to S. typhimurium and rapidly succumb to infection [37,38]. Bacterial DNA can be recognized by TLR9 within the cytoplasm. However, it is likely that these PRRs are dispensable for establishing an intestinal immune defense against S. typhimurium in vivo, because none of the mice deficient in TLR5, TLR9, or NLRC4 shows increased susceptibility to oral S. typhimurium infection [16,37,39●●]. The discrepancy between these results implies involvement of multiple cell types and upstream pathways that are responsible for caspase-1 activation and IL-1β, and IL-18 production in response to S. typhimurium infection. Moreover, the contribution of TLR signaling to virulence of the pathogens has been suggested [16,33,39●●].
TLR signaling can be a driving force of mucosal inflammation in the setting of enteric infection with non-invasive pathogens. For example, absence of TLR4 reduces intestinal inflammation and morbidity in C. rodentium infection [40]. In the H. hepaticus infection model, RAG2×MyD88 double knockout mice as well as RAG2−/− chimeric mice that carry MyD88-deficient bone marrow demonstrate no intestinal inflammation, while MyD88 sufficient RAG2−/− counterparts show chronic colitis [41●]. These findings suggest that MyD88-dependent TLR signaling in myeloid cells during the infection with non-invasive bacterial pathogens dominantly mediates intestinal inflammation.
Secretory IgA and Antimicrobial Peptides
Secretory IgA (sIgA) and antimicrobial peptides are crucial component of host immune defense against enteric pathogens. It has been suggested that sIgA prevents adherence and invasion by enteric pathogens [42,43]. Antimicrobial peptides are also known to inhibit colonization of microorganisms on epithelial surfaces [44]. Signaling through TLRs and NLRs appears to play a central role in regulation of sIgA induction and antimicrobial peptides and thus contributes to the maintenance of commensals as well as primary defense against intestinal pathogens [45].
PRR signaling seems to be involved in multiple steps in intestinal IgA secretion. In the intestine, follicular B cells in PPs are activated by direct contact with activated T cells. The activated B cells then undergo terminal differentiation to plasma cells by activation of the transcriptional factors Bimp-1 and IRF-4 and travel to the lamina propria to secrete IgA [46]. On the other hand, recruitment of B cells to the lamina propria requires expression of specific chemokines from IECs that may be induced by several types of PRR signaling [47]. This T cell-dependent intestinal sIgA secretion takes five to seven days. To compensate for this time lag, lamina propria B cells can rapidly undergo class switch recombination in a T cell-independent manner through induction of B cell-activating factors, APRIL (A proliferation-inducing ligand) and BAFF (B cell-activating factor of the TNF family) [48,49]. DCs and IECs have been shown to express these B cell-activating factors in response to bacterial recognition through TLRs [50,51]. This sIgA has been considered to have multiple cross-reactions and contributes to host defense against a variety of pathogens [52,53]. Constitutively-active expression of TLR4 in IECs results in increased lamina propria B cell number and sIgA secretion along with higher expression of mucosal APRIL [54]. Activation of TLR3 and TLR4 has been shown to induce expression of the polymeric Ig receptor (pIgR), an epithelial immunoglobulin transporter, by IECs that enhances luminal IgA secretion [55,56]. The pIgR−/− mice have more S. typhimurium colonization in PPs than wild-type (WT) mice, and succumb to infection with a lower infective dose [57]. Since the sIgA-mediated intestinal defense mechanism is associated with a reduction in local bacterial burden in the intestine, sIgA is also suggested to contribute to prevention of epidemics with enteropathogens [57].
Defensins are one of the major antimicrobial peptides in the intestine. Depending on the molecular structure, defensins are classified into two major forms i.e., α-defensins (cryptdins in mice) and β-defensins [58]. In contrast to α-defensins that are specific to intestinal Paneth cells, β-defensins are expressed by a variety of cell types including IECs. Defensins mediate non-oxidative microbial killing by inducing membrane disruption of the microorganisms. Mice deficient in the metalloprotease matrilysin (MMP7), an enzyme required for maturation of α-defensins, are highly susceptible to oral S. typhimurium infection [59]. Conversely, transgenic expression of human α-defensin HD5 by mice confers greater resistance to oral S. typhimurium infection [60]. Although some defensins are expressed constitutively, α-defensins and β-defensins may be induced by bacterial stimuli suggesting a contribution of PRR signaling. Therefore, defensins are an important component of intestinal defense against pathogenic infection which may be regulated by PRR signaling.
Conventionally raised MyD88−/− mice have been found to have an increased bacterial translocation to the MLNs due to a significant defect in production of multiple antimicrobial peptides in Paneth cells [61]. Paneth cell specific transgenic expression of MyD88 restores the bacterial burden in the MLNs to the WT levels. In addition to α-defensins, MyD88 signaling regulates the expression of another type of antimicrobial Paneth cell product, c-type lectins such as RegIIIγ and RegIIIβ [61]. TLR9−/− and NOD2−/− mice have impaired expression of Paneth cell cryptdins compared to WT mice [62,63]. Expression of functional NOD2 in IECs inhibits invasion and growth of S. typhimurium in vitro [64]. NOD2−/− mice are susceptible to oral but not systemic infection with L. monocytogenes due to defective expression of Paneth cell cryptdin-4 [62]. These NOD2−/− mice also show increased colonization of H. hepaticus compared to WT mice after oral inoculation [65]. In addition, signaling through TLR2, TLR3, TLR4, TLR5, NOD1, NOD2 and NLR (NLRP3) have all been implicated with the expression of β-defensins in IECs [16,66,67]. Therefore, multiple PRR signaling may induce several sets of antimicrobial peptides that allows the host to prevent the colonization of pathogens.
Macrophage Phagocytosis: Bacterial Killing vs. Pathogen Carrier
Elimination of infectious agents by macrophage phagocytosis is an important part of host defense. There are several phagocytic receptors that are required to initiate the uptake of pathogens into endosomes. Signaling from phagocytic receptors further facilitates phagosome maturation. Emerging evidence has demonstrated a significant contribution of TLR signaling in multiple steps of phagocytosis indicating TLRs as potent phagocytic receptors [68–70]. During the maturation process, phagosomes fuse with lysosomes to degrade internal pathogens. Formation of phagolysosomes activates NADPH oxidase and inducible nitric oxide synthase that catalyzes acidification and oxidative burst resulting in bacterial killing. Digestion of pathogens produces other PAMPs in the phagosomes that further allow activation of other PRRs, which in turn induce cytokines and chemokines forming organized innate immune responses. Secreted cytokines such as TNF-α and IFN-γ help activate other phagocytic cells preparing against further invasion of the pathogens. Finally, phagocytes that digest pathogens initiate adaptive immunity to generate pathogen-specific immune defense programs.
Another aspect of phagocytosis is that macrophage phagocytosis can be used by the pathogens to disseminate to multiple organs of the host. Many pathogens have evolved a variety of strategies to survive macrophage phagocytosis, which include avoidance of phagocytosis, disruption of phagosome trafficking, promotion of cell apoptosis, dampening of inflammation, and alteration of intercellular signaling [71]. Several pathogens have shown their ability to utilize PRR signaling for their survival in the host [16,71,72]. Pathogens surviving intracellularly may be carried to the MLNs or the spleen by macrophages, which may cause a systemic infection. Therefore, limitation of pathogens by macrophage versus virulence of pathogens that utilize phagocytosis for dissemination may determine the outcome of enteric infections.
Natural Killer Cells and Innate Lymphoid Cells (ILCs) in Intestinal Defense Mechanism
Natural Killer (NK) cells are one of the major innate immune cells that are indispensable for early host defense against pathogenic infection. Their defense mechanisms are comprised of the strong cytotoxicity and cytokine secretion, particularly IFN-γ [73]. Absence of NK cells increases colonization of S. typhimurium in the intestine and enhances dissemination to the peripheral organs after oral infection [74]. Interestingly, depletion of NK cells results in reduced susceptibility of intestinal inflammation during oral S. typhimurium infection in streptomycin pre-treated mice due to reduced IFN-γ expression in the intestine [75]. These results indicate that NK cells are important not only to block pathogen invasion but also to strongly induce inflammatory responses in the intestinal interface.
ILCs are a recently identified NK cell-related cell type that is composed of phenotypically heterogenous populations. Emerging evidence has highlighted the importance of ILCs in immuno-surveillance of intestinal mucosa [76,77]. The precise mechanism of ILCs-mediated intestinal defense against bacterial pathogens is still largely unknown, but the effector component appears to be associated with a rapid secretion of IL-17, IFN-γ, and IL-22. The cytokine phenotypes of ILCs are mainly regulated by the expression of a transcriptional factor, retinoic acid receptor-related orphan receptorγt (RORγt) [78]. IL-17 is known to contribute to host defense against enteropathogens through induction of neutrophil recruitment and antimicrobial peptides such as β-defensins in the intestine [79,80]. On the other hand, IL-22 deficient mice are highly susceptible to intestinal C. rodentium infection [81]. IL-22-mediated host defense seems to be associated with induction of RegIIIβ and RegIIIγ [77]. However, the role of IL-22 in intestinal defense may be pathogen specific as IL-22 is dispensable for the clearance of oral L. monocytogenes infection [82].
Conclusions
The recent globalization of the food supply increases the chance for wide-spread exposure to food-borne pathogens and the risk of outbreaks. Although antibiotic therapy may be effective in treating enteric pathogens, the rapid onset of host systemic inflammatory responses, the acquisition of antimicrobial resistance, and potential induction of chronic inflammatory disorders mediated by enteric pathogens have become serious concerns. In contrast to our evolutionally conserved PRRs, the diverse virulence profiles of enteric pathogens threaten to overcome host defense mechanisms. Recent studies continue to elucidate the mechanism by which host innate immunity interacts with pathogens during intestinal bacterial infection. Innate immunity is a strong host defense program yet opportunistic modification of this mechanism by pathogen seems to be associated with intestinal pathology as well as progression to systemic disease. Targeting of this mechanism will help develop effective strategies to protect host against multiple types of pathogens that infect through intestinal mucosa.
Acknowledgments
The contents of this review was supported by National Institute of Allergy and Infectious Diseases (R56 AI095255) and Senior Research Award from Crohn’s Colitis Foundation of America for M.F.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
- 1.Helms M, Vastrup P, Gerner-Smidt P, Molbak K. Short and long term mortality associated with foodborne bacterial gastrointestinal infections: registry based study. BMJ. 2003;326:357. [PMC free article] [PubMed] [Google Scholar]
- 2.Yee EL, Palacio H, Atmar RL, Shah U, Kilborn C, Faul M, Gavagan TE, Feigin RD, Versalovic J, Neill FH, et al. Widespread outbreak of norovirus gastroenteritis among evacuees of Hurricane Katrina residing in a large "megashelter" in Houston, Texas: lessons learned for prevention. Clin Infect Dis. 2007;44:1032–1039. doi: 10.1086/512195. [DOI] [PubMed] [Google Scholar]
- 3.Walton DA, Ivers LC. Responding to cholera in post-earthquake Haiti. N Engl J Med. 2011;364:3–5. doi: 10.1056/NEJMp1012997. [DOI] [PubMed] [Google Scholar]
- 4.Zarocostas J. Experts urge vaccination to try to control cholera outbreak in Haiti. BMJ. 2011;342:d23. doi: 10.1136/bmj.d23. [DOI] [PubMed] [Google Scholar]
- 5.Niedergang F, Didierlaurent A, Kraehenbuhl JP, Sirard JC. Dendritic cells: the host Achille's heel for mucosal pathogens? Trends Microbiol. 2004;12:79–88. doi: 10.1016/j.tim.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Miao EA, Miller SI. Bacteriophages in the evolution of pathogen-host interactions. Proc Natl Acad Sci U S A. 1999;96:9452–9454. doi: 10.1073/pnas.96.17.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodsky IE, Medzhitov R. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 2008;4:e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold R, Jehl A, Rattei T. Targeting effectors: the molecular recognition of Type III secreted proteins. Microbes Infect. 2010;12:346–358. doi: 10.1016/j.micinf.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006;74:625–631. doi: 10.1128/IAI.74.1.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 11.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 13.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 14.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol. 2006;176:2465–2469. doi: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- 16.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 18.Westphal S, Lugering A, von Wedel J, von Eiff C, Maaser C, Spahn T, Heusipp G, Schmidt MA, Herbst H, Williams IR, et al. Resistance of chemokine receptor 6-deficient mice to Yersinia enterocolitica infection: evidence of defective M-cell formation in vivo. Am J Pathol. 2008;172:671–680. doi: 10.2353/ajpath.2008.070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voedisch S, Koenecke C, David S, Herbrand H, Forster R, Rhen M, Pabst O. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect Immun. 2009;77:3170–3180. doi: 10.1128/IAI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 21.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 22.Hapfelmeier S, Hardt WD. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 2005;13:497–503. doi: 10.1016/j.tim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Heesemann J, Gaede K, Autenrieth IB. Experimental Yersinia enterocolitica infection in rodents: a model for human yersiniosis. APMIS. 1993;101:417–429. [PubMed] [Google Scholar]
- 24.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 25.Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol. 2008;24:32–37. doi: 10.1097/MOG.0b013e3282f2b0fb. [DOI] [PubMed] [Google Scholar]
- 26.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 29.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, Fang FC. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- 33.Sing A, Tvardovskaia N, Rost D, Kirschning CJ, Wagner H, Heesemann J. Contribution of toll-like receptors 2 and 4 in an oral Yersinia enterocolitica mouse infection model. Int J Med Microbiol. 2003;293:341–348. doi: 10.1078/1438-4221-00277. [DOI] [PubMed] [Google Scholar]
- 34.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 35.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 36.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 37.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, Jones MB, Dracheva T, Peterson SN, Monack DM, Barton GM. TLR signaling is required for Salmonella typhimurium virulence. Cell. 2011;144:675–688. doi: 10.1016/j.cell.2011.01.031. ●● This article nicely shows how TLR signaling forms host-pathogen interactions during S. typhimurium infection. Certain TLR signaling contributes to host effector immunity, but other TLR signaling may be used as virulence effector of the pathogen.
- 40.Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun. 2006;74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. doi: 10.1053/j.gastro.2010.04.045. ● This study shows distinct contributions of TLR signaling by cell type in induction of intestinal inflammation. MyD88 signaling in myeloid cells is responsible for induction of innate inflammation in the intestine.
- 42.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 43.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 44.Ouellette AJ. Paneth cell alpha-defensins in enteric innate immunity. Cell Mol Life Sci. 2011;68:2215–2229. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 47.Sundstrom P, Lundin SB, Nilsson LA, Quiding-Jarbrink M. Human IgA-secreting cells induced by intestinal, but not systemic, immunization respond to CCL25 (TECK) and CCL28 (MEC) Eur J Immunol. 2008;38:3327–3338. doi: 10.1002/eji.200838506. [DOI] [PubMed] [Google Scholar]
- 48.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 50.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Bouvet JP, Dighiero G. From natural polyreactive autoantibodies to a la carte monoreactive antibodies to infectious agents: is it a small world after all? Infect Immun. 1998;66:1–4. doi: 10.1128/iai.66.1.1-4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruno ME, Rogier EW, Frantz AL, Stefka AT, Thompson SN, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor in intestinal epithelial cells by Enterobacteriaceae: implications for mucosal homeostasis. Immunol Invest. 2010;39:356–382. doi: 10.3109/08820131003622809. [DOI] [PubMed] [Google Scholar]
- 56.Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175:376–384. doi: 10.4049/jimmunol.175.1.376. [DOI] [PubMed] [Google Scholar]
- 57.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 59.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 60.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 61.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 63.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 64.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 65.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 68.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 69.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 70.Kong L, Sun L, Zhang H, Liu Q, Liu Y, Qin L, Shi G, Hu JH, Xu A, Sun YP, et al. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6:150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberger CM, Finlay BB. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4:385–396. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 72.Wong CE, Sad S, Coombes BK. Salmonella enterica serovar typhimurium exploits Toll-like receptor signaling during the host-pathogen interaction. Infect Immun. 2009;77:4750–4760. doi: 10.1128/IAI.00545-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Santo JP. Functionally distinct NK-cell subsets: developmental origins and biological implications. Eur J Immunol. 2008;38:2948–2951. doi: 10.1002/eji.200838830. [DOI] [PubMed] [Google Scholar]
- 74.Ashkar AA, Reid S, Verdu EF, Zhang K, Coombes BK. Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues. Infect Immun. 2009;77:214–222. doi: 10.1128/IAI.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrington L, Srikanth CV, Antony R, Shi HN, Cherayil BJ. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol Med Microbiol. 2007;51:372–380. doi: 10.1111/j.1574-695X.2007.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escaliere B, Renauld JC, Dussurget O, et al. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat-lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y, Matsuzaki G. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology. 2010;131:377–385. doi: 10.1111/j.1365-2567.2010.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 82.Graham AC, Carr KD, Sieve AN, Indramohan M, Break TJ, Berg RE. IL-22 production is regulated by IL-23 during Listeria monocytogenes infection but is not required for bacterial clearance or tissue protection. PLoS One. 2011;6:e17171. doi: 10.1371/journal.pone.0017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lebeis SL, Powell KR, Merlin D, Sherman MA, Kalman D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2009;77:604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–11826. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]