Abstract
Our understanding of human cytomegalovirus (HCMV) biology was long hindered by the inability to perform efficient viral genetic analysis. This hurdle was recently overcome when the genomes of multiple HCMV strains were cloned as infectious bacterial artificial chromosomes (BACs). The BAC system takes advantage of the single-copy F plasmid of E. coli that can stably carry large pieces of foreign DNA. In this system, a recombinant HCMV virus carrying a modified F plasmid is first generated in eukaryotic cells. Recombinant viral genomes are then isolated and recovered in E. coli as BAC clones. BAC-captured viral genomes can be manipulated using prokaryotic genetics, and recombinant virus can be reconstituted from BAC transfection in eukaryotic cells. The BAC reverse genetic system provides a reliable and efficient method to introduce genetic alterations into the viral genome in E.coli and subsequently analyze their effects on virus biology in eukaryotic cells.
Keywords: human cytomegalovirus, HCMV, betaherpesvirus, herpesvirus, bacterial artificial chromosome, BAC, mutagenesis, virus reconstitution
INTRODUCTION
HCMV is the prototypic member of the human betaherpesvirus subfamily, which also includes human herpesvirus 6A (HHV-6A), human herpesvirus 6B (HHV-6B), and human herpesvirus 7 (HHV-7). HCMV is the largest human virus with an encapsidated, linear double-stranded DNA viral genome of ~240 kb. Viral particles are composed of a genome containing capsid surrounded by a protein tegument layer and a lipid bilayer envelope. Following virus entry into host cells, the viral capsid delivers the HCMV genome to the nucleus of the infected cell. After an initial acute infection the virus can latently persist for the lifetime of the host and has the potential to cause serious health problems, especially for immunocompromised individuals and unborn children. However, the molecular biology of this important pathogen has been incompletely understood, due in part to the lack of an efficient means to dissect the role of viral elements in HCMV infection. The BAC reverse genetic system provides a reliable and efficient method to introduce genetic alterations into the viral genome in E.coli bacteria and subsequently analyze their effects on virus biology in eukaryotic cells, thus providing the means to understand the mechanisms of how HCMV persists and causes disease in humans.
The BAC system takes advantage of the circular fertility factor (F factor) of E. coli that can stably carry large pieces of DNA (>300 kb) (Shizuya et al., 1992). The first step in isolating a viral genome as a BAC clone is to introduce a BAC vector into the viral genome of interest. This BAC vector is based on a mini-F plasmid which maintains the BAC clone at a single or very low copy number per bacterial cell, thus reducing the number of recombination events at repetitive viral genomic elements and greatly enhancing the stability of the cloned genome. A BAC vector pYD-C223, which is derived from pYD-C29 (Yu et al., 2002), can be modified to clone HCMV isolates as well as other herpesviruses (Fig. 1). This vector carries markers for selection of recombinant viral clones in eukaryotic cells (e.g. puromycin) as well as in E. coli bacteria (e.g. chloramphenicol). Addition of a green fluorescent protein (GFP) reporter is useful to monitor virus infection. Finally, two loxP sites bracket the BAC vector sequence to allow its subsequent removal from the viral genome via Cre/Lox recombination when virus is reconstituted from the BAC clone. While a number of methods have been used to introduce a BAC vector into viral genomes, such as direct ligation of linearized viral genome and BAC vector (Borenstein and Frenkel, 2009) and random in vitro BAC transposition (Zhou et al., 2009), the most reliable and commonly used strategy is the targeted homologous recombination of the viral genome with viral sequences cloned in a linearized BAC capture vector (Borst et al., 1999; Messerle et al., 1997; Yu et al., 2002) (Fig. 2). Recombinant viral DNA carrying the BAC sequence is then isolated and transformed into E. coli to generate viral BAC clones, which are then screened by several methods to identify candidates that have captured a complete viral genome (Fig.3).
Figure 1.
pYD-C223 BAC vector. “pac”, puromycin N-acetyltransferase conferring puromycin resistance; “IRES”, internal ribosome entry site; “cam”, chloramphenicol acetyltransferase conferring chloramphenicol resistance.
Figure 2.
BAC-based reverse genetic system for HCMV. “P”, PmeI; “N”, NotI; “S”, SacI; “L”, loxP; “SeqL” and “SeqR”, left and right viral homology arms, respectively.
Figure 3.
Primer pairs used for PCR analysis to confirm that the isolated viral BAC clone carries the BAC vector sequence at the correct locus as well as distal regions of the HCMV genome.
Once candidate viral BAC clones are isolated from E. coli, their ability to reconstitute infectious virus is tested by electroporation of the purified BAC DNA into eukaryotic cells. In addition, coexpression of the Cre recombinase allows site-specific recombination at the loxP sites to excise the BAC vector sequence. This results in reconstitution of progeny virus that essentially has the wild-type genomic configuration except for a single 34 bp loxP site located together with the GFP marker at the original BAC insertion site (Fig. 2).
Once the ability of the viral BAC clone to reconstitute virus is validated, the viral BAC clone can be subjected to well established prokaryotic genetics, allowing almost unlimited manipulation of the cloned viral genome and generation of recombinant clones of any kind. Commonly used approaches for BAC mutagenesis in E. coli are allelic exchange (Smith and Enquist, 2000; Yu et al., 2002), transposon mutagenesis (Smith and Enquist, 1999; Yu et al., 2003), and more recently, lambda Red-mediated linear recombination (Lee et al., 2001; Warming et al., 2005; Yu et al., 2000). Linear recombination, which requires only ~50 bp of homology, has become the standard method to generate targeted genetic alterations in viral BAC clones because of its efficiency and versatility.
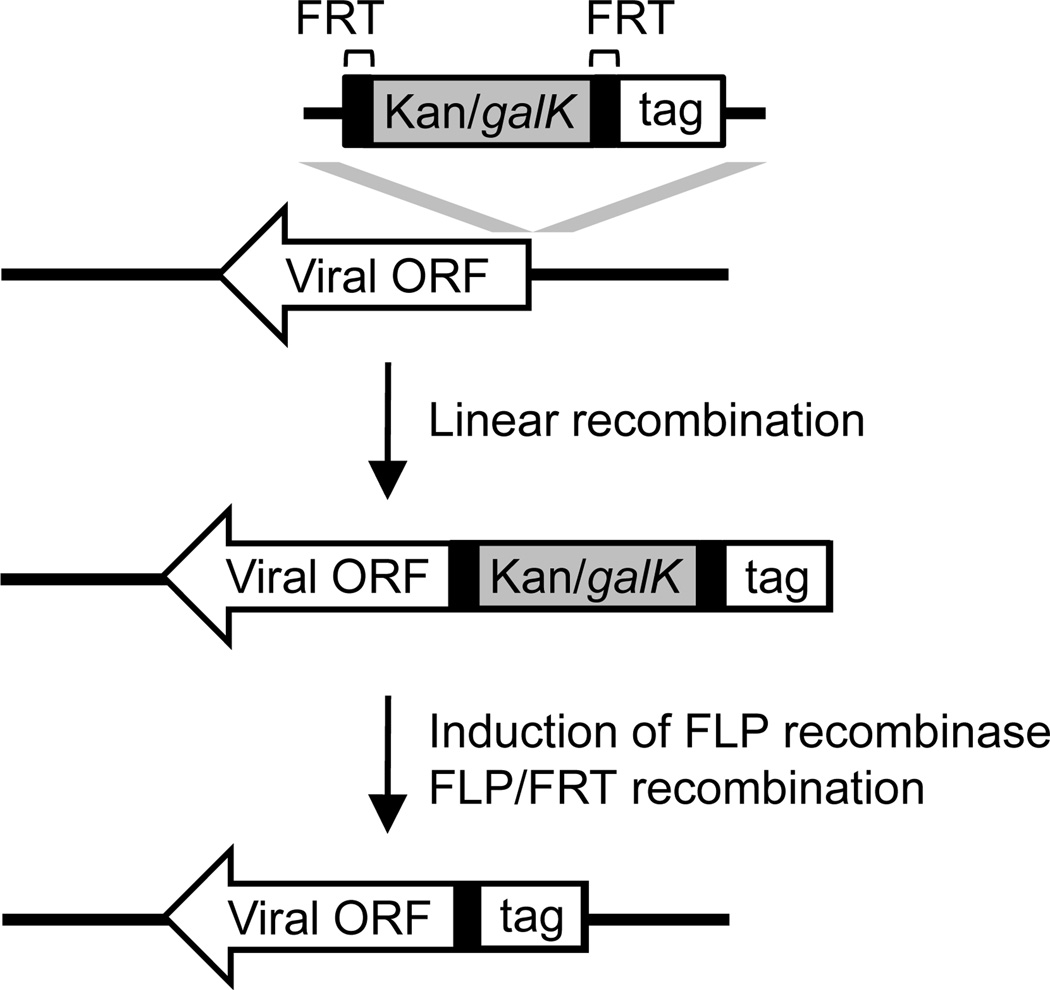
In this unit we will focus on a reliable two-step linear recombination method that uses a kanamycin/galactokinase (Kan/galK) dual selection cassette to provide both positive and negative selection (Qian et al., 2008; Warming et al., 2005) (Fig. 4). In the first step, recombination of a PCR-generated Kan/galK cassette into the viral genome is selected by kanamycin resistance. This step can be used to engineer insertion mutations (Protocol 3), introduce foreign sequences (e.g. tag) (Protocols 4 and 5), or mark the targeted locus for seamless genetic alteration in later steps If the goal is to introduce seamless genetic alteration at the locus, galK negative selection is performed in a second linear recombination step. This selects recombinants that have replaced the Kan/galK cassette with the sequence of interest (Protocol 3) (Fig. 4). If the goal is to introduce foreign sequences, such as a tag, into the viral locus, the DNA fragment used for recombination will carry the sequence of interest in addition to the selection cassette bracketed by short sequences for site-specific recombination, such as the Flippase Recognition Target (FRT) site (Fig. 5). After the sequence of interest is introduced into the viral genome by kanamycin selection, FLP/FRT site-specific recombination at the subsequent step will remove the Kan/galK cassette leaving behind a single FRT site together with the sequence of interest (Protocols 4 and 5). Once recombinant BAC clones are generated in E. coli, they are transfected into eukaryotic cells to reconstitute recombinant viruses. The consequence of engineered genetic alterations can then be investigated by analyzing the resulting recombinant virus. One additional advantage of this BAC-based genetic manipulation is to allow the rapid identification of viral elements that are essential for HCMV replication. Disruption of an essential viral element by Kan/galK insertion should result in an inability to reconstitute virus. Nonetheless, the final functional classification of an essential viral element should be validated by repairing the disrupted viral locus with the wild-type sequence (Protocol 3), or regulating its function by inducible approaches, such as ddFKBP-tagging (Protocol 5). This will ensure that the phenotype associated with the original mutant virus is the direct result of the disruption of the targeted locus rather than spontaneous mutations elsewhere in the genome. This can be readily accomplished by the second step of linear recombination using galK negative selection.
Figure 4.
Two-step lambda-mediated linear recombination in E. coli to engineer seamless genetic alteration in the viral BAC clone.
Figure 5.
Linear recombination followed by FLP/FRT recombination to tag a viral ORF in the HCMV BAC clone.
This unit describes various steps involved in isolating HCMV virus as an infectious BAC clone (Protocols 1 and 2). While several methods to manipulate viral BACs have been used by different labs, a convenient two-step linear recombination method (Protocol 3) and its variations (Protocols 4 and 5) to generate a variety of recombinant viruses are described.
CAUTION: HCMV is a Biosafety Level 2 (BSL-2) pathogen. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms. It should be emphasized that specimens from patients suspected of being infected with HCMV (immunocompromised adults) may also contain other pathogenic viruses and should be manipulated with utmost caution. All tissue culture experiments should be performed under sterile conditions in a biosafety hood.
Basic Protocol 1: Isolation of an HCMV genome as a BAC clone
The general method described in this section is adapted from the two strategies used to isolate the HCMV laboratory strains AD169 (Yu et al., 2002) and Toledo (Murphy et al., 2003) as well as the clinical isolate PH (Murphy et al., 2003) as infectious BAC clones. Many of the issues outlined in the Commentary section of this unit have been addressed in these cases. Two HCMV genomic loci have been used to accommodate insertion of the BAC sequence. In the laboratory strain AD169, the BAC sequence was inserted following the US28 open reading frame without the need for deletion of any viral sequence (Yu et al., 2002). In other strains, the BAC sequence successfully replaced the nonessential HCMV US2-US6 locus (Murphy et al., 2003) or the US29–US34 region (Stanton et al., 2010). Deleted sequences can later be repaired using prokaryotic genetics once the viral genome is isolated as a BAC clone (see Protocol 3). The locus chosen as the integration site of the BAC vector in this protocol depends on the HCMV virus to be cloned. For a clinical HCMV isolate, it may be prudent to replace nonessential genes with the BAC vector sequence followed by subsequent repair of the locus once the BAC clone is isolated (See Commentary).
This protocol includes multiple sections describing various steps required to isolate an HCMV virus as an infectious BAC clone. To generate the BAC capture vector, viral sequences flanking the locus where the BAC vector will be inserted, approximately 1 kb each, are amplified from HCMV viral DNA by PCR and cloned into pYD-C223, a pMBO131 mini-F plasmid derived BAC vector (Fig. 1). pYD-C223 encodes an SV40 promoter-driven, IRES-based bicistronic cassette that expresses both GFP and puromycin-resistance to identify and enrich recombinant virus in eukaryotic cells, respectively. This construct also contains two loxP sites that flank the entire BAC vector except for the GFP marker. The final BAC capture vector is configured so that after linearization by restriction digestion, the BAC vector sequence is flanked by viral sequence arms (Fig. 2). The linearized BAC capture vector is then cotransfected with infectious HCMV DNA isolated from virions into human fibroblast cells. The viral sequences flanking the linearized BAC capture vector (e.g. US28 and US29 sequences, or US1-2 and US7 sequences) provide the homology arms needed for recombination and insertion of the BAC capture vector into the viral genome in these transfected fibroblasts. The GFP-positive, BAC-carrying recombinant viruses are plaque purified and further enriched by puromycin selection. Circular recombinant viral genomes are isolated by the Hirt method, which specifically isolates episomal DNA from eukaryotic cells (Hirt, 1967). They are then transformed into recombination-deficient E. coli bacteria (DH10B) as BAC clones carrying the viral genome. The clones are further screened by restriction digestion, PCR analysis, Southern blotting, and direct sequencing to confirm the presence of the intact viral genome.
Materials
BAC vector, such as pYD-C223 (derived from pYD-C29 (Yu et al., 2002))
Plasmid expressing HCMV protein pp71 (e.g. pYD-C21 (Baldick et al., 1997; Yu et al., 2002))
Plasmid expressing the Cre recombinase (e.g. GS403 (Smith and Enquist, 1999))
10 cm- and 15 cm-diameter tissue culture plates
6-well tissue culture dishes
Serological pipets, sterile
Cell scraper, sterile
Wide-orifice pipet tips, sterile
Primary human fibroblasts
Dulbecco’s modified Eagles medium with 4.5 g/L of glucose (DMEM) (e.g. D5796 from Sigma)
Supplemented DMEM (see recipe)
2× DMEM (see recipe)
DMEM/agarose overlay solution, sterile (see recipe)
37°C humidified CO2 (5%) incubator
Microcentrifuge
Refrigerated multipurpose centrifuge (e.g. 5810R from Eppendorf)
15 ml and 50 ml conical polypropylene centrifuge tubes
Inverted fluorescence tissue culture microscope
Ultracentrifuge, SW28 swinging bucket rotor or equivalent, and ultra clear centrifuge tubes (Beckman)
Electroporator (e.g. Bio-Rad Gene Pulsar Xcell)
1 mm, 2 mm, and 4 mm gap electroporation cuvettes
Commercial electrocompetent E. coli DH10B cells (e.g. Invitrogen MegaX).
PCR thermocycler
High fidelity PCR enzyme (e.g. Advantage HD Polymerase from clontech)
Ethanol
Isopropanol
D-Sorbitol solution (20% w/v in 50 mM Tris-HCl, pH 7.2, 1 mM MgCl2), sterile
2× lysis buffer (see recipe)
Commercial kit for purifying BAC DNA or low-copy number plasmid DNA (e.g., Clontech Nucleobond Xtra Midi DNA purification kit)
Commercial kit for PCR purification or gel extraction (e.g. Invitrogen PureLink PCR Purification Kit or Quick Gel Extraction Kit)
10.1 TE buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.0), sterile
Tris buffer (10 mM Tris-HCl, pH 8.0), sterile
LB plates
LB broth
SOC broth (see recipe)
Chloramphenicol (1000×, 15 mg/ml in ethanol), filter sterilized
Puromycin (1000×, 2 mg/ml in ddH2O), filter sterilized
Miniprep solution I (50 mM Tris-HCl, 10 mM EDTA, pH 8.0)
Miniprep solution II (200 mM NaOH, 1% SDS)
Miniprep solution III (3.0 M KOAc, pH 5.5)
Tris-equilibrated phenol
Chloroform:isoamyl alcohol (24:1)
Phenol:chloroform:isoamyl alcohol (25:24:1)
Hirt solution I (10mM Tris-HCl, 10mM EDTA, pH 8.0), sterile
Proteinase K
1 mg/ml RNase A
10% sodium dodecyl sulfate (SDS), sterile
5M NaCl, sterile
As there are a number of feasible PCR cycling conditions and cloning strategies (i.e. use of TA cloning intermediates versus direct cloning of PCR products) to engineer homology arms of viral sequences into a BAC vector, only the general cloning strategy is discussed.
Use extreme care when handling large naked DNA (e.g. virion DNA and BAC DNA) to reduce DNA shearing. Use wide-orifice tips whenever possible and store large viral DNA at 4°C.
Isolation of infectious HCMV virion DNA
-
1.
Prepare ten 15 cm plates of nearly confluent primary human fibroblast cells.
-
2.Infect cells with HCMV at an MOI of 0.05 and add 20 ml of fresh supplemented DMEM to each plate.For HCMV clinical isolates, it may be difficult to acquire enough viral material to start infection at the MOI of 0.05. In this case, cells can be inoculated with the virus at a lower MOI but during the adsorption period, the plates are subjected to centrifugation for 1 hr at 800 × g, room temperature, and then incubated for 1 additional hr at 37°C. This inoculation method, sometimes termed "spinoculation", can enhance HCMV infectivity by up to 10-fold.
-
3.Let infection proceed for 5 more days after 100% cytopathic effect (CPE) is observed.The entire infection process usually takes 10 days for laboratory strains. For clinical isolates, it will take longer to achieve 100% CPE.
-
4.
Collect supernatant in 50 ml conical tubes and pellet cell debris in a multipurpose centrifuge for 20 min at 3,200 × g, 4°C.
-
5.
Collect the supernatant and aliquot 25 ml per tube into 1 × 3 1/2 inch ultra clear centrifuge tubes.
-
6.Underlay the supernatant with 7 ml sorbitol buffer with a 5 ml pipette.Add the sorbitol solution slowly to avoid mixing the two layers.
-
7.
Centrifuge 1 hr at 55,000 × g, room temperature, in a SW28 swinging bucket rotor (or equivalent) to pellet virus.
-
8.
Resuspend each virus pellet in 250 µl 10.1 TE buffer. Combine suspensions of all the virus pellets in a 15 ml screw-cap tube, and make up the volume to a total of 2 ml.
-
9.Gently mix virus suspension with 2 ml 2× lysis buffer by inverting the tube several times and incubate 2 hr at 37°C.From this point on, extreme care should be taken with viral DNA and wide-orifice pipet tips should be used if pipetting is needed.
-
10.
Extract viral DNA with an equal volume of Tris-equilibrated phenol by slowly rocking the tube for 2 min, spinning the tube in the multipurpose centrifuge for 2 min at 1,800 × g, and carefully transferring the aqueous phase to a fresh tube using a pipet with a wide-orifice tip.
-
11.
Repeat step 10.
-
12.
Extract viral DNA with an equal volume of a chloroform:isoamyl alcohol (24:1) mixture as described in step 10.
-
13.
Repeat step 12.
-
14.
Precipitate viral DNA with 2 volumes of ice-cold ethanol. String-like DNA precipitate should become visible immediately. Pick up DNA strings with a pipette tip and transfer them to a 1.5 ml microfuge tube.
-
15.
Pellet viral DNA in a microcentrifuge for 2 min at 2,300 × g, room temperature. Wash DNA with ice-cold 70% ethanol and air dry for 5 min.
-
16.Add 500 µl 10.1 TE buffer and incubate viral DNA overnight at 4°C. Fully resuspend viral DNA the next day by gently tapping and inverting the tube multiple times. Measure the DNA concentration by determining the OD260 in a spectrophotometer three times and take the average. Aliquot and store viral DNA at 4°C.Do not store infectious viral DNA at temperatures below 4°C. Freeze-thaw cycles will cause damage to large virion DNA and render it less infectious.
Construction of the BAC capture vector
-
17.Amplify two viral genomic fragments by PCR using purified HCMV viral DNA as the template. These two fragments should be the viral sequences immediately flanking the viral genomic site where the BAC capture vector is designed to insert. For instance, one fragment can be the sequence of the US1-2 region and the other the US7 region; or one fragment can be immediately upstream and the other immediately downstream of the stop codon of the US28 ORF. These PCR fragments should be at least 1 kb in length to ensure successful homologous recombination to introduce the BAC capture sequence into the viral genome. For the primer pairs used for PCR, one primer for each fragment should contain a unique site that can be used to conveniently linearize the final BAC capture vector (e.g. PmeI) (Fig. 2) (see below). The other primer should contain the restriction site (e.g. SacI or NotI) that allows the resulting PCR fragment to be cloned into the SacI or NotI site of the BAC vector pYD-C223 (Fig. 1). These primer restriction sites should be designed so that viral sequences are ligated into the BAC capture plasmid in the correct orientation (Fig. 2).Use a high fidelity polymerase to minimize the chance of introducing random mutations into the PCR products. The PCR products can then be cloned into a TA vector (e.g. pGEM-T (Promega)) or directly digested for cloning into the BAC vector. Sequence the cloned products to confirm that they contain no mutations.
-
18.
To generate the final BAC capture vector, ligate the two PCR fragments into the SacI/NotI sites of YD-C223 (Figs. 1 and 2). Transform the ligation reaction into E. coli DH10B cells and select for Cam+ colonies on LB agar plates.
-
19.
Isolate BAC capture vector DNA from E .coli using a commercial kit for BAC DNA purification (e.g. Nucleobond Xtra Midi DNA purification kit (Clontech)). Identify clones carrying the correct final BAC capture vector using standard molecular biology tools (restriction digestion analysis, PCR analysis, sequencing analysis, etc).
Construction of recombinant HCMV virus carrying the BAC capture plasmid
-
20.Linearize 10 µg of BAC capture vector DNA by digestion with PmeI. Clean up the digested DNA by phenol/chloroform extraction followed by ethanol precipitation. Resuspend the DNA in 30 µl of sterile ddH2O.Run a small aliquot of the digested DNA on an agarose gel to confirm complete digestion of the DNA.
-
21.Use wide-orifice tips to add 2 µg HCMV viral DNA isolated from virion particles, 2 µg purified linearized BAC capture vector DNA, and 1 µg of plasmid DNA expressing HCMV protein pp71 (e.g. YD-C21) to a 4 mm electroporation cuvette.Cotransfection of pp71-expressing plasmid markedly increases the efficiency of virus reconstitution from transfection of infectious viral DNA (Baldick et al., 1997; Yu et al., 2002).
-
22.
Add 30 ml of warm supplemented DMEM medium to a 50 ml conical tube for use in step 28.
-
23.
Trypsinize one 15 cm plate of nearly confluent human fibroblasts and transfer cells to a 15 ml conical tube. Spin cells in a multipurpose centrifuge for 5 min at 200 × g, room temperature. Aspirate the supernatant and resuspend cells in 200 µl of DMEM.
-
24.
Add 200 µl of resuspended cells to the cuvette containing DNA and make up the total volume to 275 µl with DMEM.
-
25.
Gently tap the cuvette to ensure that the solution is thoroughly mixed.
-
26.
Electroporate cells in a BioRad Gene Pulsar at 260 V and 950 µF.
-
27.
Add 1 ml of supplemented DMEM to the cuvette and gently resuspend the cells.
-
28.
Transfer cells to the prepared 50 ml conical tube containing 30 ml of supplemented DMEM. Gently invert the tube to mix cells.
-
29.
Aliquot 2 ml of resuspended cells into each well of two 6-well dishes. Gently rock the dishes to distribute cells evenly. Place the dishes in the 37°C incubator.
-
30.The following day, inspect the dishes under a light microscope to ensure that the density of surviving cells is >50% confluency. Aspirate the media and overlay each well with 2 ml of the overlay solution. Allow the overlay solution to solidify for 30 min at room temperature, and return dishes to the 37°C incubator.Fresh cells can be added to the plate to maintain at least 50% confluence if electroporation results in excessive cell death. However, the low number of surviving cells often results in low reconstitution rate.
-
31.After 2–3 weeks, inspect the dishes under a fluorescent tissue culture microscope. GFP positive virus plaques should be readily visible. Plaque-purify GFP-positive recombinant virus.GFP expression is used here as a readout for the generation of recombinant virus carrying the BAC capture vector. Multiple GFP positive plaques should be picked and tested following the procedure described below to ensure that the correct recombinant virus is acquired.
-
32.Amplify individual plaque purified recombinant virus by infecting a 10 cm plate of human fibroblasts. Once small GFP plaques become visible, aspirate the medium and replace with 10 ml of supplemented DMEM containing 2 µg/ml puromycin and incubate at 37°C to further enrich recombinant virus.The number of days needed for GFP plaques to develop varies depending on the amount of plaque purified virus used. Nonetheless, they should be visible within 10 days. To speed up infection, once plaques are forming, trypsinize and reseed infected cells to accelerate the spread of infection.
-
33.
Two days later, aspirate puromycin-containing medium, wash the plate with DMEM twice, and replace with 10 ml supplemented DMEM. Add fresh cells to reach approximately 80% confluency.
-
34.
Let infection proceed 5 days after 100% CPE is observed.
-
35.
Collect supernatant in 50 ml conical tubes and pellet cell debris in a multipurpose centrifuge for 20 min at 3,200 × g, 4°C.
-
36.
Collect and aliquot supernatant as the recombinant virus stock. Store the stock indefinitely at −80°C, thaw one aliquot, and determine virus titer by plaque assay or TCID50 assay.
-
37.Extract viral DNA from pelleted cells or cell debris and use standard molecular biology assays (e.g. PCR analysis, restriction digestion, Southern blot analysis) to analyze viral DNA and ensure that isolated recombinant virus carries the BAC capture vector sequence at the correct genomic locus (Fig 3).Virus progeny of HCMV clinical strains are mostly cell associated. In this case, recombinant virus stock can be prepared from both infected supernatant medium and cell lysate. The described protocol removes most but not all of the parental virus that does not carry the BAC capture vector. This low level of contaminating parental virus will not impact isolation of the viral BAC clone using the protocol below.
Isolate recombinant HCMV virus as a viral BAC clone in E. coli
-
38.Infect one 10 cm plate of human fibroblasts with BAC-carrying recombinant virus at an MOI of 5 for 48 hr.Circular viral DNA is isolated from infected cells using the modified Hirt method (Hirt, 1967) as described below. If necessary, let infection proceed even longer (e.g 72–120 hrs) to increase the number of circular viral DNA intermediates formed in infected cells.
-
39.
Scrape cells off the plate with a cell scraper and collect cells by centrifugation in the multipurpose centrifuge for 5 min at 200 × g.
-
40.
Wash cells by gently resuspending them in 5 ml of Hirt solution I and collect cells by centrifugation as described above.
-
41.Resuspend cells in 2 ml of Hirt solution I containing 250 µg/ml proteinase K.Once cells are lysed it is very easy to shear the large viral DNA. Mix only by gentle inversion. Use wide-orifice tips if pipetting is needed.
-
42.
Aliquot 0.5 ml of resuspended cell lysate into each of 4 microfuge tubes.
-
43.
Add SDS and NaCl to a final concentration of 0.6% and 1M respectively.
-
44.
Incubate the lysate overnight at 4°C.
-
45.
Clear the lysate by centrifugation in a microcentrifuge for 30 min at 9,300 × g, 4°C.
-
46.
Extract the supernatant with an equal volume of Tris-equilibrated phenol twice. Collect the top aqueous layer using wide-orifice pipet tips.
-
47.
Extract the supernatant with an equal volume of chloroform:isoamyl alcohol (24:1) mixture twice. Collect the top aqueous layer using wide-orifice pipet tips.
-
48.
Precipitate the DNA with 2 volumes of ice-cold ethanol.
-
49.
Wash the DNA pellet with 70% ethanol.
-
50.
Gently resuspend the DNA in 30 µl of 10 mM Tris-HCl (pH 8.0).
-
51.Electroporate the DNA into DH10B electrocompetent E. coli cells using a 2 mm electroporation cuvette with the standard setting.To obtain the maximal number of transformants, test different amounts of viral DNA for electroporation (e.g. from 1–10 µl of the DNA). Using commercial electrocompetent cells with high transformation efficiency (e.g. MegaX DH10B (Invitrogen)) also increases the success at this step.
-
52.
Recover cells in 1 ml of SOC medium for 1–3 hr at 37°C.
-
53.
Concentrate the culture by centrifugation and resuspend in 80 µl of LB medium.
-
54.Plate the entire culture on a LB agar plate containing 15 µg/ml of chloramphenicol. Incubate the plate overnight at 37°C.It can take up to 2 days for Cam+ colonies to appear.
-
55.
Pick and restreak clones to fresh chloramphenicol-containing LB plates.
-
56.
Culture individual colonies in 10 ml of chloramphenicol-containing liquid LB medium overnight at 37°C.
-
57.
Save 0.5 ml of the individual cultures as a frozen glycerol stock and store at −80°C. Extract BAC DNA from the remaining culture using the following mini prep protocol to screen for correct clones.
Miniprep of BAC DNA
-
58.
Transfer the overnight culture to a 15 ml conical tube and collect cells by centrifugation for 15 min at 1,800 × g.
-
59.
Resuspend the pellet in 200 µl of miniprep solution I. Transfer the suspension to a sterile microfuge tube.
-
60.Add 300 µl of miniprep solution II and gently invert to mix. Lyse the cells for 5 min at room temperature.Do not exceed 5 min.
-
61.
Add 300 µl of miniprep solution III to neutralize the suspension and gently invert to mix.
-
62.
Incubate for 5 min at room temperature. Centrifuge in a microcentrifuge for 20 minutes at 9,300 × g, 4°C.
-
63.
Carefully pour the supernatant into a new microfuge tube that contains 500 µl of phenol:chloroform:isoamyl alcohol (25:24:1).
-
64.
Gently mix using a tube rotator for 30 min at room temperature.
-
65.
Centrifuge the tube in the microcentrifuge for 5 min at 9,300 × g, room temperature.
-
66.
Use wide-orifice tips to transfer 600 µl of the aqueous phase to a microfuge tube containing 500 µl of isopropanol. Invert the tube to mix.
-
67.
Centrifuge the tube in a microcentrifuge for 30 min at 9,300 × g, 4°C.
-
68.
Wash the pellet with 1 ml of ice-cold 70% ethanol. Centrifuge the tube for 5 min at 4°C.
-
69.
Carefully pour off the ethanol, centrifuge the tube again briefly, and remove the residual ethanol by pippeting.
-
70.Air dry the tube for 2 min.Do not completely dry the DNA pellet or it may be difficult to resuspend.
-
71.Resuspend the DNA by adding 50 µl of 10 mM Tris-HCl (pH 8.0) containing 20 µg/ml RNase A. Gently tap the tube to help dissolve the pellet.The BAC DNA can be stored for several weeks at 4°C. Storage at lower temperatures will result in shearing of the large BAC DNA.
Analysis of BAC DNA
The BAC DNA should be carefully analyzed to confirm overall integrity of the cloned viral genomic DNA and correct insertion of the BAC vector sequence. To analyze integrity of the BAC clones, digest 15 µl of BAC DNA with appropriate restriction enzymes, and compare the restriction pattern of BAC DNA to the predicted pattern or that of linear virion DNA of parental virus. EcoRI and BamHI are commonly used for restriction digestion because they produce evenly spaced fragments upon agarose gel electrophoresis and are useful to detect any gross alteration of the viral genome. PCR analysis of viral regions distal to the BAC integration site can also be used to test if most of the viral sequence is cloned in the viral BACs (Fig. 3). To confirm correct integration of the BAC capture vector sequence, the left and right recombination junctions should be analyzed by PCR reaction. For analysis of each junction, one primer should anneal to the BAC vector and the other primer should anneal to the viral genome outside of the homology arm sequences that recombined the BAC vector into the viral genome (Fig. 3). In addition, other DNA analysis assays, such as Southern blot or direct sequencing, can be used if necessary. Any clones that pass these initial analyses are candidate viral BAC clones and should be further tested to see if they reconstitute infectious virus upon transfection back into eukaryotic cells.
Basic protocol 2: Reconstitution of infectious virus from BAC clones
Once candidate BAC clones are identified, they must be transfected back into eukaryotic cells to determine if they can reconstitute infectious virus in tissue culture. This section describes an efficient method to deliver HCMV BAC DNA into human fibroblasts by electroporation. HCMV BAC DNA is commonly co-transfected with two additional plasmids. One plasmid expresses the HCMV pp71 (UL82) tegument protein and the other expresses Cre recombinase. pp71 acts as a viral transactivator to help initiate lytic infection (Baldick et al., 1997; Yu et al., 2002), and Cre mediates site-specific recombination at the loxP sites that flank the BAC vector sequence and results in loss of the vector sequence from the viral genome. Thus, co-transfection of these three constructs reconstitutes infectious HCMV virus genetically identical to parental virus except for a GFP expression marker and a single 34 bp loxP site left in the viral genome (Fig. 2). The GFP marker allows convenient monitoring of viral reconstitution and spread. Reconstituted virus is harvested, its titer determined, and its biological traits analyzed using methods described in other chapters.
Isolation of viral BAC DNA from E.coli
-
1.
Grow 100 ml of overnight bacterial culture of the viral BAC clone. Following the manufacturer’s protocol, use the Nucleobond Xtra Midi DNA purification kit (Clontech) to isolate BAC DNA up to the step of pelleting of the final BAC DNA by centrifugation.
-
2.
Resuspend the DNA pellet in 400 µl of 10 mM Tris-HCl (pH 8.0) and transfer it to a sterile microfuge tube using a wide-orifice tip.
-
3.
Add 40 µl of 3M KOAc (pH 5.5) and 0.9 ml of 100% ethanol to the tube. Mix by inversion.
-
4.
Centrifuge the tube in a microcentrifuge for 10 min at 9,300 × g, 4°C. A small pellet should be visible.
-
5.
Carefully pour off the supernatant. Add 1 ml of ice-cold 70% ethanol and wash the DNA pellet by inverting the tube.
-
6.
Centrifuge the tube for 5 min at 9,300 × g, 4°C. Carefully pour off the supernatant.
-
7.
Centrifuge the tube again briefly, and remove the residual ethanol by pipetting.
-
8.
Allow the pellet to air dry for 5 min.
-
9.
Add 50 µl of 10 mM Tris-HCl (pH 8.0) to the tube. Dissolve the DNA overnight at 4°C.
-
10.
Determine the concentration of the BAC DNA by taking the OD260 value in a spectrophotometer.
-
11.
Determine the quality of the BAC DNA by digesting 1 µg with EcoRI and analyzing restriction fragments by gel electrophoresis using a 0.6% agarose gel.
-
12.Store the BAC DNA at 4°C until ready to use.Freshly prepared BAC DNA works best in transfection so make sure to use the DNA within 2 weeks.
Reconstitution of HCMV virus from transfection of HCMV BAC DNA
NOTE: All experiments are performed in a tissue culture biosafety hood using wide-orifice tips.
-
13.
For each transfection, you need one 15 cm plate of nearly confluent primary human fibroblasts (~ 6 × 106 cells).
-
14.
Prepare a 10 cm plate for use in step 23 by adding 10 ml of warm supplemented DMEM.
-
15.Add the following DNA to a sterile microfuge tube:
HCMV BAC DNA 2–5 µg pp71-expressing plasmid (e.g. pYD-C21) 1 µg Cre-expressing plasmid (e.g. GS403) 1 µg -
16.
Gently mix DNA by pipetting using a wide-orifice tip. Transfer DNA to a 4 mm electroporation cuvette.
-
17.
Trypsinize and collect cells in the multipurpose centrifuge for 5 min at 200 × g, room temperature.
-
18.Carefully aspirate the medium and resuspend cells in an appropriate volume of DMEM.Each plate of cells should be resuspended in 200 µl of DMEM.
-
19.
Add 200 µl of resuspended cells to the cuvette containing DNA and make up the final total volume to 275 µl with DMEM.
-
20.
Tap the cuvette to mix immediately before electroporation.
-
21.Electroporate cells in a BioRad Gene Pulsar with the following setting:
Cuvette: 0.4 cm Voltage: 260 V Capacitance 950 µF Time constant should be 30–50 ms. -
22.
Add 1 ml of supplemented DMEM to the cuvette to gently resuspend cells.
-
23.
Transfer cells to the prepared 10 cm plate containing supplemented DMEM. Swirl the plate to break up cell clumps and evenly distribute cells.
-
24.
Incubate the plate overnight at 37°C.
-
25.
Change the medium the next morning to remove floating dead cells.
-
26.Inspect the plate under a light microscope. The density of surviving cells should be >25% confluence.Fresh cells can be added to the plate to maintain at least 25% confluence if electroporation results in excessive cell death. However, the low number of surviving cells often results in a low reconstitution rate.
-
27.Monitor transfected cells every 2–3 days by GFP expression or CPE.Viral plaques should be visible within a week. Plaques from transfection of a clinical HCMV BAC clone can take longer to develop.
-
28.When cells exhibit 100% CPE and 50% of cells are detached from the plate (usually within 3 weeks), collect medium supernatant in screw cap tubes and clear cell debris by centrifugation in a multipurpose centrifuge for 20 min at 3,200 × g, 4°C.Progeny virus of HCMV clinical strains are mostly cell associated. In this case, the stock of reconstituted virus can be prepared from both infected supernatant medium and cell lysate.
-
29.
Collect and aliquot supernatant as reconstituted virus stock. Store the stock indefinitely at −80°C, thaw one aliquot, and determine virus titer by plaque assay or TCID50 assay.
-
30.Perform both single and multistep growth curve analysis in appropriate cell types to determine the growth property of reconstituted virus. Include the parental virus from which the viral BAC clone is derived as the positive control for a direct comparison.Growth analysis is routinely performed in human fibroblasts. However, clinical HCMV isolates have expanded cell tropism. The ability to replicate in other cell types that are natural targets of HCMV infection in vivo, such as endothelial cells, epithelial cells, and macrophages, is a characteristic of clinical isolates and has been used as a biomarker to differentiate clinical isolates from laboratory adapted strains. Therefore, it is essential to perform growth analysis of reconstituted virus in these cell types if the BAC is used to clone an HCMV clinical isolate.
-
31.
Examine expression of representative viral immediate early, early, and late genes (e.g. by immunoblot analysis, reverse transcriptase-coupled quantitative PCR) as well as viral DNA replication (e.g. by slot blot, quantitative PCR) at different times (e.g. 12–72 hr) post infection of reconstituted virus. Include parental virus as the positive control for a direct comparison. Please reference other chapters or literature for these assays.
-
32.Chose the viral BAC clone that reconstitutes virus with the genomic structure, growth characteristics, and infection profile most closely resembling parental virus as the wild type BAC clone for future experimentation.Once a positive clone is identified it is advisable to completely sequence the viral BAC clone to ensure the cloned viral genome is intact and to provide the sequence information for site-directed genetic alteration in E. coli in later steps. If unwanted mutations are detected by sequencing, they can be readily repaired by linear recombination in E.coli (Protocol 3).
Basic protocol 3: Seamless genetic manipulation of the BAC-cloned viral genome by linear fragment-based homologous recombination (linear recombination)
Once an HCMV BAC clone is isolated, there are many ways to manipulate the viral genome in E. coli. In this chapter, we focus on an efficient and versatile method that is based on the lambda Red recombination system (Fig. 4). This method, we termed two-step linear recombination, has many advantages over other homologous recombination-based methods. It can produce rapid and efficient recombination between the viral BAC clone and a linear DNA fragment, such as a synthetic oligo or PCR product, which contains only very short regions of homology to the target viral genome. Only ~50 bp of homology are required and can therefore be easily engineered in the oligo sequence or PCR product. It uses a Kan/galK dual selection cassette to provide both positive and negative selection (Qian et al., 2008; Warming et al., 2005). In the first step, the Kan/galK cassette is introduced into the viral genome by Kan positive selection to target a locus for gene disruption (this protocol) or tagging (Protocols 4 and 5). In the second step, replacement of the cassette with any other sequence of interest by galK negative selection generates seamless genetic alterations.
Generation of electrocompetent, recombination-ready E.coli for linear recombination
The E. coli strain SW102 contains a defective lambda prophage integrated in the bacterial genome (Warming et al., 2005). The lambda recombination genes exo, bet, and gam, are transcribed from the lambda PL promoter. exo encodes a 5'–3' exonuclease that recognizes the ends of a linear DNA and creates single-stranded overhangs. bet encodes a single-stranded DNA binding protein that protects the overhangs and is required for subsequent homologous recombination. gam prevents degradation of the linear DNA by inhibiting the E. coli RecBCD complex. The temperature-sensitive lambda cI857 repressor prevents expression from the PL promoter at 32°C so no lambda recombination enzymes are produced. The repressor becomes inactive at 42°C, allowing the production of these recombination enzymes at this temperature. The lambda exo, bet, and gam proteins can then efficiently incorporate linear DNA that carries 50 bp homology arms into the targeted region of the BAC clone by homologous recombination.
Additional Materials
SW102 E. coli cells (Warming et al., 2005) that contain the HCMV BAC clone
Plasmid pYD-C255 that carries the Kan/galK dual selection cassette (Qian et al., 2008)
Supercentrifuge and rotor
Water bath shaker
500 ml flasks, sterile
Glycerol solution (10% v/v in ddH2O), sterile
DpnI restriction enzyme
Kanamycin (1000×, 25 mg/ml in ddH2O), filter sterilized
Ampicillin (1000×, 40 mg/ml in ddH2O), filter sterilized
1× M9 solution (see recipe)
DOG negative selection plates (see recipe)
- MacConkey indicator plates (see recipe)Maintain and propagate SW102-derived bacterial cells at the temperature between 30°C–32°C unless noted otherwise. Growing SW102 at the temperature greater than 32°C will result in unwanted induction of the lambda recombination system and instability of the viral BAC clone. Always maintain and propagate bacterial cells carrying the BAC clone in the presence of 15 µg/ml chloramphenicol.
To introduce an HCMV BAC clone into the bacterial strain SW102, isolate the viral BAC DNA by miniprep (Protocol 1) and transform it into SW102 by electroporation. Analyze multiple Cam+ colonies to ensure that no unwanted genomic alterations are produced in the BAC sequence during the transformation process. Make frozen stocks of SW102 colonies carrying the correct viral BAC clone.
Large scale preparation of recombination enzyme-induced, electroporation-competent cells
A large batch of competent cells can be prepared by this protocol and stored for several months at −80°C. However, freezing cells at −80°C may reduce transformation efficiency. If needed, competent cells can be prepared using the small scale protocol described in the later section and used immediately to achieve the maximal transformation efficiency.
Pick one SW102 colony that contains the correct BAC from a fresh LB plate to make a 10 ml overnight LB culture.
The following day, inoculate 1 L LB medium with the overnight culture.
Heat a water bath shaker to 42°C. Warm three 500 ml flasks at 42°C.
- When the OD600 reaches 0.55–0.6, divide the bacterial culture into pre-warmed 500 ml flasks and incubate in the water bath shaker for 15 min at 200 rpm, 42°C.This induces expression of lambda recombination enzymes.
Prepare an ice water slurry in a large container.
Swirl the flasks in the ice water slurry for 10 min.
Pellet cells by centrifugation in a supercentrifuge for 10 min at 3,800 × g, 4°C.
- Pour off the supernatant, resuspend and combine pelleted cells in a total of 100 ml of ice-cold sterile ddH2O. Divide cells into two 50 ml conical tubes and pellet cells in a multipurpose centrifuge for 10 min at 1,800 × g, 4°C.It is convenient to collect cells using the multipurpose centrifuge instead of the supercentrifuge from this step on. Nonetheless, if the multipurpose centrifuge is not available, the super-centrifuge can be used as well.
Repeat step 8.
Resuspend the cell pellet in a total of 20 ml ice-cold 10% glycerol, and pellet cells in the multipurpose centrifuge for 10 min at 2,500 × g, 4°C.
Resuspend the cell pellet in 3 ml ice-cold 10% Glycerol. Aliquot cell suspension at 50 µl/tube, snap freeze, and store for several months at −80°C.
Small scale preparation of recombination enzyme-induced, electroporation-competent cells
This protocol describes the preparation of a small batch of competent cells. They should be used freshly to yield higher transformation efficiencies.
Pick one SW102 colony that contains the correct BAC from a fresh LB plate to make a 2 ml overnight culture.
Use 1 ml of the overnight culture to inoculate 100 ml LB medium.
Heat a water bath shaker to 42°C.
When OD600 reaches 0.6–0.8, incubate the culture in the water bath shaker for 15 min at 200 rpm, 42°C.
Prepare an ice water slurry in a small container.
Swirl the flask in the ice water slurry for 10 min.
- Aliquot the culture into two 50 ml conical tubes. Pellet cells by centrifugation in a multipurpose centrifuge for 10 min at 1,800 × g, 4°C.It is convenient to collect cells using the multipurpose centrifuge instead of the supercentrifuge. Nonetheless, if the multipurpose centrifuge is not available, the supercentrifuge can be used as well.
Resuspend each pellet in 5 ml ice-cold sterile ddH2O by swirling.
Combine cell suspensions and make up final volume to 40 ml with ice-cold sterile ddH2O.
Pellet cells by centrifugation in the multipurpose centrifuge for 10 min at 1,800 × g, 4°C.
Wash the pellet 2 more times with 40 ml ice-cold sterile ddH2O as done above.
Resuspend cells in 200–500 µl ice-cold sterile ddH2O, transfer cells to an ice-cold microfuge tube, and keep cells on ice. Cells are now ready for electroporation.
Two-step linear recombination
Step 1: Kanamycin positive selection to insert the Kan/galK cassette into the targeted viral locus
This step introduces a Kan/galK cassette for gene disruption or for marking the locus for engineering of any other genetic alterations by the second step using negative selection.
- Amplify the 2.3 kb Kan/galK cassette from pGalK-Kan (pYD-C255) (Qian et al., 2008) by PCR using a pair of primers that also contain a 50 bp sequence homologous to the targeted viral region:
- 5’ Primer = 50 bp viral homologous sequence + CCTGTTGACAATTAATCATCG
- 3’ Primer = 50 bp viral homologous sequence + CTCAGCAAAAGTTCGATTTA
- Reaction program = 94°C/15 sec; 25 cycles = 94°C/8 sec, 59°C/1 min, 68°C/2.5 min; 68°C/3 min.
This program is optimized for the Advantage HD polymerase from Clonetech. The program can be altered as necessary if other types of polymerases are used. - Treat the PCR product with 1 unit of DpnI for 3 hr at 37°C.DpnI digests and removes methylated plasmid template but not unmethylated PCR products. This step ensures Kan+ colonies isolated at later steps only result from homologous recombination of the PCR product and not from introduction of the kanamycin-resistant plasmid template.
Examine the PCR product by agarose gel electrophoresis. Depending on the purity of the product, gel purify or directly purify the 2.3 kb PCR product of the Kan/galK cassette using a commercial kit (e.g. PureLink PCR Purification Kit or Quick Gel Extraction Kit (Invitrogen)). Elute the DNA with 50 µl of sterile ddH2O.
Electroporate 23 µl of 42°C induced competent cells prepared in this protocol with 2 µl of the PCR product in a 1 mm cuvette at 25µF, 1,750V, and 200Ω.
Recover cells in 1 ml of SOC medium for 5 hr.
Transfer cells to a 1.5 ml microfuge tube and pellet cells by centrifugation. Resuspend cells in 80 µl of medium, plate them on a LB plate containing chloramphenicol (15 µg/ml) and kanamycin (25 µg/ml), and incubate overnight. Colonies will be visible in the late afternoon the following day.
- Replica-plate colonies onto a LB plate with chloramphenicol and kanamycin and a LB plate with ampicillin (40 µg/ml).Colonies that carry the correct recombinants and are free from contaminating PCR plasmid template should be Amp−, Kan+, Cam+.
- Restreak Amp−, Kan+, Cam+ colonies onto a MacConkey indicator plate and incubate for 24 hr. galK+ colonies are pink or bright red.galK tends to accumulate spontaneous inactivating mutations during Kan positive selection and therefore loses its activity. It is essential to choose the clones that retain active galK if the second step of negative selection will be needed.
- Miniprep BAC DNA (Protocol 1) from 5 galK+ candidate clones. Screen for the correct clones by restriction digestion analysis, PCR analysis, and/or Southern blotting to identify the clones that carry the Kan/galK cassette in the correct locus but are free of any other unwanted obvious genetic alterations. Correct clones can be used for subsequent negative selection.In general, >90% of clones that are Amp−, Kan+, Cam+ are correct recombinants.
Step 2: GalK negative selection to replace the Kan/galK cassette by other genetic alteration
This step seamlessly introduces any type of genetic alteration into the locus marked by the Kan/galK cassette.
The galK gene product, galactokinase, phosphorylates galactose to galactose-1-phosphate in the galactose degradation pathway. Galactokinase can also phosphorylate the galactose analog, 2-deoxy-galactose (DOG), which cannot be further metabolized. This leads to a toxic buildup of 2-deoxy-galactose-1-phosphate and death of the galK+ cells. Growing cells on minimal medium in the presence of DOG selects for the loss of the galK gene in E. coli.
Construct the DNA fragment that carries the genetic alteration to be introduced. As in step 1, the DNA fragment needs ~50 bp viral homology sequences at each end for recombination to replace the Kan/galK cassette. The DNA fragment can be made by PCR amplification (10–50 ng) or by using double-stranded synthetic oligos (200 ng). In this unit, we describe the protocol using a PCR product.
Treat the PCR product with 1 µl DpnI for 3 hr at 37°C.
Gel purify or directly purify the PCR product. Elute the DNA in 50 µl of sterile ddH2O.
- Prepare 42°C-induced, electrocompetent SW102 cells that carry the Kan/galK marked viral BAC clone engineered by the first positive selection step of linear recombination.Bacterial cells that have already undergone linear recombination may become unstable, possibly due to the detrimental effect of accumulation of the active lambda recombination enzymes. In this case, it is advisable to transfer the BAC DNA to fresh naïve SW102 cells for negative selection.
Electroporate 23 µl of 42°C induced competent cells with 2 µl of the PCR product in a 1 mm cuvette at 25µF, 1,750V, and 200Ω.
Recover cells in 1 ml of SOC medium in a 10 ml culture tube for 5 hr.
Wash cells three times by pelleting in a microcentrifuge for 15 sec at 9,300 × g and resuspending the pellet in 1 ml 1× M9 salts.
- Plate 100 µl of 100 & 10−1 dilution of recovered cells on Cam+/DOG+ negative selection plates. Incubate for 2–3 days until small colonies grow out.Pick colonies as soon as they appear because false positive colonies may also appear if the plate is incubated too long.
- Replica-plate colonies onto a Cam+ plate, Kan+ plate, and Amp+ plate.Correct recombinants should be Cam+, Kan−, and Amp−.
- Miniprep BAC DNA (Protocol 1) from 10 candidate clones. Perform restriction digestion analysis, PCR analysis, and/or Southern blotting to identify the clones that carry the engineered alteration in place of the Kan/galK cassette but are free of any other unwanted obvious genetic alterations. Confirm candidate clones by direct sequencing.In general, >50% of clones that are Cam+, Kan−, and Amp− are correct recombinants
Basic protocol 4: Rapid tagging of a viral ORF in an HCMV BAC clone by FLP-mediated site-specific recombination
In addition to gene knockouts and replacements, the BAC system can readily be used to introduce a tag at the N- or C -terminus of a viral ORF in the viral genome. This section describes a reliable method that can be adapted to create a variety of tagged viral ORFs by linear recombination using a modified Kan/galK cassette and an E. coli strain that encodes the Flippase recombination enzyme (FLP) under an arabinose-inducible promoter, such as the strain SW105 (Warming et al., 2005) (Fig. 5). FLP is a site-specific recombinase from the 2 micron plasmid of Saccharomyces cerevisiae and it recognizes a specific 34 bp DNA sequence termed Flippase Recognition Target (FRT). When two FRT sites are present on a DNA sequence, the FLP enzyme recognizes and creates double-stranded breaks at these sites. Following cleavage, the ends of one FRT site are exchanged and re-ligated with the ends of the second cleaved FRT site, resulting in a deletion of the DNA between the FRT sites if the sites have the same orientation. As with other site-specific recombinases, this system has developed into a useful tool to perform precise genetic engineering. To introduce a tag into a viral ORF using the following protocol, a tagging cassette is generated by cloning a tag of interest (e.g. 3×FLAG or HA) directly upstream of the Kan/galK cassette that is flanked by FRT sites. The tagged cassette is then amplified by PCR using a pair of primers that also contain 50 bp of sequence homologous to the targeted viral region. The tagging cassette is introduced into the BAC clone at the targeted locus by linear recombination and kanamycin selection. Induction of FLP expression by arabinose treatment promotes FLP/FRT recombination and precise excision of the Kan/galK sequence. This fuses the tag together with a single FRT site in-frame to the viral ORF of interest. In this protocol, 3×FLAG tagging is used as the example to describe the procedure.
Additional Materials
SW105 E. coli cells that encode the arabinose-inducible FLP recombinase (Warming et al., 2005) and the HCMV BAC clone
Plasmid pYD-C744 that carries the 3×FLAG-FRT-Kan/galK-FRT cassette (Perng and Yu, unpublished):
- L (+) arabinose solution (10% w/v in ddH2O), sterileMaintain and propagate SW105-derived bacterial cells at a temperature between 30°C–32°C unless noted otherwise. Growing SW105 at temperatures greater than 32°C will result in unwanted induction of the lambda recombination system and instability of the viral BAC clone. Maintain and propagate bacterial cells carrying the BAC clone in the presence of 15 µg/ml chloramphenicol.
- Amplify the 2.4 kb 3×FLAG-FRT-Kan/galK-FRT cassette from pYD-C744 by PCR using a pair of 70 bp primers described below. The primers have 50 bp of viral genomic sequences flanking the start codon or stop codon of the targeted viral ORF (for N-terminal or C-terminal tagging, respectively), followed by a 20–25 bp sequence that anneals and amplifies the cassette:
- 5’ Primer = 50bp viral homology + (ATG)* GACTACAAAGACCATGACGG
- 3’ Primer = 50bp viral homology + (CTA)* CGGGAAGTTCCTATTCTCTA*Add ATG in 5' primer or CTA in 3' primer for N-terminal or C-terminal tagging, respectively.
- Reaction program = 94°C/15 sec; 25 cycles= 94°C/8 sec, 68°C*/1 min, 68°C/3 min; 68°C/5 min.*Decrease the annealing temperature by 1 degree/cycle.This program is optimized for the Advantage HD polymerase from Clonetech. The program can be altered as necessary if other types of high fidelity polymerase are used.
Perform linear recombination as described in Protocol 3 using electrocompetent SW105 cells. Pick several transformants that are Amp−, Kan+, Cam+. Verify the recombinant BAC DNA by restriction digestion, PCR, and sequencing.
To remove the Kan/galK sequence from the recombinant BAC clone, inoculate 5 ml of LB medium with an isolated colony and incubate overnight.
- Inoculate 10 ml of fresh LB medium with 1 ml of overnight culture, and grow for 2–3 hr.The culture should grow to log phase and the OD600 should be approximately 0.5.
Induce expression of the FLP recombinase by adding 100 µl of 10% L (+) arabinose to a final concentration of 0.1% arabinose. Culture with shaking for 1 additional hr.
- Plate 100 µl of serial dilutions of the culture (10−4 – 10−6) on LB plates with 15 µg/ml chloramphenicol and incubate overnight to recover colonies.As a control, also plate the same dilutions on LB plates with 25 µg/ml kanamycin. Much fewer colonies are anticipated in the presence of kanamycin.
Replica plate 10 Cam+ colonies onto a LB plate with chloramphenicol and an LB plate with kanamycin. Incubate overnight.
Inoculate 5 colonies that are Cam+, Kan− into 10 ml of LB medium with chloramphenicol. Culture overnight.
- Miniprep viral BAC DNA as described in Protocol 1. Confirm the integrity of the BAC DNA and precise excision of the Kan/galK sequence by restriction digestion, PCR, and direct sequencing of the locus.This inducible FLP/FRT recombination is highly efficient. Usually >90% of resulting colonies are Kan−.
Basic protocol 5: Develop a protein genetics-based inducible system to manipulate viral protein stability and activity by tagging its ORF with a ddFKBP tag
The ability to readily create N- or C-terminally tagged viral proteins in the context of the viral genome can be applied to generate a protein genetics-based inducible system. This system was developed by Wandless and colleagues (Banaszynski et al., 2006), and later adapted to study the function of CMV proteins (Glass et al., 2009; Perng et al., 2011; Qian et al., 2010). In this system a viral protein is tagged with a 110-amino acid destabilization domain derived from a mutant variant of the human FKBP protein (ddFKBP) (Banaszynski et al., 2006) so that the stability and function of the tagged viral protein can be efficiently regulated (Fig. 6). The fusion protein is normally directed for rapid degradation by ddFKBP and thus loses its activity. Accordingly, the recombinant virus expressing the fusion viral protein is anticipated to display a null phenotype. However, addition of the synthetic ligand Shield-1 (Shld1) stabilizes the ddFKBP-tagged protein and therefore maintains its activity. The recombinant virus is anticipated to display a wild type phenotype, allowing propagation of the recombinant virus.
Figure 6.
ddFKBP tagging-based protein genetic approach to study the function of HCMV proteins during infection.
This protocol describes a method to engineer a ddFKBP-tagged recombinant virus using linear recombination and subsequently study the roles of the tagged protein during virus infection (Fig. 6). The viral gene of interest is tagged with the ddFKBP domain at the N-terminus and virus is reconstituted in the presence of Shld1. Subsequent comparative analysis of the recombinant virus in the presence and absence of Shld1 determines how the loss of the targeted viral protein affects HCMV infection.
Additional Materials
Plasmid pYD-C630 that carries the cassette composed of the coding sequence of ddFKBP (110aa FKBP12 F36V L106P variant) (Banaszynski et al., 2006) followed by FRT-Kan/galK-FRT (Perng et al., 2011)
1000× Shield-1 (Shld1) solution: 1 mM in ethanol, stored at −20°C. Shld1 is purchased from Cheminpharma Farmington, CT
- Anti-FKBP12 mouse monoclonal antibody (BD Bioscience #610808)Maintain and propagate SW105-derived bacterial cells at the temperature between 30°C–32°C unless noted otherwise. Growing SW105 at a temperature greater than 32°C will result in unwanted induction of the lambda recombination system and consequently instability of the viral BAC clone. Maintain and propagate bacterial cells carrying the BAC clone in the presence of 15 µg/ml chloramphenicol.
-
1.Amplify the 2.7 kb ddFKBP-FRT-Kan/galK-FRT cassette from the plasmid pYD-C630 by PCR using a pair of 70 bp primers described below. The primers have a 50 bp sequence of viral genomic sequences immediately upstream or downstream of the start codon of the viral targeted ORF, followed by a 20–25 bp sequence that anneals and amplifies the cassette:
- 5’ Primer = 50 bp viral homology sequence + ATGGGAGTGCAGGTGGAAACCATC
- 3’ Primer = 50 bp viral homology sequence + GCTGGAGCTCCACCGCGGGAAGTTC
- Reaction program = 94°C/15 sec; 25cycles = (94°C/8 sec, 68°C*/1 min, 68°C/3 min); 68°C/3min.*Decrease the annealing temperature by 1 degree/cycleThis program is optimized for the Advantage HD polymerase from Clonetech to minimize the chance of introducing random mutations into the PCR products. The program can be altered as necessary if other types of high fidelity polymerase are used. It is possible that N-terminal tagging with ddFKBP will target the fusion protein for degradation more efficiently than C-terminal tagging (Banaszynski et al., 2006).
-
2.
Perform linear recombination as described in Protocol 3 using electrocompetent SW105 cells. Pick several transformants that are Amp−, Kan+, Cam+.
-
3.
Verify the recombinant BAC DNA by restriction digestion, PCR, and direct sequencing
FLP/FRT site specific recombination to remove the Kan/galK cassette
-
4.
Perform arabinose induction and FLP/FRT recombination as described in Protocol 4 to remove the Kan/galK cassette from the ddFKBP-tagged recombinant BAC clone. This procedure fuses the ddFKBP coding sequence in-frame with the viral ORF of interest.
Reconstitution of ddFKBP-tagged recombinant virus
-
5.Reconstitute recombinant virus from electroporation of the ddFKBP-tagged recombinant BAC clone in human fibroblasts using Protocol 2 with the following modifications:Perform electroporation in duplicate to monitor virus reconstitution with and without Shld1.Twenty four hr after electroporation, replace medium in one plate with fresh medium containing 1 µM Shld1 and the other with medium without Shld1.To maintain the Shld1 concentrations during the course of virus reconstitution, supplement 1 µM Shld1 every 48 hr.Culturing transfected cells in the presence of Shld1 should stabilize the fusion protein and allow efficient production of recombinant virus. Culturing cells in the absence of Shld1 serves as the control to test the efficacy of Shld1-regulated protein stability on the function of the targeted viral protein during HCMV infection.
-
6.
Amplify viral stock by infecting multiple 15 cm plates of human fibroblasts with ddFKBP-recombinant virus at an MOI of 0.05 in the presence of Shld1.
-
7.Let infection proceed 5 days after 100% CPE is observed.This usually takes 10 days for HCMV laboratory stains. HCMV clinical isolates will take longer to develop 100% CPE
-
8.
Collect infected medium supernatant and/or infected cell lysates. Pellet cell debris by centrifugation in a multipurpose centrifuge for 20 min at 3,200 × g, 4°C.
-
9.
To remove residual Shld1 and concentrate virus, collect the supernatant and aliquot 25 ml per tube into 1 × 3 1/2 inch ultra clear centrifuge tubes.
-
10.
Underlay the supernatant with 7 ml of sorbitol buffer with a 5 ml pipette.
-
11.
To pellet virus, centrifuge in a SW28 swinging bucket rotor (or equivalent) for 1 hr at 55,000 × g, 4°C.
-
12.
Resuspend each virus pellet in 2.5 ml of DMEM, combine and mix suspensions.
-
13.Aliquot virus into microfuge tubes and store as virus stock at −80°C. Thaw one aliquot and determine virus titer by TCID50 assay.To titer ddFKBP-tagged virus, maintain the Shld1 concentration by supplementing 1 µM Shld1 every 48 hr.
-
14.Examine the phenotype and growth properties of reconstituted ddFKBP-tagged virus with or without Shld1 in human fibroblasts as well as other appropriate cell types. Include non-tagged parental virus as an additional positive control.An antibody that recognizes the ddFKBP tag can be used to monitor expression and localization of the tagged viral protein and determine the efficacy of Shld1 treatment on the stability of the tagged protein, if antibody for the targeted viral protein is unavailable.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps.
Supplemented Dulbecco’s modified Eagles medium:
| 1000 ml | Dulbecco’s modified Eagles medium with 4.5 g/L of glucose |
| 100 ml | Fetal calf serum |
| 1 ml | Penicillin (50 mg/ml)/streptomycin solution (100 mg/ml) |
2× Dulbecco’s modified Eagles medium:
Dissolve the DMEM power (Invitrogen, cat. 12100-061) in 5 liters of ddH2O and filter sterilize the solution.
Supplemented DMEM/agarose overlay solution:
Dissolve 2 g of low melting temperature NuSieve®GTG® agarose (Lonza) in 70 ml of ddH2O, autoclave, and keep warm at 42°C. Prepare 2× supplemented DMEM solution with 100 ml of 2× DMEM, 20 ml of fetal calf serum, 10 ml of 7.5% sodium Bicarbonate, and 200 µl of penicillin (50 mg/ml)/streptomycin (100 mg/ml) solution, and warm at 37°C. Mix agarose solution with 2× supplemented DMEM by vigorous swirling.
2× lysis buffer:
| 20 mM | Tris-HCl, pH 8.0 |
| 50 mM | EDTA, pH 8.0 |
| 200 mM | NaCl |
| 1.2 % | SDS |
| 200 µg/ml | Proteinase K (add just before use) |
SOC broth:
| 1 g | Yeast Extract |
| 4 g | Tryptone |
| 0.4 ml | 5N NaCl |
| 0.5 ml | 1N KCl |
| 2 ml | 1N MgCl2 |
| 2 ml | 1N MgSO4 |
Make up the total volume to 150 ml with ddH2O and autoclave. Dissolve 0.72 g of glucose in 50 ml ddH2O and filter sterilize the solution. Add the entire sterile glucose solution to the autoclaved solution once cooled.
1× M9 solution:
| 1.2 g | Na2HPO4 |
| 0.6 g | KH2PO4 |
| 0.2 g | NH4Cl |
| 0.1 g | NaCl |
Add ddH2O to 200 ml and autoclave
5× M63 solution:
| 5 g | (NH4)2SO4 |
| 34 g | KH2PO4 |
| 1.25 mg | FeSO4.7H2O |
Adjust to pH 7 with KOH, add ddH2O to 500 ml, and autoclave
DOG negative selection plates:
Autoclave 7.5 g agar in 400 ml ddH2O in a 1L flask, and let it cool to 50°C.
- Add the following, mix well, and pour plates:
100 ml 5X M63 0.5 ml 1M MgSO4.7H2O 5 ml 20% Glycerol 5 ml 20% 2-deoxy-galactose (DOG) 5 ml 0.1 mg/ml Biotin 2.5 ml 9 mg/ml Leucine 0.5 ml 15 mg/ml Chloramphenicol
MacConkey indicator plates:
Add 25 g MacConkey agar (Difco or other) into 470 ml of dd H2O, and autoclave. Let it cool to 50°C, add 25 ml of 20% D-galactose and 0.5 ml of 15 mg/ml chloramphenicol, and pour plates.
COMMENTARY
BAC-based viral genetic systems for herpesviruses
In the past, molecular analysis of HCMV biology was hindered by the inability to efficiently generate genetic mutants of the virus. Before the advent of the viral BAC system, HCMV genetic mutants were isolated by homologous recombination and allelic exchange in tissue culture. HCMV is a slowing growing virus with a life cycle of 72 hours, so construction and isolation of mutant virus in tissue culture was time-consuming and inefficient. It could take months or longer to isolate a single recombinant virus. In contrast, isolation of an array of recombinant viruses using the BAC system takes only a few weeks because mutagenesis is performed in E.coli bacteria using well established prokaryotic genetics.
F factor, the circular fertility factor of E. coli, can stably carry large pieces of DNA (>300 kb) (Shizuya et al., 1992). A modified F-factor was adapted to clone the circular baculovirus genome as the first infectious viral BAC (Luckow et al., 1993). This technology was later applied to herpesviruses to clone the linear genome of murine cytomegalovirus (MCMV) as an infectious BAC (Messerle et al., 1997). Using similar approaches, the genomes of several herpesviruses have been cloned thus providing the necessary tools to efficiently manipulate their genomes and study their molecular biology (Adler et al., 2000; Borenstein and Frenkel, 2009; Borst et al., 1999; Cui et al., 2008; Delecluse et al., 1998; Horsburgh et al., 1999; Marchini et al., 2001; McGregor and Schleiss, 2001; Saeki et al., 1998; Smith and Enquist, 1999, 2000; Tang et al., 2010; Yu et al., 2002).
The BAC technology is particularly useful to study the molecular biology of HCMV clinical isolates, which was almost impossible in the past. It is well established that HCMV accumulates many adaptive genetic alterations in its genome (point mutations, rearrangements, and deletions) during extensive passaging in human fibroblasts, the widely used standard cell type for HCMV propagation (Cha et al., 1996; Dolan et al., 2004; MacCormac and Grundy, 1999; Murphy et al., 2003; Patterson and Shenk, 1999; Skaletskaya et al., 2001; Waldman et al., 1989; Yu et al., 2002). Many biological characteristics of clinical isolates are consequently lost in laboratory adapted strains, such as the ability to replicate efficiently in other cell types that are natural targets of HCMV infection in vivo (e.g. endothelial, epithelial cells). This high probability for mutation when passaged in tissue culture limits the studies that can reliably be performed using clinical isolates, because after several passages in tissue culture the virus may acquire mutations and no longer represent the parental virus. Cloning HCMV clinical isolates as BACs from patient samples allows the preservation and propagation of their genomes in E. coli. Subsequent investigation of reconstituted virus with limited passaging in tissue culture can reveal the effect of the targeted mutation without the complications arising from unwanted spontaneous adaptive mutations.
There are a number of factors to consider when choosing the site for insertion of the BAC vector sequence into the HCMV viral genome. To avoid potentially disrupting the functions of neighboring genes, the BAC vector is routinely inserted in a locus believed to be nonessential for viral growth. Additionally, to prevent the viral genome from becoming oversized (which is a particular concern for HCMV clinical isolates), the BAC vector can be inserted to replace a nonessential viral sequence of a similar size. Several HCMV genomic loci have been used to accommodate insertion of the BAC sequence. In AD169, the BAC sequence was inserted following the US28 open reading frame without the need for deletion of any viral sequence (Yu et al., 2002). In other strains, the BAC sequence successfully replaced the nonessential HCMV US2-US6 (Murphy et al., 2003) or the US29-US34 region (Stanton et al., 2010). The replacement and deletion of nonessential viral regions may be the safest approach particularly for cloning the viral genome of clinical HCMV isolates, as these isolates contain large regions of the viral genome that have been lost in laboratory strains and may be more limited in their capacity to accommodate additional foreign sequences. This could help to stabilize the inserted BAC sequence and prevent accumulation of compensatory mutations elsewhere in the viral genome. Once the viral BAC is isolated in E. coli, the deleted sequence can be repaired using prokaryotic genetics.
In this unit recombinant HCMV virus is isolated by cotransfection of linearized BAC capture vector together with linear HCMV genomic DNA isolated from virus particles into human fibroblasts. While this method works well for HCMV which produces relatively high virus titers, alternative methods can and have been used to isolate BAC clones of other herpesviruses or other HCMV strains. For example, BAC clones of the gammaherpesviruses Epstein Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) were isolated by transfection of BAC capture vector directly into virus infected-cells (Delecluse et al., 1998; Zhou et al., 2002). Alternatively, cells transfected with the BAC capture vector can be cocultured with virus-infected cells to produce BAC-carrying recombinant virus (Tang et al., 2010).
Creation of mutagenized recombinant viruses
Several homologous recombination-based methods have been used for genetic manipulation of the BAC clones in E. coli. Allelic exchange mediated by the E. coli RecA enzyme is reliable but time consuming (Smith and Enquist, 1999; Yu et al., 2002). Lambda-mediated linear recombination is efficient, and together with the use of the galK marker for both positive and negative selections, has been used to introduce seamless genetic alterations (Yu et al., 2000). However, galK positive selection is slow and false positives tend to accumulate during the longer incubation. The Kan/galK dual selection cassette described here was developed as an alternative due to the efficiency of kanamycin positive selection (Qian et al., 2008). This Kan/galK cassette has been used to generate a variety of gene disruptions, tagged viral proteins, seamless targeted mutations, and marker rescued viruses (Fehr and Yu, 2010, 2011; Perng et al., 2011; Qian et al., 2010; Qian et al., 2008; Xuan et al., 2009). Recently, another method to efficiently create a variety of mutants uses linear recombination coupled with in vivo cleavage of BAC DNA with the I-SceI endonuclease (Tischer et al., 2006). While not described in this unit, the I-SceI mediated approach has also been used to create a variety of mutants and is an alternative approach to consider, especially for introducing mutations without foreign sequence.
FLP/FRT site-specific recombination and ddFKBP tagging for conditional regulation of viral gene function
FLP/FRT site-specific recombination provides a convenient way to tag a viral protein in one linear recombination step (Fig. 5). This approach has been used by several labs to mark viral gene products during infection and saves considerable time compared to the two-step linear recombination approach for introducing seamless tags. As FLP/FRT recombination leaves behind a single 34 bp FRT site, care must be taken to ensure that the engineered tag is in frame with the viral protein with proper start and stop codons. In most cases the remaining FRT sequence has no effect on the function of the tag and viral protein. However, if FLP/FRT recombination is also planned in later steps for other loci, it is advisable to tag the protein using the seamless two-step method described in Protocol 3.
The ddFKBP approach for conditional regulation described here has been used to study several HCMV viral proteins (Glass et al., 2009; Perng et al., 2011; Qian et al., 2008; Tandon and Mocarski, 2011), and is especially useful for analysis of essential viral genes. It offers unique advantages over traditional methods for creating mutant viruses. It allows the abrogation of protein products of essential genes during virus infection without the need to create null mutant virus and complementing cell lines. Additionally, recombinant virus can be readily propagated in the presence of Shld1. As only one virus stock is required for the comparative study of HCMV infection in the presence or absence of a viral protein product, it eliminates the need to create marker rescued virus and avoids the potential complications resulting from an altered particle-to-PFU ratio in mutant virus. Finally, it provides evidence that the protein encoded by the tagged ORF and not other potential products encoded within the locus is important for viral replication.
The synthetic ligand Shld1 is expensive and must be supplemented every 48 hours to maintain an effective concentration for regulating the stability of ddFKBP-tagged proteins in tissue culture. For this reason, a new destabilization tag that is derived from E. coli dihydrofolate reductase (DHFR) has been developed, and an inexpensive small-molecule ligand trimethoprim (TMP) can be used to regulate the stability of the tagged protein (Iwamoto et al., 2010). This new method should be readily adapted as an alternative to the ddFKBP method and provides another way to conditionally regulate the function of HCMV proteins.
Troubleshooting
Please refer to Table 1 for possible issues and suggested solutions arising from the protocols described in this unit.
Table 1.
Troubleshooting Guide for BAC Cloning and Genetic Manipulation of HCMV
| Problem | Cause | Remedy |
|---|---|---|
| BAC isolation | ||
| BAC-cloned viral genome is different from the published sequence | Natural sequence variability in parental virus; mutations arising from the BAC cloneing process | Use parental virus as the control when analyze the BAC-cloned viral genome. If they are similar, the BAC clone captures the genome of parental virus; if they are different, screen additional clones or repair mutations with BAC genetics. |
| None of the BAC clones tested contain the large viral genome | Not enough BAC clones isolated or screened | Use high throughput methods (e.g. colony hybridization) to screen a large number of BAC clones |
| Mutations in BAC-cloned genome of clinical isolates | Adaptive mutations arising from extended virus growth in tissue culture | Passage virus as little as possible during the cloning process; freeze virus stocks at critical steps in the protocol for later reference to ensure that no mutations are acquired. Repair the BAC clone if necessary. |
| Mutations accumulate after a viral genome is isolated as a BAC clone (rare) | Too many serial passages of the BAC clone in E. coli | Use the original stock of the BAC clone if possible; keep the BAC clone in recombination-defective E. coli (DH10B) for long-term storage. |
| Virus reconstitution from BAC | ||
| Excessive cell death following BAC transfection | BAC DNA is low in purity or high in bacterial endotoxin | Reduce amount of BAC DNA transfected; isolate fresh batch of the BAC DNA for electroporation; pool multiple transfections into one plate. |
| Slow virus reconstitution from a recombinant BAC clone | Engineered genetic alteration affects virus growth | Trypsinize plate when virus plaques appear, reseed to redistrubute infected cells, and accelerate virus spread; reconstitute virus on complementing cells. |
| No virus reconstituted from a recombinant BAC clone transfection | Transfection fails; an essential viral element is disrupted | Examine plate 2–3 days after transfection for GFP+ cells to assess the transfection efficiency; use an inducible approach (e.g. ddFKBP tagging) to construct recombinant virus; reconstitute virus on complementing cells. |
| Kan/galK two-step linear recombination | ||
| Unexpected alterations elsewhere in the BAC | Unwanted mutations introduced because of elevated recombination | Do not heat-induce lambda recombination enzymes for more than 15 min |
| Unexpected alterations elsewhere in the BAC | Repetitive elements present in the linear fragment or BAC DNA | Redesign the linear fragment so the 50 bp viral homology arms contain unique sequences. |
| Too many false positive colonies from galK negative selection | Inefficient induction of lambda recombination enzymes; | Use fresh 42°C induced, electrocompetent cells prepared by the Small Scale protocol;. |
| Too many false positive colonies from galK negative selection | Incubation time on the DOG negative selection plates is too long | Pick colonies that appear early on the DOG plate. |
| The viral genome is unstable or lost from the BAC clone after linear recombination | Multiple rounds of induction of lambda Red enzymes is detrimental to bacteria | Transfer BAC DNA into fresh cells for next round of linear recombination. |
| FLP/FRT-mediated protein tagging | ||
| N-terminal tagged protein not detected | Tag is not in frame | Ensure that the tag is in frame with the viral ORF |
| N-terminal tagged protein not detected | Protein is translated from a downstream methionine | Tag the ORF at the downstream methionine or at the C-terminus. |
| Stable accumulation of ddFKBP-tagged protein in the absence of Shld1 | ddFKBP fails to destabilize the fusion protein | Test ddFKBP tagging at the C-terminus; Use an alternative destabilizing tag (e.g. DHFR). |
| ddFKBP-tagged virus is defective even in the presence of Shld1 | Tag impairs protein function | Move the ddFKBP tag to the C-terminus. |
Anticipated Results
The strategy to isolate HCMV as infectious BAC clones involves multiple complex steps, each of which needs to be carefully planned and carried out. While there are several methods to construct recombinant virus containing the BAC vector sequence, cotransfection of linear BAC capture vector and infectious viral DNA is most efficient for HCMV. Monitoring the formation of GFP+ plaques should reveal if recombinant virus has been successfully generated. While GFP+ plaque purification and puromycin selection enrich for recombinant HCMV virus, the key to isolate a BAC clone carrying the complete HCMV genome is to analyze a large number of potential BAC clones in E. coli. Many of these potential BAC clones may be incorrect and therefore careful screening using multiple methods, including high throughput ones (e.g. colony hybridization, high throughput PCR), may be necessary. If no unwanted mutations are present in candidate clones, infectious virus should be successfully reconstituted from BAC transfection using Protocol 2. While the titer of reconstituted virus can give an indication of its replicating ability, poor transfection efficiency can also result in decreased viral titer. For this reason, the growth kinetics of BAC-derived virus should be carefully compared to that of parental virus by single or multistep growth curves before a particular clone is chosen for further use.
Linear recombination to introduce the Kan/galK cassette into the BAC clone is efficient and >90% of the resulting Kan+ BACs will have the correct mutation. galK negative selection usually takes longer than Kan positive selection but it can also be expected that > 50% of the resulting Kan− clones will have the correct sequence. Generating tagged proteins using the FRT-Kan/galK-FRT cassette and FLP/FRT-mediated recombination is also efficient. After induction of the FLP recombinase, usually >90% of the resulting clones have the Kan/galK sequence excised. The FRT sequence left behind after excision of the Kan/galK sequence is not anticipated to affect the function of the tagged protein but it needs to be taken into consideration to ensure the tag is fused in frame to the protein of interest.
While ddFKBP tagging is a powerful tool, in our hands only approximately 25% of tagged HCMV proteins can be regulated by Shld1 treatment. In some cases the ddFKBP-tagged recombinant virus retains the wild-type phenotype even in the absence of Shld1. In other cases, tagged virus is defective regardless of Shld1 treatment. While it is possible to troubleshoot some of these issues (Table 1), it is expected that some viral proteins will not tolerate the ddFKBP tag or be regulated by Shld1. In these cases, traditional approaches (e.g. complementing cells) or alternative tagging (e.g. DHFR) should be explored.
Time Considerations
Isolation of HCMV virion DNA will take approximately 2 weeks for a laboratory strain but longer for a clinical isolate because of its slower replication in tissue culture. Once the BAC capture vector is made, the amount of time needed to isolate an HCMV genome as BAC clones depends on a number of factors, but mostly on the ability to construct and acquire sufficient amounts of recombinant virus. Additionally, the time for completion will also be determined by how many Cam+ E. coli colonies need to be screened to identify a correct clone, and if there are any deletions in the viral BAC clone that need to be repaired. Finally, full genome sequencing will validate the viral BAC clone and facilitate downstream genetic manipulation but requires additional time to complete.
Virus reconstitution from a wild-type BAC clone of an HCMV clinical isolate should take 3 weeks. Determination of virus titer by TCID50 or plaque assay takes approximately two weeks. One round of amplification of BAC-derived clinical virus at an MOI of 0.05 needs approximately 16 days. The time needed to characterize BAC-derived wild-type or mutant virus varies depending on the assays used.
Two-step linear recombination (Protocol 3) is relatively routine and requires approximately 1 week to complete. The tagging approaches described in Protocols 4 and 5 can be completed in 4 days but will require additional time for characterization.
Literature Cited
- Adler H, Messerle M, Wagner M, Koszinowski UH. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J Virol. 2000;74:6964–6974. doi: 10.1128/jvi.74.15.6964-6974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick CJ, Jr, Marchini A, Patterson CE, Shenk T. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J Virol. 1997;71:4400–4408. doi: 10.1128/jvi.71.6.4400-4408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein R, Frenkel N. Cloning human herpes virus 6A genome into bacterial artificial chromosomes and study of DNA replication intermediates. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19138–19143. doi: 10.1073/pnas.0908504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst EM, Hahn G, Koszinowski UH, Messerle M. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J Virol. 1999;73:8320–8329. doi: 10.1128/jvi.73.10.8320-8329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, McGregor A, Schleiss MR, McVoy MA. Cloning the complete guinea pig cytomegalovirus genome as an infectious bacterial artificial chromosome with excisable origin of replication. Journal of virological methods. 2008;149:231–239. doi: 10.1016/j.jviromet.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A, Cunningham C, Hector RD, Hassan-Walker AF, Lee L, Addison C, Dargan DJ, McGeoch DJ, Gatherer D, Emery VC, et al. Genetic content of wild-type human cytomegalovirus. The Journal of general virology. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- Fehr AR, Yu D. Human cytomegalovirus gene UL21a encodes a short-lived cytoplasmic protein and facilitates virus replication in fibroblasts. J Virol. 2010;84:291–302. doi: 10.1128/JVI.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr AR, Yu D. Human cytomegalovirus early protein pUL21a promotes efficient viral DNA synthesis and the late accumulation of immediate-early transcripts. J Virol. 2011;85:663–674. doi: 10.1128/JVI.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Busche A, Wagner K, Messerle M, Borst EM. Conditional and reversible disruption of essential herpesvirus proteins. Nature methods. 2009;6:577–579. doi: 10.1038/nmeth.1346. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Horsburgh BC, Hubinette MM, Qiang D, MacDonald ML, Tufaro F. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 1999;6:922–930. doi: 10.1038/sj.gt.3300887. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Bjorklund T, Lundberg C, Kirik D, Wandless TJ. A general chemical method to regulate protein stability in the mammalian central nervous system. Chemistry & biology. 2010;17:981–988. doi: 10.1016/j.chembiol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCormac LP, Grundy JE. Two clinical isolates and the Toledo strain of cytomegalovirus contain endothelial cell tropic variants that are not present in the AD169, Towne, or Davis strains. Journal of medical virology. 1999;57:298–307. doi: 10.1002/(sici)1096-9071(199903)57:3<298::aid-jmv14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Marchini A, Liu H, Zhu H. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J Virol. 2001;75:1870–1878. doi: 10.1128/JVI.75.4.1870-1878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Schleiss MR. Molecular cloning of the guinea pig cytomegalovirus (GPCMV) genome as an infectious bacterial artificial chromosome (BAC) in Escherichia coli. Mol Genet Metab. 2001;72:15–26. doi: 10.1006/mgme.2000.3102. [DOI] [PubMed] [Google Scholar]
- Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski UH. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Yu D, Grimwood J, Schmutz J, Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM, Shenk TE. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Shenk T. Human cytomegalovirus UL36 protein is dispensable for viral replication in cultured cells. J Virol. 1999;73:7126–7131. doi: 10.1128/jvi.73.9.7126-7131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng YC, Qian Z, Fehr AR, Xuan B, Yu D. The human cytomegalovirus gene UL79 is required for the accumulation of late viral transcripts. Journal of virology. 2011;85:4841–4852. doi: 10.1128/JVI.02344-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Leung-Pineda V, Xuan B, Piwnica-Worms H, Yu D. Human cytomegalovirus protein pUL117 targets the mini-chromosome maintenance complex and suppresses cellular DNA synthesis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000814. e1000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Xuan B, Hong TT, Yu D. The full-length protein encoded by human cytomegalovirus gene UL117 is required for the proper maturation of viral replication compartments. J Virol. 2008;82:3452–3465. doi: 10.1128/JVI.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Ichikawa T, Saeki A, Chiocca EA, Tobler K, Ackermann M, Breakefield XO, Fraefel C. Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors. Hum Gene Ther. 1998;9:2787–2794. doi: 10.1089/hum.1998.9.18-2787. [DOI] [PubMed] [Google Scholar]
- Shizuya H, Birren B, Kim UJ, Mancino V, Slepak T, Tachiiri Y, Simon M. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8794–8797. doi: 10.1073/pnas.89.18.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Enquist LW. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J Virol. 1999;73:6405–6414. doi: 10.1128/jvi.73.8.6405-6414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Enquist LW. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4873–4878. doi: 10.1073/pnas.080502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton RJ, Baluchova K, Dargan DJ, Cunningham C, Sheehy O, Seirafian S, McSharry BP, Neale ML, Davies JA, Tomasec P, et al. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J Clin Invest. 2010;120:3191–3208. doi: 10.1172/JCI42955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Mocarski ES. Cytomegalovirus pUL96 is critical for the stability of pp150-associated nucleocapsids. Journal of virology. 2011;85:7129–7141. doi: 10.1128/JVI.02549-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Kawabata A, Yoshida M, Oyaizu H, Maeki T, Yamanishi K, Mori Y. Human herpesvirus 6 encoded glycoprotein Q1 gene is essential for virus growth. Virology. 2010;407:360–367. doi: 10.1016/j.virol.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Tischer BK, von Einem J, Kaufer B, Osterrieder N. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques. 2006;40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- Waldman WJ, Sneddon JM, Stephens RE, Roberts WH. Enhanced endothelial cytopathogenicity induced by a cytomegalovirus strain propagated in endothelial cells. Journal of medical virology. 1989;28:223–230. doi: 10.1002/jmv.1890280405. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic acids research. 2005;33 doi: 10.1093/nar/gni035. e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B, Qian Z, Torigoi E, Yu D. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol. 2009;83:3463–3474. doi: 10.1128/JVI.02307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Silva MC, Shenk T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12396–12401. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Smith GA, Enquist LW, Shenk T. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol. 2002;76:2316–2328. doi: 10.1128/jvi.76.5.2316-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Li Q, Gao SJ. A sequence-independent in vitro transposon-based strategy for efficient cloning of genomes of large DNA viruses as bacterial artificial chromosomes. Nucleic acids research. 2009;37 doi: 10.1093/nar/gkn890. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. Journal of virology. 2002;76:6185–6196. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]