Abstract
Peroxidation of cardiolipin in mitochondria is essential for the execution of apoptosis. We suggested that integration of oleic acid into cardiolipin generates non-oxidizable cardiolipin species hence protects cells against apoptosis. We synthesized mitochondria-targeted triphenylphosphonium oleic acid ester. Using lipidomics analysis we found that pretreatment of mouse embryonic cells with triphenylphosphonium oleic acid ester resulted in decreased contents of polyunsaturated cardiolipins and elevation of its species containing oleic acid residues. This caused suppression of apoptosis induced by actinomycin D. Triacsin C, an inhibitor of acyl-CoA synthase, blocked integration of oleic acid into cardiolipin and restored cell sensitivity to apoptosis.
Keywords: Cardiolipin, apoptosis, mitochondria, cardiolipin remodeling, cardiolipin oxidation, mitochondria-targeted triphenylphosphonium oleic acid ester
1. Introduction
Cardiolipin (CL) is a mitochondria-specific doubly charged anionic phospholipid with four fatty acid residues [1]. In normal cells, it is exclusively localized to the inner mitochondrial membrane (IMM) where it accounts for ~25% of all phospholipids [2]. CL is synthesized on the matrix side of the IMM [3,4] as a premature form that is deacylated by phospholipases A to generate monolyso-CL [5,6,7]. The latter can be re-acylated by tafazzin, a mitochondrial phospholipid trans-acylase [6,8] predominantly present in the IMM [9], to yield mature species of CL [10]. In addition, mitochondria contain a coenzyme A-(CoA)-dependent acyltransferase, mono-lyso-CL acyltransferase (MLCL AT), that acylates monolyso-CL to CL [11]. Finally, endoplasmic reticulum (ER) acyl-CoA lyso-CL acyltransferase-1 (ALCAT 1), known to be upregulated by oxidative stress [12,13], can also catalyze synthesis of polyunsaturated CL species [14].
In addition to CL’s multiple structural and signaling functions in normal cell physiology, it is also an important player in apoptotic cell death pathways. Changes of the CL content and oxidation status have been associated with the execution of extrinsic and intrinsic apoptosis [15,16]. The early stage of intrinsic apoptosis is characterized by the formation of CL/cytochrome c (cyt c) complexes in mitochondria that exhibit a potent peroxidase activity towards polyunsaturated CL [17]. Polyunsaturated species of CL have been identified as a preferred oxidation substrate of cyt c catalyzed reactions in vitro [18,19] and in vivo [20,21,22,23]. Accumulation of CL oxidation products in mitochondria of apoptotic cells has been found essential for the release of pro-apoptotic factors into the cytosol [17]. We suggested that integration of mono-unsaturated oleic acid residues into mitochondrial CL – via its remodeling pathways in mitochondria - will generate non-oxidizable CL species thus protecting cells against apoptosis. We synthesized a poorly-peroxidizable triphenylphosphonium (TPP) octadecaenoic acid (C18:1) ester (TPP-C18:1) and used it for targeted delivery into mitochondria of mouse embryonic cells (MEC). Using liquid chromatography/mass spectrometry (LC/MS) based lipidomics analysis we established that pro-apoptotic stimulation with actinomycin D (AcD) was accompanied by selective oxidative consumption of CL molecular species containing polyunsaturated octadecadienoic (C18:2), eicosatetraenoic (C20:4), and docosahexaenoic (C22:6) acids. Pretreatment of MEC with TPP-C18:1 resulted in: i) significant decrease of CL polyunsaturated molecular species and simultaneous elevation of poorly-oxidizable CL molecular species containing C18:1 and ii) suppression of AcD induced apoptosis. An inhibitor of long chain acyl-CoA synthase (ACSL), triacsin C, blocked integration of C18:1 into CL molecules and restored MEC’s sensitivity to AcD-induced apoptosis.
2. Methods
Synthesis of TPP-C18:1
3-[(Z)-octadec-9-enoyl]oxypropyl-triphenyl-phosphonium chloride: A suspension of C18:1 (1 mmol) and silver nitrate (2 mmol) was stirred at 25 °C for 2 hrs. (3-bromopropyl)triphenylphosphonium bromide (1 mmol) was added and the reaction mixture was further stirred at 25 °C for 12 hrs. Thereafter, the mixture was filtered and the filtrate evaporated to dryness under reduced pressure. The remaining residue was re-dissolved in 50% methanol containing 1% NaHCO3 and 1% NaCl. The TPP ester was extracted with ethyl acetate and the extract dried over Na2SO4. Evaporation of the organic solvent afforded 0.55 mmol of 3-[(Z)-octadec-9-enoyl]oxypropyl-triphenyl-phosphonium chloride (ESI-MS analysis revealed a single peak with m/z = 585.4).
Cell culture
Mouse embryonic cells (MEC) were grown in Dulbecco’s Modified Eagle Medium containing 15% fetal bovine serum, 25 mM HEPES, 0.05 mg/ml uridine, 0.05mM 2-mercaptoethanol, 1x MEM (Invitrogen, Carlsbad, CA) and 100 U/ml penicillin/streptomycin in a humidified atmosphere (5% CO2 plus 95% air). Cells were pretreated with TPP-C18:1 (1–50μM) at 37°C for 2 hrs and after that exposed to AcD (100 ng/ml) at 37°C for 16 hrs. To block ASCL cells were treated with triacsin C (10 μM) at 37°C for 30 min. Cell viability was measured using AlamarBlue assay (Invitrogen, Carlsbad, CA). Apoptosis was evaluated by phosphatidylserine (PS) externalization using Annexin V–FITC apoptosis detection kit (Biovision, Mountain View, CA) and caspase 3/7 with a luminescence Caspase–Glo™ 3/7 assay kit (Promega, Madison, WI).
Analysis of CL
Lipids were extracted using the Folch procedure [24]. Lipid phosphorus was determined by a micro-method [25]. LC/MS was performed using a Dionex Ultimate™ 3000 HPLC coupled on-line to a linear ion trap mass spectrometer (LXQ Thermo-Fisher) as described [21]. CL was separated by 2D-HPTLC [26] and fatty acids were analyzed by LC/MS after hydrolysis of CL with porcine pancreatic phospholipase A2 (PLA2) as described [21].
Analysis of TPP-C18:1
Mitochondria were isolated from MEC treated with TPP-C18:1 (50 μM, for 2h at 37°C) as described [27]. TPP-C18:1 was extracted from mitochondria by Folch procedure [24] and LC/MS in positive mode was performed using a Dionex Ultimate™ 3000 HPLC coupled on-line to a linear ion trap mass spectrometer (LXQ Thermo-Fisher). TPP-C18:1 and TPP were separated on a normal phase column (Luna 3 μm Silica 100A, 150x2 mm, (Phenomenex, Torrance CA)) with flow rate 0.2 mL/min using gradient solvents containing 5 mM CH3COONH4 (A – n-hexane:2-propanol:water, 43:57:1 (v/v/v) and B - n-hexane:2-propanol:water, 43:57:10 (v/v/v). At these conditions the retention times for TPP-C18:1 and TPP were 27.9 and 50.6 min, respectively.
Statistics
The results are presented as mean ± S.E.M. values from at least three experiments, and statistical analyses were performed by either paired/unpaired Student's t-test or one-way ANOVA. The statistical significance of differences was set at p< 0.05
3. Results
AcD-induced apoptosis and oxidative consumption of CL in MEC
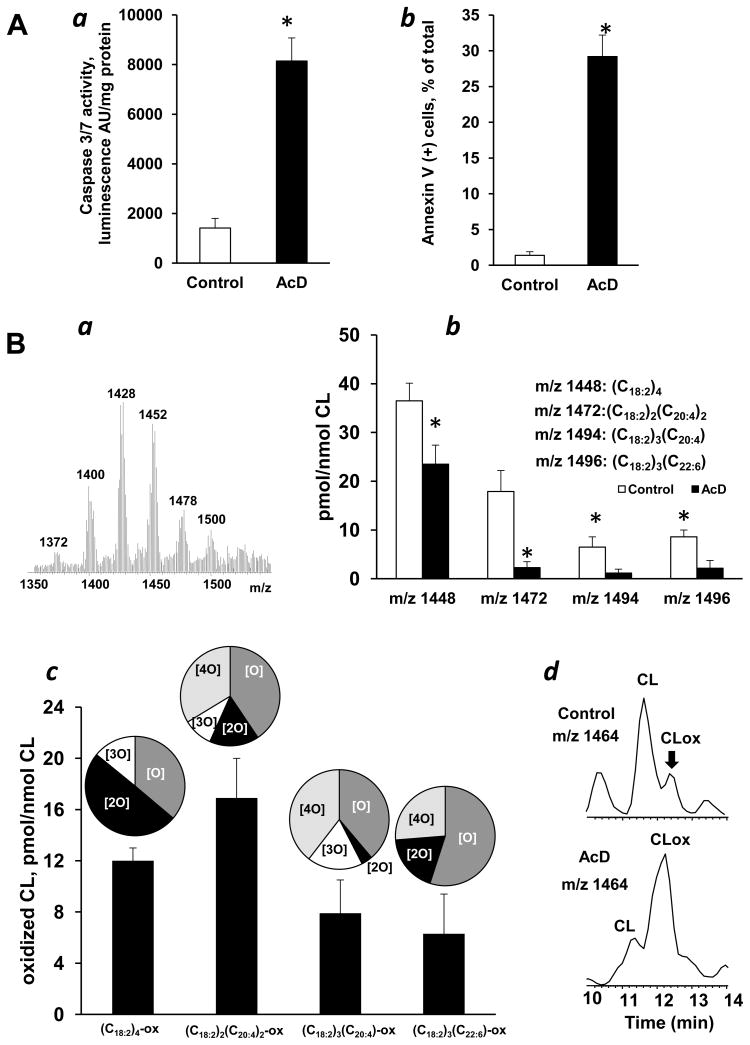
To avoid complications possibly associated with non-specific oxidative events commonly accompanying the execution of apoptotic program triggered by redox-cycling agents (eg, paraquat, tetracyclines antibiotics, quinones) as well as sources of oxidizing equivalents like H2O2 we used a known non-oxidant inducer of mitochondria-dependent intrinsic cell death pathway, AcD [28]. Accordingly, AcD induced apoptosis in MEC as evidenced by caspase 3/7 activation (Fig. 1Aa) and PS externalization (Fig. 1Ab). To assess the role of CL peroxidation in AcD-induced apoptosis we employed LC/MS analysis of CL molecular species. In a typical negative mode MS spectrum of CL from MECs, six clusters were detectable (Fig. 1Ba). Thirty six molecular species of CLs contained oxidizable polyunsaturated fatty acid residues [19]. We found a significant decrease in the amount of polyunsaturated CLs in cells challenged with AcD (vs control cells) – in line with likely peroxidative “consumption” of polyunsaturated molecular species of CL. The oxidation was selective: significant decreases of the contents occurred only in four molecular species of CL - with m/z 1448, m/z 1472, m/z 1494 and m/z 1496 - containing C18:2, C20:4, and C22:6 fatty acid residues and corresponding to (C18:2)4, (C18:2)3(C20:4), (C18:2)2(C20:4)2 and (C18:2)3(C22:6), respectively (Fig. 2Bb). Quantitative assessment of oxidized CL revealed accumulation of oxidation products formed from these CL molecular species (Fig. 2Bc,d) that were represented by species containing 1–4 oxygens in their fatty acid residues (Fig. 2Bc, inserts).
Figure 1. Apoptosis and CL oxidation induced by AcD in MEC.
A - Apoptosis induced by AcD in MEC. (a) Caspase 3/7 activation and (b) PS externalization in MEC exposed to AcD (100 ng/ml). Data are means ± S.E., n=10, *p<0.05 vs control. B - AcD-induced oxidation of CL in MEC. (a) Typical negative mode ESI-MS spectrum of CL obtained from MEC, LC/MS quantitative assessment of oxidizable (b) and oxidized (c) CL molecular species. CL oxidation products with 1–4 oxygens in each oxidized CL molecular species were detected and shown on inserts. (d) Base peak chromatogram of CL molecular species with m/z 1464. A higher intensity of the peak with m/z 1464 corresponding to oxygenated CL molecular species ((C18:2)3(C18:2-OH), retention time 12.2 min) was detected in AcD treated MEC. Data are means ± S.E., n=7, *p<0.03 vs control.
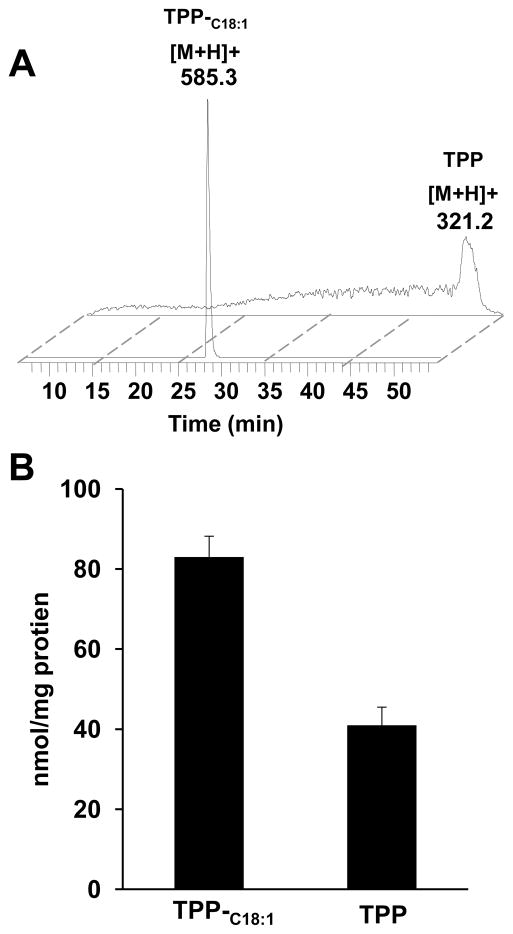
Figure 2. LC/MS analysis of TPP-C18:1 and its hydrolysis product, TPP, in mitochondria of MEC.
A – LC/MS base peak profiles of TPP-C18:1 and TPP in mitochondria from MEC exposed to TPP-C18:1. B - Quantitative assessment of TPP-C18:1 and TPP in mitochondria. Data are means ± S.E. n=6.
Effect of TPP-C18:1 on AcD-induced apoptosis and CL oxidation in MEC
We reasoned that delivery of TPP-conjugated monounsaturated C18:1 into mitochondria would favor its integration into CL through acyl-CoA-dependent remodeling pathways by MLCL AT or ALCAT 1 [6,12,13]. Using an uncoupler might be diagnostic of potential-driven accumulation of TPP-C18:1 in mitochondria. However, experiments with uncouplers in cells are complicated by their cytotoxic effects. A recent study discovered another intricacy in interactions of TPP-based penetrating ions with anions of fatty acids that can act as mitochondria-targeted protonophorous uncouplers [29]. Therefore, we chose to employ LC/MS to assess the content of TPP-C18:1 and its de-esterified product, TPP, in mitochondria of cells exposed to TPP-C18:1. Direct LC/MS analysis demonstrated the presence of TPP-C18:1 and its hydrolysis product, TPP in mitochondria of treated cells (Fig. 2a). Quantitative assessment revealed that the amount of TPP-C18:1 and TPP was 83.0 ± 5.2 and 41.0 ± 4.5 nmol/mg mitochondrial protein, respectively (Fig.2b). Given that accumulation of TPP-conjugated non-modified and modified fatty acids such as dodecyl-TPP, TPP-imidazole-substituted C18:0 and C18:1 in mitochondria has been demonstrated in previous studies [27,29] - it is conceivable, although still hypothetic, that TPP-C18:1 employed in the present work also accumulated in mitochondria.
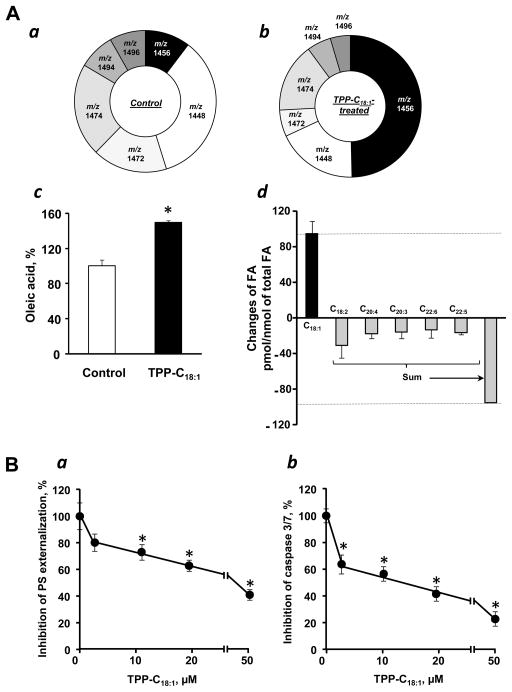
Interestingly, LC/MS analysis of lipids in TPP-C18:1 treated MEC revealed significant decrease in the levels of exactly those CL species with polyunsaturated fatty acids (m/z 1448, m/z 1472, m/z 1494 and m/z 1496) that underwent oxidation in cells exposed to AcD. At the same time, the content of C18:1-containing species of CL, particularly CL-(C18:1)4 (m/z 1456) was markedly increased (Fig. 3Aab). In line with this, LC/MS analysis of fatty acids after CL hydrolysis by PLA2 demonstrated the increase of C18:1 (Fig. 3Ac) and decrease of C18:2, C20:4, C20:3, C22:6 and C22:5 fatty acid residues in CL molecules (Fig. 3Ad).
Figure 3. Modification of CL molecular species affects apoptosis induced by AcD in TPP-C18:1 treated MEC.
A- Effect of TPP-C18:1 on the content of CL molecular species in MEC. Content of CL molecular species in control (a) and TPP-C18:1 treated cells (b). Content of C18:1 (c) and changes in CL fatty acid composition (d) in MEC treated with TPP-C18:1. The amounts of CL molecular species with m/z 1448, m/z 1472, m/z 1494 and m/z 1496 in TPP-C18:1 treated cells were dropped to 21.8 ± 3.3, 7.2 ± 2.0, 6.5 ± 2.3 and 5.4 ± 1.8 pmol/nmol of total CL from 36.5 ± 3.6, 17.9 ± 4.3, 8.7 ± 1.6 and 8.6 ± 1.4 pmol/nmol of total CL in control cells, respectively. Consequently, the amount of CL molecular species with m/z 1456 was increased from 10.8 ± 2.7 to 58.6 ± 7.2 pmol/nmol of total CL. Data are means ± S.E., n = 3–10. B - Effect of TPP-C18:1 on AcD-induced phosphatidylserine externalization (a) and caspase 3/7 activation (b) in MEC. Data are means ± S.E., n=8, *p<0.05 vs control.
Assuming that CL peroxidation is essential for the execution of the mitochondrial segment of apoptotic program, TPP-C18:1 should exert anti-apoptotic effect. Indeed, treatment with TPP- C18:1 markedly increased resistance of MEC to AcD induced apoptosis as evidenced by inhibition of PS externalization (Fig. 3Ba) and caspase 3/7 activity (Fig.3Bb). In contrast, C18:1 without the TPP-moiety did not change cell viability and was not effective in suppression of apoptosis in these cells (data not shown). Importantly, the AcD-induced “oxidative consumption” of polyunsaturated CL molecular species was significantly less pronounced in cells pre-treated with TPP-C18:1 (Fig. 4A). These results indicate that selective CL remodeling leading to the accumulation of its non-peroxidizable species is associated with the protection of cells against AcD-induced apoptosis.
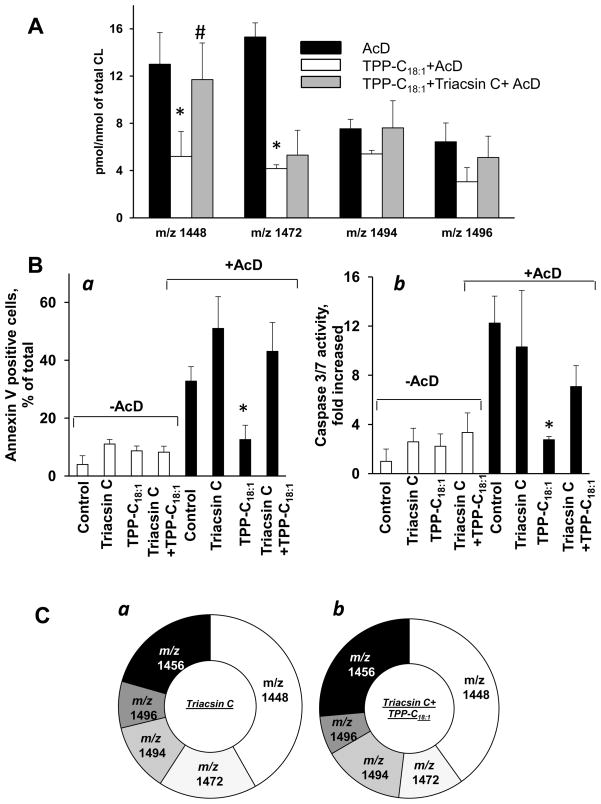
Figure 4. Effect of triascin C on apoptosis and CL oxidation induced by AcD in MEC treated with TPP-C18:1.
A -Effect of AcD, TPP-C18:1 and triacsin C on CL oxidation in MEC. Data are presented as increase of oxidized CL molecular species. Data are means ± S.E., n=3–7, *p<0.05 vs control. B - Effect of TPP-C18:1 and triacsin C on AcD-induced phopsphatidylserine expernalization (a) and caspase 3/7 activation (b) in MEC. Data are means ± S.E., n=3–10, *p<0.05 vs control. C- Content of CL molecular species in triacsin C treated (a) and triacsin C treated TPP-C18:1 exposed cells (b). The amounts of CL molecular species with m/z 1448, m/z 1472, m/z 1494 and m/z 1496 were estimated as 33.3 ± 1.9, 13.8 ± 2.0, 9.6 ± 1.8 and 6.5 ± 1.6 pmol/nmol of total CL in triacsin C pre-treated and 37.6 ± 3.9, 11.0 ± 3.1, 6.6 ± 4.3 and 3.9 ± 3.1 pmol/nmol of total CL in triacsin pre-treated/TPP-C18:1 exposed cells, respectively. Consequently, the amount of CL molecular species with m/z 1456 was 16.4 ± 3.4 to 24.7 ± 4.2 pmol/nmol of total CL. Data are means ± S.E., n=3–5.
Effect of triacsin C on AcD-induced apoptosis and CL oxidation in TPP-C18:1 treated MEC
To further verify that CL remodeling is responsible for anti-apoptotic effect of TPP-C18:1 , we utilized triacsin C – an inhibitor of ACSL, an enzyme that generates acyl-CoAs [30]. We found that triacsin C treated cells exhibited high sensitivity to AcD-induced apoptosis and abolished the anti-apoptotic effect of TPP-C18:1 (Fig.4B). Neither significant enrichment of CL with C18:1 (Fig.4Cab) nor changes in CL oxidation (Fig. 4A) were detected in triacsin C treated/TPP- C18:1 exposed cells compared to control. Thus, CL re-acylation pathway – dependent on ACSL-driven formation of acyl-CoA - is the likely major contributor to the anti-apoptotic effect of TPP-C18:1 in MEC.
4. Discussion
MEC are commonly used as a normal cell culture model for studies of cell death pathways including apoptosis [31,32,33]. It has been demonstrated that MEC exhibit typical apoptotic features such as DNA fragmentation, PS externalization, caspase 3/7 activation as well as cyt c release in response to different stimuli including AcD [17,19,34]. Because the goal of this work was to develop protective strategies against apoptosis, we chose to employ a model of normal, rather than tumor, cells for the current study.
Accumulation of CL oxidation products - predominantly CL-hydroperoxides – is a required early stage of apoptosis essential for the release of pro-apoptotic factors - including cyt c – from mitochondria into the cytosol [17]. This was uniformly characteristic of different pro-apoptotic stimuli in cultured cells in vitro [18,19] and several types of exposures in vivo such as total body irradiation [21,22], hyperoxia [20] and traumatic brain injury [23]. This CL and its peroxidation may act as a mitochondrial switch regulating sensitivity of cells to apoptosis [17] hence represent a new target for drug discovery. The peroxidation process is catalyzed by an intermembrane space protein of mitochondria – cyt c - that forms a high affinity peroxidase complex with polyunsaturated CLs [17]. Inhibitors of the peroxidase activity effective in suppressing CL peroxidation have been shown to exert potent anti-apoptotic effects [19,20,35]. Among those, mitochondria-targeted imidazole-substituted fatty acids displayed a potent anti-apoptotic effect in vitro and in vivo resulting in significant protection against acute radiation damage [27].
This work demonstrates, for the first time, that mitochondrial delivery of poorly peroxidizale mono-unsaturated TPP-C18:1 resulted in remodeling of endogenous pool of CLs such that CLs containing polyunsaturated fatty acid residues becomes less abundant while the content of C18:1-containing species increased. This remodeling conferred increased resistance of MEC to AcD induced apoptosis. Given that saturated fatty acid residues do not undergo peroxidative modifications, they may represent an alternative group of compounds for CL remodeling with potential anti-apoptotic consequences. However, it has been reported that saturated fatty acids are poor substrates for CL reacylation reactions [13] and the rates of their turnover in CLs are very low [36].
The diversity of CLs is defined by two major metabolic pathways - its biosynthesis and remodeling. De novo synthesis via condensation of one molecule of phosphatidylglycerol and one molecule of cytidine-5’-diphosphate-1,2-diacylglycerol catalyzed by CL synthase (CLS1) takes place in mitochondria [3,4]. It is believed that de novo synthesis is responsible for non-specific diversification of CL molecular species while acyl-specific remodeling limits and fine-tunes the number of CL molecular species in different tissues [37]. At least three enzymes are involved in remodeling of CL. Tafazzin, a mitochondrial phospholipid transacylase - presents exclusively in the IMM [6,8,9] - transfers acyl residues, predominantly linoleic acid, from PC and PE into monolyso-CL [38,39,40] independently of CoA [41]. CoA-dependent remodeling of CLs can occur in both mitochondria and ER [12,13]. It is catalyzed by MLCL AT and ALCAT 1 with high specificity towards poly- and monounsaturated fatty acids [13]. Both enzymes utilize acyl-CoA as a substrate in the reaction for acylation of mono-lyso-CL [11,13] and can be specifically down regulated through inhibition of ACSL by triascin C [30]. In line with this, our results demonstrated that triascin C effectively inhibited TPP-C18:1-driven remodeling of CL in MEC. Notably, triascin C also restored sensitivity of TPP-C18:1-supplemented cells to AcD-induced apoptosis suggesting that CoA-dependent remodeling is involved in modification of CL in MEC exposed to TPP-C18:1.. This is further supported by our oxidative lipidomics measurements which revealed that only four out of thirty six polyunsaturated molecular species of CL were selectively oxidized in MEC during apoptosis triggered by AcD. By metabolically manipulating these CL species and decreasing their susceptibility to peroxidation we were be able to decrease the sensitivity of cells to apoptosis.
Our previous work established that by suppressing CLS1 by specific RNAi we were able to cause CL-deficiency, decrease CL peroxidation thus increase resistance of HeLa cells challenged with AcD to apoptosis [28]. Here, we experimentally tested an alternative approach based on manipulations of CL oxidizability using poorly-oxidizable C18:1. To minimize C18:1 toxicity and deliver C18:1 into cells and mitochondria without damaging them, we conjugated C18:1 with an organic cation TPP - commonly used for delivery different payloads into mitochondria via “electrophoretic mechanism” based on negative inside mitochondrial membrane potential [42,43,44]. TPP conjugated fatty acids can be rapidly hydrolyzed intracellularly by esterases in mitochondria and ER [27]. Therefore, unesterified C18:1 can be quickly activated by ACSL that present in the ER or mitochondria [45,46] to C18:1-CoA and utilized for CL remodeling.
It is possible that enrichment of mitochondria with oleoyl-containing CL molecular species may affect interactions of this essential anionic phospholipid with a number of mitochondrial proteins such as cyt c oxidase [47,48], creatine kinase [49,50], ATP synthase [51,52] mitochondrial ADP carrier [53] resulting in altered functional status. Our previous work, however, demonstrated that depletion of 55% of endogenous CL in HeLa cells did not affect their growth rate, mitochondria biomarker proteins and levels of ATP and mitochondrial membrane potential [28]. Further, cyt c binding constant for tetra-oleoyl-CL was similar to that of tetra-linoleoyl-CL [54].
In summary, we developed a new approach to regulate cell’s sensitivity to apoptosis via manipulations of their CL’s oxidizability. While our findings on regulation of sensitivity to apoptosis via manipulations of the CL’s oxidizability have been obtained using MEC as a normal cell culture model, we suggest that this approach may be applicable to other cell types as well. Indeed, oxygenation of CL molecular species during apoptosis of different normal primary cell cultures, including lung epithelial cells, lung endothelial cells, rat cortical neurons has been reported [18,20,21]. However, metabolic remodeling of CLs can be utilized as an effective tool not only for suppressing (by non-oxidizable fatty acid precursors) but also for enhancing (via highly oxidizable polyunsaturated fatty acid precursors) apoptotic death pathway. In this way, this approach can be utilized for the development of new therapeutic modalities to prevent excessive apoptosis or stimulate it in cancer cells.
Highlights.
Generation of non-oxidizable oleate-enriched cardiolipins via mitochondrial targeting
Desensitization to apoptosis via suppression of cardiolipjn oxidation
Inhibition of long chain acyl-CoA synthase by triacsin C reinstates polyunsaturation of cardiolipin and sensitivity to apoptosis.
Acknowledgments
Supported by NIH: U19AIO68021, HL70755, HL094488, ES020693, by NIOSH OH008282.
Abbreviations
- CL
Cardiolipin
- PS
phosphatidylserine
- IMM
inner mitochondrial membrane
- CoA
coenzyme A-
- MLCL AT
mono-lyso-CL acyltransferase
- ER
endoplasmic reticulum
- ALCAT
1acyl-CoA lyso-CL acyltransferase-1
- ACSL
long chain acyl-CoA synthase
- PLA2
phospholipase A2
- CLS1
CL synthase
- TPP
triphenylphosphonium
- C18,1
octadecaenoic acid
- TPP-C18:1
triphenylphosphonium octadecaenoic acid ester
- MEC
mouse embryonic cells
- LC-MS
liquid chromatography-mass spectrometry
- AcD
actinomycin D
- C20,4
eicosatetraenoic acid
- C22,6
docosahexaenoic acid acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daum G, Lees ND, Bard M, Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancuso DJ, Kotzbauer P, Wozniak DF, Sims HF, Jenkins CM, et al. Genetic ablation of calcium-independent phospholipase A2{gamma} leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction. J Biol Chem. 2009;284:35632–35644. doi: 10.1074/jbc.M109.055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houtkooper RH, Turkenburg M, Poll-The BT, Karall D, Perez-Cerda C, et al. The enigmatic role of tafazzin in cardiolipin metabolism. Biochim Biophys Acta. 2009;1788:2003–2014. doi: 10.1016/j.bbamem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Beranek A, Rechberger G, Knauer H, Wolinski H, Kohlwein SD, et al. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J Biol Chem. 2009;284:11572–11578. doi: 10.1074/jbc.M805511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011;286:899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bione S, D'Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, et al. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, et al. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma BJ, Taylor WA, Dolinsky VW, Hatch GM. Acylation of monolysocardiolipin in rat heart. J Lipid Res. 1999;40:1837–1845. [PubMed] [Google Scholar]
- 12.Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 13.Taylor WA, Hatch GM. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1) J Biol Chem. 2009;284:30360–30371. doi: 10.1074/jbc.M109.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Romestaing C, Han X, Li Y, Hao X, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crimi M, Esposti MD. Apoptosis-induced changes in mitochondrial lipids. Biochim Biophys Acta. 2011;1813:551–557. doi: 10.1016/j.bbamcr.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 18.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, et al. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem. 2008;107:1614–1633. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, et al. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L73–85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, et al. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat Res. 2011;175:610–621. doi: 10.1667/RR2297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, et al. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Bottcher CJF, Van Gent CM, Pries C. A rapid and sensitive sub-micro phosphorus determination. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- 26.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation induced death. Nat Commun. 2011 doi: 10.1038/ncomms1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Jiang J, Tyurin VA, Zhao Q, Mnuskin A, et al. Cardiolipin deficiency leads to decreased cardiolipin peroxidation and increased resistance of cells to apoptosis. Free Radic Biol Med. 2008;44:1935–1944. doi: 10.1016/j.freeradbiomed.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Severin FF, Severina, Antonenko YN, Rokitskaya TI, Cherepanov DA, et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc Natl Acad Sci U S A. 2010;107:663–668. doi: 10.1073/pnas.0910216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vessey DA, Kelley M, Warren RS. Characterization of triacsin C inhibition of short-, medium-, and long-chain fatty acid: CoA ligases of human liver. J Biochem Mol Toxicol. 2004;18:100–106. doi: 10.1002/jbt.20009. [DOI] [PubMed] [Google Scholar]
- 31.Kukat A, Edgar D, Bratic I, Maiti P, Trifunovic A. Random mtDNA mutations modulate proliferation capacity in mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2011;409:394–399. doi: 10.1016/j.bbrc.2011.04.145. [DOI] [PubMed] [Google Scholar]
- 32.Sakao K, Singh SV. D,L-sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66(Shc) J Cell Biochem. 2011 doi: 10.1002/jcb.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moujalled DM, Cook WD, Lluis JM, Khan NR, Ahmed AU, et al. In mouse embryonic fibroblasts, neither caspase-8 nor cellular FLICE-inhibitory protein (FLIP) is necessary for TNF to activate NF-kappaB, but caspase-8 is required for TNF to cause cell death, and induction of FLIP by NF-kappaB is required to prevent it. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, et al. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 35.Kagan VE, Wipf P, Stoyanovsky D, Greenberger JS, Borisenko G, et al. Mitochondrial targeting of electron scavenging antioxidants: Regulation of selective oxidation vs random chain reactions. Adv Drug Deliv Rev. 2009;61:1375–1385. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahjudi PN, Yee J, Martinez SR, Zhang J, Teitell M, et al. Turn-over of non-essential fatty acid in cardiolipin in rat heart. J Lipid Res. 2011 doi: 10.1194/jlr.M015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatch GM. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells (Review) Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Kelley RI, Blanck TJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 40.Malhotra A, Xu Y, Ren M, Schlame M. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta. 2009;1791:314–320. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Testet E, Laroche-Traineau J, Noubhani A, Coulon D, Bunoust O, et al. Ypr140wp, 'the yeast tafazzin', displays a mitochondrial lysophosphatidylcholine (lyso-PC) acyltransferase activity related to triacylglycerol and mitochondrial lipid synthesis. Biochem J. 2005;387:617–626. doi: 10.1042/BJ20041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 43.Rokitskaya TI, Sumbatyan NV, Tashlitsky VN, Korshunova GA, Antonenko YN, et al. Mitochondria-targeted penetrating cations as carriers of hydrophobic anions through lipid membranes. Biochim Biophys Acta. 2010;1798:1698–1706. doi: 10.1016/j.bbamem.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Lyamzaev KG, Pustovidko AV, Simonyan RA, Rokitskaya TI, Domnina LV, et al. Novel mitochondria-targeted antioxidants: plastoquinone conjugated with cationic plant alkaloids berberine and palmatine. Pharm Res. 2011;28:2883–2895. doi: 10.1007/s11095-011-0504-8. [DOI] [PubMed] [Google Scholar]
- 45.Digel M, Ehehalt R, Stremmel W, Fullekrug J. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol Cell Biochem. 2009;326:23–28. doi: 10.1007/s11010-008-0003-3. [DOI] [PubMed] [Google Scholar]
- 46.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta. 2010;1801:246–251. doi: 10.1016/j.bbalip.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinzawa-Itoh K, Aoyama H, Muramoto K, Terada H, Kurauchi T, et al. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosca M, Minkler P, Hoppel CL. Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta. 2011;1807:1373–1382. doi: 10.1016/j.bbabio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Schlattner U, Tokarska-Schlattner M, Ramirez S, Bruckner A, Kay L, et al. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochim Biophys Acta. 2009;1788:2032–2047. doi: 10.1016/j.bbamem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 50.Maniti O, Lecompte MF, Marcillat O, Vial C, Granjon T. Mitochondrial creatine kinase interaction with cardiolipin-containing biomimetic membranes is a two-step process involving adsorption and insertion. Eur Biophys J. 2010;39:1649–1655. doi: 10.1007/s00249-010-0600-4. [DOI] [PubMed] [Google Scholar]
- 51.Acehan D, Malhotra A, Xu Y, Ren M, Stokes DL, et al. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys J. 2011;100:2184–2192. doi: 10.1016/j.bpj.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou M, Morgner N, Barrera NP, Politis A, Isaacson SC, et al. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science. 2011;334:380–385. doi: 10.1126/science.1210148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, et al. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]