Abstract
Fertilization results from the fusion of male and female gametes in all sexually reproducing organisms. Much of nematode fertility work was focused on Caenorhabditis elegans and Ascaris suum. The C. elegans hermaphrodite produces a limited number of sperm initially, and then commits to the exclusive production of oocytes. The post-meiotic differentiation called spermiogenesis converts sessile spermatids into motile spermatozoa. The motility of spermatozoa depends on dynamic assembly and disassembly of a MSP (Major Sperm Protein)-based cytoskeleton uniquely found in nematodes. Both self-derived and male-derived spermatozoa are stored in spermatheca, the site of fertilization in hermaphrodites. The oocytes are arrested in meiotic prophase I until a sperm derived signal relieves the inhibition allowing the meiotic maturation to occur. Oocyte undergoes meiotic maturation, enters into spermatheca, gets fertilized, completes meiosis and exits into uterus as a zygote. This review focuses on our current understanding of the events around fertilization in nematodes.
Keywords: Spermatid, spermatozoa, Major Sperm Protein, spermiogenesis, meiotic maturation, oocyte
1. Introduction
Nematodes inhabit diverse ecological niche and are well adapted to survive under extreme environmental conditions. For example, the nematode Cryonema crassum lives in ice (Tchesunov and Hope, 1997) and various species of nematodes inhabits hot desert (Pen-Mouratov and Steinberger, 2005). Nematodes are found in soil, fresh water, and marine water; exist as a free living organism, parasitize plants, animals and humans (De Ley, 2006). Studying the mechanism of fertilization in nematodes will help us understand the universal features of fertilization as well as specialized features unique to nematodes. In this review we summarize our current understanding of nematode fertilization that is largely derived from studying the model organisms Caenorhabditis elegans and Ascaris suum.
Nematodes exhibit enormous diversity with respect to the mode of reproduction; some nematodes reproduce sexually, whereas others reproduce asexually. Sexually reproducing nematodes relies on the union of sperm and oocytes for generating zygote. Most of the sexually reproducing nematodes exist as male and female (gonochorism), wherein the sperm from the male are ejaculated in to the female during mating. The reproductive success of gonochoristic species thus depends on the presence of both male and female E.g. Caenorhabditis remanei, Caenorhabditis brenneri. In contrast, some of the sexually reproducing nematodes exhibit androdioecy, where in majority of the population exists as hermaphrodites and rare males. The males produce exclusively sperm whereas self-fertile hermaphrodites produce both sperm and oocytes. Examples of androdioecious nematodes include, Caenorhabditis elegans, Caenorhabditis briggsae, Oscheius myriophila (Kiontke et al., 2004). Sexual and asexual (parthenogenesis) reproduction occurs in some nematodes such as Meloidogyne hapla (Liu et al., 2007) and Strongyloides (Streit, 2008).
The hermaphrodite gonad of C. elegans is shaped like the letter `U'. For the detailed description of anatomy and development of gonad, readers are referred to several excellent papers (Hirsh et al., 1976; Hubbard and Greenstein, 2000; Klass et al., 1976; Schedl, 1997).
The sequences of events that precede the formation of gametes in hermaphrodites are specification of germ cells (Hubbard and Greenstein, 2005; Strome, 2005), mitotic proliferation of germ cells, meiotic entry of germ cells, differentiation of gametes (Kimble and Crittenden, 2005; Kimble and Crittenden, 2007). All these events are spatially and temporally coordinated in C. elegans. During the early embryonic development, posterior cells of successive division asymmetrically receive specialized granules called P granules which are chiefly composed of a cocktail of maternally supplied proteins and RNAs. One of the proteins associated with P granule, PIE-1 plays a crucial role in specifying germ cell. In pie-1 mutants, the P2 blastomere fails to acquire germ cell fate and develops like its sister cells to produce excessive pharyngeal and intestinal tissues (and hence the name pie-1 which stands for pharynx and intestine excess) (Mello et al., 1992). PIE-1 is a CCCH zinc finger proteins that represses the transcription of genes in germ cells. Ectopic expression of PIE-1 in somatic blastomeres causes the repression of mRNA accumulation in somatic cells (Seydoux et al., 1996). The germ cell precursors migrate to the distal ends of the gonad. The somatic cell located at the distal end of the gonad, called distal tip cell (DTC) promotes mitotic proliferation of germ cell precursors. Removal of DTC by laser ablation causes the germ cell progenitors to exit from mitosis and enter into meiosis (Kimble and White, 1981). During the early larval stages, the germ cells differentiate into limited number of sperm. Spermatogenesis refers to the process in which the germ cell precursors undergo two rounds of meiotic divisions and produce haploid spermatids. From L4 stage onwards, the germ cell switches to produce exclusively oocytes (Ellis and Schedl, 2007).
2. Spermiogenesis
Spermatogenesis results in the production of haploid spermatids (L'Hernault, 2006; L'Hernault, 2009; Nishimura and L'Hernault). Spermatids are round in shape, measuring about 4 μm in diameter, lack the ability to move and are incompetent to fertilize oocytes. Through a process known as sperm activation or spermiogenesis, immotile spermatids are converted into motile spermatozoa which projects pseudopod from one side of the spermatozoa (See Figure 1). Polarized formation of the pseudopod enables the spermatozoa to move by protrusion of the cytoskeletons at the leading edge and retraction near the cell body. Spermiogenesis is accompanied by fusion of Membranous Organelles (MOs) with plasma membrane, leading to the release of MO contents to the extracellular space. The overall morphology, organization of cellular organelles of spermatids and spermatozoa is similar in three closely related species, C. elegans, Caenorhabditis briggsae and Caenorhabditis remanei (Geldziler et al., 2006).
Figure 1.
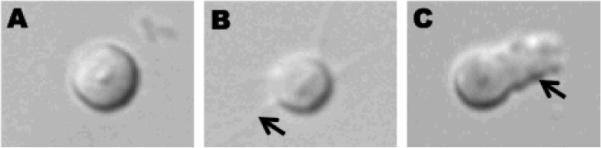
DIC images of spermatid (A), partially activated spermatid showing spikes (arrow indicates spike/filopodium in B) and fully activated spermatozoa (arrow indicates pseudopod in C).
Activation of sperm needs to be tightly controlled, both spatially and temporally. Complete failure to undergo spermiogenesis renders the resultant spermatids incompetent to fertilize oocytes, where as precocious activation of sperm in male reproductive tract thwarts their efficient transfer into hermaphrodite (Argon and Ward, 1980; Stanfield and Villeneuve, 2006). In hermaphrodites, the first ovulation pushes the spermatids into spermatheca where they get activated. Activation of most or all of the spermatids is essential in hermaphrodites, since when the zygote exits spermatheca, most of the sperm are swept away into uterus. These sperm migrate back to the spermatheca to fertilize succeeding oocytes and to do so, all motility apparatus need to be fully activated. Hence, activating almost all of the spermatids is advantageous to the reproductive success of hermaphrodites. In contrast, male derived spermatids are activated only in the uterus of hermaphrodite. The seminal fluid ejaculated along with the spermatids presumably contains sperm activator(s). Controlled sperm activation in males might offer fitness in two ways: first, being round in shape, spermatids can be densely packed within the male reproductive tract, thereby maximizing the ability of the males to sire many progeny. Second, activated spermatozoa constantly treadmills its cytoskeleton, and doing so, spends ATP. Hence, in evolutionary perspective, it would be favorable for the male to maximize the storage of quiescent form of gametes and activate them as and when needed (Singson, 2006).
2.1.Regulation of spermiogenesis in vivo
Hermaphrodites and males utilize partially different pathways to activate spermatids. This conclusion is based on the observation of “spe-8 class” mutants. The spe-8 class mutant comprises of spe-8 (L'Hernault et al., 1988; Shakes and Ward, 1989), spe-12 (L'Hernault et al., 1988; Nance et al., 1999; Shakes and Ward, 1989), spe-19 (Geldziler et al., 2005), spe-27 (Minniti et al., 1996) and spe-29 (Nance et al., 2000). The spe-8 class mutant hermaphrodites are self-sterile. Sterility is caused by inability of the mutant spermatids to undergo successful spermiogenesis in the spermatheca of hermaphrodite. Since the motility of the sperm depends on successful formation of psudopod, the mutant spermatids are incapable of moving back to spermatheca once they exit from it. Interestingly, mating spe-8 class mutant hermaphrodite with sterile males could restore self-fertility to the hermaphrodite. When the sterile males inseminate, a male derived sperm activator is released into the spe-8 class hermaphrodite, which then transactivates spe-8 class mutant spermatids into spermatozoa. This result suggests that hermaphrodite-derived factors(s) activate spermatids in a SPE-8 class proteins-dependent manner and activation by male-derived factor(s) is largely independent of the action of SPE-8 class proteins. In other words, SPE-8 class proteins are dispensable for spermiogenesis, as long as male derived factor(s) can be supplemented. Hence, the males from spe-8 class mutants are fertile and can be crossed with spe-8 class mutant hermaphrodites to give rise to progeny.
The phosphoinositide (PI) 5-phosphatase, CIL-1 is required for full activation of sperm. The cil-1 mutant spermatids do not fully extend their pseudopods in response to both in vivo and in vitro activators. As a result, the cil-1 mutant spermatozoa do not move efficiently (Bae et al., 2009).
A genetic screen for male fertility factor uncovered the essential role of protease inhibitor, swm-1 (Stanfield and Villeneuve, 2006). The seminal vesicle of wild type male contains spermatids which get activated only after their ejaculation into hermaphrodite reproductive tract. In contrast, the seminal vesicle of swm-1 males harbors several activated spermatozoa. These activated spermatozoa are not efficiently transferred by males, presumably because these ectopically activated spermatozoa are more adhesive than spermatids in male reproductive tract. The swm-1 spermatozoa from male successfully fertilize oocytes when introduced into recipient hermaphorodite by artificial insemination, suggesting that swm-1 spermatozoa are functionally active and the sterility observed in swm-1 males is due to sperm-transfer defect. Consistent with this idea, self-fertility is uncompromised in swm-1 hermaphrodites, since the hermaphrodite do not depend on sperm-transfer from extraneous source for its self-fertility. SWM-1 is predicted to function as a secreted serine protease inhibitor. Unknown proteases(s) are capable of activating spermatids in male present in the seminal vesicles. SWM-1 binds and inhibits these protease(s), to prevent ectopic activation of spermatids in males (Stanfield and Villeneuve, 2006).
The casein kinase I homolog, SPE-6 regulate spermiogenesis in response to male derived sperm-activation signals as well as hermaphrodite derived activation signals. A small proportion of spermatids from spe-6 males undergo precocious activation, suggesting that normal function of SPE-6 is to negatively regulate spermiogenesis machinery. Furthermore, mutation in spe-6 suppresses all the spe-8 class mutants (Muhlrad and Ward, 2002), (Geldziler et al., 2005) which suggests the following model: in the absence of sperm activation signal, SPE-6 negatively regulates spermiogeneis by phosphorylating one or more key proteins that in turn inhibit spermiogenesis. Exposure of sperm-activation signal(s) inhibits SPE-6 through SPE-8 class proteins. Inhibition of SPE-6 relieves its negative regulation over spermiogenesis machinery, thereby promotes the activation of sperm.
2.2. In vitro activation of sperm
Currently the molecular identities of in vivo activator(s) of spermiogenesis are not known. However, spermatids can be activated to spermatozoa by treatment with various pharmacological agents. Ascaris spermatids were initially activated in vitro by addition of vas deference extract or protease (Abbas and Cain, 1979). C. elegans spermatids can be activated by treatment with Monensin (Nelson and Ward, 1980), Triethanolamine(TEA), pronase (Ward et al., 1983), chloride channel inhibitors (Machaca et al., 1996), or calmodulin inhibitors (Shakes and Ward, 1989). Artificial insemination of TEA-activated spermatozoa can successfully fertilize oocyte (LaMunyon and Ward, 1994), suggesting that in vitro activated spermatozoa is functionally active. Not surprisingly, pronase activated spermatozoa do not fertilize oocyte, as the protease exposure is expected to cleave several cell surface proteins participating in fertilization. The precise mechanism of how these various activators induce spermiogenesis is unclear. The likely mechanism of sperm activation by ionophore monensin and weak acid TEA might be due to the increase of intracellular pH. Sperm activation is accompanied by rapid increase in intracellular pH which in turn favors MSP(Major Sperm Protein) fiber assembly (King et al., 1994). Fully activated spermatozoa exhibits a gradient intracellular pH in which the pseudopodal end has higher intracellular pH, the locus where MSP assembly takes place.
2.3. Formation of filopodia and psudopod
The morphological changes accompanying spermiogenesis are similar after treating the C. elegans spermatids with either monensin (Nelson and Ward, 1980) or TEA (Shakes and Ward, 1989). Within a few minutes spikes starts appearing which eventually coalesce to form pseodopod. Similarly, spermatids from C. briggsae and C. remanei can also be activated by pronase treatment in vitro (Geldziler et al., 2006). Treatment of Ascaris spermatids with vas deference extract causes the extension of spikes or filopodia within 30 seconds (Miao et al., 2007). The spikes or filopodia appears to represent intermediate stage of the sperm activation. Like C. elegans, extension of filopodia is immediately succeeded by the formation of pseudopod in Ascaris as well. Miao et al. (2007) reconstituted filopodial growth in vitro and analyzed their dynamics and ultra structure. Electron microscopy of in vitro reconstituted filopodia revealed that the MSP fibers are bundled together to form a filament and the tip of the filaments are covered with membrane envelope. The filopodia grows faster than the MSP fibers that are eventually formed in mature spermatozoa. Filopodia and MSP fibers share many common features. Several proteins found in MSP fibers, such as MFP1, MFP2, MFP3 (See sperm motility) are also found in filopodia; agents that interfere with fiber growth likewise affects filopodia assembly (Miao et al., 2007).
3. Sperm motility
Our current understanding of the mechanism of sperm motility is largely derived from the studies of sperm from Ascaris suum. Large number of sperm can be isolated from this parasitic worm which makes it feasible to purify proteins and perform variety of sophisticated biochemical analysis (L'Hernault and Roberts, 1995). The amoeboid sperm moves by protrusion at its leading edge and retraction near the cell body. Close examination of a motile spermatozoon shows that bundle of filaments are progressively formed at the leading edge while the filaments are taken apart near the cell body (Miao et al., 2003).
The nematode motility apparatus is composed of Major Sperm Protein (MSP), which occupies 40% of the cytosolic protein in sperm. The three dimensional structure of MSP has been solved by X-ray crystallography (Bullock et al., 1996a; Bullock et al., 1996b) and NMR method (Haaf et al., 1996). MSP is made of seven strands of beta pleated sheath, which collectively resembles immunoglobulin-like fold. MSP occurs as a stable dimer in solution at various pH. Investigation of purified MSP using gel filration, NMR and sedimentation equilibrium ultracentrifugation suggest that MSP occurs as a dimer. The three dimensional structure of C. elegans MSP exhibit very similar folding pattern (Baker et al., 2002). Yeast two-hybrid analysis confirmed the dimerization of C. elegans MSP and further investigation of mutagenized library of MSP revealed the importance of several critical residues that participate in dimerization of MSP (Smith and Ward, 1998). The symmetric dimers of MSP are the building blocks of MSP fibers which polymerizes into higher order structure (Baker et al., 2002).
3.1. In vitro reconstitution of motility apparatus
Italiano et al. (1996) reconstituted the motile apparatus of Ascaris sperm in vitro. Italiano et al. isolated spermatids from Ascaris suum, activated the spermatids into spermatozoa; lysed spermatozoa and centrifuged to get a fraction called S100. Addition of ATP to this S100 fraction was sufficient enough to assemble and elongate the MSP fiber in vitro. Examination of the in vitro assembled fiber reveals the presence of two components: the leading edge has vesicle, followed by an elongating fiber. The vesicle is derived from the leading edge of the pseudopod, since an anti-phospho tyrosin antibody that stains the leading edge of pseudopods stains these vesicles as well. Apparently the vesicles are turned “inside out”, such that the outer part of the vesicle reconstituted in vitro corresponds to the inner side of the plasma membrane that is normally found in vivo. Hence the ability of the growing fibers to push the vesicle recapitulates protrusion of the pseudopod at the leading edge. The fiber is composed of higher order structure of MSP polymer. The S100 can be further centrifuged to get two sub-fractions: vesicular and cytosolic fraction. Both fractions are necessary to reconstitute motility in vitro. A research group led by Thomas Roberts uncovered the identity of several proteins that function in vesicle or cytosol to influence fiber assembly. MPOP seems to be the only component present in the vesicle that influences MSP nucleation. MFP1, MFP2 and MPAK are the hitherto identified cytosolic proteins that regulate MSP assembly and/or integrity.
3.2. Proteins influencing the assembly of MSP fiber
A 48 kDa membrane protein (p48 or MPOP) was identified as a vesicular component that initiates the assembly of MSP fibers (LeClaire et al., 2003). Several lines of evidences suggest that phospho-MPOP (MSP Polymerization Organizing Protein) is involved in the assembly of MSP fibers. First, anti-phosphotyrosin antibody stains the leading edge of the pseudopod and the vesicle of MSP fibers grown in vitro, the loci where the assembly of MSP takes place. Second, treatment of tyrosin phosphatase abolished the ability of the vesicle to support fiber growth. Third, supplementing phospho-MPOP that is immunoprecipitated from vesicle with cytosol and ATP is sufficient to induce fiber formation in vitro. Phosphorylation of MPOP appears to be mediated by a yet unidentified tyrosin kinase. Only upon the incubation of cytosol that MPOP could be phosphorylated, precluding the possibility that MPOP could be an auto-phosphorylated proein. Unfortunately, the gene encoding MPOP is not yet cloned, owing to the practical difficulties. Identifying MPOP would shed more light on the mechanism of MSP nucleation at the leading edge.
The MPOP is distributed throughout the plasma membrane, as judged by staining the sperm with anti-MPOP antibody. An unidentified tyrosin kinase phosphorylates MPOP at the leading edge. Apparently, this tyrosin kinase is sensitive to change in the pH, as phosphorylation of MPOP is abolished at lower pH and promoted at higher pH. The pH dependent phosphorylation of MPOP has an important correlation: MSP polymerizes and depolymerizes at relatively higher and lower pH, respectively (King et al., 1994). Furthermore, cytoplasms of spermatozoa establish a pH gradient such that the leading edge of the pseudopod has higher pH than the base of the pseudopod. The MPOP is localized throughout the plasma membrane of sperm. Apparently, the higher pH at leading edge activates an unidentified tyrosin kinase, which in turn selectively phosphorylates MPOP located at the leading edge of the pseudopod (LeClaire et al., 2003). Phospho-MPOP recruits a protein kinase, MPAK (MSP polymerization-activation Kinase) at the leading edge of the pseudopod to facilitate the nucleation of MSP fibers. Several evidences support the role of MPAK in MSP assembly (Yi et al., 2007). First, MPAK is localized to the periphery of the vesicle from which MSP fiber grows in vitro. Second, addition of anti-MPAK abolishes the in vitro assembly of MSP fiber from S100 supplemented with ATP. Third, selective depletion of MPAK from S100 rendered the resultant fraction refractory to support in vitro fiber growth. Addition of purified MPAK back to the MPAK-depleted S100 fraction restored its ability to support fiber growth. Several lines of evidences indicate that Phospho-MPOP physically interacts with MPAK (Yi et al., 2007). First, native gel electrophoresis shows the presence of phospho-MPOP and MPAK complex. Second, phospho-MPOP can be detected from the proteins immunoprecipitated by anti-MPAK antibody and vice versa. Third, the vesicles from in vitro grown fibers contain both phospho-MPOP and MPAK. Finally, the dephosphorylated form of MPOP fails to bind with MPAK. One of the substrates of MPAK has been identified as MFP2 (MSP fiber protein 2). Purified MPAK incorporates radio labeled phosphate group to purified MFP2 in vitro. MPAK phosphorylates threonine residue(s) of MFP2. Phosphorylated form of MFP2, and not unphosphorylated MFP2, can bind with MSP fibers (see figure 2). MFP2 is found on the MSP fiber complexes in sperm and in vitro grown fibers (Buttery et al., 2003). Addition of MFP2 purified from sperm and bacterially expressed MFP2 can increase the rate of fiber formation in vitro (Buttery et al., 2003; Grant et al., 2005). X-ray crystallography of MFP2 reveals that the protein is composed of two domains and each domain is composed of three sub-domains (Grant et al., 2005).
Figure 2.
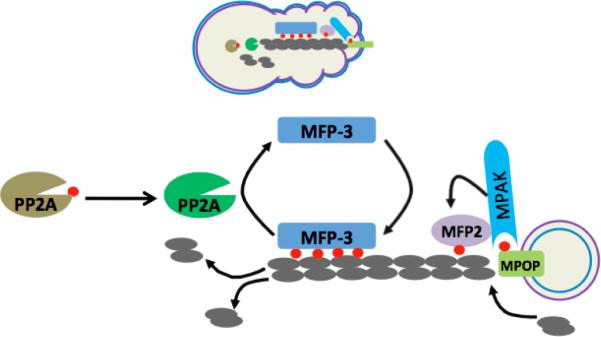
Schematic diagram depicting the motility machinery of Ascaris sperm. (Top) Cartoon of spermatozoan shows the in vivo location of the proteins participating in the assembly and disassembly of MSP fibers. The outer and inner leaflets of plasma membrane are represented as blue line and violet line, respectively. (Bottom) The MSP dimer (grey) constitutes the building block of MSP fiber. The MSP fiber grown in vitro is composed of two parts: vesicular part and fibrous part. Note that the plasma membrane of the vesicle that supports the assembly of MSP fiber is turned `inside out'. Unknown tyrosin kinase activates MPOP at the leading edge. Phospho-MPOP recruits MPAK which in turn phosphorylates MFP2. Phospho-MFP2 increases the rate of MSP fiber assembly. Unknown kinase phosphorylates cytosolic protein, MFP3 at multiple sites. Phospho-MFP3 binds with MFP3 to stabilize MSP fibers. The Ser/Thr phosphatase, PP2A is localized near the cell body which gets activated upon dephosphorylation by unknown phosphatase. Active PP2A dephosphorylates phospho-MFP3 and dephosphorylated MFP3 detaches from MSP fibers, thereby favoring the disassembly of MSP fibers near the cell body. Phospho group appended to the proteins are depicted as small red circle.
MFP1 is another protein present in the cytosolic fraction of S100 which negatively regulates fiber elongation. Addition of purified MFP1 to ATP supplemented S100 decreases the rate of fiber elongation in vitro (Buttery et al., 2003).
3.3. Proteins influencing the disassembly of MSP fibers
Motility of pseudopod depends on two forces: MSP filaments are polymerized and bundled at the leading edge, while the opposite end of MSP filaments- the one near the cell body- gets dissembled. Perfusion of MSP fibers assembled in vitro with tyrosin phosphatase elicited two effects on the fiber; the fiber stopped growing at the leading edge and started shrinking from the opposite end (Miao et al., 2003). The former effect is attributed to the dephosphorylation of MPOP which in its dephosphorylated form no longer able to support the nucleation of MSP fibers (LeClaire et al., 2003). The later effect - shrinking of fibers from the opposite end - suggests the involvement of a tyrosin phosphatase in actively retracting MSP filaments. Retraction of fibers is a reversible process; removing the tyrosing phosphatase followed by reperfusion of S100 and ATP enabled fiber growth once again. Retraction is accompanied by decrement in optical density of the fiber. Electron microscopy of retracted segment of the fiber exhibit decreased fiber diameter and filament density (Miao et al., 2003). In order to identify the endogenous tyrosin phosphatase responsible for the retraction of MSP fibers, Yi et al. (2009) screened for a variety of phosphatase inhibitors to block the effect of exogenously added commercial preparation of tyrosin phosphatase (YOP) and found that addition of PP2A inhibitors, such as calyculin A and okadaic acid, prevented the retraction of fibers in vitro (Yi et al., 2009). Several lines of evidences support that Ser/Thr phosphatase, PP2A plays a role in fiber retraction. First, commercial preparations of PP2A can induce retraction of fibers in vitro. Second, some preparation of S100, so called retraction-competent S100 (RC S100), underwent spontaneous retraction, which can be prevented by concomitant addition of a PP2A specific inhibitor, okadaic acid. Third, depletion of PP2A from the S100 fraction dramatically reduced retraction rate and adding back the PP2A to PP2A depleted S100 restored the retraction rate. Fourth, PP2A is localized near cell body, the locus where retraction occurs. The PP2A is found in two forms: phosphorylated and dephosphorylated form. The dephosphorylated form of PP2A seems to regulate the retraction reaction. The batch of S100 that underwent spontaneous retraction contained dephosphorylated form of PP2A where as the batch of S100 that didn't undergo spontaneous retraction contained phosphorylated PP2A. Retraction of fibers triggered by YOP is accompanied by dephosphorylation of PP2A. Collectively, an unidentified tyrosin phosphatase dephosphorylates and thereby activates PP2A. Activated PP2A dephosphorylates target protein(s) to facilitate retraction of fibers. In order to identify the substrate of PP2A, Yi et al incubated the fibers in the presence or absence of PP2A and isolated the protein band that failed to stain with anti-threonine antibody in PP2A-treated sample. Sequencing the resultant band enabled the identification of MFP3 as one of the substrates of PP2A. Immunofluorescence microscopy revealed that MFP3 is localized to the MSP fibers in vivo and in vitro. Addition of PP2A dephosphorylates MFP3 in a time dependent manner and the dephosphorylation is accompanied by detachment of MFP3 from the fiber. Concomitant inclusion of PP2A inhibitor stabilizes phospho-MFP3 and its association with fiber. Taken together, MFP3 is phosphorylated at threonine residue(s) by an unknown Ser/Thr kinase. Phospho-MFP3 binds with MSP fibers to stabilize the bundles. PP2A is activated near the cell body which then dephosphorylates MFP3. Dephosphorylated MFP3 lose its affinity with MFP fibers, leading to the destabilization of MSP fibers and eventual dissociation of MSP dimers from the fibers (See figure 2).
3.4. Potential regulators of sperm motility in C. elegans
Reconstruction of in vitro motility machinery enabled the identification of several key factors orchestrating protrusion and retraction of MSP bundles at leading edge and cell body of the Ascaris sperm. While Ascaris offers the luxury of sophisticated biochemical analysis, only limited genetic analysis can be performed on this organism. Interestingly, most of the proteins identified in Ascaris, have homologs in C. elegans. Hence, studying the C. elegans homologs of these proteins would complement and advance our understanding of the motility of nematode sperm. Table 1 summarizes the list of proteins hitherto identified in Ascaris suum that regulate the motility of sperm and their counterpart in C. elegans. Most of the homologs of Ascaris motility machinery are expected to execute similar function in C. elegans. The overall structures - and therefore functions of two proteins - with more than 40% identity are expected to be similar (Lesk, 2001). Ascaris MPAK and its C. elegans homologs share 67% identity along the entire length and exhibit highly conserved kinase domain, increasing the likelihood that C. elegans MPAK might function analogous to Ascaris MPAK to facilitate the assembly of MSP fibers. The C. elegans MPAK homologs encoded by C39H7.1 and Y38H8A.3 share 99% identity and 100% similarity with each other, raising a possibility that these proteins might function redundantly in C. elegans. Interestingly the transcripts of C39H7.1 and Y38H8A.3 were previously shown to be enriched during spermatogenesis (Reinke et al., 2000) and the corresponding proteins were identified as abundantly enriched during spermatogenesis (Chu et al., 2006). C39H7.1 is located in fourth chromosome at 1.97cM, one of the three regions in C. elegans genome where spermatogenesis-genes are clustered together in a non-random manner (Miller et al., 2004). MSD-4 is the C. elegans homolog of MFP1 that contains MSP domain. Several proteins containing MSP domains, including MFP1/MSD-4 are expressed at higher level in male germ line of Ascaris and C. elegans (Tarr and Scott, 2004).
Table 1.
Summary of the list of proteins that regulate MSP bundle assembly and disassembly in Ascaris suum and their C. elegans homologs
| Ascaris proteins | Function | C. elegans homolog(s) | % of identity (%of similarity) |
|---|---|---|---|
| Tyrosin Kinase* | Phosphorylates and activates MPOP | ||
| MPOP* | Nucleates MSP filaments at leading edge | ||
| MPAK (Casein Kinase) | Binds with phospho-MPOP, phosphorylates MFP2 | C39H7.1 and Y38H8A.3 | 67% |
| MFP1 | Decreases rate of fiber formation | MSD-4 SSP-9 to SSP-32 | 47–51% (64–65%) |
| MFP2 | Increases rate of fiber formation | ZK265.3 | 52% (67%) |
| Tyrosin Kinase* | Phosphorylates and inhibits PP2A | ||
| Tyrosin Phosphatase* | Dephosphorylates and activates PP2A | ||
| Ser/Thr Kinase* | Phosphorylates MFP3 | ||
| MFP3 | Phospho-MFP3 binds with MSP filaments and stabilizes it. Dephosphorylated MFP3 detaches from fibers. | SSQ-1 to SSQ-4 | |
| PP2A | Dephosphorylates MFP3 | LET-92 |
Identity of the proteins not known
4. Directed migration of sperm to spermatheca
The sperm ejaculated by males must travel through uterus and enter into spermatheca. While the motility of the sperm depends on protrusion and retraction of pseudopod, the navigation of sperm to the right direction – to spermatheca – relies on the cues from oocytes (Kubagawa et al., 2006). In the mutants that do not produce any oocyte, the sperm inseminated from a wild type male do not reach spermatheca. The active components in the C. elegans oocyte that attract sperm were identified as Poly Unsaturated Fatty Acids (PUFA). The PUFA synthesis is mediated by fat genes in the intestine and in fat-2 mutant hermaphrodites, the sperm ejaculated from wild type male fails to reach spermatheca (Kubagawa et al., 2006). Transportation of PUFAs from intestine to oocyte is mediated through RME-2, an LDL receptor present on oocyte that uptakes yolk by endocytosis (See figure(Edmonds et al., 2010) 3). The rme-2 null mutant hermaphrodite exhibited severe sperm movement defect. The PUFAs are further converted into eicosanoids by variety of enzymes. Knock down of the genes enriched in oocytes that are predicted to play a role in lipid metabolism or lipid transport caused sperm migratory defect. Recent report suggests that PUFAs are converted into F-series prostaglandins in the oocytes (Edmonds et al., 2010). The F-series prostaglandins are implicated in guiding the sperm to spermatheca (Edmonds et al., 2010). The other species of Caenorhabditis exhibit similar spemathecal targeting when mated with con-specific male (Hill and L'Hernault, 2001). Even in most of the heterospecific crosses, sperm from the males of one species can successfully migrate in to the spermatheca of other species. Thus, the signal(s) that attract sperm to spermatheca must be conserved across the genus Caenorhabditis (Hill and L'Hernault, 2001). Given the identification of PUFA as a sperm attractant in C. elegans, it is tempting to speculate that the genus Caenorhabditis utilizes PUFA to direct male derived sperm into spermatheca. Synthesis of eicosanoids has been reported from other nematodes as well. For example, the GST (Glutathione-S-Transferase) of Oesophagostomum dentatum is capable of converting Prostaglandin H2 to Prostaglandin G2 (Joachim and Ruttkowski); filarial worm, Onchocerca volvulus encodes prostaglandin D synthase (Perbandt et al., 2008). Oocytes of Onchocerca volvulus are strongly labeled with prostaglandin E2 (Brattig et al., 2006). Whether eicosanoid produced by other nematodes functions to attract sperm needs further investigation.
Figure 3.
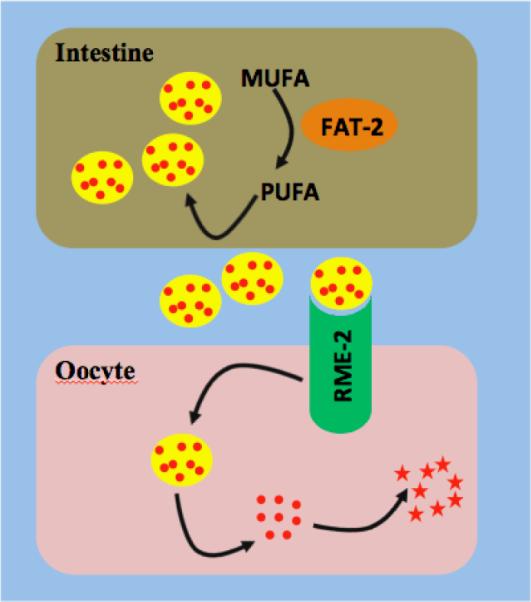
Cartoon depicting the pathway of sperm attractant production in C. elegans Dietary Mono Unsaturated Fatty Acids (MUFA) are converted into Poly Unsaturated Fatty Acid (PUFA) through a fat metabolic pathway in which FAT-2 plays an important role. PUFA(Red dots) are incorporated into yolk (yellow circle). The yolk enters into psedocoelom and reaches the gonad, where LDL receptor RME-2 mediates endocytosis of yolk. PUFAs are further metamolised to form a `sperm attractant' (Red stars) which promotes the directional migration of sperm to spermatheca.
5. Meiotic maturation of oocytes
5.1. MSP triggers oocyte maturation
Oocytes are arrested in meiotic prophase I in many animals, including C. elegans. The resumption of meiosis depends on the cues from sperm. In a mutant worms that do not produce any sperm, the oocytes are arrested for prolonged period in meiotic prophase I. When the sperm are supplied to these sperm less worms through males, the oocytes resume meiosis and undergo normal meiotic maturation. What component in the sperm triggers meiotic maturation in oocytes? Towards answering this question, Miller et al. performed a series of experiments and found that MSP (Major Sperm Protein) is the sperm derived factor that triggers meiotic maturation in oocytes (Miller et al., 2001). First, Miller et al. let the isolated sperm stay in a buffer for few hours and injected the cell-free supernatant (sperm-conditioned medium) into the uterus of female worms. The female worms supplemented with sperm-conditioned medium underwent normal meiotic maturation. Prior incubation of the sperm-conditioned medium with protease abolished its ability to trigger meiotic maturation and gave them the clue that the active component is protein. They then fractionated sperm-conditioned medium by HPLC and analyzed a fraction that retained its activity by MALDI-TOF mass spectrometry and identity of the active component was revealed as MSP. Two lines of evidences suggest that MSP is indeed the sperm-derived factor that triggers oocyte maturation (Miller et al., 2001): First, injection of anti-MSP antibody reduced ovulation rate in wild type worms. Second, injection of recombinant MSP can restore meiotic maturation in females.
5.2. Secretion of MSP
The finding that MSP as a signaling protein emanating from sperm posed an important question: How MSP is secreted? When C. elegans spermatocyte develops and differentiate into spermatozoa, most of the cellular organelles such as ER, golgi apparatus are discarded in to a residual body and hence C. elegans sperm is free from any secretory organelle. Besides, MSP is a cytoplasmic protein and apparently doesn't have any secretory signal. Transmission electron microscopy of adult hermaphrodite revealed the presence of novel vesicles, measuring 150–300nm, in the extracellular space of proximal gonad (Kosinski et al., 2005). Electron microscopy of the serial section of these vesicles showed that they are made of two concentric lipid bilayers and when probed with anti-MSP antibody, presence of MSP could be detected in these vesicles. The MSP containing vesicles arise from sperm by an unconventional budding process. The vesicle budding seems to be regulated by cues from hermaphrodites, because the male spermatids do not spontaneously form MSP vesicles, but can be induced to form MSP vesicles by adding female extract (Kosinski et al., 2005). Biochemical fractionation followed by identification of the active component(s) in the female extract that promote budding of male sperm-derived MSP vesicles in vitro would help us better understand the identity of the cue(s). Alternatively genome wide RNAi screens can be carried out to find the gene products essential for budding of MSP vesicles. The existence of MSP vesicles could only be detected by high pressure freezing and freeze substitution microscopy, not by conventional electron microscopy. Ultrarapid freezing step employed in the former technique instantaneously fixes all molecules in their close-to-native form within millisecond (McDonald and Auer, 2006). Inability to detect MSP vesicles through conventional electron microscopy led to the suggestion that MSP vesicles might form an instable and labile structure. Rupture of these delicate vesicles might release MSP for binding with its target receptor(s).
5.3. MSP binds with VAB-1 to promote meiotic maturation
Through a series of calculated guesses and experiments, Miller et al. identified that the Eperin receptor, VAB-1 is one of the MSP receptors (Miller et al., 2003). First, fluorescently labeled, purified MSP can specifically bind on the wild type oocyte. The intensity of the fluorescence is dramatically reduced when probed on the vab-1 null mutant oocyte. Second, COS-7 cells transfected with VAB-1, but not the control protein, can bind with purified MSP. Finally, ectodomain of VAB-1 receptor can bind with purified MSP in vitro (Govindan et al., 2006). In addition to VAB-1, there must be other, yet unidentified protein(s), which serve as a MSP receptor(s), as the labeled MSP still binds with vab-1 null mutant oocyte and vab-1 mutants do respond to MSP. VAB-1 is an epherin receptor. C. elegans genome encodes four eherins, EFN-1/VAB-2, EFN-2, EFN-3 and EFN-4. Among these four epherins, EFN-2 binds with VAB-1 in the absence of MSP and negatively regulates meiotic maturation of oocytes. Purified EFN-2 protein can physically interact with VAB-1 in vitro (Wang et al., 1999). The source of EFN-2 seems to be germ cells, since knocking down the efn-2 selectively in the germ line is sufficient enough to increase ovulation rate (Miller et al., 2003). MSP regulates the trafficking of its own receptor, VAB-1. Absence of sperm in the oviduct leads to the sequestration of VAB-1 receptors primarily into recycling endosomes. Injection of MSP is sufficient to exclude the VAB-1 localization from recycling compartment (Cheng et al., 2008). Meiotic maturation in response to MSP signal is controlled, at least, in part by such regulated trafficking of VAB-1. Epherin homologs from mammals are known to be associated with membrane through glycosyl phosphatidyl inositol (GPI)-anchors. All epherins from C. elegans contain putative GPI-modification signals in their C-termini (Wang et al., 1999) and EFN-1/VAB-2 has been experimentally proven to be a GPI-anchored protein (Chin-Sang et al., 1999). We hypothesize that like other epherins, EFN-2 might also be GPI-anchored on the oocyte membrane and might bind with adjacent VAB-1 receptor to actively repress meiotic maturation in the absence of MSP. It would be interesting to validate whether EFN-2 is GPI-anchored as well. Future research is needed to address how MSP inhibits VAB-1 signal mediated by EFN-2 binding. Does MSP compete with EFN-2 to bind with VAB-1 receptor? Or, is the mode of inhibition non-competitive one?
5.4. MSP activates MAP Kinase
Activation of MAP Kinase pathway is one of the hallmarks of meiotic maturation in C. elegans (Miller et al., 2001) and possibly in other nematodes as well (Heger et al., 2010). Activated form of MAP Kinase can be detected only in the proximal oocytes that receive signal from sperm, and not in the mutants that lack sperm which consequently fail to undergo meiotic maturation (Miller et al., 2001). Activation of MAP Kinase and subsequent meiotic maturation depends on the signal from MSP. Injection of purified MSP is sufficient to activate MAP Kinase and promote oocyte maturation in mutant females. MSP induced MAP kinase activation is mediated through a tyrosine phosphatase, PTP-2 (Yang et al., 2010). In ptp-2 mutant hermaphrodite, barely any activated form of MAPKinase was detected in proximal most oocyte. Furthermore, unlike wild-type females, feminized ptp-2 mutant females fail to undergo meiotic maturation in response to the injection of purified MSP.
6. Sperm-oocyte interaction and fertilization
Successful fertilization requires that the sperm need to recognize, bind and eventually fuse with oocyte. The sperm need to specifically bind with oocyte, and not abortively with number of other somatic cells that it encounters in the reproductive tract. Such precise recognition of sperm-oocyte pair, often in a species specific manner, is achieved at molecular level through the interaction between ligands and their cognate receptors present on the surface of gametes.
The “SPE-9 class proteins” are sperm proteins that participate in sperm-oocyte interactions. Hitherto identified SPE-9 class proteins include SPE-9 (Singson et al., 1998), SPE-38 (Chatterjee et al., 2005), SPE-41/TRP-3 (Xu and Sternberg, 2003) and SPE-42 (Kroft et al., 2005). Mutation in all spe-9 class genes exhibits very similar phenotype; they all produce spermatids which undergo perfect spermiogenesis, extend pseudopod, navigate to the spermatheca, come in physical contact with oocyte, but fail to fertilize oocyte. The morphologies of the spermatozoa from spe-9 class mutants are indistinguishable from wildtype spermatozoa. The spe-9 is predicted to encode a single-pass transmembrane protein with N-terminal large extracellular domain and short cytoplasmic tail. The prediction was validated by using antibody raised against an epitope that is part of putative extracellular domain. Spermatids could be stained with such antibody without having to permeabilize the membrane, corroborating in silico prediction that SPE-9 is a cell surface protein (Zannoni et al., 2003). The extracellular domain of SPE-9 contains ten epidermal growth factor (EGF) repeats. Two main observations corroborate the idea that SPE-9 functions as a signal transducer, rather than as an adhesive molecule, and it is the impairment in adhesion and/or fusion with oocyte that causes sterility in spe-9 mutants (Putiri et al., 2004). First, introduction of transgene into spe-9 mutants that encodes truncated form of SPE-9 lacking cytoplasmic tail is sufficient enough to rescue the mutants, suggesting that the extracellular domain confers fertility. Second, introduction of transgene encoding extracellular domain of SPE-9 in secreted form does not rescue spe-9 mutants, indicating that SPE-9 need to be anchored to the membrane. Third, expression of very small amount of SPE-9 could efficiently rescue spe-9 mutants. The localization of SPE-9 is dynamically altered during the progression from spermatids to spermatozoa. In spermatids, SPE-9 is localized uniformly distributed throughout the membrane. Following activation, SPE-9 is redistributed primarily to pseudopod (Zannoni et al., 2003).
SPE-38 and SPE-41/TRP-3 exhibit very similar pattern of redistribution following sperm activation. SPE-38 is a novel four pass trans-membrane protein apparently restricted to nematodes (Chatterjee et al., 2005); SPE-41/TRP-3 (Xu and Sternberg, 2003) is a one of the members of canonical transient receptor potential channel in C. elegans. In spermatids, the localization of SPE-38 and SPE-41/TRP-3 are restricted to membranous organelle. As the spermatids differentiate into spermatozoa, both of these proteins are redistributed to the cell surface. The SPE-41 is distributed all over the plasma membrane, whereas the localization of SPE-38 is confined to pseudopod. The redistribution of SPE-41/TRP-3 from MO to pseudopod requires SPE-38, but not vice versa. Furthermore, these two proteins can physically interact with each other (Singaravelu, G, Chatterjee I, Rahimi, S, Druzhinina MK, Singson, A, unpublished results).
EGG-1 and EGG-2 are single pass transmembrane proteins containing Low Density Lipoprotein (LDL) receptor repeats. EGG-1 and EGG-2 are present on the oocytes plasma and are 67% identical at amino acid level, suggesting that these two proteins may be functionally redundant. Owing to the high percent of similarity, targeting egg-1 by RNAi knocks down both egg-1 and egg-2. The oocytes of the hermaphrodites treated with egg-1 RNAi appear morphologically normal, enter into spermatheca, contact sperm but fail to undergo fertilization (Kadandale et al., 2005; Lee and Schedl, 2001; Maeda et al., 2001). Based on the knock out and/or knock down phenotype, localization to the oocyte mebrane and structural resemblance with other proteins that is known to mediate cell-cell interaction, the EGG-1/EGG-2 proteins are more likely to participate in sperm-oocyte interaction.
Identifying the sperm protein(s) that bind with EGG-1/EGG-2 and the oocyte proteins that bind with SPE-9 group proteins would shed more light on the mechanism of sperm-oocyte interaction at molecular level.
7. Activation of egg and oocyte-to-embryo transition
Fertilization triggers egg activation which is characterized by completion of meiotic division, extrusion of polar bodies, and block to polyspermy followed by egg shell formation to encase developing embryo. Activation of egg requires both paternal and maternal components.
7.1. Paternal factors influencing oocyte-to-embryo transition
Sperm delivers a haploid nucleus, a pair of centrioles and few proteins to the oocyte during fertilization. The haploid nuclei from sperm and oocytes unite together to form diploid zygote. The centrosome is required for the formation of mitotic spindle. Centrosomes are selectively eliminated during oogenesis (Kim and Roy, 2006) which ensures that the mitotic division will occur only after fertilization.
Mutation in emb-27 and emb-30 genes causes chromosome segregation defect leading to the formation of anucleate meiotic products. Sadler and Shakes (2000) utilized emb-27 and emb-30 mutant males as a source of anuleate sperm and crossed with feminized worms to understand the role of nucleus in fertilization. Interestingly, spermatids devoid of nucleus can successfully undergo spermiogenesis, crawl, fertilize oocyte and establish anterior-posterior polarity, suggesting that sperm nucleus is not required to carry out any of these functions (Sadler and Shakes, 2000). Not surprisingly, the oocytes fertilized by anucleate sperm are arrested as embryo, indicating that the nucleus supplied by sperm is important for the development.
SPE-11 is a novel protein present throughout the development of C. elegans sperm which is delivered from sperm to oocyte upon fertilization (Browning and Strome, 1996; Hill et al., 1989). The spe-11 mutants can successfully fertilize oocyte. However, many processes are impaired following fertilization such as completion of meiosis, formation of egg shell, orientation of mitotic spindle. Crossing spe-11 hermaphrodite with wildtype male can fully rescue the spe-11 phenotype, suggesting that SPE-11 is a paternally supplied factor that acts following fertilization. Interestingly, ectopic expression of SPE-11 in oocyte is sufficient enough to rescue spe-11 mutants, indicating that regardless of the source, SPE-11 supplied to the oocyte is sufficient to activate oocyte.
Point of sperm entry specifies posterior axis in C. elegans (Goldstein and Hird, 1996). Anterior-posterior polarity is established soon after fertilization and is accompanied by asymmetric partitioning of several proteins and RNAs within the cytoplasm of egg. Asymmetric partitioning of proteins and RNAs are partly achieved by differential contractility of actomyosin network. CYK-4 is a paternally supplied protein that can be detected near the site of sperm fusion in the oocyte following fertilization. CYK-4 is a Rho GTPase-activating protein that establishes anterior-posterior polarity, at least partly by down-regulating actomyosin network near the site of its delivery, thereby generating a gradient of actomyosin network (Jenkins et al., 2006).
7.2. Maternal factors influencing oocyte-to-embryo transition
CHS-1 (Zhang et al., 2005), EGG-3 (Maruyama et al., 2007), EGG-4 and EGG-5 (Parry et al., 2009) are essential for activating egg following fertilization. EGG-4 and EGG-5 are 99% identical and functions redundantly. Although fertilization occurs normally in the oocytes depleted of chs-1, egg-3 or egg-4/egg-5, egg activation is impaired. Extrusion of polar body, formation of egg shell and polarized dispersal of F-action – the events associated with egg activation – are impaired in egg-3 mutants and egg-4/egg-5 RNAi worms. CHS-1 catalyzes the polymerization of UDP-N-acetyl-glucosamine to synthesize chitin (Zhang et al., 2005). EGG-3, EGG-4 and EGG-5 belongs to Protein Tyrosin Phosphatase-like protein family, which lacks critical amino acid at active center (Cheng et al., 2009; Maruyama et al., 2007; Parry et al., 2009; Stitzel et al., 2007). Hence these “pseudophosphatases” bind with specific phosphoproteins and protect them from phosphatases. EGG-3 and CHS-1 proteins are localized to the plasma membrane/cortex of oocyte (Maruyama et al., 2007). Depletion of EGG-3 drives CHS-1 to cytoplasm and vice versa, suggesting that locatization of EGG-3 and CHS-1 is interdependent. EGG-4/5 and MBK-2 are localized to the cortex in EGG-3 dependent manner (Cheng et al., 2009; Parry et al., 2009). MBK-2 is a homolog of MiniBrain-Kinase that regulates at least five proteins involved in oocyte-to-embryo transition: MEI-1, OMA-1, OMA-2, MEX-5 and MEX-6. MBK-2 is activated by autophosphorylation of tyrosin residue located in `activation loop'. In oocytes, EGG-3 anchors MBK-2 to the cortex, preventing the access of MBK-2 to its substrates (see figure 4). EGG-3 simultaneously binds with EGG-4/5 and brings it to the proximity of MBK-2 (Cheng et al., 2009; Parry et al., 2009). Being a psudophosphase, EGG-4/5 binds with activated MBK-2 and inhibits it. Following the degradation of EGG-3 and possibly EGG-4/5, MBK-2 is released from cortex and phosphorylates multiple proteins to execute oocyte-to-embryo transition. The transition of oocytes (where meiotic division is completed) into embryo (where mitotic division is initiated) is accompanied by myriad of changes such as, redistribution of proteins, selective degradation of proteins and modifying the activity of proteins to take newer roles.
Figure 4.
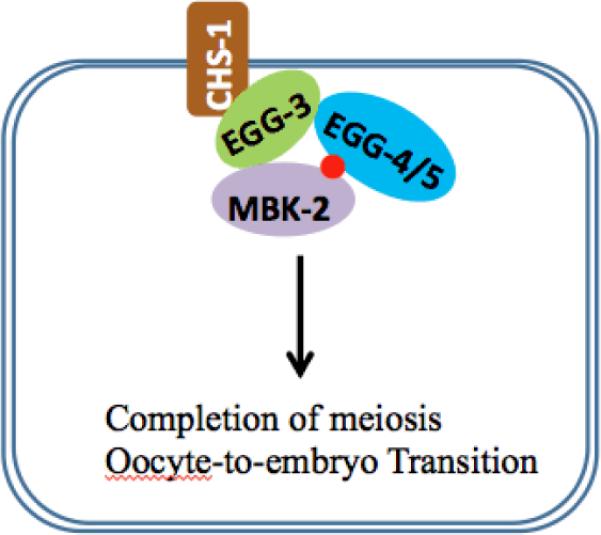
Proteins complexes present at the cell cortex of oocyte required for oocyte-to-embryo transition. EGG-3 binds with CHS-1, EGG-4/5 and MBK-2. The phospho group appended to the MBK-2 by itself during translation of MBK-2 is depicted as a small red circle. Pseudophospatase EGG-4/5 binds with phospho-MBK-2 and inhibits its activity in oocyte.
The meiotic division differs from mitotic division in many aspects. For example, the anastral meiotic spindles are shorter in length compared to astral mitotic spindles. The microtubule severing protein MEI-1 is localized to meiotic spindle in oocytes and is specifically required for meiotic division. Loss-of-function mutation in mei-1 leads to the disorganization of meiotic spindle (Clark-Maguire and Mains, 1994b). However, MEI-1 should be cleansed from the oocyte soon after the completion of meiosis, failure to do so would lead to the inappropriate execution of microtubule severing activity over mitotic spindle (Clark-Maguire and Mains, 1994a). In mei-1 gain-of-function mutant, meiotic spindles are normal, but mitotic spindles are shorter than wild type. Timely degradation of mei-1 is very crucial; both precocious degradation of MEI-1 in oocyte undergoing meiotic division and failure to degrade MEI-1 before the initiation of mitotic spindles are detrimental. MBK-2 phosphorylates MEI-1 to mark it for timely degradation(Stitzel et al., 2006).
The zinc finger proteins, OMA-1 and OMA-2 functions redundantly to regulate meiotic maturation in oocytes (Detwiler et al., 2001). The oocytes of oma-1;oma-2 double mutants fail to complete meiotic maturation. Phosphorylation of OMA-1/OMA-2 enables the sequestration of general transcription factor, TAF-4 in the cytoplasm of the zygote, achieving global transcriptional repression (Guven-Ozkan et al., 2008). In wild-type embryos, the earliest transcriptional activity can be detected at four-cell stage. However, the oma-1;oma-2 double mutant starts expressing genes as early as one cell stage. Timely degradation of OMA-1 is equally important; persistence of OMA-1 in cleavage division causes ectopic accumulation of several maternal proteins including, germ cell specific transcription factor PIE-1 in the somatic blastomeres (Lin, 2003).
The EGG-3 anchors activated MBK-2 to the cell cortex to prevent precocious phosphorylation of its substrates (see figure 4). Cell cycle progression signals promote degradation of EGG-3, EGG-4/5 to release MBK-2 from cortex to cytosol. Timely release of MBK-2 from cortex into cytosol ensures that the MBK-2 substrates such as MEI-1, OMA-1/2 are not precociously phosphorylated.
8. Concluding remarks
Understanding the mechanistic details of nematode fertilization will help us dissect evolutionarily conserved pathways. For example, oocytes of most organisms are arrested at one or two points during meiosis and sperm often signals to promote further development (Tripathi et al., 2010). Similarly, the C. elegans oocytes are arrested in meiotic prophase I and the MSP released from sperm promotes meiotic maturation of oocytes (Miller et al., 2001). In many animals, oocytes secrete chemo-attractants that serve to lure the sperm towards oocyte (Kaupp et al., 2008). Likewise, the eicosanoids secreted by C. elegans oocytes attract sperm to migrate to spermatheca (Kubagawa et al., 2006). Evolutionarily conserved MAP Kinase pathways are often utilized during the gamete development and/or differentiation in many animals, including C. elegans (Ferrell, 1999; Yang et al., 2010). Thus, a detailed investigation of C. elegans reproductive biology is expected to bring several conserved molecules to limelight that play a role around the event of fertilization in higher animals, including humans. Studying C. elegans will also shed light on the reproductive strategies employed uniquely in nematodes. For example, sperm from almost all nematodes move using psedopods and require MSP based cytoskeleton for motility (Fraire-Zamora and Cardullo, 2010). Insight into nematode-specific components will help us identify target(s) that can be manipulated to control the reproduction of parasitic nematodes.
Acknowledgements
We would like to thank Singson lab members for useful discussions. We would like to thank Bhuvaneswari Murugaiyan, Kasinath Kuravi and Sina Rahimi for assistance with the preparation of the manuscript. This work was supported by grants from NIH (R01 HD054681).
References
- Abbas M, Cain GD. In vitro activation and behavior of the ameboid sperm of Ascaris suum (Nematoda) Cell Tissue Res. 1979;200:273–84. doi: 10.1007/BF00236419. [DOI] [PubMed] [Google Scholar]
- Argon Y, Ward S. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics. 1980;96:413–33. doi: 10.1093/genetics/96.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YK, Kim E, L'Hernault S W, Barr MM. The CIL-1 PI 5-phosphatase localizes TRP Polycystins to cilia and activates sperm in C. elegans. Curr Biol. 2009;19:1599–607. doi: 10.1016/j.cub.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AM, Roberts TM, Stewart M. 2.6 A resolution crystal structure of helices of the motile major sperm protein (MSP) of Caenorhabditis elegans. J Mol Biol. 2002;319:491–9. doi: 10.1016/S0022-2836(02)00294-2. [DOI] [PubMed] [Google Scholar]
- Brattig NW, Schwohl A, Rickert R, Buttner DW. The filarial parasite Onchocerca volvulus generates the lipid mediator prostaglandin E(2) Microbes Infect. 2006;8:873–9. doi: 10.1016/j.micinf.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Browning H, Strome S. A sperm-supplied factor required for embryogenesis in C. elegans. Development. 1996;122:391–404. doi: 10.1242/dev.122.1.391. [DOI] [PubMed] [Google Scholar]
- Bullock TL, Parthasarathy G, King KL, Kent HM, Roberts TM, Stewart M. New crystal forms of the motile major sperm protein (MSP) of Ascaris suum. J Struct Biol. 1996a;116:432–7. doi: 10.1006/jsbi.1996.0061. [DOI] [PubMed] [Google Scholar]
- Bullock TL, Roberts TM, Stewart M. 2.5 A resolution crystal structure of the motile major sperm protein (MSP) of Ascaris suum. J Mol Biol. 1996b;263:284–96. doi: 10.1006/jmbi.1996.0575. [DOI] [PubMed] [Google Scholar]
- Buttery SM, Ekman GC, Seavy M, Stewart M, Roberts TM. Dissection of the Ascaris sperm motility machinery identifies key proteins involved in major sperm protein-based amoeboid locomotion. Mol Biol Cell. 2003;14:5082–8. doi: 10.1091/mbc.E03-04-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee I, Richmond A, Putiri E, Shakes DC, Singson A. The Caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development. 2005;132:2795–808. doi: 10.1242/dev.01868. [DOI] [PubMed] [Google Scholar]
- Cheng H, Govindan JA, Greenstein D. Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr Biol. 2008;18:705–14. doi: 10.1016/j.cub.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KC, Klancer R, Singson A, Seydoux G. Regulation of MBK-2/DYRK by CDK-1 and the pseudophosphatases EGG-4 and EGG-5 during the oocyte-to-embryo transition. Cell. 2009;139:560–72. doi: 10.1016/j.cell.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Sang ID, George SE, Ding M, Moseley SL, Lynch AS, Chisholm AD. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell. 1999;99:781–90. doi: 10.1016/s0092-8674(00)81675-x. [DOI] [PubMed] [Google Scholar]
- Chu DS, Liu H, Nix P, Wu TF, Ralston EJ, Yates JR, 3rd, Meyer BJ. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature. 2006;443:101–5. doi: 10.1038/nature05050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Maguire S, Mains PE. Localization of the mei-1 gene product of Caenorhaditis elegans, a meiotic-specific spindle component. J Cell Biol. 1994a;126:199–209. doi: 10.1083/jcb.126.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Maguire S, Mains PE. mei-1, a gene required for meiotic spindle formation in Caenorhabditis elegans, is a member of a family of ATPases. Genetics. 1994b;136:533–46. doi: 10.1093/genetics/136.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley P. A quick tour of nematode diversity and the backbone of nematode phylogeny. WormBook. 2006:1–8. doi: 10.1895/wormbook.1.41.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Dev Cell. 2001;1:187–99. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, Kubagawa HM, Miller MA. Insulin/FOXO Signaling Regulates Ovarian Prostaglandins Critical for Reproduction. Dev Cell. 2010;19:858–71. doi: 10.1016/j.devcel.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Schedl T. Sex determination in the germ line. WormBook. 2007:1–13. doi: 10.1895/wormbook.1.82.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE., Jr. Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–42. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fraire-Zamora JJ, Cardullo RA. The physiological acquisition of amoeboid motility in nematode sperm: is the tail the only thing the sperm lost? Mol Reprod Dev. 2010;77:739–50. doi: 10.1002/mrd.21193. [DOI] [PubMed] [Google Scholar]
- Geldziler B, Chatterjee I, Kadandale P, Putiri E, Patel R, Singson A. A comparative study of sperm morphology, cytology and activation in Caenorhabditis elegans, Caenorhabditis remanei and Caenorhabditis briggsae. Dev Genes Evol. 2006;216:198–208. doi: 10.1007/s00427-005-0045-4. [DOI] [PubMed] [Google Scholar]
- Geldziler B, Chatterjee I, Singson A. The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev Biol. 2005;283:424–36. doi: 10.1016/j.ydbio.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–74. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- Govindan JA, Cheng H, Harris JE, Greenstein D. Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr Biol. 2006;16:1257–68. doi: 10.1016/j.cub.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Grant RP, Buttery SM, Ekman GC, Roberts TM, Stewart M. Structure of MFP2 and its function in enhancing MSP polymerization in Ascaris sperm amoeboid motility. J Mol Biol. 2005;347:583–95. doi: 10.1016/j.jmb.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Guven-Ozkan T, Nishi Y, Robertson SM, Lin R. Global transcriptional repression in C. elegans germline precursors by regulated sequestration of TAF-4. Cell. 2008;135:149–60. doi: 10.1016/j.cell.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaf A, Butler PJ, Kent HM, Fearnley IM, Roberts TM, Neuhaus D, Stewart M. The motile major sperm protein (MSP) from Ascaris suum is a symmetric dimer in solution. J Mol Biol. 1996;260:251–60. doi: 10.1006/jmbi.1996.0396. [DOI] [PubMed] [Google Scholar]
- Heger P, Kroiher M, Ndifon N, Schierenberg E. Conservation of MAP kinase activity and MSP genes in parthenogenetic nematodes. BMC Dev Biol. 2010;10:51. doi: 10.1186/1471-213X-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DP, Shakes DC, Ward S, Strome S. A sperm-supplied product essential for initiation of normal embryogenesis in Caenorhabditis elegans is encoded by the paternal-effect embryonic-lethal gene, spe-11. Dev Biol. 1989;136:154–66. doi: 10.1016/0012-1606(89)90138-3. [DOI] [PubMed] [Google Scholar]
- Hill KL, L'Hernault SW. Analyses of reproductive interactions that occur after heterospecific matings within the genus Caenorhabditis. Dev Biol. 2001;232:105–14. doi: 10.1006/dbio.2000.0136. [DOI] [PubMed] [Google Scholar]
- Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–19. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hubbard EJ, Greenstein D. Introduction to the germ line. WormBook. 2005:1–4. doi: 10.1895/wormbook.1.18.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano JE, Jr., Roberts TM, Stewart M, Fontana CA. Reconstitution in vitro of the motile apparatus from the amoeboid sperm of Ascaris shows that filament assembly and bundling move membranes. Cell. 1996;84:105–14. doi: 10.1016/s0092-8674(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science. 2006;313:1298–301. doi: 10.1126/science.1130291. [DOI] [PubMed] [Google Scholar]
- Joachim A, Ruttkowski B. Prostaglandin D(2) synthesis in Oesophagostomum dentatum is mediated by cytosolic Glutathione S-transferase. Exp Parasitol. doi: 10.1016/j.exppara.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Kadandale P, Stewart-Michaelis A, Gordon S, Rubin J, Klancer R, Schweinsberg P, Grant BD, Singson A. The egg surface LDL receptor repeat-containing proteins EGG-1 and EGG-2 are required for fertilization in Caenorhabditis elegans. Curr Biol. 2005;15:2222–9. doi: 10.1016/j.cub.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Kashikar ND, Weyand I. Mechanisms of sperm chemotaxis. Annu Rev Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- Kim DY, Roy R. Cell cycle regulators control centrosome elimination during oogenesis in Caenorhabditis elegans. J Cell Biol. 2006;174:751–7. doi: 10.1083/jcb.200512160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Germline proliferation and its control. WormBook. 2005:1–14. doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–33. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–19. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- King KL, Essig J, Roberts TM, Moerland TS. Regulation of the Ascaris major sperm protein (MSP) cytoskeleton by intracellular pH. Cell Motil Cytoskeleton. 1994;27:193–205. doi: 10.1002/cm.970270302. [DOI] [PubMed] [Google Scholar]
- Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci U S A. 2004;101:9003–8. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Wolf N, Hirsh D. Development of the male reproductive system and sexual transformation in the nematode Caenorhabditis elegans. Dev Biol. 1976;52:1–18. doi: 10.1016/0012-1606(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Kosinski M, McDonald K, Schwartz J, Yamamoto I, Greenstein D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development. 2005;132:3357–69. doi: 10.1242/dev.01916. [DOI] [PubMed] [Google Scholar]
- Kroft TL, Gleason EJ, L'Hernault SW. The spe-42 gene is required for sperm-egg interactions during C. elegans fertilization and encodes a sperm-specific transmembrane protein. Dev Biol. 2005;286:169–81. doi: 10.1016/j.ydbio.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol. 2006;8:1143–8. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- L'Hernault SW. Spermatogenesis. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault SW. The genetics and cell biology of spermatogenesis in the nematode C. elegans. Mol Cell Endocrinol. 2009;306:59–65. doi: 10.1016/j.mce.2009.01.008. [DOI] [PubMed] [Google Scholar]
- L'Hernault SW, Roberts TM. Caenorhabditis elegans: Modern biological analysis of an organism. Academic press; 1995. Cell biology of nematode sperm; pp. 273–301. [DOI] [PubMed] [Google Scholar]
- L'Hernault SW, Shakes DC, Ward S. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics. 1988;120:435–52. doi: 10.1093/genetics/120.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMunyon CW, Ward S. Assessing the viability of mutant and manipulated sperm by artificial insemination of Caenorhabditis elegans. Genetics. 1994;138:689–92. doi: 10.1093/genetics/138.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClaire LL, 3rd, Stewart M, Roberts TM. A 48 kDa integral membrane phosphoprotein orchestrates the cytoskeletal dynamics that generate amoeboid cell motility in Ascaris sperm. J Cell Sci. 2003;116:2655–63. doi: 10.1242/jcs.00469. [DOI] [PubMed] [Google Scholar]
- Lee MH, Schedl T. Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev. 2001;15:2408–20. doi: 10.1101/gad.915901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesk AM. Introduction to Protein Architecture. Oxford University Press; 2001. [Google Scholar]
- Lin R. A gain-of-function mutation in oma-1, a C. elegans gene required for oocyte maturation, results in delayed degradation of maternal proteins and embryonic lethality. Dev Biol. 2003;258:226–39. doi: 10.1016/s0012-1606(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Liu QL, Thomas VP, Williamson VM. Meiotic parthenogenesis in a root-knot nematode results in rapid genomic homozygosity. Genetics. 2007;176:1483–90. doi: 10.1534/genetics.107.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K, DeFelice LJ, L'Hernault SW. A novel chloride channel localizes to Caenorhabditis elegans spermatids and chloride channel blockers induce spermatid differentiation. Dev Biol. 1996;176:1–16. doi: 10.1006/dbio.1996.9999. [DOI] [PubMed] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–6. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Maruyama R, Velarde NV, Klancer R, Gordon S, Kadandale P, Parry JM, Hang JS, Rubin J, Stewart-Michaelis A, Schweinsberg P, Grant BD, Piano F, Sugimoto A, Singson A. EGG-3 regulates cell-surface and cortex rearrangements during egg activation in Caenorhabditis elegans. Curr Biol. 2007;17:1555–60. doi: 10.1016/j.cub.2007.08.011. [DOI] [PubMed] [Google Scholar]
- McDonald KL, Auer M. High-pressure freezing, cellular tomography, and structural cell biology. Biotechniques. 2006;41:137, 139, 141. doi: 10.2144/000112226. passim. [DOI] [PubMed] [Google Scholar]
- Mello CC, Draper BW, Krause M, Weintraub H, Priess JR. The pie-1 and mex-1 genes and maternal control of blastomere identity in early C. elegans embryos. Cell. 1992;70:163–76. doi: 10.1016/0092-8674(92)90542-k. [DOI] [PubMed] [Google Scholar]
- Miao L, Vanderlinde O, Stewart M, Roberts TM. Retraction in amoeboid cell motility powered by cytoskeletal dynamics. Science. 2003;302:1405–7. doi: 10.1126/science.1089129. [DOI] [PubMed] [Google Scholar]
- Miao L, Yi K, Mackey JM, Roberts TM. Reconstitution in vitro of MSP-based filopodium extension in nematode sperm. Cell Motil Cytoskeleton. 2007;64:235–47. doi: 10.1002/cm.20177. [DOI] [PubMed] [Google Scholar]
- Miller MA, Cutter AD, Yamamoto I, Ward S, Greenstein D. Clustered organization of reproductive genes in the C. elegans genome. Curr Biol. 2004;14:1284–90. doi: 10.1016/j.cub.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–7. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minniti AN, Sadler C, Ward S. Genetic and molecular analysis of spe-27, a gene required for spermiogenesis in Caenorhabditis elegans hermaphrodites. Genetics. 1996;143:213–23. doi: 10.1093/genetics/143.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad PJ, Ward S. Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics. 2002;161:143–55. doi: 10.1093/genetics/161.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Davis EB, Ward S. spe-29 encodes a small predicted membrane protein required for the initiation of sperm activation in Caenorhabditis elegans. Genetics. 2000;156:1623–33. doi: 10.1093/genetics/156.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Minniti AN, Sadler C, Ward S. spe-12 encodes a sperm cell surface protein that promotes spermiogenesis in Caenorhabditis elegans. Genetics. 1999;152:209–20. doi: 10.1093/genetics/152.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GA, Ward S. Vesicle fusion, pseudopod extension and amoeboid motility are induced in nematode spermatids by the ionophore monensin. Cell. 1980;19:457–64. doi: 10.1016/0092-8674(80)90520-6. [DOI] [PubMed] [Google Scholar]
- Nishimura H, L'Hernault SW. Spermatogenesis-defective (spe) mutants of the nematode Caenorhabditis elegans provide clues to solve the puzzle of male germline functions during reproduction. Dev Dyn. 239:1502–14. doi: 10.1002/dvdy.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry JM, Velarde NV, Lefkovith AJ, Zegarek MH, Hang JS, Ohm J, Klancer R, Maruyama R, Druzhinina MK, Grant BD, Piano F, Singson A. EGG-4 and EGG-5 Link Events of the Oocyte-to-Embryo Transition with Meiotic Progression in C. elegans. Curr Biol. 2009;19:1752–7. doi: 10.1016/j.cub.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pen-Mouratov S, Steinberger Y. Spatio-temporal Dynamic Heterogeneity of Nematode Abundance in a Desert Ecosystem. J Nematol. 2005;37:26–36. [PMC free article] [PubMed] [Google Scholar]
- Perbandt M, Hoppner J, Burmeister C, Luersen K, Betzel C, Liebau E. Structure of the extracellular glutathione S-transferase OvGST1 from the human pathogenic parasite Onchocerca volvulus. J Mol Biol. 2008;377:501–11. doi: 10.1016/j.jmb.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Putiri E, Zannoni S, Kadandale P, Singson A. Functional domains and temperature-sensitive mutations in SPE-9, an EGF repeat-containing protein required for fertility in Caenorhabditis elegans. Dev Biol. 2004;272:448–59. doi: 10.1016/j.ydbio.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–16. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Sadler PL, Shakes DC. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development. 2000;127:355–66. doi: 10.1242/dev.127.2.355. [DOI] [PubMed] [Google Scholar]
- Schedl T. Developmental genetics of the germ line. Cold Spring Harbor Laboratory Press; New York: 1997. [PubMed] [Google Scholar]
- Seydoux G, Mello CC, Pettitt J, Wood WB, Priess JR, Fire A. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382:713–6. doi: 10.1038/382713a0. [DOI] [PubMed] [Google Scholar]
- Shakes DC, Ward S. Initiation of spermiogenesis in C. elegans: a pharmacological and genetic analysis. Dev Biol. 1989;134:189–200. doi: 10.1016/0012-1606(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Singson A. Sperm activation: time and tide wait for no sperm. Curr Biol. 2006;16:R160–2. doi: 10.1016/j.cub.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Singson A, Mercer KB, L'Hernault SW. The C. elegans spe-9 gene encodes a sperm transmembrane protein that contains EGF-like repeats and is required for fertilization. Cell. 1998;93:71–9. doi: 10.1016/s0092-8674(00)81147-2. [DOI] [PubMed] [Google Scholar]
- Smith HE, Ward S. Identification of protein-protein interactions of the major sperm protein (MSP) of Caenorhabditis elegans. J Mol Biol. 1998;279:605–19. doi: 10.1006/jmbi.1998.1793. [DOI] [PubMed] [Google Scholar]
- Stanfield GM, Villeneuve AM. Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males. Curr Biol. 2006;16:252–63. doi: 10.1016/j.cub.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Cheng KC, Seydoux G. Regulation of MBK-2/Dyrk kinase by dynamic cortical anchoring during the oocyte-to-zygote transition. Curr Biol. 2007;17:1545–54. doi: 10.1016/j.cub.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Pellettieri J, Seydoux G. The C. elegans DYRK Kinase MBK-2 Marks Oocyte Proteins for Degradation in Response to Meiotic Maturation. Curr Biol. 2006;16:56–62. doi: 10.1016/j.cub.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Streit A. Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology. 2008;135:285–94. doi: 10.1017/S003118200700399X. [DOI] [PubMed] [Google Scholar]
- Strome S. Specification of the germ line. WormBook. 2005:1–10. doi: 10.1895/wormbook.1.9.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr DE, Scott AL. MSP domain proteins show enhanced expression in male germ line cells. Mol Biochem Parasitol. 2004;137:87–98. doi: 10.1016/j.molbiopara.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Tchesunov AV, Hope WD. Thalassomermis megamphis n. gen., n. sp. (Mermithidae: Nemata) from the Bathyal South Atlantic Ocean. J Nematol. 1997;29:451–64. [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Kumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol. 2010;223:592–600. doi: 10.1002/jcp.22108. [DOI] [PubMed] [Google Scholar]
- Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, Pawson T. Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell. 1999;4:903–13. doi: 10.1016/s1097-2765(00)80220-8. [DOI] [PubMed] [Google Scholar]
- Ward S, Hogan E, Nelson GA. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev Biol. 1983;98:70–9. doi: 10.1016/0012-1606(83)90336-6. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Sternberg PW. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114:285–97. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Han SM, Miller MA. MSP hormonal control of the oocyte MAP kinase cascade and reactive oxygen species signaling. Dev Biol. 2010;342:96–107. doi: 10.1016/j.ydbio.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Buttery SM, Stewart M, Roberts TM. A Ser/Thr kinase required for membrane-associated assembly of the major sperm protein motility apparatus in the amoeboid sperm of Ascaris. Mol Biol Cell. 2007;18:1816–25. doi: 10.1091/mbc.E06-08-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Wang X, Emmett MR, Marshall AG, Stewart M, Roberts TM. Dephosphorylation of major sperm protein (MSP) fiber protein 3 by protein phosphatase 2A during cell body retraction in the MSP-based amoeboid motility of Ascaris sperm. Mol Biol Cell. 2009;20:3200–8. doi: 10.1091/mbc.E09-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannoni S, L'Hernault SW, Singson AW. Dynamic localization of SPE-9 in sperm: a protein required for sperm-oocyte interactions in Caenorhabditis elegans. BMC Dev Biol. 2003;3:10. doi: 10.1186/1471-213X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Foster JM, Nelson LS, Ma D, Carlow CK. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Dev Biol. 2005;285:330–9. doi: 10.1016/j.ydbio.2005.06.037. [DOI] [PubMed] [Google Scholar]


