To be able to differentiate into any cell type, embryonic stem cells need a sophisticated mechanism to cope with DNA damage to minimize mutations. Indeed, mouse embryonic stem cells (mESCs) have about 10 times less DNA mutation rate than their differentiated counterparts1. This observation can be explained by the fact that mESCs’ ultra-sensitivity to DNA damage helps mESCs efficiently remove cells that have the potential to develop tumorigenic DNA mutations. Although the hypersensitivity of mESCs to DNA damage serves as an excellent mutation-proof mechanism, it comes at a cost of losing the population of mESCs quickly and in turn the failure of embryogenesis. As a guardian of the genome, the tumor suppressor p53 seems to play important roles to reconcile the conundrum.
While it is undisputed that p53 is critical for inducing apoptosis of somatic cells, it is still under debate that whether p53 plays a role in regulating the apoptosis in embryonic stem cells. On the one hand, earlier work by Aladjem et al., clearly showed that p53 does not play a role in the apoptosis of mESCs after DNA damage2. On the other hand, de Vries et al., demonstrated that p53-dependent apoptosis exists in mESCs3. The causes for these contradictory observations remain unclear. Possible reasons could be that genetic background of mESCs and/or dosages of DNA damaging agents used in these two studies are different. Regardless of whether p53 induces apoptosis in mESCs, a report by Lin et al., revealed a novel function of p53, which is to drive the differentiation of mESCs by repressing the expression of Nanog4. This pro-differentiation role of p53 not only fits well with its tumor suppressive function, but also nicely explains why p53 is highly expressed in mESCs. Just as we thought we knew the clear picture of p53 in mESCs, results from a study by Lee et al. provided a new twist 5. In this article, the authors used an integrated genome-wide approach to identify the p53 target genes in mESCs. Surprisingly, the Wnt signaling pathway was identified as one of the major downstream pathways of p53 in mESCs upon DNA damage.
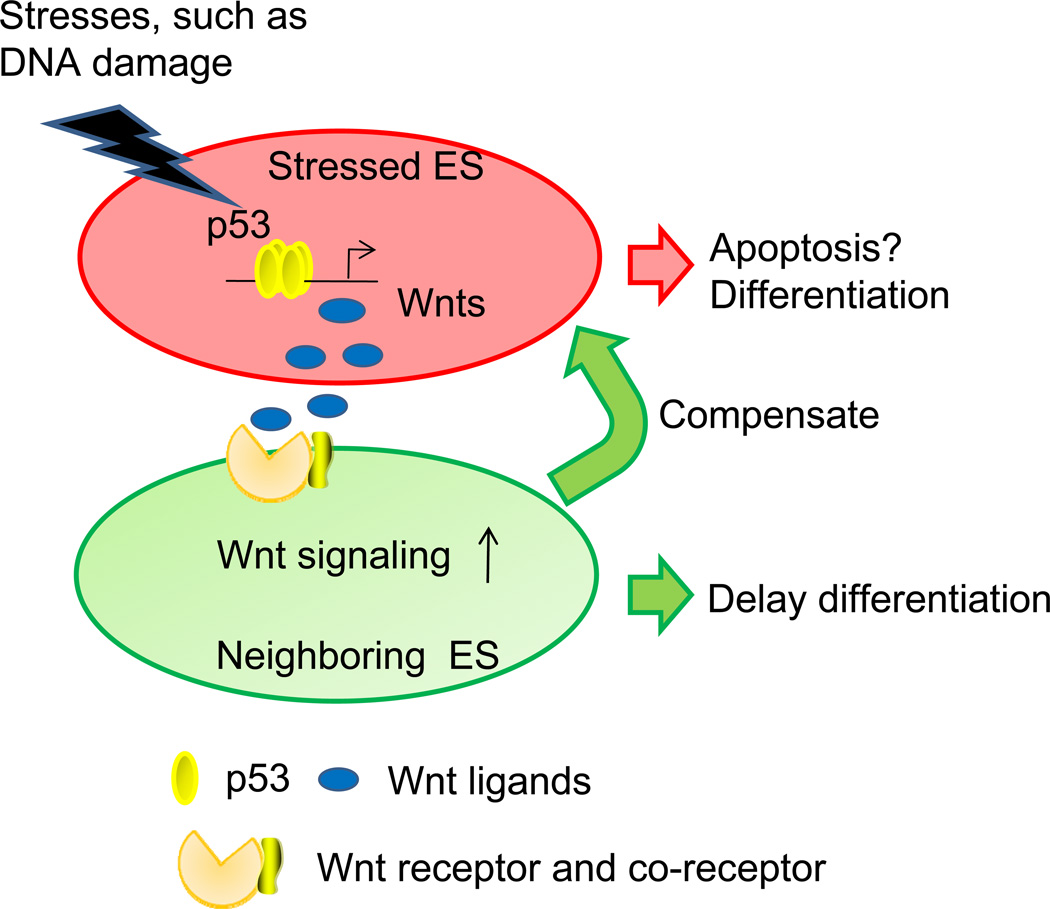
Initially, the observation that p53 induced five Wnt ligands, the anti-differentiation signal for mESCs,6 appears to be inconsistent with previously reported pro-differentiation role of p53 in mESCs. However, this seemingly inconsistency indicates that p53 has dual functions in mESCs. At the single cell level, p53 facilitates the differentiation of cells with DNA damages, and probably induces their apoptosis as well. At the whole population level, damaged mESCs secrete the Wnt ligands to act on neighboring cells to prevent their differentiation, presumably serving as a compensatory mechanism to stabilize the cell number in the population (Figure 1). This intriguing model can explain how mESCs can utilize p53 to maintain the genomic and population stability at the same time. Another important finding is that this Wnt-p53 connection is tightly associated with mESCs because the induction of Wnt ligands by p53 is greatly attenuated in somatic cells, such as murine embryonic fibroblasts and neural progenitor cells. During the early developmental stage, when nutrition is almost unlimited, the strategy of generating new mESCs to replace the damaged ones is probably the best “choice” to minimize the risk of passing mutation to offspring cells. As mESCs develop into other cell types, cells start to utilize other mechanisms, such as cell cycle arrest and DNA repair machinery, to deal with DNA damage. Given that these cells have less developmental potentials than mESCs and nutrition is generally limited by the surrounding environment, it is more energy economical for the damaged cells to repair DNA than utilize the compensatory mechanism in mESCs. It is noteworthy that p53 knockout mice have high frequency of embryogenesis failure7, 8. Whether this is related to the Wnt-p53 connection needs further study.
Figure 1.
The compensatory model that is mediated by the Wnt signaling pathway and p53 in mESCs. Facing various stresses, p53 is activated and induces the production of Wnt ligands. Wnt ligands are secreted to delay the differentiation of neighboring cells in a paracrine manner, presumably giving them more time to divide and compensate the loss of stressed mESCs.
Since over-activation of the Wnt signaling in somatic tissues generally leads to cancer9, does the Wnt induction by p53 suggest that p53 can be tumorigenic under certain conditions? To unlock the tumorigenic potential of p53, at least two barriers need to be overcome through potential genetic and epigenetic mechanisms. First, somatic cells need to be “reprogrammed” into embryonic stem-like cells because the Wnt induction by p53 is tightly restricted to mESCs. This possibility has been supported by emerging evidence that gene expression signature in certain cancer cells is similar to that in mESCs 10. In addition, recent progress of generating iPS cells from somatic cells further suggests that this barrier can be circumvented. Second, cells need to shed the pro-apoptotic function of p53 and simultaneously keep its ability to induce the Wnt ligands. This possibility can be realized through various mutations of p53. This attractive concept can be further tested by more future in vivo studies.
Acknowledgement
We thank Dr. Nan Roche for critically reading of the manuscript.
References
- 1.Cervantes RB, Stringer JR, Shao C, Tischfield JA, Stambrook PJ. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc Natl Acad Sci U S A. 2002;99:3586–3590. doi: 10.1073/pnas.062527199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–155. doi: 10.1016/s0960-9822(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 3.de Vries A, Flores ER, Miranda B, Hsieh HM, van Oostrom CT, Sage J, Jacks T. Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc Natl Acad Sci U S A. 2002;99:2948–2953. doi: 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 5.Lee KH, Li M, Michalowski AM, Zhang X, Liao H, Chen L, Xu Y, Wu X, Huang J. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci U S A. 107:69–74. doi: 10.1073/pnas.0909734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Curr Biol. 1995;5:931–936. doi: 10.1016/s0960-9822(95)00183-7. [DOI] [PubMed] [Google Scholar]
- 8.Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995;10:175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- 9.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 10.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]