Abstract
Background
Clostridium difficile infection (CDI) is a common hospital-acquired infection with increasing incidence, severity, recurrence and associated morbidity and mortality. There is emerging data on the occurrence of CDI in non-hospitalized patients. However, there is a relative lack of community-based CDI studies, as most of the existing studies are hospital-based, potentially influencing the results by referral or hospitalization bias by missing cases of community-acquired CDI.
Methods
To better understand the epidemiology of community-acquired Clostridium difficile infection, a population-based study was conducted in Olmsted County, Minnesota using the resources of the Rochester Epidemiology Project. Data regarding severity, treatment response, and outcomes were compared in community-acquired versus hospital-acquired cohorts, and changes in these parameters, as well as in incidence, were assessed over the study period.
Results
Community-acquired Clostridium difficile infection cases accounted for 41% of 385 definite CDI cases. The incidence of both community-acquired and hospital-acquired Clostridium difficile infection increased significantly over the study period. Compared to those with hospital-acquired infection, patients with community-acquired infection were younger (median age 50 years compared to 72 years), more likely to be female (76% versus 60%), had lower comorbidity scores, and were less likely to have severe infection (20% versus 31%) or have been exposed to antibiotics (78% versus 94%). There were no differences in the rates of complicated or recurrent infection in patients with community-acquired compared to hospital-acquired infection.
Conclusions
In this population-based cohort, a significant proportion of cases of Clostridium difficile infection occurred in the community. These patients were younger and had less severe infection than those with hospital-acquired infection. Thus, reports of Clostridium difficile infection in hospitalized patients likely underestimate the burden of disease and overestimate severity.
Keywords: Clostridium difficile infection, community-acquired, epidemiology
Introduction
Clostridium difficile is recognized as the primary infectious cause of pseudomembranous colitis (1) and the principal cause of infectious diarrhea in hospitalized patients (2). Recent studies have shown increasing incidence, severity and recurrence rates of CDI (3–9). For example, the incidence of healthcare acquired CDI increased 2 to 2.5 fold from the late 1990s to the early 2000s, and even more in the elderly (7, 10). There also has been a significant increase in severe cases, colectomies, and death related to CDI (7, 11, 12). However, most of the recent literature assessing these trends in CDI is based on hospitalized patients, and hence is potentially influenced by hospitalization or referral biases. A few studies have described the emergence of community-acquired CDI (13–15) but there is a lack of population-based studies describing CDI. We assessed CDI incidence, risk factors, and outcomes in a population-based cohort, including community-acquired infections in outpatients, as well as hospitalized inpatients.
Methods
Study population
All potential cases of CDI occurring in residents of Olmsted County, MN between 1991 and 2005 were identified using the resources of the Rochester Epidemiology Project (REP) (16, 17). The REP diagnostic index was searched for the ICD-9 code for CDI (008.45), along with CDI as microbiologic or clinical diagnoses (for both inpatients and outpatients). All medical records, from all sources of care available to Olmsted County residents, are linked and accessible through the REP. These medical records contain all information about diagnoses, hospital admissions, surgical procedures, results of hematologic and laboratory tests, imaging, vaccinations, and drug prescriptions for all county residents. The REP therefore allows investigators to follow subjects through their outpatient and hospitalization contacts across all local medical facilities, regardless of where the care was delivered and of insurance status. A central diagnostic index maintains records from all outpatient visits, emergency room visits, hospitalizations, nursing home visits, surgical procedures, autopsy examinations, and death certificates for all residents since 1908.
After identification of possible cases of CDI, records of patients who had provided permission (according to Minnesota state law) for their medical records to be used in research were reviewed. Clinical notes, laboratory results, endoscopy and histopathology reports were reviewed to confirm diagnoses. Records were reviewed to identify separate cases for individual patients, and determine acquisition modality (community vs. hospital). Co-morbidities for all patients were assessed by calculating the crude Charlson co-morbidity index (18). The Charlson co-morbidity index is comprised of 19 co-morbid conditions in 4 categories, and each category has a weighted-score based on the adjusted risk of one and ten-year mortality (18). A higher Charlson score reflects a more severe co-morbidity burden and an increased likelihood of one and ten-year mortality. Acid-suppressing medication use was defined as the use of either a proton pump inhibitor or a histamine-2 receptor blocker at the time of CDI diagnosis. Antibiotic exposure was defined as the use of oral or parenteral antibiotics in the 90 days preceding CDI diagnosis. Inflammatory bowel disease was defined as the presence of diagnosed ulcerative colitis, Crohn’s disease or indeterminate colitis. The presence of a malignancy was defined as a current or previous diagnosis of a localized or metastasized solid tumor, lymphoma or leukemia. The Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved the study.
Case Definitions
Based on practice recommendations (19, 20), "definite" CDI was defined as ≥ 3 loose stools / 24 hours with a positive Clostridium difficile stool toxin assay or the presence of pseudomembranous colitis on endoscopy or histology. Recurrent CDI was defined if these diagnostic criteria were met within 8 weeks of initial diagnosis after documented symptom resolution (5, 8, 19). Severe disease was defined by a white blood cell count ≥ 15,000 / mm3 or a serum creatinine rise of more than 50% from baseline (20). CDI was classified as ‘severe complicated’ if the infection was associated with hypotension, sepsis, ileus, toxic megacolon, perforation, need for ICU admission, surgery for a CDI-related complication, or death (20).
Infection was defined as hospital-acquired if symptoms onset occurred more than 48 hours after admission to, or less than 4 weeks after discharge from, a health care facility (20). Infection was defined as community-acquired if symptom onset occurred in the community or within 48 hours of admission to a hospital, provided symptom onset was more than 12 weeks after the last discharge from a hospital. Infection was defined as indeterminate if symptom onset occurred between 4 and 12 weeks from a hospital dismissal (20). Indeterminate cases (n=20) were included as community-acquired for the purposes of this study. Secular trends were analyzed to assess for changes in severity (based on change in complication rates), initial treatment and response, and likelihood to relapse over time.
Statistical Analysis
The incidence of confirmed cases of CDI in Olmsted County residents at the time of their diagnosis was calculated per 100,000 person-years. The 2000 US census data was used to calculate age- and sex-adjusted rates (direct adjustment to the age and gender distribution of US whites, 2000). Poisson regression models were used to evaluate the association of age, sex, and calendar period with crude incidence rates and mode of infection acquisition (community-acquired vs. hospital-acquired). The univariate associations of demographic and clinical characteristics with mode of acquisition were assessed via contingency tables analyses (chi square test). Since the REP captures data on both inpatients and outpatients, and data on annual hospital days for county residents is available, the incidence rates for both community-acquired CDI (per 100,000 person years) and, separately, hospital-acquired CDI (per 100,000 bed-days) were calculated. When calculated separately, hospital-acquired rates were not adjusted to US census data as described above, but hospital-acquired cases were included in the overall incidence calculations and were adjusted appropriately.
In Olmsted County, certain nursing homes are coupled with assisted living facilities. Thus, some residents of these facilities are similar to hospitalized patients, whereas others are closer to community dwellers. Therefore, for the purposes of this study, patients residing in long term care facilities were included in the overall results, but not in sub-analyses of community- versus hospital-acquired infection.
Results
Incidence of Community-acquired CDI
A total of 416 possible cases of CDI were identified; 31 (7.5%) were excluded; 30 due to lacked of a confirmatory stool assay and 1 due to lack of authorization to access records for research. Thus, 385 (92.5%) cases met criteria for "definite" CDI and were included in this study. Patient classification into community- and hospital-acquired CDI is shown in Figure 1. The median age was 67.6 years (range 1 month to 102 years), and 65.7% patients were female. The majority (53%) of cases occurred in elderly patients (65 years of age or older); 50% were hospital-acquired, 9% were nursing home acquired and a substantial proportion (41%) of cases were community-acquired. Of all CDI patients, 87% had documented antibiotic exposure in the preceding 90 days. Other patient characteristics are shown in Table 1.
Figure 1.
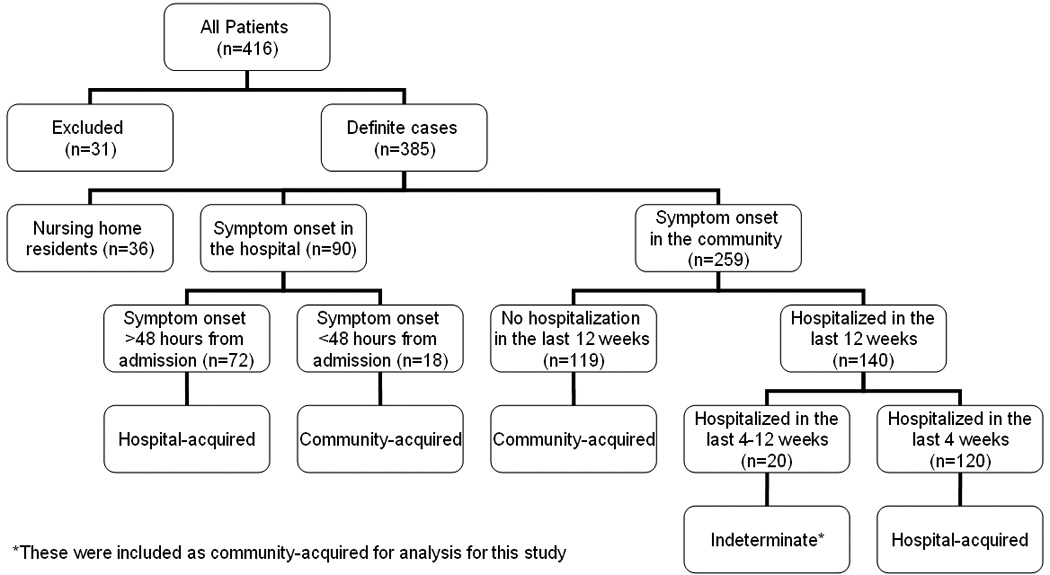
Patient classification
Table 1.
Comparison of community-acquired and hospital-acquired Clostridium difficile infection
| Characteristic | Community- acquired (n=157) |
Hospital-acquired (n=192) |
p-value |
|---|---|---|---|
| Age, median (range) | 50 (0.1–102) | 72 (0.1–99) | <0.001 |
| < 18, n (%) | 21 (13) | 8 (4) | |
| 18–65, n (%) | 87 (55) | 63 (33) | |
| >65, n (%) | 49 (31) | 121 (63) | |
| Female gender, n (%) | 119 (76) | 115 (60) | 0.002 |
| Antibiotic exposure, n (%) | 123 (78) | 181 (94) | <0.001 |
| Acid suppression use, n (%) | 35 (22) | 90 (47) | <0.001 |
| Mean Charlson co-morbidity index | 1.3 | 3.3 | <0.0001 |
| Inflammatory bowel disease, n (%) | 8 (5) | 5 (3) | 0.22 |
| Malignancy diagnosis, n (%) | 26 (17) | 61 (32) | <0.0001 |
| Severe CDI‡, n/N (%) | 32/106 (30) | 60/162 (37) | 0.25 |
| Severe CDI*, n/N (%) | 32/157 (20) | 60/192 (31) | <0.01 |
| Severe complicated CDI, n (%) | 7 (5) | 14 (7) | 0.27 |
| Recurrent CDI, n (%) | 44 (28) | 58 (30) | 0.66 |
CDI = Clostridium difficile infection. Nursing home patients were not included in the comparison of community and hospital-acquired CDI.
Defined as white blood cell count ≥ 15,000 / mm3 or ≥ 50% rise in serum creatinine. Denominators indicate the number of subjects who had at least one of the severity markers assessed.
Secondary data analysis assuming that patients for whom the markers of severity were not available as not having severe CDI.
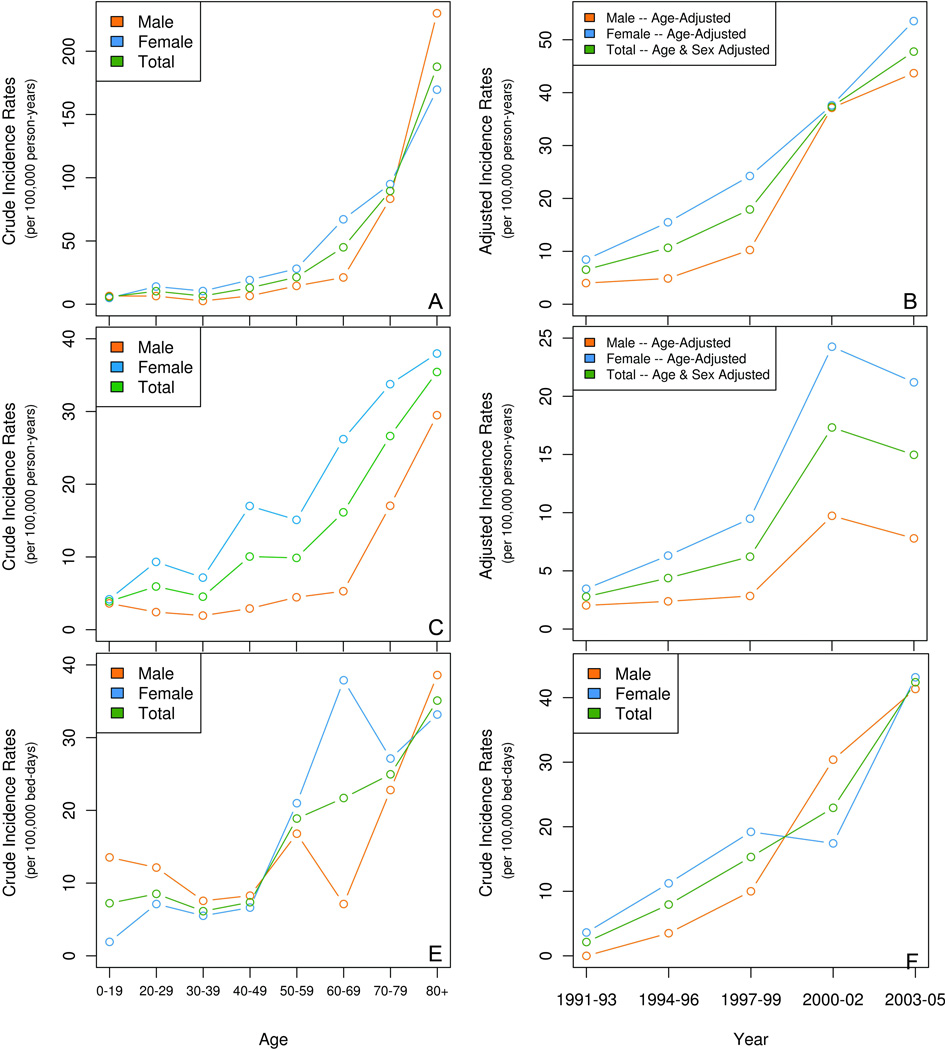
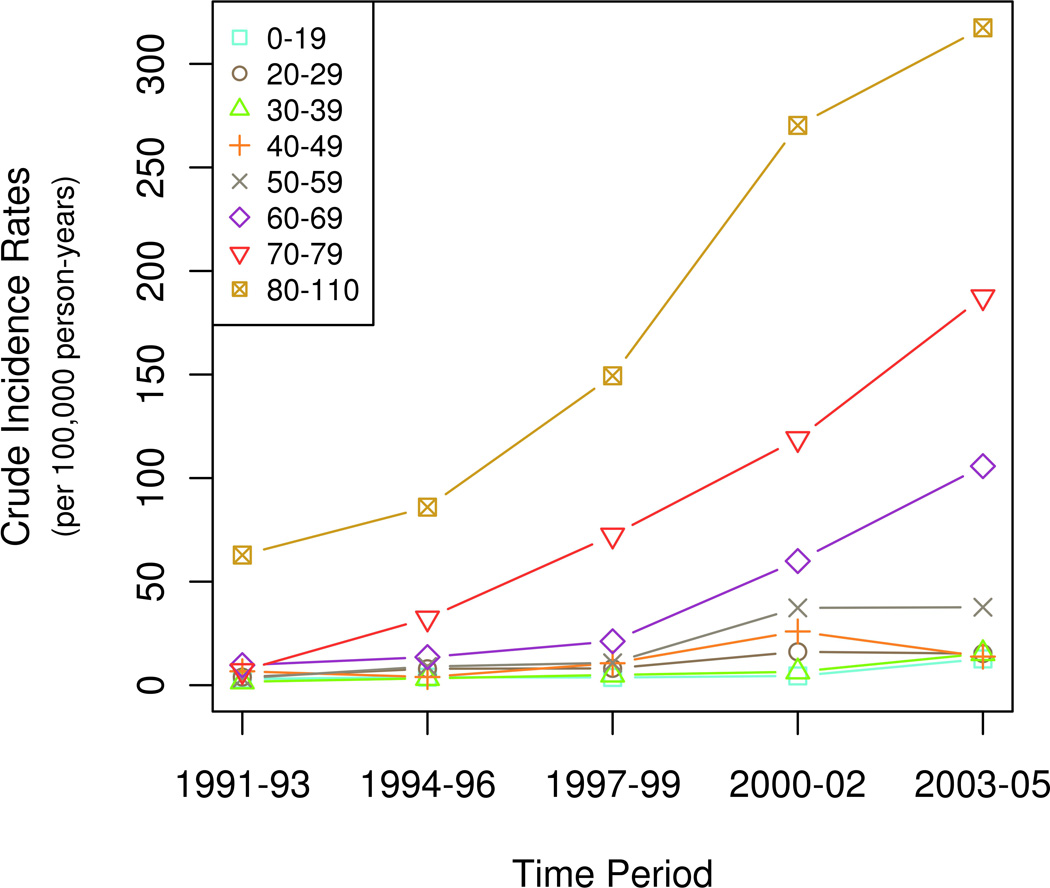
The overall age- and sex-adjusted incidence of CDI was 25.2 / 100,000 person-years. A significant increase in incidence rates by age (p < 0.001) and calendar period (p < 0.001) was observed (Figure 2A & B). The increase in incidence was most striking in the elderly (Figure 3). The effect of age on incidence rates did not change over the different calendar periods.
Figure 2. Incidence rates of Clostridium difficile infection in Olmsted County, Minnesota.
The top two panels show the incidence data for the overall study population, both age-specific (2A), and age and gender adjusted rates over time (2B). The middle two panels show the incidence of community-acquired infection, both age-specific (2C), and age and gender adjusted rates over time (2D). The lower two panels show the incidence of hospital-acquired infection, both age-specific (2E) and by calendar period (2F).
Figure 3. Age-specific incidence rates of Clostridium difficile infection over time in Olmsted County, Minnesota.
The curves depict changes in the incidence of CDI in specific age groups over the duration of the study.
The age and sex-adjusted incidence for community-acquired CDI was 9.6 per 100,000 person-years. The incidence of community-acquired CDI increased with age (Figure 2C, p < 0.001) and calendar period, with a 5.3-fold increase over the study (from 2.8 per 100,000 person-years in 1991–93 to 14.9 per 100,000 person-years in 2003–05) (Figure 2D, p < 0.001). The crude incidence for hospital-acquired CDI was 16.6 per 100,000 bed-days. The incidence of hospital-acquired CDI also increased with age (Figure 2E, p < 0.001) and calendar period, with a 19.3-fold increase over the study period (from 2 per 100,000 person-years in 1991–93 to 40.2 per 100,000 person-years in 2003–05, Figure 2F, p < 0.001).
Among cases of CDI in the elderly, 24% were community-acquired, 60% were hospital-acquired and 16% were nursing home acquired. Also, the overall incidence of CDI in the elderly was higher than in younger patients in all time intervals, and the incidence in elderly subjects increased significantly over the study period (p < 0.01) (Figure 3).
Community-acquired CDI: Clinical Features
Characteristics of the community-acquired and hospital-acquired cohorts are shown in Table 1. Older age was associated with mode of acquisition (60% of elderly cases were hospital-acquired compared with 39% of younger cases, p < 0.001). Community-acquired patients were younger and more likely to be female than hospital-acquired patients. Antibiotic use was also associated with mode of acquisition, with community-acquired CDI patients being less likely to have used antibiotics in the 90-day period prior to diagnosis. Community-acquired CDI patients were less likely to have co-morbidities (as demonstrated by a significantly lower mean Charlson index scores) and were less likely to be on an acid suppressing medication (Table 1).
Outcomes of Community acquired CDI
Laboratory markers of severity were not available in 91 CDI cases (24%). In the patients who had these markers assessed, 106 (36%) had severe infection. Complications from CDI occurred in 27 cases (7%). The proportion of severe (p=0.30) and complicated cases (p=0.94) did not change significantly over the study period.
Upon initial analysis, the proportion of patients with severe infection was not associated with mode of acquisition (Table 1). However, 32% of patients with community-acquired and 16% with hospital-acquired CDI did not have the markers of severity (serum creatinine and white blood cell count) measured. Repeating data analysis considering all the patients for whom the markers of severity were not available as not having severe infection indicated that community-acquired infection was significantly less likely to be severe than hospital-acquired infection (20% vs. 31%, p < 0.01). Excluding the patients who acquired CDI while in a nursing home, mode of acquisition was not significantly associated with severe-complicated CDI (Table 1).
Elderly patients were more likely than younger patients to have severe infection (43% versus 28%, p=0.008), and severe complicated infection (11% vs 3%; p=0.002). Colectomy was required in 2 patients secondary to CDI. There were 16 deaths (4.2%) attributed to CDI in our cohort, with 9 deaths in hospital-acquired CDI, 4 in community-acquired and 3 in nursing home-acquired CDI.
The risk of recurrent CDI in patients with community-acquired infection was 28%, which was not significantly different than in hospital-acquired patients (p=0.66), and did not vary significantly over the study period.
Hospitalization was required in 63 cases (40%) with community-acquired infection. This proportion did not change significantly over the study period. The need for hospitalization was associated with age; among the elderly community-acquired cases, 61% required hospitalization, compared to 31% in younger patients (p < 0.001). Elderly patients with community-acquired infection were twice as likely to need hospitalization as younger patients.
Discussion
Clostridium difficile infection has been traditionally thought of as a hospital-acquired or healthcare-associated infection. However, data from this population-based cohort suggests that a substantial fraction of CDI cases was acquired in the community. We also found a significant increase in the incidence of CDI in both inpatients and outpatients over the study period and, as seen previously, this increase in CDI incidence was most striking in the elderly. Most importantly, our results demonstrate that CDI impacts populations previously thought to be at low-risk, including young adults and children, and those who lack the traditional risk factors of hospitalization or antibiotic exposure. In this cohort, community-acquired CDI was common in younger patients (61% of younger patients acquired infection in the community), the majority of patients were females, and many of them (22%) were not exposed to antibiotics in the 90-day period before acquiring CDI. A recent epidemiological study showed a similar gender distribution and 98.3% of community-acquired CDI cases occurred in patients less than 65 years of age (15). In a case-control study of community-acquired CDI, antibiotic exposure was not present in 52% of CDI patients in the 4 week time period prior to onset (13). In our study, there were no differences in acid-suppression medication use in the community- and hospital-acquired CDI patients. Other studies of community-acquired CDI have reported that a similar lack of antibiotic and proton-pump inhibitor exposure (14, 15). These observations suggest that there may be additional or different risk factors for community-acquired CDI.
The difference in age based on mode of acquisition was expected (4, 21), as hospitalized patients tend to be older than community dwellers in general. However, the community-acquired CDI cohort also was more likely to be female than the hospital-acquired cohort. The reason for the difference in gender is not clear. We considered that it might be related to differential exposure to antibiotics, with females being more likely to seek medical attention (and therefore be exposed to antibiotics) in the outpatient setting than men, while in hospitalized patients, exposure to antibiotics is less likely to be related to gender. However, we found no significant association between gender and antibiotic use, and the effect of gender on acquisition mode was not modified by antibiotic use.
In our cohort, there were no differences in severity or recurrence rates between community-acquired and hospital-acquired CDI on initial analysis. However, the lack of difference in severity should be interpreted with caution. A significant proportion of patients did not have the markers of severity (serum creatinine and white blood cell count) measured. Assuming that these patients were not so severely ill as to warrant a clinician to order these tests, we reanalyzed the data considering patients in whom these markers were not available as having mild-moderate infection. This secondary analysis suggested that community acquired infection was significantly less likely to be severe than hospital-acquired infection.
As reported previously, we also found that CDI was more common and more severe in the elderly and that the increase in incidence over the study period was most striking in the oldest subset of our cohort. The majority of the elderly cases were hospital-acquired, and were more likely to have severe or severe complicated infection or required hospitalization in the community-acquired elderly patients.
There has been a significant increase in severe cases, colectomies, and death related to CDI in recent reports (7, 12). In one of these studies, severe disease was present in 12% of patients, but was 2.7 fold higher in the elderly (22). The rates for colectomy and mortality secondary to CDI has increased from 2.5-fold to 5-fold in different studies from 1993–2004 (23–25). Using the recent consensus definitions (20), severe disease occurred in 36% of our cohort and severe complicated occurred in 7%, with no significant change over the study period. As reported previously, both occurred in a significantly higher proportion of the elderly in our study.
Our findings are consistent with several other reports which show an increase in the incidence and severity of CDI world-wide since the mid to late 1990s (3, 5, 7, 10, 12, 15, 26–29). For example, there was a large outbreak in Quebec, with a four-fold increase in CDI, increasing severity and, unlike in our study, a 2.3-fold increased risk of recurrence (47% compared to 21%) over a 13-year period, with a mortality of 6.9% (5, 8, 27, 30). The Canadian Nosocomial Infection Surveillance Program Study estimated the incidence rate for CDI in adult patients admitted to hospitals as 4.6 cases per 1000 patient admissions and 65 per 100,000 patient-days (31) which was 4-fold higher than the overall crude incidence rate observed in our cohort. However, these studies did not include community-dwelling subjects with CDI, which would lead to an over-estimate of overall incidence since the incidence in community-dwellers is lower than in hospitalized subjects. In addition, we chose to exclude 30 patients with probable CDI due to lack of a confirmatory stool assay.
In large retrospective studies in the United States, the incidence of hospital-acquired CDI increased by 2 to 2.5 fold from the late 1990s to the early 2000s, especially in the elderly (7, 10). The rate of hospitalization due to CDI increased by about 23% per year from 2000 to 2005 (25), but from 2005 through 2006, the rate increased by only 6.7% (28). In contrast, in our cohort, the rate of hospitalization for community-acquired CDI did not vary significantly over the study period.
The majority of CDI epidemiological data is derived from hospital-based reports and administrative databases, such as the US National inpatient sample data and the national mortality data for CDI surveillance (24, 25, 29). There have been relatively few studies describing the epidemiology and characteristics of community-acquired CDI (4, 13, 15, 21, 32, 33). Most of the CDI studies use hospital discharge data and laboratory data as the primary source of case identification. The use of hospital discharge data has the potential to miss community-acquired cases, and laboratory databases have the potential to include false positive CDI as these patients might be colonized with Clostridium difficile but not have symptomatic infection.
The major strength of our study is that data has been collected over a period of 15 years from a stable population. Collecting data from a population-based cohort allowed us to study and compare the epidemiology of CDI in both hospital- and community-acquired cohorts. The resources of the Rochester Epidemiology Project allowed the identification of all cases of CDI in county residents and access to all the medical record information for each case. However, we were unable to analyze of trends in the use of Clostridium difficile stool testing in county residents over the study period due to unavailability of this data, thus limiting our ability to assess for diagnostic detection bias. Other limitations of this study include some missing data, such as laboratory tests used for defining severe CDI, and lack of information on Clostridium difficile strain, which was not being collected in our laboratory during the time period of this study. Finally, during the course of this study, the diagnostic test for CDI transitioned from stool cytotoxicity assay to enzyme immunoassay (EIA). Since EIA has a lower sensitivity than the cytotoxicity assay, it is likely that this change in diagnostic test underestimated the true increase in incidence that we saw over the study period.
Conclusions
The incidence of CDI increased significantly over time in both community-dwelling and hospitalized Olmsted county residents. A significant proportion of CDI occurred in community dwellers who were younger, less likely to have been exposed to antibiotics, and less likely to have severe infection than patients with hospital-acquired infection. A significant proportion of CDI cases would be missed and demographic and severity data would be skewed if hospital data were used as the only source for CDI epidemiological studies. Given the significant proportion of our cohort who had community-acquired infection, CDI should be considered in all outpatients with acute diarrhea.
Study highlights.
What is current knowledge?
Clostridium difficile infection is increasing worldwide with hospitalization and antibiotic exposure as the most common risk factors.
The epidemiology and characteristics of community-acquired Clostridium difficile infection are not well defined.
What is new here?
A major proportion of Clostridium difficile infection patients is community-acquired.
These patients are younger, often lack traditional risk factors, and have less severe disease than patients with hospital-acquired infection.
Acknowledgments
Disclosures: This research was supported in part by an unrestricted research grant from ViroPharma and made possible by the Rochester Epidemiology Project (Grant number R01 AG034676 from the National Institute of Aging).
Footnotes
Conflict of Interest
Guarantor of the article: Darrell S. Pardi, MD, MS, FACG.
Specific author contributions: Study design, data collection, statistical analysis and drafting the manuscript: Sahil Khanna, Darrell Pardi, Scott Aronson and Patricia Kammer; study design and drafting the manuscript: Robert Orenstein; study design, statistical analysis and drafting the manuscript: Jennifer L. St. Sauver, W. Scott Harmsen and Alan R. Zinsmeister.
Financial support: Data collection and statistical analysis were supported in part by an unrestricted research grant from ViroPharma. The Rochester Epidemiology Project is funded via grant number R01 AG034676 from the National Institute of Aging.
Potential competing interests: None
References
- 1.Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. New Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk--four states 2005. Morb Mortal Wkly Rep. 2005;54:1201–1205. [PubMed] [Google Scholar]
- 5.Loo VG, Poirier L, Miller MA, et al. A Predominantly Clonal Multi-Institutional Outbreak of Clostridium difficile-Associated Diarrhea with High Morbidity and Mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 6.McDonald LC, Killgore GE, Thompson A, et al. An Epidemic, Toxin Gene-Variant Strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 7.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 8.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 9.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 10.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals 1996–2003. Emerg Infect Dis. 2006;12:409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oake N, Taljaard M, van Walraven C, et al. The Effect of Hospital-Acquired Clostridium difficile Infection on In-Hospital Mortality. Arch Intern Med. 2010;170:1804–1810. doi: 10.1001/archinternmed.2010.405. [DOI] [PubMed] [Google Scholar]
- 12.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox MH, Mooney L, Bendall R, et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62:388–396. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 14.Naggie S, Miller BA, Zuzak KB, et al. A Case-control Study of Community-associated Clostridium difficile Infection: No Role for Proton Pump Inhibitors. Am J Med. 2011;124:276 e1–276 e7. doi: 10.1016/j.amjmed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Fellmeth G, Yarlagadda S, Iyer S. Epidemiology of community-onset Clostridium difficile infection in a community in the South of England. J Infect Public Health. 2010;3:118–123. doi: 10.1016/j.jiph.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for Surveillance of Clostridium difficile-Associated Disease. Infect Control Hosp Epidemiol. 2007;28:140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Surveillance for community-associated Clostridium difficile--Connecticut, 2006. Morb Mortal Wkly Rep. 2008;57:340–343. [PubMed] [Google Scholar]
- 22.Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States 1999–2004. Emerg Infect Dis. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciardi R, Rothenberger DA, Madoff RD, et al. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–631. doi: 10.1001/archsurg.142.7.624. discussion 631. [DOI] [PubMed] [Google Scholar]
- 25.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States 2000–2005. Emerg Infect Dis. 2008;14:929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30:526–533. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 27.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zilberberg MD. Clostridium difficile-related hospitalizations among US adults 2006. Emerg Infect Dis. 2009;15:122–124. doi: 10.3201/eid1501.080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States 2000–2005. Pediatr Infect Dis J. 2008;27:1111–1113. doi: 10.1097/inf.0b013e31817eef13. [DOI] [PubMed] [Google Scholar]
- 30.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravel D, Miller M, Simor A, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48:568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 32.Dubberke ER, Butler AM, Hota B, et al. Multicenter study of the impact of community-onset Clostridium difficile infection on surveillance for C. difficile infection. Infect Control Hosp Epidemiol. 2009;30:518–525. doi: 10.1086/597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaugerie L, Flahault A, Barbut F, et al. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment Pharmacol Ther. 2003;17:905–912. doi: 10.1046/j.1365-2036.2003.01531.x. [DOI] [PubMed] [Google Scholar]