Abstract
Ca2+ signaling is an important determining factor in many cellular processes, especially in cancer cell proliferation, motility and invasion. Glioblastoma is the deadliest brain cancer with its average survival time of less than a year, with the most prominent cellular feature being the ability of these cells to migrate to and invade the neighboring tissue. We hypothesized that disturbing the Ca2+ signaling pathway would decrease the propensity for these cells to migrate. Thus, we investigated the detailed Ca2+ signaling pathway of the glioblastoma cells in response to various receptor tyrosine kinases (RTK) and G-protein coupled receptor (GPCR) agonists. Here we report that caffeine, which is a well-known activator of ryanodine receptors (RyRs), paradoxically inhibits inositol-1, 4, 5-triphospate receptor(IP3R)-mediated Ca2+ increase by selectively targeting IP3R subtype 3(IP3R3), whose mRNA expression is significantly increased in glioblastoma cells. Consequently, by inhibiting IP3R3-mediated Ca2+ release, caffeine was found to inhibit the invasion and migration of various glioblastoma cell lines in scrape motility, Matrigel invasion, soft agar, and brain slice implantation assays. In a mouse xenograft model of glioblastoma, caffeine intake via drinking water greatly increased mean survival duration of subject animals. These findings propose IP3R3 as a novel target for glioblastoma treatment and that caffeine may be a useful adjunct therapy that slows glioblastoma invasion and migration by selectively targeting IP3R3.
Introduction
Glioblastoma, the most frequent and malignant tumor in the central nervous system, has a very poor prognosis, with a median survival of only one year after diagnosis (1, 2). Total surgical removal of glioblastoma is rarely possible because of the widespread infiltration of brain by neoplastic cells and nearly all tumors will ultimately fail adjuvant therapy and recur. Thus, the fundamental source of treatment failure is the insidious propensity of tumor cells to invade normal brain structures (2, 3). A host of extracellular signaling molecules activate glioblastoma cells to affect proliferation, motility, and invasiveness. These signaling molecules include various growth factors such as EGF and PDGF, and GPCR agonists such as ATP, bradykinin, lysophosphatidic acid, S1P, thrombin, and plasmin (2). They in turn activate cell surface receptors such as EGFR, PAR1, B2, P2Y, LPA, S1P receptors (4–6) and modulate downstream effectors of the intracellular signaling pathway. An important consequence of the intracellular signaling is an increase in intracellular Ca2+ concentration ([Ca2+]i), which is well known to be a critical signal for gene expression, motility, differentiation, and survival. Furthermore, many GPCRs are known to transactivate and converge onto EGF receptors in various cancer cells (4), aberrantly exacerbating the Ca2+ signaling and other signaling cascades.
Cancer cell migration depends mainly on actin polymerization and intracellular organization of various cytoskeletal proteins, which are influenced by a variety of actin binding proteins (7, 8). Regulation of actin binding protein activity is mediated by second messengers such as phosphoinositides and calcium (7, 8). Therefore, the precise mechanism of receptor-mediated Ca2+ increase in glioblastoma cells is an important factor for controlling proliferation, motility, and invasiveness of these cells (9, 10). However, to date, only a limited number of studies have been conducted with regard to Ca2+ signaling in glioblastoma cells.
Caffeine, a well known activator of RyR, has been reported to display anticancer effects (11, 12). Caffeine and its analogs have diverse effects on pain, Alzheimer’s disease, asthma, cancer, diabetes, and Parkinson’s disease (13). Recent studies have shown that caffeine inhibits metastasis in a mouse mammary tumor model and UV-induced skin cancer in nude mouse (11, 12). Therefore, we investigated the detailed Ca2+ signaling pathway of the glioblastoma cells in response to various RTK and GPCR agonists and examined the possible target of caffeine.
Materials and Methods
Human surgical tissue samples
All the fresh, surgically removed tissue samples examined in this study were histologically diagnosed as glioblastoma according to WHO classification. The primary human glioblastoma cells and astrocytes were obtained from brain tissue of the Brain Bank of Seoul National University Hospital. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB Approval #: H-0B05-036-243).
Cell culture
The primary human glioblastoma cells and astrocytes from the Brain Bank of Seoul National University Hospital were enzymatically dissociated to single-cell from mechanically dissected glioblastoma and temporal lobe tissues. The cells were then suspended in DMEM (Gibco) supplemented with 20% FBS (Gibco). Cultured human glioblastoma cell lines (U178MG, U87MG, T98G, U373MG, and M059K) were maintained in DMEM supplemented with 10% FBS, penicillin (50 units/mL), and streptomycin (50 units/mL). wtEGFR and ΔEGFR are the U87MG cell line that has a wild type EGFR or EGFRvIII (ΔEGFR) mutation, which causes a constitutively active tyrosine kinase activity. These cells were maintained in the same medium containingm 200 ug/ml G418 as described previously (14–16).
Calcium imaging
For imaging, all cell lines were cultured as monolayers on 12 mm glass coverslips coated with poly-D-lysine (Sigma). Glioblastoma cells were incubated with 5 μM Fura 2-AM plus 1 μM pluronic acid (Molecular Probes) for 30 min at room temperature. External solution contained (in mM): 150 NaCl, 10 HEPES, 3 KCl, 2 CaCl2, 1 MgCl2, 22 sucrose, 10 glucose; pH adjusted to 7.4 and osmolarity to 325 mOsm. INDEC Imaging Workbench version 5.2.10 was used for acquisition of intensity images and conversion to ratios. Thrombin (Sigma), S1P (Sigma), LPA (Sigma), TFLLR (Peptron), 2-MT-ATP (Sigma), Bradykinin (Sigma), or EGF (Sigma) were used as GPCR and RTK agonists.
Scrape motility assay
All cell lines were grown as monolayers in 12-well culture plates in serum-containing media. Scrapes were made with a 10 μL pipet tip. After Caffeine (Sigma), Thapsigargin (Tocris), or Ryanodine (Tocris) were added, plates were returned to the incubator. To prevent proliferation, 10 μM fluorodeoxyuridine/uridine (FdU/U; Sigma) was added. After incubation for 24 hours, the cells were fixed in 4% paraformaldehyde. The areas of repopulation of three 100 X fields within the scrape areas were determined and the mean percentage of scrape area wound closure was determined.
Matrigel invasion assay
Cell invasion was assayed using transwell inserts containing 8μm pore size (Corning, NY, USA) in 24-well culture plates. For invasion assay, inserts were coated with 2 mg/ml basement membrane Matrigel (BD Bioscience, Bedford, MA, USA). 1 × 105 cells in serum-free medium were plated onto the upper side of insert and complete medium was placed in the lower chamber to act as a chemoattractant. After 24 h of incubation at 37 °C, the cells on the upper side of insert were removed by wiping with a cotton swab. The cells migrated to the lower side of membrane were stained with DAPI and randomly photographed under microscope at 200 × magnification. The mean number of untreated cells was considered as 100% invasion. Each condition was duplicated and five fields randomly selected and counted for each assay. Caffeine (Sigma), DPCPX (Tocris), Bicuculline (Tocris), or IBMX (Sigma) were added at the time of cell plating.
Soft agar colony formation assay
Cells (1 × 104) were seeded into 6-well plates in a soft agar (0.3%) overlaying a 0.6% base agar. The solidified cell layer was covered with medium containing caffeine which was replaced every 4 days. Cells were incubated at 37 °C for 14~17 days to allow colonies to develop. Afterward colonies were stained with 0.05% cresyl violet and photographed. Each experiment was done in triplicate.
Transfection
HEK 293T cells were transfected with plasmids carrying various IP3R subunits using Effectene Transfection Reagent (Qiagen) according to the manufacturer’s instructions. U178MG cells were transfected with plasmids carrying GFP or IP3R3-shRNA-GFP by using electroporation. A single voltage (1100V) was given for 30 ms by Microporator (Seltagen).
IP3 uncaging
U178MG cells were co-labeled with 200 μM NPE-caged Ins 1,4,5-P3(Invitrogen) and 100 μM Oregon green 488 BAPTA-2 (Invitrogen) by using elecetroporation. Co-labeled U178MG cells were visualized using Olympus Fluoview FV1000 confocal microscope. Caged IP3 was uncaged by 50% of 405 laser for 100 ms. Increases in fluorescence intensity over baseline within ROI were analyzed using Fluoview ver. 1.7a software.
Organotypic brain slice invasion model
Organotypic hippocampal slice cultures were prepared and maintained as described previously (17). Organotypic glioma invasion model was followed by some modification of the procedure as previously described (18). Briefly, DiI-stained U178MG cells (~5000 cells) were gently placed on the slices in the absence or presence of caffeine 6 days after slice preparation. After 1 h and 120 h, movement of the glioma cell in the slices was detected with an inverted confocal laser scanning microscope (Zeiss LSM5, Carl Zeiss). Image J software (NIH) was used to calculate the invasion area of DiI-stained cells. Invasion Area (%) = (Area of DiI-stained cells at 120 h/Area of DiI-stained cells at 1h) × 100.
Tumor xenografts in nude mice
We used U87MG cell line in the tumor xenograft models because this cell line has been widely used due to their high tumorigenicity in nude mouse. The U178MG cells did not show any tumorigenicity in nude mouse in the initial attempts to establish xenograft model. Five-week-old athymic mice (Balb/c nu/nu) were obtained from Central Laboratory Animal Inc. (Japan). For the xenograft tumor growth assay, U87MG cells (3 × 106 cells/150 μl PBS) were injected subcutaneously into the right flanks of the mice (n=5~10 mice per group). From the day 7 after injection and on, caffeine (Sigma) was given as drinking water (1 mg/ml). The control animals were given distilled water. Balb/c nude mice bearing U87MG were randomized into two groups (control and caffeine; n=13 per group) on day 7 after tumor inoculation. Caffeine (1 mg/ml) was delivered orally on the day of ramdomization. Tumor size was measured weekly for 28 days with the use of caliper. Tumor size was measured twice per week for 4 weeks, and tumor volumes were calculated by the formula: volume = length × width2/2. The effect of the caffeine was determined by the growth delay. To investigate the effects of caffeine on the survival, an orthotopic implantation model was established with U87MG. U87MG cells (2.5 × 105 cells/5μl PBS) were implanted by intracranial injections in the left frontal lobe at coordinates 2 mm lateral from the bregma, 0.5 mm anterior, and 3.5 mm intraparenchymal. Caffeine (1 mg/ml) was given to mice from 1 week before inoculation. Mice were monitored daily for general appearance, behavioral changes, and neurological deficits. Mice were sacrificed when moribund. All prototols were approved by the Gyeongsang National University IACUC. The survival data were analyzed by log rank Kaplan-Meier method using SigmaStat (Version 3.0).
Caffeine concentration in serum and brain
After 2 weeks administration of caffeine through drinking water (1 mg/ml), animals were sacrificed. Serum and brain tissue contents of caffeine were measured by HPLC as previously described (19).
Microarray analysis
The microarray analysis was performed on tissue samples of 34 normal brain tissue from lobectomy patients and 51 glioma from human glioblastoma patients. The detailed method is available in the Supplementary Materials & Methods.
Results
Ca2+ signaling in glioblastoma cells
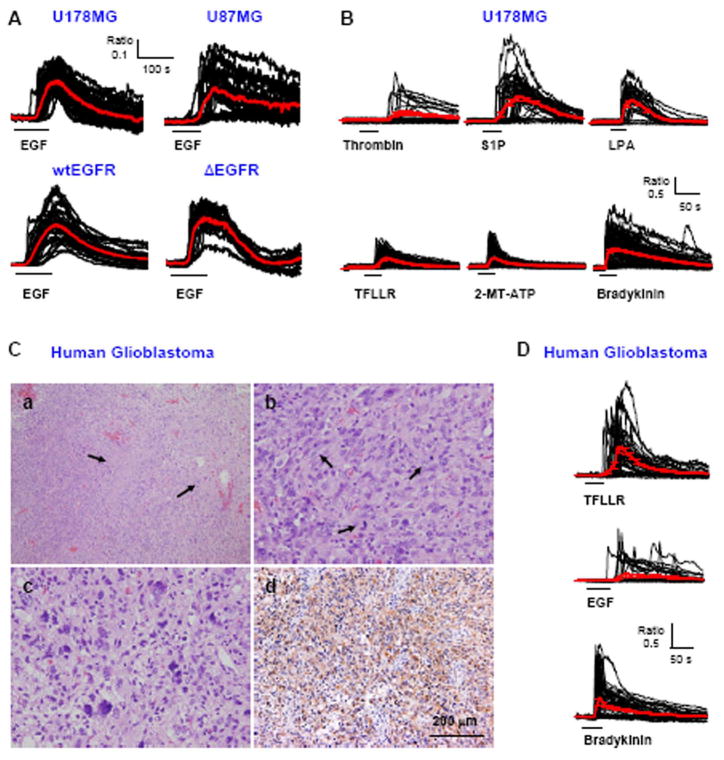
We first examined the effect of activating RTKs and GPCRs on Ca2+ signaling pathway by performing Ca2+ imaging experiments from Fura2-AM loaded, cultured human glioblastoma cell lines and acutely dissociated glioblastoma cells prepared from surgically removed tissue. Abath application of 100 ng/ml EGF to cultured U178MG and U87MG glioblastoma cell lines induced a robust [Ca2+]i increases (Figure 1A). EGF induced Ca2+ responses regardless of the status of EGFR- whether it was wild type or the constitutively active deletion mutant ΔEGFR of U87MG cell line (Figure 1A). U178MG cells also showed robust Ca2+ responses by various GPCR agonists (Figure 1B). The acutely dissociated glioblastoma cells prepared from surgically removed tissue (Figure 1C) also showed robust Ca2+ responses by TFLLR, EGF, and bradykinin (Figure 1D). These results demonstrate the functional expression of various RTKs and GPCRs, whose activation leads to increases in [Ca2+]i.
Fig. 1. Ca2+ responses by various GPCR and RTK agonists.
A. Traces from Ca2+ imaging recordings performed in U178MG, U87MG, wtEGFR-U87MG, or Δ EGFR-U87MG cells. Each trace represents a Ca2+ response in one cell (n=36–83 per cell line). Red lines represent average responses with error bars indicating standard error of mean (SEM). Black horizontal bars show time and duration of 100 ng/ml EGF application. B. Ca2+ responses by 100 nM Thrombin, 10 μM S1P, 30 μM LPA, 30 μM TFLLR, 100 μM 2-MT-ATP, and 10 μM Bradykinin in U178MG. C. (a) Low magnification view of this tumor showing cellular glial tumor with two foci of pseudopalisading necrosis (arrows) (H&E). (b) The tumor shows frequent mitotic cells (arrows) (H&E), (c) Mutinulcleated pleomorphic nuclei are present. (H&E). (d) Most of the tumor cells are immunoreactive for GFAP. (GFAP immunostaining). D. Ca2+ responses induced by GPCR and RTK agonists in the primary human glioblastoma cells.
We found that an increase in [Ca2+]i in these cells was contributed in part by a release of Ca2+ from intracellular release pools and subsequently by a Ca2+ entry through the store operated channels (Supplementary Figure 1A,B,C). The release of Ca2+ from intracellular stores was completely inhibited by 1μM U73122, an inhibitor of phospholipase C (PLC), which produces IP3 by metabolism of phosphoinositol-4,5-bisphosphate (PIP2) in response to an activation of GPCRs and RTKs (Supplementary Figure 1D). From these results we concluded that glioblastoma cells express various surface receptors that are coupled to the common phosphoinositide pathway that leads to Ca2+ release from intracellular stores and subsequent Ca2+ influx through store operated channels.
Caffeine inhibits IP3- mediated Ca2+ release
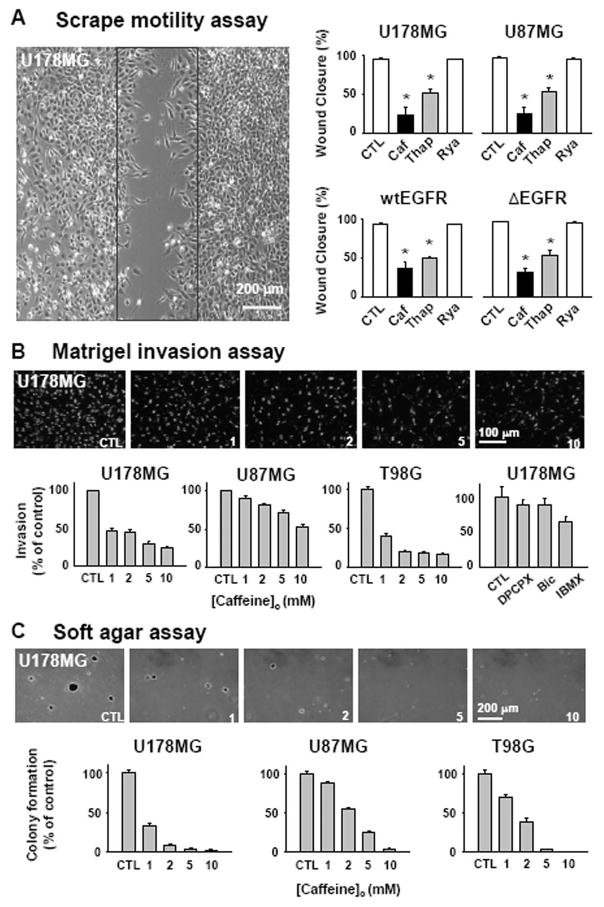
There are two known ion channels primarily responsible for release of Ca2+ from intracellular stores; IP3Rs and RyRs. Caffeine has been classically known to induce a release of Ca2+ from intracellular stores by opening RyRs, especially in muscle cells and cardiac myocytes (13). Thus, we tested caffeine along with other agents that enhance or disturb the Ca2+ release machinery in various assays for glioblastoma motility, invasion, and proliferation. Contraryto our expectations, we found that caffeine significantly and concentration-dependently (1~10 mM) inhibited the motility, invasion, and proliferation of various human glioblastoma cell lines, including U178MG, U87MG, and T98G cells (Figure 2A,B,C), while minimally affecting the cell viability at this concentration range (data not shown). This paradoxical effect of caffeine was mimicked by other agents that are known to disturb intracellular concentration of Ca2+, such as 1 μM thapsigargin (Figure 2A), 10 μM 2-APB, 20 μM CPA, and 50 μM BAPTA-AM (data not shown). However, 10μM ryanodine, an agonist of RyRs at this concentration, did not demonstrate this inhibitory effect on migration and invasion (Figure 2A). Furthermore, the blocking concentration of ryanodine at 100 μM was not able to inhibit the bradykinin induced Ca2+ increase (Supplementary Figure 1E). These results suggested that caffeine’s mode of action might not be related to opening of RyRs
Fig. 2. Caffeine slows motility, invasion, and colony formation of glioblastoma cells.
A. Monolayers of glioblastoma cells were wounded by a scrape (black box) and treated with 10 mM caffeine (Caf), 1 μM thapsigargin (Thap), or 10 μM ryanodine (Rya). All error bars represent SEM. (*p <0.01, ANOVA with Newman-Keuls post hoc.). B. (Top) representative pictures of DAPI labeled cells that invaded through 8 μm holes in the Matrigel inserts in the presence of indicated caffeine concentration. (Bottom) percentage of invaded cells respect to the control condition. Similar experiment was done with 800nM DPCPX, 10 μM Bicuculline, 100 μM IBMX and % of invasion was plotted (last panel). C. Caffeine effect on the anchorage-independent growth of glioblastoma cells in vitro was tested. (Top) representative photographs of colonies grown in indicated caffeine concentration. (Bottom) percentage of number of colony normalized to the control condition.
Caffeine has been shown to inhibit adenosine receptors, GABAA receptors, phosphodiesterase activity, and G2 checkpoint for repair of damaged DNA (13). We tested whether modulating these target molecules affects the invasiveness of glioblastoma cells. The A1 adenosine receptor blocker, 800 nM DPCPX, GABAA receptor blocker 10μM bicuculline, and phosphodiesterase inhibitor, 100μM IBMX had no significant effect on invasion (Figure 2B last panel), suggesting that the caffeine’s mode of action might be at other targets.
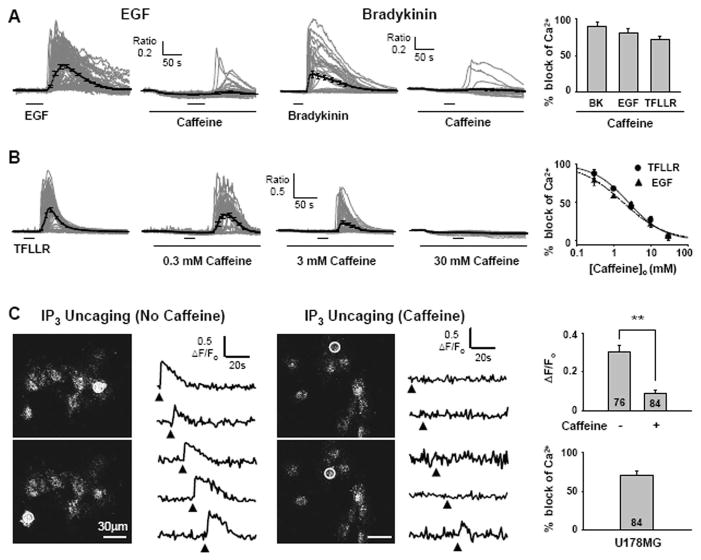
Caffeine has been also reported to inhibit IP3Rs without affecting IP3 binding at millimolar concentrations (20), possibly by competing at thew ATP binding site of IP3Rs (21). Therefore, we tested whether caffeine can inhibit the increase in [Ca2+]i upon activation of GPCRs and RTKs in cultured U178MG cells. We found that caffeine significantly inhibited the bradykinin-, EGF-, and the PAR1 agonist TFLLR-induced increase in [Ca2+]i (Figure 3A,B) in a concentration-dependent manner with half maximal concentration of 2.45 mM and 1.81 mM for TFLLR- and EGF-induced responses, respectively (Figure 3B). This inhibitory action of caffeine was not due to an inhibition of store operated channels (SupplementaryFigure 2A), a depletion of Ca2+ stores (Supplementary Figure 2B,C,D), or an activation of RyRs (Supplementary Figure 1E), leaving an inhibition of IP3Rs as the most likely mode of action by caffeine.
Fig. 3. Caffeine reduces GPCR and RTK induced Ca2+ increase by inhibiting IP3R.
A. EGF or bradykinin induced Ca2+ responses in the absence or presence of caffeine in U178MG cells. 10 mM caffeine was treated 100 s before stimulation with indicated agonist. % block of various agonists induced Ca2+ release by 10 mM caffeine in U178MG cells. The peak of average Ca2+ response trace in the presence of caffeine was divided by the average of Ca2+ response in the absence of caffeine. (N=3) B. TFLLR-induced Ca2+ responses in the presence of 0.3 mM, 3 mM, and 30 mM caffeine. Concentration-effect curve of Ca2+ increase evoked by TFLLR (IC50: 2.45 mM) or EGF (IC50: 1.87 mM). C. Left panels show intensity images of U178MG cells loaded with caged IP3 and Oregon green 488 BAPTA-2. White circles indicate region of interest exposed to a 405 nm laser for uncaging of IP3. Right traces are representative fluorescence changes upon laser stimulation (filled triangle) over time in the absence of caffeine or in the presence of 10 mM caffeine. The average fluorescence intensity changes in the presence or absence of caffeine at the peak of Ca2+ transients (**p <0.001 by student’s t-test). Summary of IP3 uncaging experiment. Caffeine blocks 71% of Ca2+ release in U178MG cells.
To determine if caffeine’s inhibitory action on Ca2+ increase is due to a direct action on IP3R’s, we performed IP3 uncaging experiment on U178MG cells that were electroporatically loaded with caged-IP3 and Ca2+ indicator dye, Oregon Green 488 BAPTA-2. UV flashing of each cell induced uncaging of IP3 and subsequent increase in [Ca2+]i. This effect was significantly inhibited in the presence of caffeine (Figure 3C), supporting the conclusion that caffeine directly inhibits IP3R mediated Ca2+ release.
IP3R3 is required for caffeine sensitivity
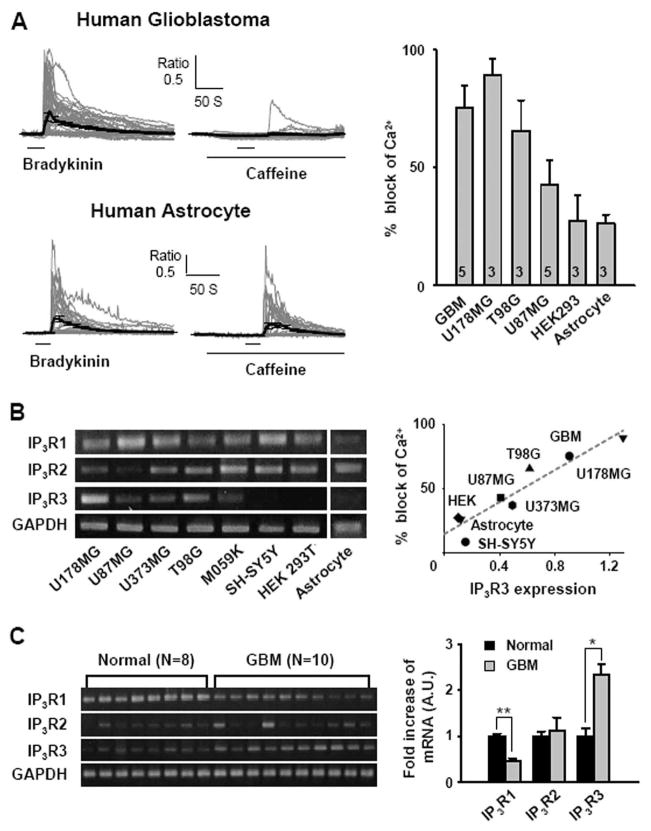
We then tested whether caffeine’s inhibitory action on Ca2+ responses was also observed in other cell types. We found that a variety of cell types displayed varying degree of inhibition of Ca2+ responses by caffeine, with the highest block in U178MG and the lowest block in human astrocytes (Figure 4A). To see if the degree of block by caffeine is correlated with IP3R expression, we performed semi-quantitative RT-PCR for three subtypes of IP3R mRNA for each cell type (Figure 4B). Of the three subtypes of IP3R, expression of IP3R subtype 3 (IP3R3) showed the highest correlation with the percent block of Ca2+ responses (coefficient of correlation r2=0.891, p<0.001; Figure 4B), suggesting that caffeine’s effect on Ca2+ increase might be linked to the expression level of IP3R3. We also performed semi-quantitative RT-PCR for the three subtypes of IP3R on glioblastoma patient tissue samples and compared with normal tissue samples from lobectomy surgery. We found that glioblastoma tissue displayed on average more than 2-fold increase in expression of IP3R3 mRNA compared to the normal tissue, whereas IP3R2 mRNA was unchanged and IP3R1 significantly decreased (Figure 4C). This is consistent with the high percentage of block of Ca2+ responses by caffeine in acutely prepared human glioblastoma cells (Figure 4A). These results indicate that the expression of IP3R3 is significantly correlated with the caffeine’s inhibitory action on Ca2+ responses.
Fig. 4. Correlation between IP3R3 and block of Ca2+ release by caffeine.
A. Block of bradykinin-induced increase in [Ca2+]i by caffeine on the primary human glioblastoma cells and astrocytes. Summary of block of GPCR agonist-induced Ca2+ responses by caffeine on various cell types. B. mRNA expression of IP3Rs and GAPDH was tested by semi-quantitative RT-PCR in various human glioblastoma cell lines (U87MG, U178MG, U373MG, T98G, M059K), human neuroblastoma cell line (SH-SY5Y), human embryonic kidney cell line (HEK293T), and human astrocyte. Correlation between expression of IP3R subtype 3 normalized to GAPDH in each cell type and caffeine block of agonist induced Ca2+ responses. (r 2=0.891, p<0.001) C. Semi-quantitative RT-PCR of IP3R subtypes in normal human brain and human glioblastoma tissue samples. Averages of densitometric measurement of IP3R mRNA expression in human samples, normalized to the normal human brain tissue sample (*p<0.001, **p<0.0001 by student’s t-test).
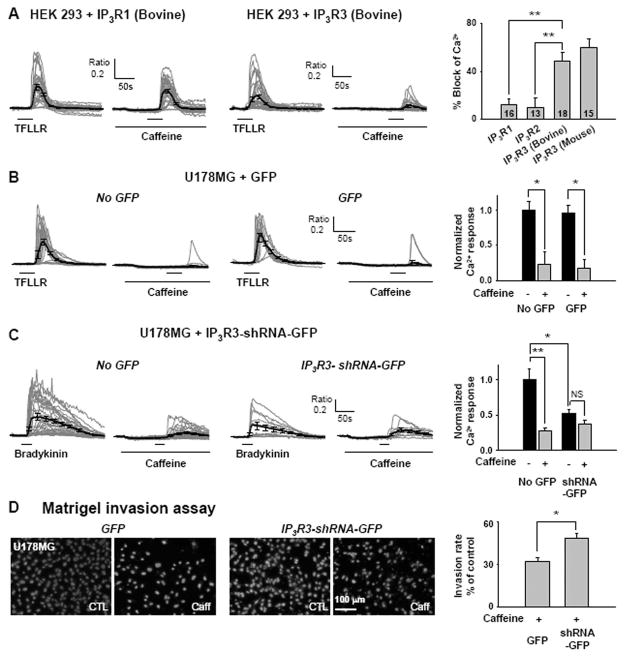
To confirm that caffeine’s inhibitory action on Ca2+ response is specific to IP3R3, we either over-expressed IP3R3 into HEK 293T cells which normally lack IP3R3, or inhibited the expression of IP3R3 using small hairpin-forming RNA (shRNA) in U178MG cells which highly express IP3R3. In HEK 293T cells heterologously expressing IP3R3, the block percentage of TFLLR-induced Ca2+ responses by caffeine was significantly increased, compared to the IP3R1 or IP3R2 over-expressing HEK 293T cells (Figure 5A). To silence the IP3R3 mRNA in U178MG cells, we first developed a shRNA that is specific to IP3R3 mRNA by screening several candidate hairpin-forming oligomers, one of which (candidate #3) selectively reduced the IP3R3 mRNA expression without affecting mRNA levels for other IP3R subtypes (Supplementary Figure 3A). Subsequent testing of the shRNA on U178MG cells revealed that gene-silencing of IP3R3 in U178MG suppressed the caffeine sensitivity of Ca2+ responses in these cells (Figure 5C, Supplementary Figure 3B), but not by the control vector containing GFP only (Figure 5B, Supplementary Figure 3B). Interestingly, gene-silencing of IP3R3 in U178MG caused a general reduction in TFLLR- or bradykinin-induced Ca2+ responses (Figure 5C), suggesting that IP3R3 is the major Ca2+ release channels in these cells. These results indicate that IP3R subtype 3 is critical for the caffeine’s inhibitory action. Moreover, glioblastoma cells show aberrant increase in expression of this subtype (Fig 4C), providing a potential target for treatments aimed at altering glioblastoma migration and invasion.
Fig. 5. IP3R 3 is required for caffeine sensitivity.
A. Block of TFLLR-induced increase in [Ca2+]i by 10 mM caffeine in HEK293T cells transfected with IP3R1(Bovine) and IP3R3(Bovine). Summary of % block by caffeine in HEK293T cells transfected with IP3R1(Bovine), IP3R2(Bovine), IP3R3(Bovine), or IP3R3(Mouse) (**p<0.001 by Student’s t-test). B. Ca2+ responses of GFP negative and positive cells on U178MG transfected with vector containing only GFP. Ca2+ responses in each condition are normalized to Ca2+ responses in GFP negative without caffeine treatment (*p<0.05 by Student’s t-test). C. Ca2+ responses of GFP negative and positive cells on U178MG transfected with vector containing IP3R3-shRNA and GFP. Ca2+ responses in each condition are normalized to Ca2+ responses in GFP negative without caffeine treatment (*p<0.05, **p<0.001 by Student’s t-test). D. Results of the Matrigel invasion assay on IP3R3-shRNA expressing cells and GFP expressing cells with or without caffeine treatment. Summary of the Matrigel invasion assay with IP3R3-shRNA expressing cells significantly less inhibition of invasion by caffeine (*p<0.01, student’s t-test).
Next, we examined whether gene silencing by shRNA for IP3R3 in U178MG cells would make these cells less sensitive to caffeine in Matrigel invasion assay. As expected, gene silencing of IP3R3 by shRNA rendered these cells significantly less sensitive to caffeine treatment in Matrigel invasion assay compared to the GFP vector expressing cells (t-test, p<0.01; fig. 5D, transfection efficiency: ~30%). These results indicate that IP3R3 confers caffeine sensitivityon invasion of U178MG cells.
Caffeine inhibits invasion and increases survival rate
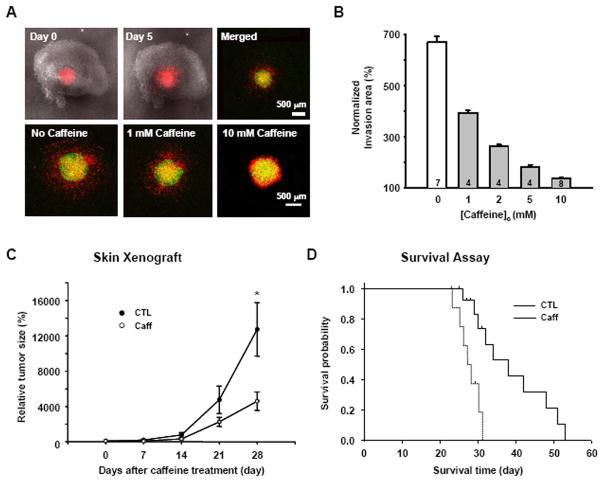
To translate the results from the in vitro experiments, we examined the effect of caffeine on brain slices and in in vivo animal models, in which local microenvironments could compromise the effect of caffeine. In mouse brain slices in culture, we placed 1μl of DiI labeled U178MG cells (~5000 cells) in hippocampal region after 6 days in culture and examined the radial progression of these cells to neighboring regions 5 days after the placement. We found that the invasion of DiI labeled U178MG cells was significantly lower in the brain slices that were treated with various concentration of caffeine, compared to the untreated slices (Figure 6A,B). Next, in a skin xenograft model, U87MG cells that are known to exhibit high tumorigenicity were injected into skin of nude mice and progression of tumor mass was followed. We found that those mice supplied with caffeine containing drinking water [1 mg/ml (equivalent to 5 mM), starting from 7 days after implantation] showed significantly reduced tumor mass compared to control mice at 35 days after implantation (Figure 6C). In an intracranial xenograft model of glioblastoma using U87MG cells, the same dose of caffeine improved the survival of tumor-bearing mice (mean survival time: 38 days) compared to control (mean survival time: 28 days) (Figure 6D). It has been reported that when caffeine (0.44 mg/ml) was treated in the drinking water for 2 weeks, serum concentration in mice was near 6 μg/ml (22), which was approximately the same concentration in people drinking 2~5 cups of coffee per day (23). Since the brain concentration of caffeine was highly correlated with serum concentration (19) and our experimental mice received 1 mg/ml drinking water for more than 2 weeks, we predicted that the brain concentration should be at least 6 μg/ml (equivalent to 0.03 mM). We then directly measured the serum and brain content of caffeine after caffeine treatment using HPLC as previously described (19). The measured caffeine concentrations were not very different from the predicted values (serum content: 7.134±1.089 μg/ml, brain content: 6.135±0.368μg/ml).
Fig. 6. Caffeine inhibits invasion and increases survival rate.
A. DiI-stained U178MG cells were placed on the surface of slices in the absence or presence of caffeine (0~10 mM) 6 days after slice preparation. The first two merged DIC and red fluorescence images show the hippocampal brain slice and DiI-stained U178MG cells. After 1 h (Green) and 120 h (Red), movement of the glioblastoma cells in the slices was detected with an inverted confocal laser scanning microscope. B. Invasion area (%) was calculated from the formula, Area of DiI-stained cells at 120 h/Area of DiI-stained cells at 1h) × 100. C. Effect of caffeine on the growth of U87MG cells in vivo skin xenograft model (*p<0.01). D. Kaplan-Meier survival curves of nude mouse bearing intracranial U87MG tumors. Log rank, p=0.001, control versus caffeine.
Molecules in Ca2+ signaling pathway are up-regulated in glioblastoma
To see if the molecules in the phosphoinositide and Ca2+ signaling pathway in glioblastoma are changed, we have performed genome wide DNA chip microarray analysis on 51 tissue samples from glioblastoma patients and compared with 34 normal tissue samples from lobectomy surgery (Supplementary Figure 4A). We found that most of molecules that are in the signaling pathway of phosphoinositide production and Ca2+ release showed an average of more than 2 fold increase in mRNA expression in glioblastoma patient samples. These include PAR1(7.7 fold increase), EGFR(3.1), PLCβ3(1.4), PLCγ1(1.4), IP3R3(2.3) and TRPC6(2.1). However, RyRs did not show any significant increase. The gene tree analysis also shows clusters of genes related to upstream or downstream signaling pathway whose expression is significantly increased (Supplementary Figure 4B). The enhanced Ca2+ signaling provides a source of Ca2+ that is sensitive to caffeine and critical for motility, invasion, and possibly cell division of glioblastoma cells (Supplementary Figure 4C).
Discussion
We have initially started with a simple hypothesis that inhibiting the Ca2+ signaling pathway would inhibit the invasive and motile behavior of glioblastoma cells. Similar to normal glial cells, glioblastoma cells show robust Ca2+ increases upon activation of various GPCR and RTK agonists (Figure 1). The major source of Ca2+ increase was found to be the release of intracellular Ca2+ stores through IP3Rs and subsequent trigger of influx through the store operated channels upon depletion of the intracellular stores (Supplementary Figure 1A,B). Of the three subtypes of IP3R, we found that subtype 3 is overexpressed in various glioblastoma cell lines and glioblastoma patient samples, whereas normal astrocytes showed virtually no expression of IP3R3 (Figure 4B,C). On the other hand, IP3R1 was significantly decreased in glioblastoma tissues (Figure 4C), making IP3R3 as the major contributor of Ca2+ signaling in these cells.
IP3Rs are known to be difficult to study especially due to the lack of suitable inhibitors and subtype specific blockers. We found that caffeine paradoxically inhibited IP3R-mediated Ca2+ responses in a subtype 3 specific manner (Figure 5). Using caffeine as a tool to inhibit IP3R3-mediate Ca2+ release, we have demonstrated that inhibiting IP3R3 effectively reduced the migration, invasion, and survival of glioblastoma cells (Figure 2). The gene silencing of IP3R3 by shRNA also effectively reduced the caffeine sensitivity of Ca2+ signaling and invasiveness in the Matrigel invasion assay (Figure 5). Our results are the first to demonstrate the involvement of IP3R3 in glioblastoma Ca2+ signaling and invasion. Furthermore, we suggest that IP3R3 can be specifically targeted for therapeutic intervention in glioblastoma patients with minimal influence on normal glial as well as neuronal functions.
Whether caffeine can directly affect the gating of IP3R3 channels or not is still unknown. However, according to previous studies demonstrating that caffeine can compete with ATP binding to IP3Rs (21) at millimolar concentrations (20), caffeine could selectively bind to IP3R3 and affect the gating of IP3R3. Further work is required to investigate the direct role of caffeine on IP3R3 gating in comparison to other subtypes of IP3R.
In summary our study provides IP3R3 as a novel therapeutic target for glioblastoma treatment. Our study also provides new insights into the detailed molecular mechanism of caffeine action on migration and invasion of glioblastoma. The apparent beneficial effect of caffeine suggested by our study should trigger future investigations of the therapeutic potential for caffeine to treat this deadly disease that otherwise has no cure.
Supplementary Material
Acknowledgments
This work was supported by KIST Core Competency Program (C.J.L.), Korea Research Foundation KRF-2005-070-C00096 (C.J.L.), NIH NS43875 (C.J.L.), NS39419 (S.F.T.), by the grant (M103KV010016-06K2201-01610) from Brain Research Center of the Twenty-First Century Frontier Research Program funded by the Ministry of Science and Technology of the Republic of Korea(S.H.P), and by the MRC program of MOST/KOSEF (S.S.K) R13-2005-012-02001-0).
References
- 1.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 2.Nakada M, Nakada S, Demuth T, et al. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–78. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–9. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 5.Junge CE, Lee CJ, Hubbard KB, et al. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Weydt P, Moller T, Labrakakis C, Patt S, Kettenmann H. Neuroligand-triggered calcium signalling in cultured human glioma cells. Neurosci Lett. 1997;228:91–4. doi: 10.1016/s0304-3940(97)00366-2. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 9.Ishiuchi S, Tsuzuki K, Yoshida Y, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–8. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 11.Lu YP, Lou YR, Xie JG, et al. Caffeine and caffeine sodium benzoate have a sunscreen effect, enhance UVB-induced apoptosis, and inhibit UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2007;28:199–206. doi: 10.1093/carcin/bgl112. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Rouse J, Lukes L, et al. Caffeine suppresses metastasis in a transgenic mouse model: a prototype molecule for prophylaxis of metastasis. Clin Exp Metastasis. 2004;21:719–35. doi: 10.1007/s10585-004-8251-4. [DOI] [PubMed] [Google Scholar]
- 13.Daly JW. Caffeine analogs: biomedical impact. Cell Mol Life Sci. 2007;64:2153–69. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HS, Nagane M, Klingbeil CK, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 15.Klingler-Hoffmann M, Fodero-Tavoletti MT, Mishima K, et al. The protein tyrosine phosphatase TCPTP suppresses the tumorigenicity of glioblastoma cells expressing a mutant epidermal growth factor receptor. J Biol Chem. 2001;276:46313–46318. doi: 10.1074/jbc.M106571200. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Simoni A, Yu LM. Preparation of organotypic hippocampal slice cultures: interface method. Nat Protoc. 2006;1:1439–45. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- 18.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–9. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan GB, Greenblatt DJ, Leduc BW, Thompson ML, Shader RI. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J Pharmacol Exp Ther. 1989;248:1078–83. [PubMed] [Google Scholar]
- 20.Brown GR, Sayers LG, Kirk CJ, Michell RH, Michelangeli F. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J. 1992;282(Pt 2):309–12. doi: 10.1042/bj2820309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maes K, Missiaen L, De Smet P, et al. Differential modulation of inositol 1,4,5-trisphosphate receptor type 1 and type 3 by ATP. Cell Calcium. 2000;27:257–67. doi: 10.1054/ceca.2000.0121. [DOI] [PubMed] [Google Scholar]
- 22.Conney AH, Zhou S, Lee MJ, et al. Stimulatory effect of oral administration of tea, coffee or caffeine on UVB-induced apoptosis in the epidermis of SKH-1 mice. Toxicol Appl Pharmacol. 2007;224:209–13. doi: 10.1016/j.taap.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.de Leon J, Diaz FJ, Rogers T, et al. A pilot study of plasma caffeine concentrations in a US sample of smoker and nonsmoker volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:165–71. doi: 10.1016/s0278-5846(02)00348-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.