Abstract
Background
Physicochemical characteristics of liposome/DNA complexes influence transfection efficiency and affect each other in a very intricate way. The result of this is discrepancies in conclusions drawn about the individual influence of each one.
Methods
Aiming to elucidate the influence of liposome/DNA charge ratio and size on transfection efficiency and on each other, we used liposome/DNA complexes with charge ratio (+/−) in the range of 1–50 and extruded through membranes of 400, 200, and 100 nm. Plasmid DNA encoding green fluorescent protein was used to measure transfection efficiency by flow cytometry. Sizes of liposome/DNA complexes were measured by dynamic light scattering.
Results
Liposome size was reduced after extrusion but this was mainly driven by the charge ratio and not by the size of the membrane pores. Reduction of complex size at each charge ratio positively correlated with transfection efficiency. When the size of the complexes was approximately constant, increasing the charge ratio was found to promote transfection efficiency. Cationic lipid N-(1-(2,3-dioleoyloxy)propyl)N,N,N trimethylammonium chloride was used for modulation of positive charge and a cytotoxicity test showed that increasing its amount increases cytotoxicity.
Conclusion
It can be concluded that charge ratio dictates the size of the complex whereas overall size reduction and higher charge ratios promote transfection efficiency in vitro.
Keywords: transfection efficiency, liposome charge, liposome size
Introduction
Gene therapy is defined as induction or inhibition of genes by means of introducing various forms of nucleic acids into cells. Since the entry of nucleic acids into cells is a very inefficient process, successful gene therapy requires an efficient drug delivery system. Viral vectors and nonviral delivery systems are used for delivery of nucleic acids into cells. Viral vectors are very efficient in introducing genes into cells but have limitations regarding the size of the genes that can be delivered and the safety of such formulations. Alternatively, nonviral delivery systems are much safer and not limited in their delivery of large pieces of DNA but are often not sufficiently efficient.1–3 Nonviral delivery systems include various physical modes of delivery (eg, gene gun, electroporation, hydrodynamic delivery, and ultrasound) whereas chemical systems include various cationic polymers and cationic liposomes.4,5 The most frequently used nonviral systems are liposomes as can be seen from the proportions of vectors used in clinical trials.6 Cationic liposomes interact with negatively charged nucleic acids and these complexes enter the cell by endocytosis, then fuse with endosomal membranes and release nucleic acids into the cytoplasm.7–11 Liposomes have been investigated for over 20 years as delivery systems for nucleic acids, but the process is not fully understood and depends on various physicochemical characteristics of the liposome/DNA, such as size,12–15 lamellarity,16 structure,17 fusogenicity,18 charge ratio,13,14,19 and charge density.20 Furthermore, these properties often influence each other, further complicating the picture.21,22 Also, various studies have been conducted with the aim of synthesizing new cationic lipids with improved properties and studying structure-activity relationships.23,24 However, since various factors influence the transfection efficiency, it is difficult to draw a definite conclusion about the influence of structural characteristics of lipids and physicochemical properties of liposome/DNA complexes on transfection efficiency. Finding an effective formulation thus remains mostly a process of trial and error.
The aim of this study was to systemically investigate liposome/DNA formulations differing in lipid composition, size, and charge ratio and to define the most effective liposome/DNA characteristics promoting transfection efficiency in vitro. Such a study was undertaken as a preliminary screening for the best formulation to be studied in a future design of a liposome/DNA vaccine and investigation of its efficacy in vivo. To monitor transfection efficiency we used plasmid DNA encoding-enhanced green fluorescent protein (pEGFP) and the percentage of green fluorescent protein (GFP)-expressing cells was determined by flow cytometry. It was incorporated into three differently composed liposomal formulations: (1) phosphatidylcholine (PC), 1,2-dioleoyl-sn-glycero-3 phosphatidylethanolamine (DOPE) and N-(1-(2,3-dioleoyloxy)propyl)N,N,N trimethylammonium chloride (DOTAP); (2) DOPE and DOTAP; (3) PC, cholesterol (CHOL), and DOTAP. In addition, the charge ratio was varied from 1 to 50 by changing the quantities of positive lipid (DOTAP) in the process of liposome preparation. Lipoplexes were sized using extrusion through a membrane of appropriate pore size. Charge ratios well above those usually studied (1–10) were investigated here since it has already been shown that liposome/DNA complexes have better transfection efficiency with higher charge ratios (+/− ≈ 15),25 presumably due to the existence of free liposomes. 26 Interestingly, as far as we are aware, the higher ratios have not been studied before, so this is the first such study. The obtained charge and size of the formulated liposome complexes were subsequently monitored by dynamic light scattering. In addition, lipoplexes were prepared by hydrating lipid films directly with DNA solution and not by mixing of the preformed liposomes and DNA as is usually the method employed in preparing complexes. Our results show that after extrusion of liposome complexes, their size is decreased, but is mainly governed by the charge ratio. Also, increase in charge ratio and decrease in size of complexes improved transfection efficiency. Cytotoxicity of liposomal preparations was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) cell viability assay and it was found that with increasing amount of DOTAP cytotoxicity also increased.
Materials and methods
Chemicals
The DOPE, PC, and DOTAP were from Avanti Polar Lipids (Alabaster, AL). CHOL and MTT were from Sigma (St Louis, MO). Aliquots of lipids were stored as a chloroform stock solution at −20°C. Dimethyl sulfoxide was from Merck (Darmstadt, Germany). All other chemicals were from Kemika (Zagreb, Croatia).
Plasmid DNA (pDNA) encoding pEGFP was from Clontech (Mountain View, CA). The pDNA was purified on a DEAE CIM disk from BIA Separation (Ljubljana, Slovenia) as previously described.27 The concentration and purity of the pDNA was determined by the ratio A260/280 and by agarose gel electrophoresis.
Liposome/DNA preparation
Thin lipid films were obtained by rotary evaporation of chloroform lipid solution in round-bottom flasks. Evaporation and rehydration of lipid films (8.44 mg) was performed at 37°C. Rehydration was performed with 1.35 mL pEGFP solution (30 μg/mL) in phosphate-buffered saline (PBS) (10 mM phosphate buffer pH = 7.4 with 0.15 M NaCl). In three component systems the amount of PC was twice that of the other helper lipid and the amount of DOTAP was directed by the charge ratio. This setup resulted in the following lipid molar ratios (charge ratios are denoted in square brackets): PC-DOPE-DOTAP [1] 2-1-0.04, PC-DOPE-DOTAP [10] 2-1-0.5, PC-DOPE-DOTAP [25] 2-1-1.3, PC-DOPE-DOTAP [50] 2-1-4.3, PC-DOPE [50] 1-1.4, PC-CHOL-DOTAP [50] 1-1-2.25. To reduce the size of the lipoplexes, some of the lipoplex preparations were extruded (29×) through polycarbonate membranes of specific pore size using a LipoFast extruder from Avestin (Ottawa, Canada).
Cell culture
The following cell lines were used: 293T cells (SV40 T-antigen expressing human kidney cells), COS7 (SV40 transformed African green monkey kidney cells), and Vero cells (normal African green monkey kidney cells). All cell lines were purchased from the American Type Culture Collection (ATCC). The cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (Moregate Biotech, Australia) and neomycin 50 μg/mL (Gibco Invitrogen Corporation, CA) and incubated at 37°C in a humidified atmosphere of 5% CO2. The cells were trypsinized (0.25%) every 3–4 days and further subcultivated by splitting them in a ratio of 1:6.
Lipofection procedure
Cells were seeded in six well plates (2.5 × 105 cells/well) in complete medium at 37°C in a humidified atmosphere containing 5% CO2. After 24 hours cells were washed with DMEM once and liposome/DNA complexes (500 μL) plus 500 μL DMEM were added. After 3 hours at 37°C in a humidified atmosphere containing 5% CO2, the transfection solution was replaced with complete medium and cells were further cultured for 48 hours. As a positive control, 5 × 105 cells were electroporated at (700 V, 450 μs, 1×) in a 4 mm gap cuvette (Multiporator, Eppendorf, Germany) with pEGFP and cultivated for 48 hours.
Measurement of transfection efficiency by flow cytometry
Electroporated or lipofected cells were trypsinized, washed once with complete medium, and then once with PBS. Cells were resuspended in 500 μL of PBS supplemented with 0.1% sodium azide and 2% fetal calf serum and analyzed on a FACSCalibure flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Cytotoxicity
293T cells were seeded in a 96-well plate (1 × 104 cells/well) and cultured in complete medium at 37°C in a humidified atmosphere containing 5% CO2. After 24 hours, liposome/DNA complexes (50 μL) plus 50 μL DMEM were added. After 3 hours complexes were removed and 200 μL of complete medium was added. Cells were cultivated for a further 48 hours at 37°C in a humidified atmosphere containing 5% CO2. Medium was removed and subsequently 50 μL of MTT (0.5 mg/mL) was added and cells were cultivated for another 4 hours. Formed formazan crystals were dissolved by addition of 200 μL dimethyl sulfoxide. The plates were read on a ThermoScientific microplate spectrophotometer (Thermo Fisher Scientific, MA) at 580 nm and at 680 nm as a reference wavelength. Samples were assayed in tetraplicate.
Size and charge of liposome/DNA complexes
Liposome size and zeta potential were measured by dynamic light scattering using Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) equipped with 532 nm “green” laser. Liposomal formulations for zeta and size measurements were diluted with PBS to a final concentration 0.125 mg/mL. Size of lipoplexes is expressed as an average diameter (z-average) that is obtained from the Zetasizer Nano software using intensity derived size distribution. For comparison of results, the diameter of the major population obtained from number-derived size distribution was taken into account. Three separate preparations were analyzed, each measured three times.
Results
Influence of the charge ratio on the size of extruded liposome/DNA complexes
Size and charge ratio (ratio of number of positive charges originating from cationic lipid and number of negative charges from DNA’s phosphates) of liposome/DNA complexes are considered very important physicochemical characteristics for transfection efficiency of formulation.12–15,19 We prepared lipoplexes (PC-DOPE-DOTAP) with charge ratios 1, 10, 25, and 50 by varying the amount of DOTAP. To investigate the influence of the size of liposome/DNA complexes on the transfection efficiency we extruded lipoplexes through membranes with various pore sizes: 100, 200, and 400 nm. Sizes of liposome/DNA complexes were measured by dynamic light scattering. Size measured by dynamic light scattering can be given as a number distribution, volume distribution, or intensity distribution and mean diameter (z-average) which is calculated from intensity distribution using a non-negatively least squares algorithm (software provided by the manufacturer) and these results can differ from each other. Dynamic light scattering results revealed that tested liposomal formulations were not 100% homogenous in size but rather contained one major vesicle population (generally comprising more than 90% of all vesicles). Therefore, we processed results from number distributions (we took into account the largest size population which was generally above 90%) and compared them to z-average. It should be noted that three measurements of the same sample expressed as z-average had lower standard deviations then the mean size of the major population from number distributions. The trend of liposome size in respect to charge ratio was the same for both, mean diameter and number distribution, but the values of mean diameters were always higher (Figure 1). Liposome size of extruded formulations was reduced in comparison to unextruded, however it was not determined by the size of membrane pores but by the charge ratio. The mean diameters of all preparations (PC-DOPE-DOTAP), regardless of membrane pore size, with charge ratio 50, 25, 10, and 1 were 561 ± 29.7, 727 ± 74.0, 1477 ± 100.3, and 7121 ± 942.2 nm, respectively. Increase in charge ratio resulted in decrease in the size of liposome/DNA complex. Sizes of complexes that were not extruded (Figure 1B) did not show any trend but this might be because they were very large and on the upper limit of the instrument measurement ability (in some runs the instrument could not even measure the sizes of these large complexes) so precision of obtained results is quite low. Interestingly, liposome/DNA complexes with charge ratio 50 and 25 (regardless of composition or extrusion) after ultracentrifugation (300, 000 × g) and also after a period of no disturbance in a container floated at the surface whereas lipoplexes with charge ratio 10 and 1 settled at the bottom of the cuvette. Zeta potential of liposomal preparations was also measured. It can be seen that the zeta potential was governed by the charge ratio and was not changed by extrusion as could be expected (Table 1).
Figure 1.
Size of PC-DOPE-DOTAP/pEGFP complexes measured by dynamic light scattering.
Notes: Results are expressed as z-average (av) or as size of major population from number distribution (nu). (A) Size of extruded complexes. (B) Size of unextruded complexes. Results are expressed as mean ± standard deviation of three separate experiments. Each sample was measured three times.
Abbreviations: PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; pEGFP, plasmid (encoding) enhanced green fluorescent protein.
Table 1.
Zeta potential of PC-DOPE-DOTAP/pEGFP complexes before and after extrusion. Complexes were prepared by hydration of thin lipid film with pEGFP solution, which were extruded through membranes with 100 nm pores
| Charge ratio | Zeta potential/mV | |
|---|---|---|
|
|
||
| Unextruded | 100 nm | |
| 50 | 38.7 ± 0.89 | 37.7 ± 0.66 |
| 25 | 26.1 ± 2.05 | 27.1 ± 2.38 |
| 10 | 14.8 ± 1.49 | 15.2 ± 2.97 |
| 1 | −2.8 ± 0.85 | 0.3 ± 1.69 |
Notes: Measurements were performed in PBS, and the final concentration was 0.125 mg/mL. Results are expressed as mean ± standard deviation of three separate experiments. Each sample was measured three times.
Abbreviations: PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; pEGFP, plasmid (encoding) enhanced green fluorescent protein; PBS, phosphate-buffered saline.
Influence of liposome composition, charge ratio, and size of the liposome/DNA complex on transfection efficiency
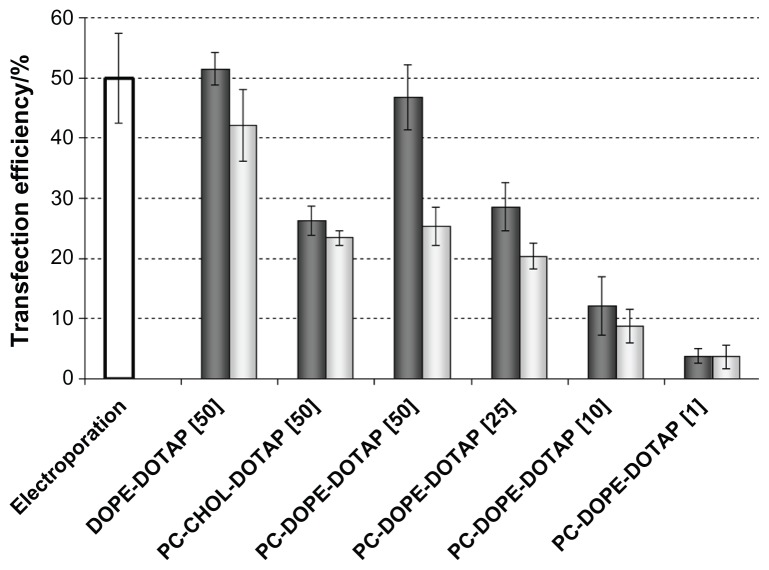
Since it is well known that lipid composition and structure of lipids can influence transfection efficiency we investigated three formulations; PC-DOPE-DOTAP, DOPE-DOTAP, and PC-CHOL-DOTAP. We used pEGFP to asses transfection efficiency of liposomal formulations which was measured by flow cytometry. Results were expressed as a percentage of cells expressing GFP. We have tested liposome/pEGFP formulations in three different types of cells (Vero, COS7, and 293T). The best transfection results were obtained in 293T cells (Figure 2) so they were used throughout this work. We used DOTAP as cationic lipid as it is the most widely used lipid for lipofection.28 As neutral (helper) lipids we used PC, DOPE, and CHOL. DOPE is known to be fusogenic and promotes transfection efficiency in vitro29,30 whereas CHOL decreases fluidity of the bilayer, increases stability, and is considered to be more efficient in vivo;25,31 and in the presence of serum.32 Also, we used three-component systems since it has been shown that multicomponent systems may also promote transfection efficiency of liposome/DNA complexes. 33 PC-DOPE-DOTAP formulation was prepared with charge ratios 1, 10, 25, and 50 while DOPE-DOTAP and PC-CHOL-DOTAP were prepared only at ratio 50. Liposome/pEGFP complexes were prepared by hydration of thin lipid film which yields large vesicles and these preparations were extruded to reduce the size. Zeta potential and size of prepared formulations are shown in Figure 1 and Tables 1 and 2. PC-DOPE-DOTAP/pEGFP complexes showed increase of transfection efficiency upon increase of charge ratio and reduction of the size of liposome/DNA complexes (Figure 3). Lipoplexes composed of DOPE-DOTAP were investigated regarding size but only at charge ratio 50 and showed the best efficiency of all formulations tested (Figure 3). We did not investigate DOPE-DOTAP formulations at other charge ratios in more detail because preliminary experiments showed that these formulations strongly adhered to flasks and were very hard to hydrate and resuspend with DNA solution, although these experiments also showed a trend of increase in transfection efficiency with increase in charge ratio (data not shown). Results from transfection efficiency experiments with DOPE-DOTAP formulations showed that size reduction of complexes resulted in enhancement of transfection efficiency (Figure 3). In the case of formulations consisting of PC-CHOL-DOTAP it was shown that decrease in size resulted in just a slight increase in transfection efficiency (Figure 3). To conclude, for different liposome compositions, size reduction (from micrometer to nanometer sizes, Figure 1 and Table 2) improved transfection efficiency which was most evident for PC-DOPE-DOTAP complexes while slightly lower for DOPE-DOTAP and the lowest for PC-CHOL-DOTAP.
Figure 2.
Transfection efficiency of lipoplexes and electroporation in different cell types.
Notes: Liposomal formulation: PC-DOPE-DOTAP/pEGFP complex, charge ratio 50, extruded through 100 nm membranes (light gray columns); electroporation (dark gray columns). Results are expressed as mean ± standard deviation of at least three separate experiments.
Abbreviations: PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; pEGFP, plasmid (encoding) enhanced green fluorescent protein.
Table 2.
Size and zeta potential of liposome/pEGFP complexes
| DOPE-DOTAP | PC-CHOL-DOTAP | |
|---|---|---|
| Zeta potential/mV | ||
| Unextruded | 37.7 ± 0.66 | 39.0 ± 9.60 |
| 100 nm | 28.4 ± 2.79 | 39.8 ± 4.26 |
| Z-average/nm | ||
| Unextruded | 2664 ± 254.6 | 2690 ± 1137.5 |
| 100 nm | 559 ± 56.3 | 410 ± 88.4 |
Notes: Lipoplexes were prepared by hydration of thin lipid film with pEGFP solution, which were extruded through membranes with 100 nm pores. Measurements were performed in PBS, and the final concentration was 0.125 mg/mL. Results are expressed as mean ± standard deviation of three separate experiments. Each sample was measured three times.
Abbreviations: pEGFP, plasmid (encoding) enhanced green fluorescent protein; PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; CHOL, cholesterol; PBS, phosphate-buffered saline.
Figure 3.
Influence of composition, size, and charge ratio on transfection efficiency of complexes in 293T cells.
Notes: Lipoplexes were prepared by hydration of thin lipid film with pEGFP solution (light gray columns), which were extruded through membranes with 100 nm pores (dark gray columns). Results are expressed as mean ± standard deviation of at least three separate experiments. Number in brackets denotes the charge ratio.
Abbreviations: PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; pEGFP, plasmid (encoding) enhanced green fluorescent protein; CHOL, cholesterol.
Cytotoxicity of liposome/DNA complexes
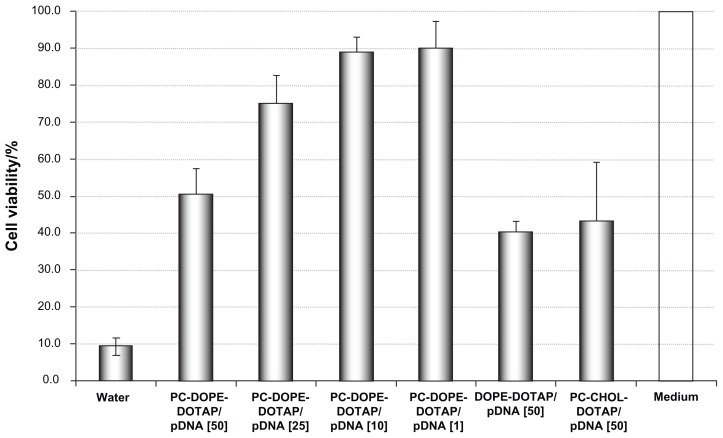
Cytotoxicity of liposome/DNA complexes was determined using MTT assay. The viability of untreated cells (positive control) was set to 100%. The viability of negative control cells (treated with water) was only 10%. Increasing charge ratio of liposomal formulations ie, higher amount of DOTAP (in PC-DOPE-DOTAP) increased cytotoxicity of liposome/DNA complexes and caused cell viability decrease from 90% for charge ratio 1 to 50% for charge ratio 50 (Figure 4). Also, in two other formulations, DOPE- DOTAP and PC-CHOL-DOTAP with charge ratio 50, toxicity was 40% and 43%, respectively. Furthermore, we observed that liposome/DNA complexes exhibit greater cytotoxicity than liposomes alone (data not shown) as previously published by Nguyen et al.34
Figure 4.
Cytotoxicity of complexes determined by MTT assay.
Notes: Results are expressed as mean ± standard deviation of at least three separate experiments. Number in brackets denotes the charge ratio.
Abbreviations: PC, phosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, N-(1-(2,3-dioleoyloxy)propyl),N,N,N-trimethylammonium chloride; pDNA, plasmid DNA; CHOL, cholesterol.
Discussion
A large amount of research has been conducted with the aim of defining physicochemical characteristics of liposome/DNA complexes leading to better transfection efficiency. Overall, it can be only concluded that characteristics of liposome/DNA complexes influence the efficiency of liposomes as delivery systems and additionally influence each other so it is very difficult to unambiguously define superior characteristics leading to superior efficiency. In the presented work we wanted to explore the efficiency of liposome/DNA complexes with higher charge ratios up to 50 since usually only charge ratios in the range from 1 to 10 have been investigated. Furthermore, we wanted to characterize and define liposomal formulations with respect to charge and size.
Regardless of the method used for mammalian cell transfection, it is important to optimize transfection conditions. This includes the optimization of the liposome/DNA formulation and the ratio of the formulation to medium for every cell type used. In order to avoid a protracted optimization process we tested three robust cell lines (Vero, COS7, and 293T), which are well known for their susceptibility to transfection and are therefore often used experimentally for generation of either transient or stable transformants. As shown in Figure 2 these cell lines display different susceptibilities for transfection when electroporation and lipofection were conducted under conditions described herein. 293T cells were transfected to an almost identical level by both methods so for further experiments we decided to use this cell line.
Results shown in Figure 1 demonstrate that the size of liposome/DNA complexes was reduced after extrusion but is very dependent on the charge ratio and not so much on the pore size of extrusion membrane since regardless of the pore size (400, 200, or 100 nm) liposome size was about the same for certain charge ratios. Increase in the size of complexes approaching equivalence point and decrease at higher and lower charge ratios (8–0.5) have already been observed by Xu et al, except that only charge ratios up to 8 were used.35 Furthermore, we have observed that lipoplexes with higher charge ratios floated after ultracentrifugation and those with smaller charge ratios sedimented. Xu et al found that on sucrose gradient position of the band corresponding to lipoplexes was determined by charge ratio.35 Increase in complex size when the zeta potential approached zero was also observed by Birchall et al. This group studied charge ratios in the range of 2.8–0.8 (+/−).36 The same effect was also reported for charge ratios 6–0.125.13,14 Taken together it can be concluded that charge ratio influences the size of complexes and moreover it does so even after extrusion through membranes.
Influence of charge ratio on transfection efficiency of lipoplexes has also been investigated. Some report a peak of the transfection efficiency being above a charge ratio of 2.3 but only in one type of cell whereas in the other type the transfection efficiency seemed not to be influenced by the charge ratio.36 There are some reports that the highest transfection efficiency is at a charge ratio of 1.5 and decreasing at higher and lower charge ratios.13 Liu et al reported that the charge ratio of 15.9 (corresponding to 48 nmol: 1 μg) is best in the range 0.33 to 15.9 when studied in vivo.19 Some reports imply that increase in size (above 1000 nm) of the complex is the major determinant of in vitro transfection leading to improvement of efficiency.12 Others showed that the high transfection activity correlates with the presence of complexes of sizes in the range 650–1500 nm.37 Also it has been shown that the type of endocytosis (clathrin or caveolae mediated) is size dependent and influences the type of processing in the cell. Particles about 500 nm in size are endocytosized by a caveolae-mediated process which does not lead to a degradative pathway in contrast to particles smaller than 200 nm that undergo clathrin-dependent endocytosis leading to lysosomal degradation.11 This means that complexes smaller than 500 nm would be able to deliver nucleic acids to cytoplasm only if they were able to exit the endosome, whereas those larger than 500 nm would be more favorable for delivery. Our results show that the transfection efficiency in vitro increased with the increasing charge ratio and decreasing size in the case of PC-DOPE-DOTAP and DOPE-DOTAP (Figure 3), but it is important to say that size was decreased only to about 500 nm. The effect of charge ratio is however evident with unextruded PC-DOPE-DOTAP lipoplexes where mean diameter is above 4 μm for all charge ratios and the highest transfection efficiency is achieved with the highest charge ratio. In addition, transfection efficiency of extruded lipoplexes is also charge ratio dependent in the same manner (Figure 3), although size differences are more pronounced in this case and should be considered. Apparently, both the effect of size reduction and increase in charge ratio influence the transfection efficiency but also each other as shown in Figure 1. Taken together, it might be concluded that increase in charge ratio ie, charge density, is contributing to transfection efficiency most probably due to stronger interaction with the cell membrane or easier escape from the endosome (since charge density was shown to correlate with endosome escape).20 Formulations containing CHOL showed the smallest transfection efficiency in comparison to DOPE-containing formulations as is usually the case in in vitro experiments. In addition, CHOL-containing formulations showed only slight enhancement of transfection efficiency following size reduction. It can be concluded that, in the experimental conditions used, increase in charge ratio leads to enhancement of transfection efficiency and together with size reduction (also influenced by charge ratio) leads to more efficient formulations for delivery of nucleic acids. Finally, we tested cytotoxicity of the formulations used for transfection and found that PC-CHOL-DOTAP, PC-DOPE-DOTAP, and DOPE-DOTAP all at charge ratio 50 are the most toxic probably due to the large amounts of DOTAP. These formulations had much higher concentrations of DOTAP (5 mM) than those shown to be nontoxic for cells (CaSki) 40 μM but this was required for obtaining high charge ratios.38
Conclusion
Our results indicate that the reduction of the size and increase in charge ratio of liposome/DNA complexes promotes transfection efficiency but unfortunately also induces higher cytotoxicity due to the higher amounts of cationic lipid DOTAP. Also, we have demonstrated that the size of liposome/DNA complexes after extrusion through certain pore sizes is reduced to some extent but is strongly governed by the charge ratio. This emphasizes the complex relationship of liposome properties and activity and the necessity for further experiments studying mechanism of action of liposome- based transfection and liposome characteristics promoting transfection to achieve effective and safe nucleic acids delivery.
Acknowledgments
This work was supported by the Croatian Ministry of Science, Education and Sports through projects 021-0212432-2033, 021-0212432-3123, and 021-0212432-2431. The authors thank the Laboratory for Radiochemistry for kindly allowing the use of the Malvern Zetasizer located at the Institute Rudjer Bošković and Dr Maja Dutour Sikirić for useful discussion regarding size measurement.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questiones, and future promises. Med Res Rev. 2007;27:696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 3.Gascon AR, Pedraz JL. Cationic lipids as gene transfer agents: a patent review. Expert Opin Ther Pat. 2008;18:515–524. [Google Scholar]
- 4.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61–77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007 – an update. J Gene Med. 2007;9:833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 7.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated, DNA-trensfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Szoka FC. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 10.Zelphati O, Szoka FC. Mechanism of oligonucleotide release from cationic liposomes. Proc Nat Acad Sci U S A. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and calveolae-mediated endocytosis. J Liposome Res. 2006;16:237–247. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- 12.Ross PC, Hui SW. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999;6:651–659. doi: 10.1038/sj.gt.3300863. [DOI] [PubMed] [Google Scholar]
- 13.Almofti MR, Harashima H, Shinohara Y, Almofti A, Li W, Kiwada H. Lipoplex size determines lipofection efficiency with or without serum. Mol Membr Biol. 2003;20:35–43. doi: 10.1080/09687680210035104. [DOI] [PubMed] [Google Scholar]
- 14.Almofti MR, Harashima H, Shinohara Y, Almofti A, Baba Y, Kiwada H. Cationic liposome-mediated gene delivery: biophysical study and mechanism of internalization. Arch Biochem Biophys. 2003;410:246–253. doi: 10.1016/s0003-9861(02)00725-7. [DOI] [PubMed] [Google Scholar]
- 15.Ramezani M, Khoshhamdam M, Dehshahri A, Malaekeh-Nikouei B. The influence of size, lipid composition and bilayer fluidity of cationic liposomes on the transfection efficiency of nanolipoplexes. Colloids Surf B. 2009;72:1–5. doi: 10.1016/j.colsurfb.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Zuidam NJ, Hirsch-Lerner D, Marguiles S, Barenholz Y. Lamellarity of cationic liposomes and mode of preparation of lipoplexes affect transfection efficiency. Biochim Biophys Acta. 1999;1419:207–220. doi: 10.1016/s0005-2736(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 17.Safinya CR. Structures of lipid-DNA complexes: supramolecular assembly and gene delivery. Curr Opin Struct Biol. 2001;11:440–448. doi: 10.1016/s0959-440x(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 18.Koynova R, Tarahovsky YS, Wang L, MacDonald RC. Lipoplex formulation of superior efficacy exhibits high surface activity and fusogenicity, and readily releases DNA. Biochim Biophys Acta. 2007;1768:375–386. doi: 10.1016/j.bbamem.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Qi H, Huang L, Liu D. Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration. Gene Ther. 1997;4:517–523. doi: 10.1038/sj.gt.3300424. [DOI] [PubMed] [Google Scholar]
- 20.Lin AJ, Slack NL, Ahmad A, George CX, Samuel CE, Safinya CR. Three-dimensional imaging of lipid gene-carriers: membrane charge density controls universal transfection behavior in lamellar cationic liposome-DNA complexes. Biophys J. 2003;84:3307–3316. doi: 10.1016/S0006-3495(03)70055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boktov J, Hirsch-Lerner D, Barenholz Y. Characterization of the interplay between the main factors contributing to lipoplex-mediated transfection in cell cultures. J Gene Med. 2007;9:884–893. doi: 10.1002/jgm.1079. [DOI] [PubMed] [Google Scholar]
- 22.Masoti A, Mossa G, Cametti C, et al. Comparison of different commercially available cationic liposome-DNA lipoplexes: parameters influencing toxicity and transfection efficiency. Colloids Surf, B. 2009;68:136–144. doi: 10.1016/j.colsurfb.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Niculescu-Duvaz D, Heyes J, Springer CJ. Structure-activity relationship in cationic lipid mediated gene transfection. Curr Med Chem. 2003;10:1233–1261. doi: 10.2174/0929867033457476. [DOI] [PubMed] [Google Scholar]
- 24.Kearns MD, Donkor A-M, Savva M. Structure transfection activity studies of novel cationic cholesterol-based amphiphiles. Mol Pharm. 2008;5:128–139. doi: 10.1021/mp700131c. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Mounkes LC, Liggitt HD, et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol. 1997;15:167–173. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- 26.Song YK, Liu D. Free liposomes enhance the transfection activity of DNA/lipid complexes in vivo by intravenous administration. Biochim Biophys Acta. 1998;1372:141–150. doi: 10.1016/s0005-2736(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 27.Branovic K, Forcic D, Ivancic J, et al. Application of short monolithic columns for fast purification of plasmid DNA. J Chromatogr B. 2004;801:331–337. doi: 10.1016/j.jchromb.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Simberg D, Weisman S, Talmon Y, Barenholz Y. DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Crit Rev Ther Drug Carrier Syst. 2004;24:257–317. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.10. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher S, Ahmad A, Perouzel E, Jorgensen MR, Miller AD. A dialkynoyl analogue of DOPE improves gene transfer of lower-charged, cationic lipoplexes. Org Biomol Chem. 2006;4:196–199. doi: 10.1039/b514532e. [DOI] [PubMed] [Google Scholar]
- 30.Koltover I, Salditt T, Rädler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 31.Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15:647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 32.Crook K, Stevenson BJ, Dubouchet M, Porteous DJ. Inclusion of cholesterol in DOTAP transfection complexes increases the delivery of DNA to cells in vitro in the presence of serum. Gene Ther. 1998;5:137–143. doi: 10.1038/sj.gt.3300554. [DOI] [PubMed] [Google Scholar]
- 33.Caracciolo G, Pozzi D, Caminiti R, et al. Transfection efficiency boost by designer multicomponent lipoplexes. Biochim Biophys Acta. 2007;1768:2280–2292. doi: 10.1016/j.bbamem.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen LT, Atobe K, Barichello JM, Ishida T, Kiwada H. Complex formation with plasmid DNA increases the cytotoxicity of cationic liposomes. Biol Pharm Bull. 2007;30:751–757. doi: 10.1248/bpb.30.751. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Hui S-W, Frederik P, Szoka FC. Physicochemical characterization and purification of cationic lipoplexes. Biophys J. 1999;77:341–353. doi: 10.1016/S0006-3495(99)76894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birchall JC, Kellaway IW, Mills SN. Physico-chemical characterisation and transfection efficiency of lipid-based gene delivery complexes. Int J Pharm. 1999;183:195–207. doi: 10.1016/s0378-5173(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 37.Rakhmanova VA, Pozharski EV, MacDonald RC. Mechanisms of lipoplex formation: dependence of the biological properties of transfection complexes on formulation procedures. J Membrane Biol. 2004;200:35–45. doi: 10.1007/s00232-004-0689-4. [DOI] [PubMed] [Google Scholar]
- 38.Lappalainen K, Jääskeläinen I, Syrjänen K, Urtti A, Syrjänen S. Comparison of cell proliferation and toxicity assays using two cationic liposomes. Pharm Res. 1994;11:1127–1131. doi: 10.1023/a:1018932714745. [DOI] [PubMed] [Google Scholar]