Abstract
Gas embolism is a serious complication of decompression events and clinical procedures, but the mechanism of resulting injury remains unclear. Previous work has demonstrated that contact between air microbubbles and endothelial cells causes a rapid intracellular calcium transient and can lead to cell death. Here we examined the mechanism responsible for the calcium rise. Single air microbubbles (50–150 μm), trapped at the tip of a micropipette, were micromanipulated into contact with individual human umbilical vein endothelial cells (HUVECs) loaded with Fluo-4 (a fluorescent calcium indicator). Changes in intracellular calcium were then recorded via epifluorescence microscopy. First, we confirmed that HUVECs rapidly respond to air bubble contact with a calcium transient. Next, we examined the involvement of extracellular calcium influx by conducting experiments in low calcium buffer, which markedly attenuated the response, or by pretreating cells with stretch-activated channel blockers (gadolinium chloride or ruthenium red), which abolished the response. Finally, we tested the role of intracellular calcium release by pretreating cells with an inositol 1,4,5-trisphosphate (IP3) receptor blocker (xestospongin C) or phospholipase C inhibitor (neomycin sulfate), which eliminated the response in 64% and 67% of cases, respectively. Collectively, our results lead us to conclude that air bubble contact with endothelial cells causes an influx of calcium through a stretch-activated channel, such as a transient receptor potential vanilloid family member, triggering the release of calcium from intracellular stores via the IP3 pathway.
Keywords: gas embolism; inositol 1,4,5-trisphosphate
gas embolism is a serious complication of surgery, diving, and aviation (45). Depending on their size and rate of delivery, air bubbles can circulate, be deposited into, and cause damage in the microcirculation of any organ, obstruct blood vessels, or air-lock the heart (31). Great strides have been made in preventing macroscopic gas embolism from occurring during surgery (3), but the risk of microembolism remains and the vast majority of microemboli, as small as 3 μm, are gaseous (1). This is particularly true for cardiac procedures utilizing cardiopulmonary bypass (CPB), and the incidence of cognitive deficit following such surgeries is high (3). Similarly, decompression illness is also caused by the intravascular formation of gaseous microemboli (46). Gas microemboli have vascular sequelae that include endothelial cell damage or dysfunction, platelet activation, and leukocyte adhesion (45, 46). Furthermore, gas microemboli that did not obstruct blood flow caused changes in both cerebral blood flow and depressed neural function in a rabbit model (25). However, the consequences of air bubble-endothelial cell contact and the mechanisms of microvascular injury resulting from gas microembolism have been little studied (28) outside of computer modeling (32, 41). Likewise, despite gas embolism being a well-documented problem, there are very few clinical treatment options available aside from prevention. The “gold standard” hyperbaric oxygen therapy is both limited in effectiveness and potentially difficult and dangerous to administer, while the availability of pharmacological therapies has been limited (31).
Thus, to develop novel preventive or therapeutic approaches to treating gas embolism injury, it is necessary to understand the signaling pathways evoked by air bubbles in the microvasculature. Our group has developed a platform that enables us to examine the consequences of endothelial cell interactions with air microbubbles (28). Our method involves the generation of physiological-sized micro air bubbles, manipulation of these air bubbles into contact with single endothelial cells, and subsequent recording of the cellular response in real time using phase contrast and epifluorescence microscopy. Previous work in our laboratory (28) has shown that an intracellular calcium transient is elicited by bubble-cell contact, and that this transient was associated with lethality. Here, we focus on the mechanism responsible for the intracellular calcium increase following air bubble contact with endothelial cells, because calcium is known to be a crucial regulator of many endothelial cell functions including nitric oxide production (14), barrier function (44), and mitochondrial function (11).
MATERIALS AND METHODS
Cell culture and dye loading.
As an in vitro cell culture model, human umbilical cord vein endothelial cells (HUVECs) (27, 33) were obtained from Lifeline Cell Technology (Walkersville, MD) and cultured in VascuLife VEGF Cell Culture Media (Lifeline Cell Technology). Media samples were checked for mycoplasma contamination using MycoAlert Kit (Lonza, Rockland, ME). Cells between passage 2 and 6 were plated in BD Primeria 35-mm cell culture dishes (BD, Franklin Lakes, NJ) ∼48 h before planned experiments at a density of ∼3,000 cells/cm2. Cells were dye loaded with the calcium-sensitive dye 1 μmol/l Fluo-4 AM (Invitrogen, Carlsbad, CA) plus 0.005% Pluronic F-127 (Invitrogen) for 15 min at room temperature, then washed three times and incubated for an additional 15 min while protected from light to allow for deesterification. All experiments were carried out at room temperature in recording HBSS (pH 7.4 with 1.3 mmol/l CaCl2, 0.9 mmol/l MgCl2, 2 mmol/l glutamine, 0.1 g/l heparin, 5.6 mmol/l glucose, and 1% FBS). An alternate low calcium recording HBSS was prepared by diluting recording HBSS 50-fold with calcium-free HBSS to examine the role of extracellular calcium.
Pharmacological agents.
To determine the contributions of various pathways and cellular components to the intracellular calcium response elicited by bubble-cell contact, dye-loaded HUVECs were treated with various pharmacological agents. Unless otherwise noted, the agents were handled per manufacturer's instructions, prepared as 100× stocks in the vehicle noted, and were applied individually at room temperature 20 min before bubble experiments, with no removal or wash step after exposure. Briefly, to examine the role of mechanosensitive channels, cells were pretreated with 25 μmol/l gadolinium chloride (prepared in recording HBSS; Sigma Aldrich, St. Louis, MO) or 1 μmol/l ruthenium red (RuR; prepared in recording HBSS; Sigma Aldrich). To examine the role of the actin cytoskeleton, cells were pretreated with 100 nmol/l cytochalasin D (prepared in DMSO; Sigma Aldrich). To interfere with the release of calcium from intracellular stores via the inositol 1,4,5-trisphosphate (IP3) pathway, cells were treated with 1 μmol/l xestospongin C (prepared in DMSO; Enzo Lifesciences, Farmingdale, NY) or 10 mmol/l neomycin (prepared as 4× stock in recording HBSS, which was sonicated for 15 min; Enzo Lifesciences). Additionally, 1% DMSO was used as a vehicle control and 2 μmol/l ionomycin (prepared in DMSO, Enzo Lifesciences) or 10 μmol/l ATP (prepared in distilled water; Sigma Aldrich) were used as positive controls. The dosages selected were the maximum doses tolerated by cells while maintaining solubility with no more than 1% vehicle, and, as a result, did not always yield complete inhibition. For example, the 20-min pretreatment with 1 μmol/l xestospongin C resulted in 75% of cells not responding to stimulus with the 10 μmol/l ATP, a positive control.
Air bubble-cell contact experiments.
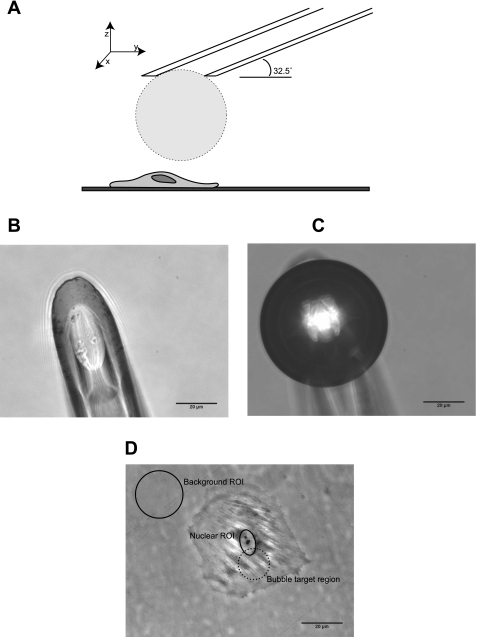
Air bubble-cell contact experiments were performed in similar fashion to previous work by Kobayashi et al. (28), with additional refinements. Drummond Nanoliter micropipettes (Drummond Scientific, Broomall, PA) were pulled using a Sutter model P-97 micropipette puller (Sutter Instruments, Novato, CA) and ground at a 32.5 degree angle using Narishige EG-44 grinder (Narishige, East Meadow, NY) to a diameter of 30–40 μm. A micropipette was then mounted on a Nanoject II injector (Drummond Scientific), backfilled alternately with recording HBSS and air, and manipulated with a PPM500 micromanipulator (WPI, Sarasota, FL). The micropipette tip was oriented such that the orifice was parallel to cell culture dish bottom, immersed in the HBSS recording buffer, and positioned ∼700 μm above the cells. Air was expelled using the Nanoject II until a bubble formed at the tip of the micropipette, followed by gradual aspiration until the bubble was 50–150 μm in diameter (Fig. 1). The bubble was then manipulated into position 100–200 μm above the perinuclear region of an isolated target cell (see Fig. 1D) and then lowered at a rate of ∼5 μm/s (z-axis manipulation only) until contact was observed in the phase contrast image capture. After bubble-cell contact, the bubble was gently lifted and manipulated out of the field of view, and the recording was continued for a total of 6 min.
Fig. 1.
A: diagram of experimental setup (approximately to scale). Micropipette with a microbubble is immersed in buffer and positioned above the perinuclear area of an isolated endothelial cell. B: representative photomicrograph of a pulled and ground Drumond Nanoliter micropipette immersed in buffer. C: representative photomicrograph of an air microbubble at the micropipette orifice. D: representative phase-contrast photomicrograph of an isolated human umbilical vein endothelial cell (HUVEC). The background and nuclear regions of interest (ROIs) are indicated, as well as the perinuclear region typically targeted for bubble contact.
Microscopy and image analysis.
Cells were imaged using a SensiCam QE camera (The Cooke, Romulus, MI) (2 × 2 binning, 688 × 520) attached to Olympus IX70 microscope (Olympus, Melville, NY) with an Olympus LUCPlanFL N ×40 0.6 numerical aperture objective (Olympus) and Photofluor light source (89 North, Burlington, VT). Computer control of the microscope was facilitated by LUDL programmable filter wheels, shutters, and focus control (Ludl Electronic Products, Hawthorne, NY) and both phase contrast (10 ms exposure) and fluorescent images (100 ms exposure) were collected every 1 s using IPL 3.7 software (BD, Rockville, MD) and saved as image stacks. ImageJ software (NIH, Bethesda, MD) was used to analyze the collected image stacks. First, regions of interest (ROI) for background fluorescence (cell-free region) and around the nucleus were defined (Fig. 1D). Next, the Time Series Analyzer plugin was used to calculate the mean fluorescence intensity of the ROIs at each time point. Fluorescence ratio (FR) was then calculated for each time point as: FR=(F − Fbg)/(F0 − Fbg), where F is intensity of the nuclear ROI, Fbg is the intensity of a background ROI, and F0 intensity of the nuclear ROI before bubble impact. Typical F0 was 65 to 75 arbitrary fluorescence units compared with typical Fbg of 55 to 65, with the exception of dishes containing neomycin, which contributed an additional ∼15 units of autofluorescence. A cell was considered a “responder” if the FR exceeded 1.5 within 100 s of bubble-cell contact. SigmaPlot (SysStat, San Jose, CA) was used for data plotting and statistical analysis. Where appropriate, data are reported as means ± SD. When comparing the peak response elicited by bubble-cell contact under two different conditions, unpaired Student's t-test was used to test significance, with P < 0.05 being considered significant.
RESULTS
HUVECs respond to bubble contact with a calcium transient.
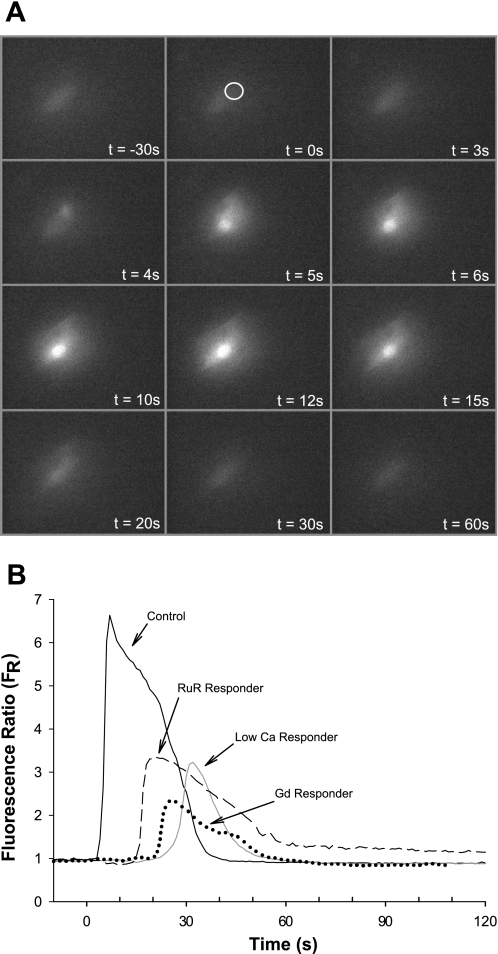
After contact with an air bubble, individual HUVECs respond with a calcium transient that typically achieves a maximum FR of 5.9 ± 1.5 (n=21) within 10–20 s postcontact and then decays over 30–120 s, returning to baseline [see Fig. 2 for a montage of fluorescence micrographs (A) and representative trace (B)]. [By comparison, stimulation of the cells with 10 μmol/l ATP results in a FR of 8.1 ± 0.6 (n=6) and 2 μmol/l ionomycin, a calcium ionophore, results in a FR of >12.] Spatially, the calcium transient originates at the bubble contact point and rapidly spreads throughout the cell. Brief, perinuclear bubble-cell contact does not result in any discernable change in morphology over the course of 6 min recordings.
Fig. 2.
A: montage of fluorescence micrographs illustrating the spatial and temporal progression of the calcium transient following air bubble contact. The time stamps indicate time relative to bubble impact and the location indicated by the white circle. B: representative traces of fluorescence signal (FR) obtained from HUVEC loaded with Fluo-4 dye responding to air bubble contact at t=0, under control recording HBSS, low calcium recording HBSS, and following pretreatment with calcium channel blockers gadolinium chloride (Gd, 25 μmol/l) or ruthenium red (RuR, 1 μmol/l).
Extracellular Ca, entering via calcium channel, is necessary for bubble-cell contact response.
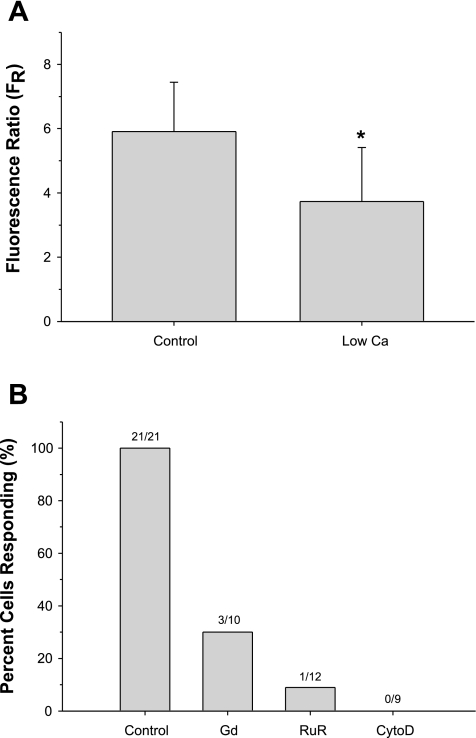
To determine the origin of the calcium transient, air bubble contact experiments were conducted with low calcium recording HBSS (∼26 μmol/l calcium), resulting in 6 of 13 cells (46%) not responding to bubble contact (see Fig. 2B for representative traces of responding cells). Furthermore, the peak of the calcium response in the responding cells was significantly reduced, 3.7 ± 1.7 (n=7, P < 0.004) compared with the normal recording HBSS experiments (Fig. 3A).
Fig. 3.
A: comparison of maximum FR in HUVECs responding to bubble contact in recording HBSS (n=21) or low calcium recording HBSS (n=7). *P < 0.005. B: percentage of HUVECs responding to bubble contact under control conditions (recording HBSS), following 20 min pretreatment with calcium channel blockers Gd (25 μmol/l) or RuR (1 μmol/l) or after disruption of the actin cytoskeleton (CytoD, 100 nmol/l).
After the finding that extracellular calcium concentration affected the response of the cell-to-bubble contact, we used pharmacological interventions to examine the role of calcium channels, which may be responsible for the calcium influx from the extracellular medium to the intracellular region. After dye loading, HUVEC cells were incubated at room temperature for 20 min with recording HBSS containing 25 μmol/l gadolinium chloride (Gd), a nonspecific stretch-activated channel (SAC) inhibitor (8), or 1 μmol/l RuR, a transient receptor potential (TRP) vanilloid (TRPV) family of channel inhibitor (48). Both inhibitors had a marked effect (see Fig. 2 for representative traces of responding cells), with Gd resulting in 7 of 10 cells (70%) not responding to bubble contact and RuR abolishing the calcium response in 11 of 12 cells (91%) (Fig. 3B). As a positive control, cells were treated with 10 μmol/l ATP, resulting in a normal calcium response. The role of SACs was further examined by disrupting the actin cytoskeleton with 100 nmol/l cytochalasin D (20 min, room temperature, disruption was confirmed via phalloidin staining), which resulted in all nine cells (100%) not responding to bubble contact (Fig. 3B). Again, the response of these cells to ATP was not affected in this case. Collectively, these findings strongly suggest that bubble contact causes extracellular calcium to enter via a calcium channel, such as a mechanosensitive TRPV family member, and that this entry is necessary for the generation of the calcium transient.
Intracellular Ca release via IP3 receptor is also necessary for cell response to air bubble contact.
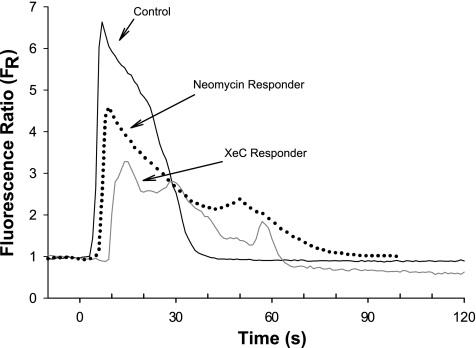
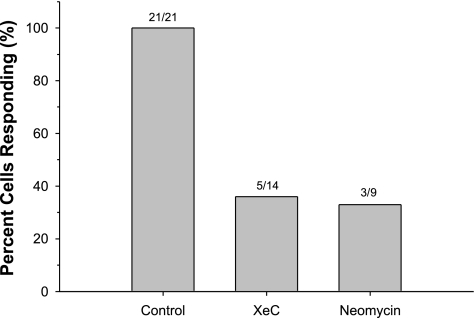
We next examined the possible role of calcium release from intracellular stores as a result of bubble contact. In endothelial cells, calcium is primarily stored in the endoplasmic reticulum (ER) and released via IP3 receptor (IP3R) calcium channels (43). To block this pathway, we pretreated HUVECs for 20 min with either 1 μmol/l xestospongin C, an IP3R blocker (18), or 10 mmol/l neomycin, which binds phosphatidylinositol 4,5-bisphosphate (PIP2) (17), thus inhibiting phospholipase C (PLC). Both inhibitors had a marked effect (see Fig. 4 for representative traces of responding cells). Treatment with xestospongin C resulted in 9 of 14 cells (64%) not responding to bubble contact, whereas neomycin abolished the response in 6 of 9 cells (66%) (Fig. 5). These results strongly suggest that the release of intracellular calcium stores is also necessary for the generation of the calcium transient following bubble contact, and that the influx of extracellular calcium alone is not sufficient to produce a detectable calcium transient.
Fig. 4.
Representative traces of fluorescence signal (FR) obtained from HUVEC loaded with Fluo-4 dye responding to air bubble contact at t=0, under control recording HBSS and following pretreatment with inositol 1,4,5-trisphosphate (IP3) receptor blocker xestospongin C (XeC, 1 μmol/l) or phospholipase C inhibitor neomycin (10 mmol/l).
Fig. 5.
Percentage of HUVECs responding to bubble contact under control conditions (recording HBSS) or following 20 min pretreatment with IP3 receptor blocker XeC (1 μmol/l) or phospholipase C inhibitor neomycin (10 mmol/l).
DISCUSSION
To develop effective preventative or therapeutic approaches to treating gas embolism, we require a greater understanding of the molecular signaling events elicited in endothelial cells by contact with air bubbles. The initial experiments of Kobayashi et al. (28) identified an increase in intracellular calcium as an early response to bubble-cell contact and associated this increase with cell injury. Those findings, combined with the fact that intracellular calcium is a crucial regulator of many endothelial cell functions, led us to pursue mechanistic studies to identify the pathways involved in generating the intracellular calcium increase following bubble-cell contact. Our studies are similar to those conducted during the early study of endothelial response to shear stress (5) and mechanical stimulation (12, 40). Ultimately, we intend to identify targets for therapeutic interventions aimed at preventing or treating gas embolism injury.
Our studies with HUVECs confirm the findings of Kobayashi et al. (28) that large calcium transients result from air bubble-cell contact. We selected HUVECs for this study to be able to more readily compare our results to existing endothelial cell biology research (33). We find that HUVECs respond with calcium transients that are similar to those observed in bovine aortic endothelial cells (BAECs) used by Kobayashi et al. (28), with the primary difference being that we observe cells rapidly returning to precontact baseline calcium levels, within 2 min, as opposed to a more gradual reduction in cytosolic calcium levels. However, this temporal difference may be due to differences between the two endothelial cell lines or arise from changes in stimulation technique or length of recording window. Thus further work is required to determine whether endothelial cells from different vascular beds respond differently to air bubble contact. Like Kobayashi, we attempted to minimize cell-cell signaling and cross-talk by studying individual, isolated cells. However, in contrast to Kobayashi, our experiments involved only single, brief, nonlethal/injurious bubble stimulations in an attempt to isolate the response to bubble contact from other pathways (such as apoptosis and/or necrosis). Furthermore, we refined the experimental technique to allow us to generate bubbles of uniform size, thus reducing variability in the contact area between air bubbles and cells. Our current technique also allows us to manipulate bubbles with a controlled rate of descent, thus reducing the role of any fluid shear component and minimizing the range of the force of impact and the degree of cellular deformation incurred. As a result of our efforts to minimize these potential confounding effects, the intracellular calcium transients we observe following air bubble contact under controlled conditions (recording HBSS) are very reproducible, with all 21 cells tested responding to bubble contact with a relative standard deviation of 26%. Thus, by carefully standardizing the bubble-related factors, we are confident that we were able to isolate the cell response to bubble contact, allowing us to use pharmacological interventions to help identify the signaling cascade behind this process. However, we acknowledge that variations in bubble parameters, including size and rate of descent, are likely to have an effect on the cellular response and may warrant additional investigation in future work.
Having essentially reproduced the basic findings of Kobayashi et al. (28) with another endothelial cell line, we proceeded to examine the role that extracellular calcium plays in generating the observed calcium transients. First, we confirmed the dependence of the air bubble contact response on extracellular calcium by lowering the extracellular calcium concentration 50-fold and observing reduced calcium signaling by HUVECs in response to air bubble contact. Next, we investigated how the extracellular calcium may be entering the cell following bubble-cell contact. We used two different cation channel blockers to determine whether extracellular calcium was entering via a channel. We first tested the effect of gadolinium, a nonspecific SAC inhibitor (8), on the bubble contact response. We found that this blocker had a marked effect, abolishing the calcium transient response of the cell to bubble contact in all but three cells. The lack of efficacy of gadolinium in these few experiments was likely due to the well-documented ability of various inorganic anions, such as the phosphate and bicarbonate present in our recording HBSS, to bind gadolinium, which can result in false negatives (8). To confirm our gadolinium results, we also used RuR, a TRPV family of channel inhibitor (48), which also consistently and completely inhibited the intracellular calcium signal in all but one cell. Furthermore, because actin has been associated with the function of SACs (26) and TRPV channels specifically (7), we confirmed our findings by pretreating cells with cytochalasin D, which disrupted the actin cytoskeleton and abolished the intracellular calcium response upon bubble air contact in all cells. Collectively, these results indicate that the entry of intracellular calcium through a TRPV channel is necessary for the generation of the calcium transients by bubble-cell contact. TRPV channels are a subfamily of the TRP cation channels that are gated by esoteric stimuli such as light, heat, osmolarity, stretch, or various chemical species (38). Four of the six known TRPV channels are expressed by endothelial cells, with TRPV1, TRPV3, and TRPV4 having known functional roles (6). Of particular interest is the possible involvement of TRPV4 in the response to bubble contact, as calcium entry through this channel has been shown to play a role in both vascular physiology [shear-dependent nitric oxide production and vasodilation (24, 29)] as well as pathology [ventilator-induced lung edema (22)]. Furthermore, exogenous activation of TRPV4 using a chemical agonist has been shown to cause microcirculatory collapse and vascular leak (47). This introduces the possibility that inhibitors of TRPV channels may have potential as pharmacological therapies for gas embolism.
The involvement of a TRPV channel in the response to air bubble contact is a key difference between the way cells respond to a mechanical stimulus, such as touching with a micropipette (12, 40) and contact with an air bubble. Briefly, Sigurdson et al. (40) found that the intracellular calcium transient resultant from micropipette stimulation required some extracellular calcium, and Diamond et al. (12) demonstrated that the transients could be blocked by cytochalasin B or manoalide, a phospholipase A and PLC inhibitor. However, the calcium signal was not affected by lowering the extracellular calcium concentration and was not blocked by gadolinium (40). Whereas the mechanical deformation in our experiments is similar to those of Sigurdson and Diamond, an air bubble is substantially different from a glass pipette tip in that it presents an air-liquid interface that can interact with biological macromolecules (30). At present, the mechanism of activation of TRPV channels, including TRPV4, in response to mechanical stimuli remains unclear (10): they may be activated directly or indirectly through a mechanosensitive partner, possibly via a second messenger. Ongoing work has demonstrated that proteins can adsorb to air-water interfaces (30) and that this adsorption can cause conformational changes, including protein denaturation (19, 20). The precise interactions between any specific protein and the air-liquid interface depend on the nature and structure of the protein (20, 35), as well as the presence of other species, including other proteins or surfactants (30). The endothelial cell surface is rich in biomolecules, both membrane bound and adsorbed (37). We hypothesize that the air-liquid interface presented by an air bubble is capable of directly interacting with endothelial cell surface biomolecules (TRPV channels and/or partner sensor molecules), causing a conformational change, resulting in a calcium influx via TRPV channel opening. Possible sensor partners include the mechanosensors implicated in sensing fluid shear stress, such as components of the glycocalyx (42), cell adhesion molecules (16), G proteins (21), or G protein-coupled receptors (9). Our conclusions are supported by the findings of Kobayashi that protein (5% BSA) and a surfactant (0.1% wt/vol Pluronic F-127) were protective in BAEC cells touched with bubbles, reducing both the peak of the calcium and lethality (compared with plain buffer). Protein present in the media will interact with both the bubble, adsorbing to the surface (23), as well as with the glycocalyx (2), effectively thickening the endothelial surface layer (37). Likewise, Pluronic F-127 has been shown to be capable of out-competing proteins at the air-liquid interface, minimizing protein adsorption (30). Both of these effects may serve to minimize direct interactions between the air bubble and membrane-associated proteins, such as a TRPV channels or partner sensor proteins, thus minimizing channel opening and calcium transient generation. Thus our findings support the concept of surfactant therapy for gas embolism (13).
While the influx of calcium through a channel is necessary to generate the observed intracellular calcium transients following bubble-cell contact, our results show that it alone is not sufficient to do so. The primary pathway responsible for calcium release in endothelial cells is the IP3-dependent release of ER calcium stores. Briefly, IP3 is produced by the enzymatic hydrolysis of PIP2, a membrane-associated phospholipid, by PLC. Once produced, IP3 can bind to the IP3 receptor at the ER, opening the calcium channel (43). We examined the contribution of intracellular calcium to the signal by either inhibiting the activity of PLC with neomycin, which binds PIP2 (17), or blocking the IP3 receptor using xestospongin C (18). Both of these inhibitors had similar and marked effects, abolishing the calcium transients in response to air bubble contact in about two-thirds of cells. Importantly, specifically blocking IP3 receptors alone, as opposed to PLC inhibition, should unmask any other PLC-dependent processes such as diacylglycerol- or IP3-dependent TRP calcium channel opening (38). However, both treatments resulted in similar results, leading us to conclude that IP3 receptor-mediated intracellular release of calcium from the ER is necessary and is the primary component of the observed calcium transients evoked by air bubble contact. The incomplete inhibition observed with xestospongin C and neomycin is likely due to dosage limitations, as we observed similar inhibition rates in response to the ATP-positive control. In the case of both inhibitors, we used the highest dose that we could expose the cells to without exceeding 1% DMSO vehicle, in the case of xestospongin C, or exceeding the solubility in aqueous media, in the case of neomycin. In pilot studies, we tested two alternative PLC inhibitors edelfosine and U73122, which also abolished the calcium response to air bubble contact (data not shown). However, these compounds were poorly tolerated by our cells. The use of an alternative IP3 receptor inhibitor 2-aminoethoxydiphenyl borate to confirm the xestospongin C results was considered; however, this antagonist also modulates TRP channels, which our earlier results had already determined to play an important role in the cellular response to air bubble contact.
Because our prior experiments had shown the importance of an extracellular calcium influx, we hypothesize that air bubble contact causes an initial calcium influx, resulting in a localized increase in calcium at the plasma membrane, sufficient to activate or enable the activation of PLC, which requires calcium for catalytic function (39). It is likely that we cannot detect the calcium influx alone due to the brief air bubble-contact resulting in a small, localized influx and the actions of the plasma membrane calcium ATPases and ER calcium ATPases, which serve to maintain cytosolic calcium homeostasis (43). Several isoforms of PLC are expressed by endothelial cells (15), but of particular relevance here are isoforms β and δ. In the presence of calcium, PLCβ is activated by G proteins (39), which may be involved in the transduction of air bubble contact. Alternately, PLC isoform δ can be stimulated directly by physiological calcium levels (0.1–10 μmol/l) (4), such as those resulting from air bubble contact. Our findings appear similar to those of Diamond et al. (12), who found that the calcium transient resultant from micropipette stimulation could be eliminated by inhibiting phospholipase A and PLC, while the addition of arachidonic acid had no effect, suggesting PLC activation as the dominant pathway. However, our results with channel blockers and IP3 receptor blockade suggest that air bubble contact triggers channel opening first, followed by PLC activation. Thus air bubble and micropipette contact differ in way the responses are triggered, but there appears to be a commonality in the ultimate intracellular source of the calcium transients. Furthermore, endothelial cells also generate IP3 in response to shear stress (34) and this can be inhibited by neomycin (36). Given the importance of the IP3 pathway in the response to such a variety of stimuli, and the fact that it appears to be downstream of the initial trigger, it may not be a particularly attractive target for pharmacological intervention specific to gas embolism injury.
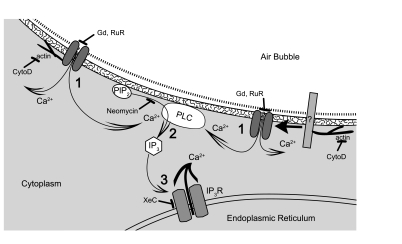
We have examined both the role of extracellular calcium influx and intracellular calcium release through a carefully considered series of experiments utilizing various pharmacological agents alone and in combination as a mechanistic means of revealing the critical components of HUVEC calcium signaling in response to bubble contact as can occur during intravascular gas embolism. It is our interpretation that endothelial cell mechanostimulation resulting from bubble contact causes the following sequence of events (Fig. 6): 1) contact between the air-liquid interface presented by the air bubble and the endothelial surface layer causes calcium channel opening (directly or indirectly) followed by calcium entry into the cell; 2) the localized increase in calcium concentration activates or facilitates the activation PLC, causing the hydrolysis of PIP2 and releasing IP3; and 3) the IP3 activates IP3R calcium channels, causing the release of calcium from ER stores, resulting in the large calcium transient we observe. The subsequent signaling caused by this calcium transient is likely to be responsible for the cell injury caused by air bubbles (28), thus mitigating this cellular response to air bubble contact is an attractive target for therapeutic intervention. Our work here clarifies the molecular pathways involved and suggests that further examination of the air-liquid-cell interface is most likely to yield a novel therapeutic target, particularly for preventive care.
Fig. 6.
Illustration of proposed mechanism by which air bubble contact elicits a calcium transient in HUVECs, with pharmacological interventions indicated by blunt arrows. (For clarity, the glycocalyx and endothelial surface layer have been not been drawn.) 1) Calcium influx following air bubble contact induced channel opening. [The left side of the illustration demonstrates possible direct interaction between air bubble and calcium channel, leading to channel opening and calcium influx, while the right side of the illustration demonstrates the interaction of the air bubble with a sensor protein (labeled “?”), which then causes to the calcium channel to open.] 2) PLC activation caused or facilitated by local increase in calcium, resulting in IP3 production. 3) IP3-dependent release of endoplasmic reticulum calcium stores.
GRANTS
This work was supported by Office of Naval Research Grant N00014-08-1-0436 (to D. M. Eckmann) and National Heart, Lung, and Blood Institute Grant R01 HL-067986 (to D. M. Eckmann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Benjamin Pichette for micropipette preparation, Dr. Donald Joseph and Dr. Ge Liang for helpful discussion of technical aspects of IP3 receptor blockage and donation of xestospongin C reagent, and Karen Sobolewski for illustration and help with figure preparation.
REFERENCES
- 1. Abu-Omar Y, Balacumaraswami L, Pigott DW, Matthews PM, Taggart DP. Solid and gaseous cerebral microembolisation during off-pump, on-pump and open cardiac surgery. Circulation 108: 549, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Adamson RH, Clough G. Plasma-proteins modify the endothelial-cell glycocalyx of frog mesenteric microvessels. J Physiol 445: 473–486, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahonen J, Salmenpera M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand 48: 4–19, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J 327: 545–552, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol 24: 871–877, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2010. November 10 doi:10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol 88: 141–152, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol Cell Physiol 275: C619–C621, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Davidson SM, Duchen MR. Endothelial mitochondria-contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Diamond SL, Sachs F, Sigurdson WJ. Mechanically induced calcium mobilization in cultured endothelial cells is dependent on actin and phospholipase. Arterioscler Thromb 14: 2000–2006, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Eckmann DM, Zhang J, Lampe J, Ayyaswamy PS. Gas embolism and surfactant-based intervention: implications for long-duration space-based activity. Ann NY Acad Sci 1077: 256–269, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Arch 459: 923–939, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Fu Y, Cheng JX, Hong SL. Characterization of cytosolic phospholipases C from porcine aortic endothelial cells. Thromb Res 73: 405–417, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259: 373–380, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta 979: 105–112, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Graham DE, Phillips MC. Proteins at liquid interfaces.1 kinetics of adsorption and surface denaturation. J Colloid Interface Sci 70: 403–414, 1979 [Google Scholar]
- 20. Graham DE, Phillips MC. Proteins at liquid interfaces. 3. Molecular-structures of adsorbed films. J Colloid Interface Sci 70: 427–439, 1979 [Google Scholar]
- 21. Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Hansen FK, Myrvold R. The kinetics of albumin adsorption to the air/water interface measured by automatic axisymmetric drop shape analysis. J Colloid Interface Sci 176: 408–417, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2: e827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helps SC, Parsons DW, Reilly PL, Gorman DF. The effect of gas emboli on rabbit cerebral blood-flow. Stroke 21: 94–99, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Ito S, Suki B, Kume H, Numaguchi Y, Ishii M, Iwaki M, Kondo M, Naruse K, Hasegawa Y, Sokabe M. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol 43: 26–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins-identification by morphologic and immunological criteria. J Clin Invest 52: 2745–2756, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi S, Crooks SD, Eckmann DM. In vitro surfactant mitigation of gas bubble contact-induced endothelial cell death. Undersea Hyperb Med 38: 59–71, 2011 [PMC free article] [PubMed] [Google Scholar]
- 29. Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Lampe JW, Liao Z, Dmochowski IJ, Ayyaswamy PS, Eckmann DM. Imaging macromolecular interactions at an interface. Langmuir 26: 2452–2459, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mirski MA, Lele AV, Fitzsimmons L, Toung TJK. Diagnosis and treatment of vascular air embolism. Anesthesiology 106: 164–177, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Mukundakrishnan K, Ayyaswamy PS, Eckmann DM. Bubble motion in a blood vessel: shear stress induced endothelial cell injury. J Biomech Eng 131: 074516, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Nachman RL, Jaffe EA. Endothelial cell culture: beginnings of modern vascular biology. J Clin Invest 114: 1037–1040, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun 170: 281–287, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Phillips MC, Sparks CE. Properties of apolipoproteins at the air-water interface. Ann NY Acad Sci 348: 122–137, 1980 [Google Scholar]
- 36. Prasad AR, Logan SA, Nerem RM, Schwartz CJ, Sprague EA. Flow-related responses of intracellular inositol phosphate levels in cultured aortic endothelial cells. Circ Res 72: 827–836, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflügers Arch 440: 653–666, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sigurdson WJ, Sachs F, Diamond SL. Mechanical perturbation of cultured human endothelial-cells causes rapid increases of intracellular calcium. Am J Physiol Heart Circ Physiol 264: H1745–H1752, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Swaminathan TN, Ayyaswamy PS, Eckmann DM. Surfactant properties differentially influence intravascular gas embolism mechanics. Ann Biomed Eng 38: 3649–3663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Tran QK, Watanabe H. Calcium signalling in the endothelium. Handbook Exp Pharmacol 145–187, 2006 [DOI] [PubMed] [Google Scholar]
- 44. van Hinsbergh VWM, Amerongen GPV. Intracellular signalling involved in modulating human endothelial barrier function. J Anat 200: 549–560, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Hulst RA, Klein J, Lachmann B. Gas embolism: pathophysiology and treatment. Clin Physiol Functional Imaging 23: 237–246, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet 377: 153–164, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse. J Pharmacol Exp Ther 326: 443–452, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys 56: 1–18, 2010 [DOI] [PubMed] [Google Scholar]