Abstract
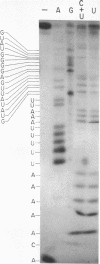
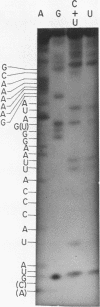
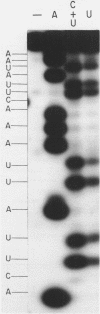
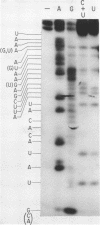
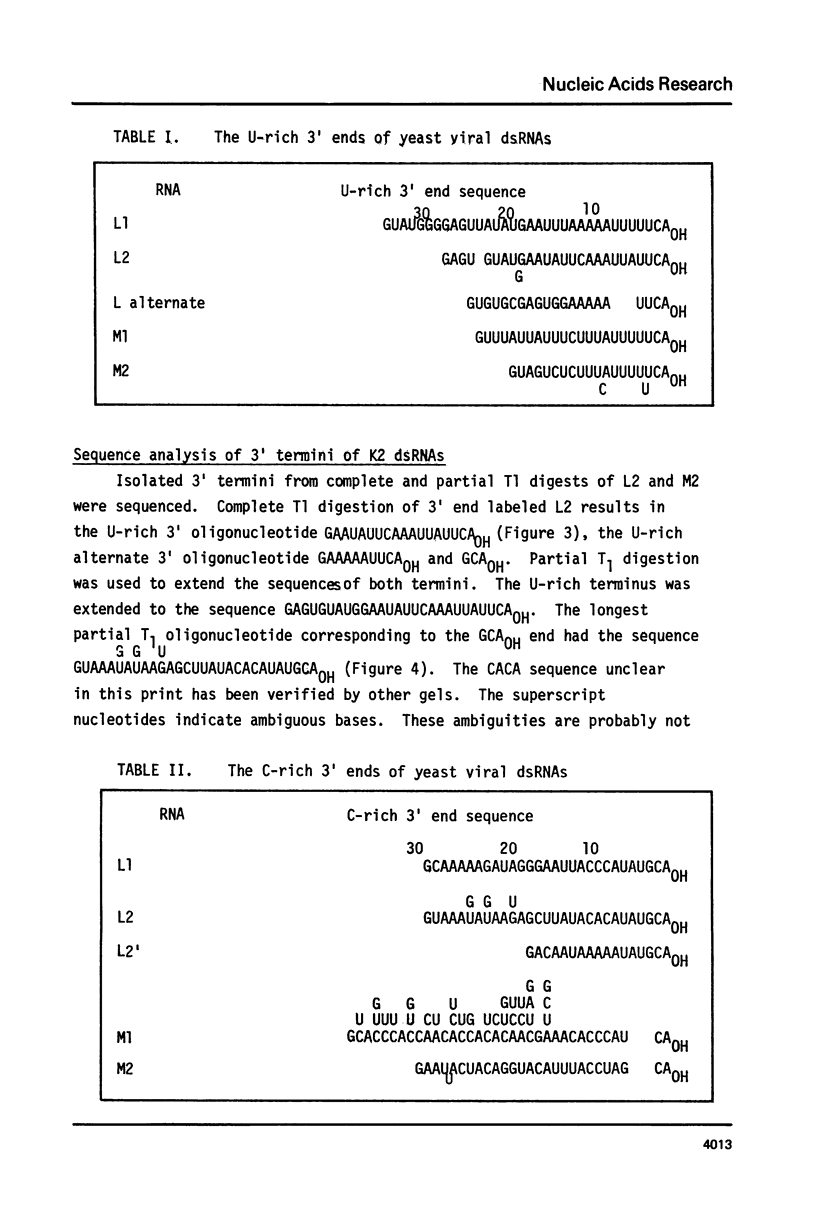
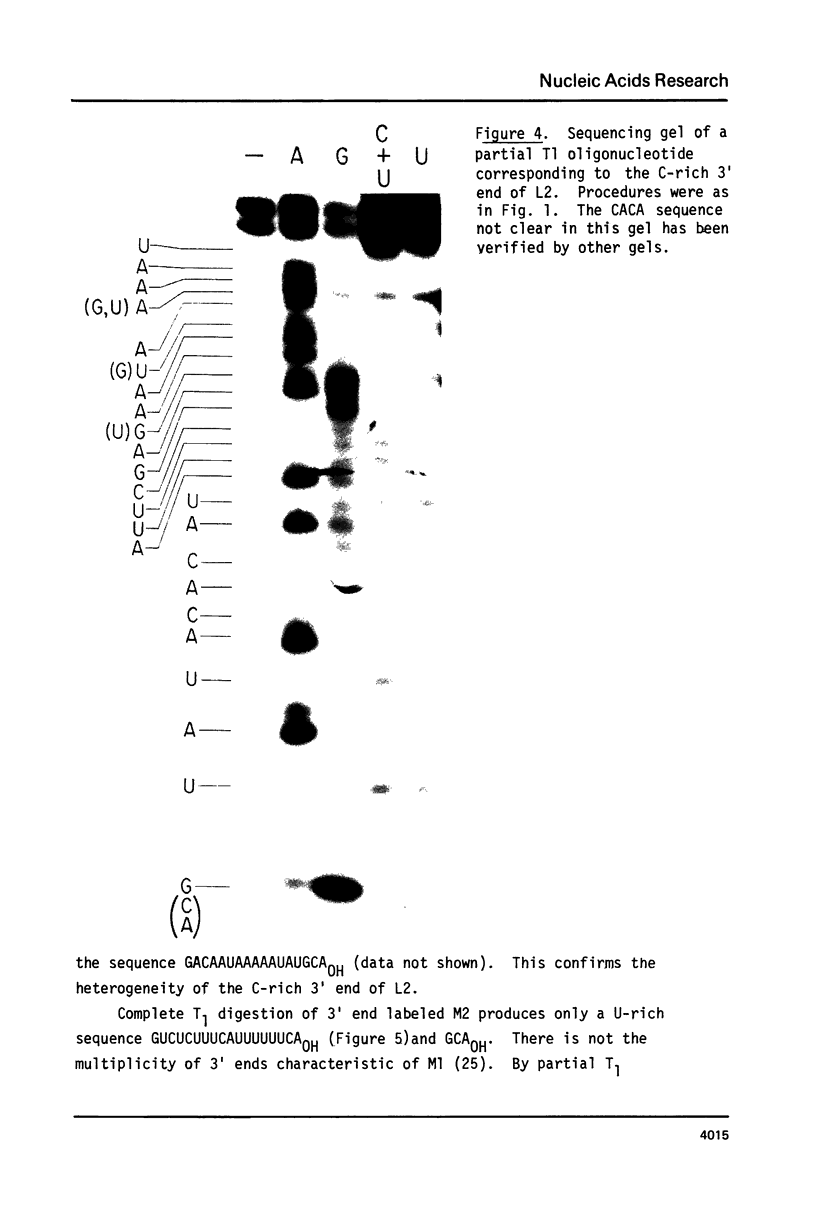
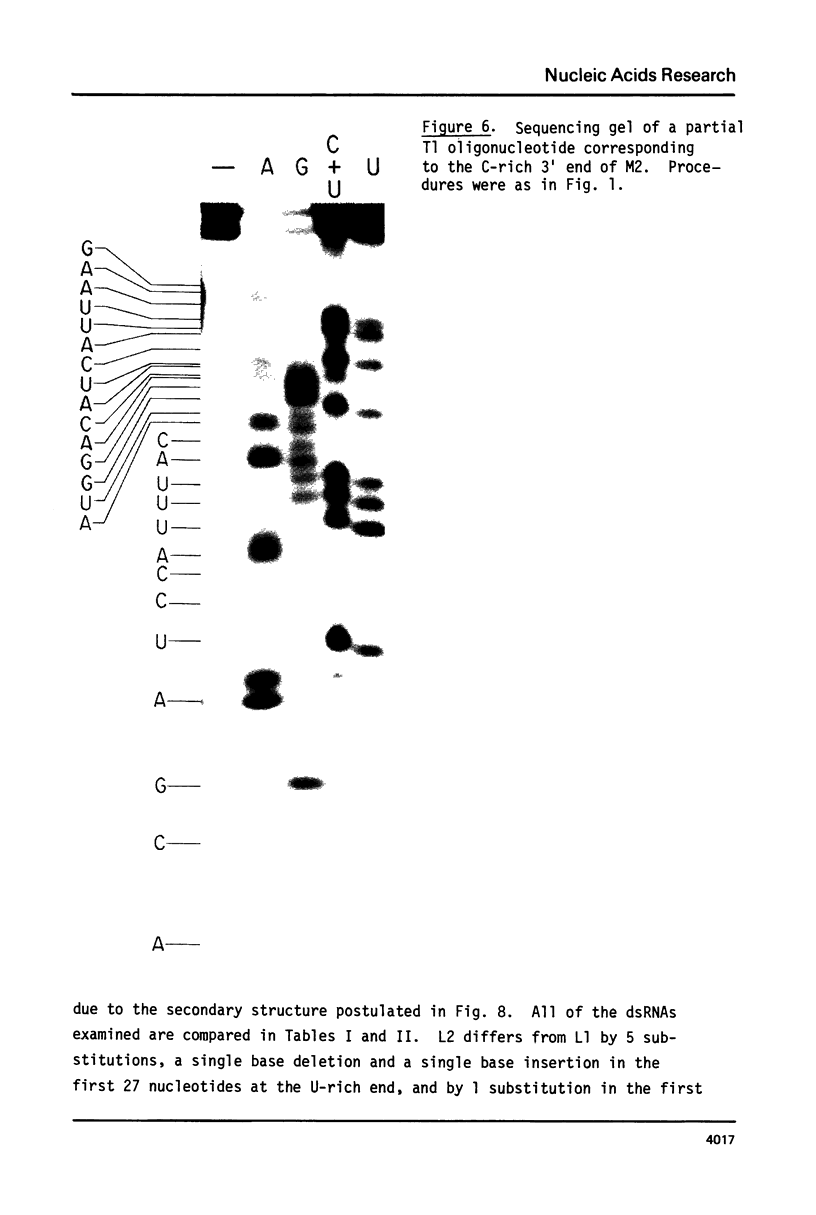
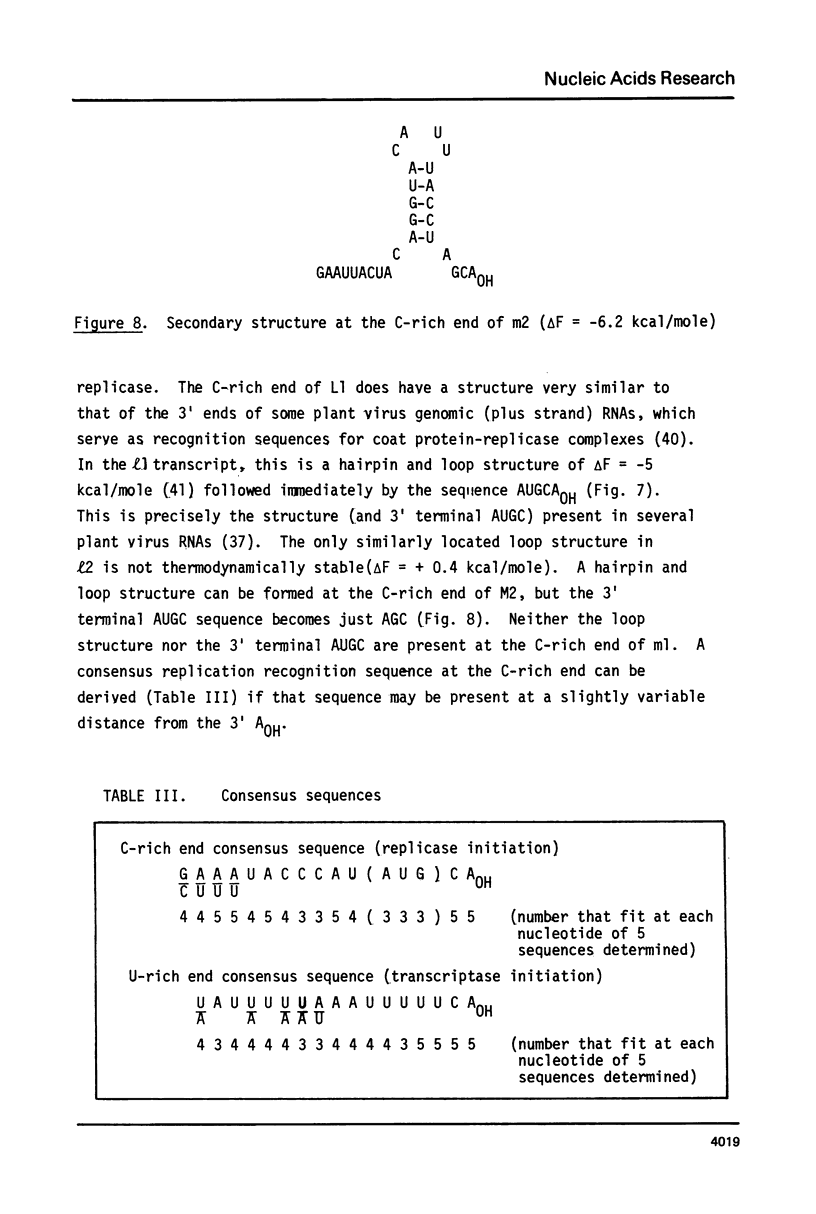
ScV is a double-stranded RNA virus of yeast consisting of two separately encapsidated dsRNAs (L and M). ScV-1 and ScV-2 are two dsRNA viruses present in two different yeast killer strains, K1 and K2. Our 3' end sequence analysis shows that the two sets of viral dsRNAs from ScV-1 and ScV-2 are very similar. Consensus sequences for transcriptase and replicase initiation are proposed. A stem and loop structure with a 3' terminal AUGC sequence, like that of several plant virus plus strand RNAs, is present at the putative replicase initiation site of one of the yeast viral RNA plus strands.
Full text
PDF



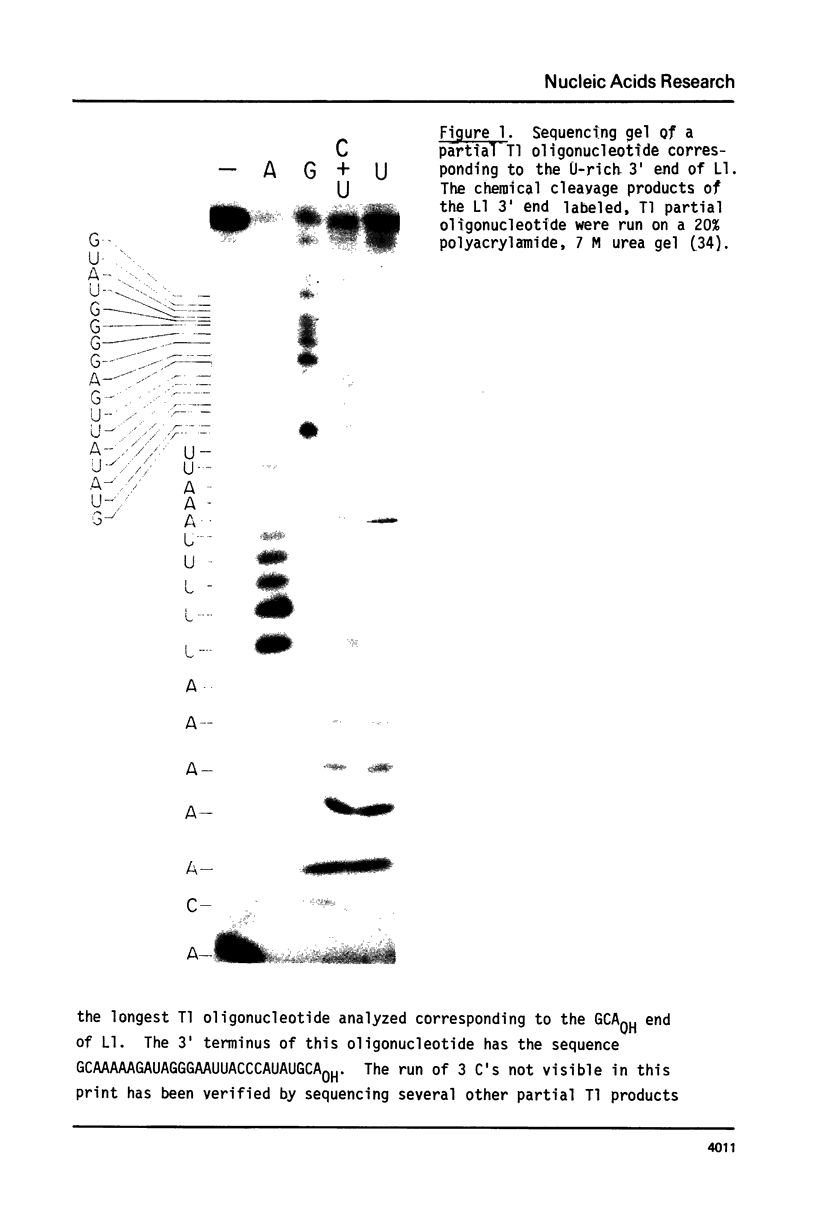
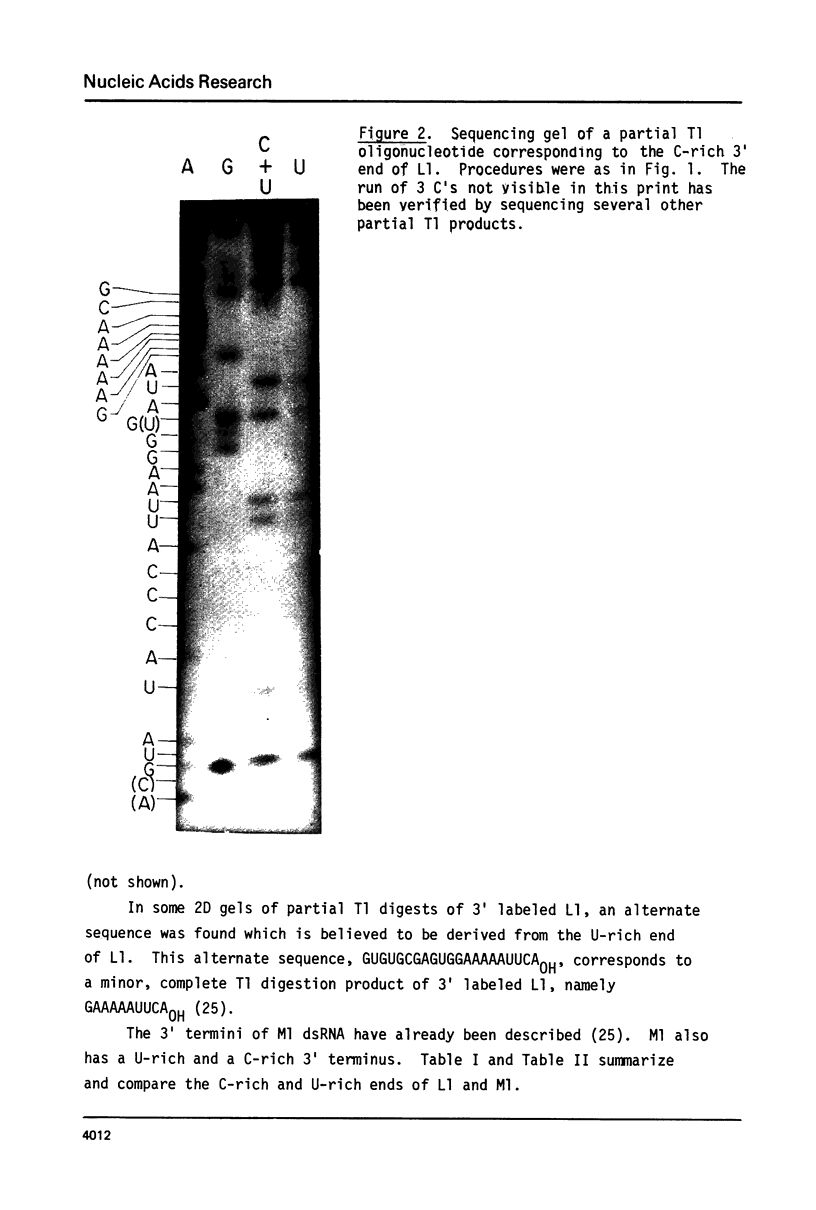





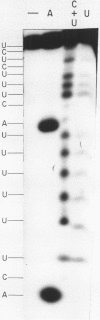
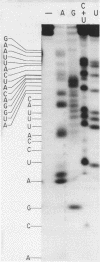
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Kane W. Relatedness of the double-stranded RNAs present in yeast virus-like particles. J Virol. 1978 Jun;26(3):762–772. doi: 10.1128/jvi.26.3.762-772.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K. W. Replication of double-stranded RNA in particles of Penicillium stoloniferum virus S. Nucleic Acids Res. 1975 Oct;2(10):1889–1902. doi: 10.1093/nar/2.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. S. Virus-like particles and double stranded RNA from killer and non-killer strains of Saccharomyces cerevisiae. Microbios. 1978;21(85-86):161–176. [PubMed] [Google Scholar]
- Hastie N. D., Brennan V., Bruenn J. A. No homology between double-stranded RNA and nuclear DNA of yeast. J Virol. 1978 Dec;28(3):1002–1005. doi: 10.1128/jvi.28.3.1002-1005.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Yeast virus-like particles possess a capsid-associated single-stranded RNA polymerase. Nature. 1977 Aug 4;268(5619):464–466. doi: 10.1038/268464a0. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Kane W. P., Pietras D. F., Bruenn J. A. Evolution of defective-interfering double-stranded RNAs of the yeast killer virus. J Virol. 1979 Nov;32(2):692–696. doi: 10.1128/jvi.32.2.692-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Bol J. F. Nucleotide sequence of the putative recognition site for coat protein in the RNAs of alfalfa mosaic virus and tobacco streak virus. Nucleic Acids Res. 1980 Aug 11;8(15):3307–3318. doi: 10.1093/nar/8.15.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Brederode F. T., Veeneman G., van Boom J. H., Bol J. F. Nucleotide sequences at the 5'-termini of the alfalfa mosaic virus RNAs and the intercistronic junction in RNA 3. Nucleic Acids Res. 1980 Dec 11;8(23):5635–5647. doi: 10.1093/nar/8.23.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova G. I., Naumova T. I. Sravitel'naia genetika drozhzhei. Soobshchenie XIII. Sravitel'noe izuchenie sakharomitsetov-ubiits iz razlichnykh kollektsii. Genetika. 1973 Nov;9(11):140–145. [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Somers J. M. Isolation of Suppressive Sensitive Mutants from Killer and Neutral Strains of SACCHAROMYCES CEREVISIAE. Genetics. 1973 Aug;74(4):571–579. doi: 10.1093/genetics/74.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T. K., Tate A., Fink G. R. A study of the transmission and structure of double stranded RNAs associated with the killer phenomenon in Saccharomyces cerevisiae. Genetics. 1976 Sep;84(1):27–42. doi: 10.1093/genetics/84.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. Induction of yeast killer factor mutations. J Bacteriol. 1977 Oct;132(1):346–348. doi: 10.1128/jb.132.1.346-348.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Weissmann C. The 3'-termini of bacteriophage Q-beta plus and minus strands. J Mol Biol. 1970 Jul 28;51(2):215–224. doi: 10.1016/0022-2836(70)90138-5. [DOI] [PubMed] [Google Scholar]
- Welsh D., Leibowitz M. J. Transcription of killer virion double-stranded RNA in vitro. Nucleic Acids Res. 1980 Jun 11;8(11):2365–2375. doi: 10.1093/nar/8.11.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Killer of Saccharomyces cerevisiae: a double-stranded ribonucleic acid plasmid. Bacteriol Rev. 1976 Sep;40(3):757–773. doi: 10.1128/br.40.3.757-773.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. W., Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Van Leeuwenhoek. 1978;44(1):59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]