Abstract
Canonical and non-canonical wnt signals often have opposed roles. In this report, we use developing Xenopus embryos to demonstrate a novel anti-proliferative role for non-canonical wnt signals in the very earliest stages of kidney development. Non-canonical wnt signals were down-regulated using PDZ domain mutants of dishevelled 2 and up-regulated using wild-type vang-like 2, while canonical signals were manipulated using dominant-negative forms of lef1 or treatment with lithium. When non-canonical signals are down-regulated in the developing Xenopus pronephros, cell proliferation rates increased and when canonical signals were shutdown the opposite occurred. Treatment with lithium chloride has a powerful pro-proliferative effect on the forming nephric primordium. Together these data show that in addition to previously documented antagonisms between these distinct wnt signaling pathways, they also have opposing effects on cell division.
Keywords: planar cell polarity, PCP, canonical wnt, signal transduction, mitosis, proliferation
INTRODUCTION
Multiple wnt ligands acting by means of canonical signaling are essential for the reciprocal inductive interacts patterning the mammalian adult kidney, the metanephros, and in its precursor organs, the pronephros and mesonephros (Carroll and McMahon, 2003; Lyons et al., 2009). In all vertebrates, the adult kidney develops from and uses elements of the preceding nephric organs, which are transient, architecturally more simple versions of the adult organ (Saxén, 1987; Vize et al., 1997; Jones, 2005). While their overall structure may be more simple, the embryonic kidneys have a similar level of physiological complexity (Zhou and Vize, 2004, 2005; Wingert et al., 2007, Wingert and Davidson, 2008) and serve as powerful model systems in which to explore general processes critical to all kidney development. In essence, the pronephros is a single giant nephron while adult kidneys have between thousands and hundreds of thousands of nephrons organized into elaborate arborized networks (Goodrich, 1930; Saxén, 1987; Vize et al., 2003).
Both canonical and non-canonical wnt signals have been shown to play important roles in kidney organogenesis. Canonical wnt signaling through beta-catenin-mediated transcription plays important roles in pronephric (Lyons et al., 2009), mesonephric (Marose et al., 2008), and metanephric kidney development (Stark et al., 1994; Carroll and McMahon, 2000; Carroll et al., 2005). In the pronephros, canonical signals play an essential role in the initial specification of the kidney primordium that gives rise to all nephric organs (Lyons et al., 2009). This pathway has also been shown to participate in metanephric specification and to be involved in balancing differentiation status (Marose et al., 2008). Although the canonical pathway has been shown to enhance cell proliferation rates in other systems (e.g., Megason and McMahon, 2002), it has not previously been demonstrated to regulate cell division in the kidney.
Multiple methodologies are available to selectively manipulate different branches of the wnt signaling pathway. The first described method was treatment with lithium chloride (Klein and Melton, 1996). The key canonical mediator gsk3 is inhibited by lithium, thus promoting canonical signaling. Dishevelled (dvl) participates in all known branches of wnt signaling (reviewed by van Amerongen and Nusse, 2009), but different domain deletions of dvl have selective differences in their effects on different signaling branches. In this report, two different domain deletions are used, dvl2-D2/dsh-D2 which lacks the entire PDZ domain (Rothbacher et al., 2000) and dvl2-D4/dsh-D4/XDD1 which lacks a portion of the PDZ domain (Sokol, 1996; Rothbacher et al., 2000). Dvl2-D2 inhibits non-canonical signaling but promotes canonical signaling (Rothbacher et al., 2000), although the relative efficacy of the impacts on the different branches is not well characterized. Dvl2-D4 appears to only inhibit non-canonical signaling (Rothbacher et al., 2000), although it can block exogenous canonical signaling (Sokol, 1996). Other reagents available include neomorphic forms of the lef1 transcription factor that effectively block only canonical signaling (Deroo et al., 2004), and vangl2, which only promotes non-canonical signaling (Darken et al., 2002). While the exact effects of these reagents are always difficult to determine, by testing them all and correlating experimental results with these different treatments, differential effects on one branch or another can be determined.
Non-canonical wnt signals are also important in kidney development. Wnt11 regulates branching of the ureteric bud (Majumdar et al., 2003; Chi et al., 2004) and has also been implicated in pronephric patterning (Tetelin and Jones, 2009). Wnts 4, 7b and 9b signal through both canonical and non-canonical pathways (Lewandoski et al., 1997; Lyons et al., 2004; Chang et al., 2007; Karner et al., 2009; Yu et al., 2009) and all are essential to normal kidney development (Stark et al., 1994; Carroll et al., 2005). Of these, both wnts 7b and 9b have been shown to act on the non-canonical planar cell polarity (PCP) pathway during kidney morphogenesis where they control the orientation of cell division planes in developing nephrons (Karner et al., 2009; Yu et al., 2009). As the genetic network patterning the adult metanephros appears to be an elaboration of that used to pattern the embryonic pronephros (Vize et al., 1997; Jones, 2005), exploring the role of non-canonical wnt signals in the pronephros may lead to broad insights into kidney development in general.
RESULTS
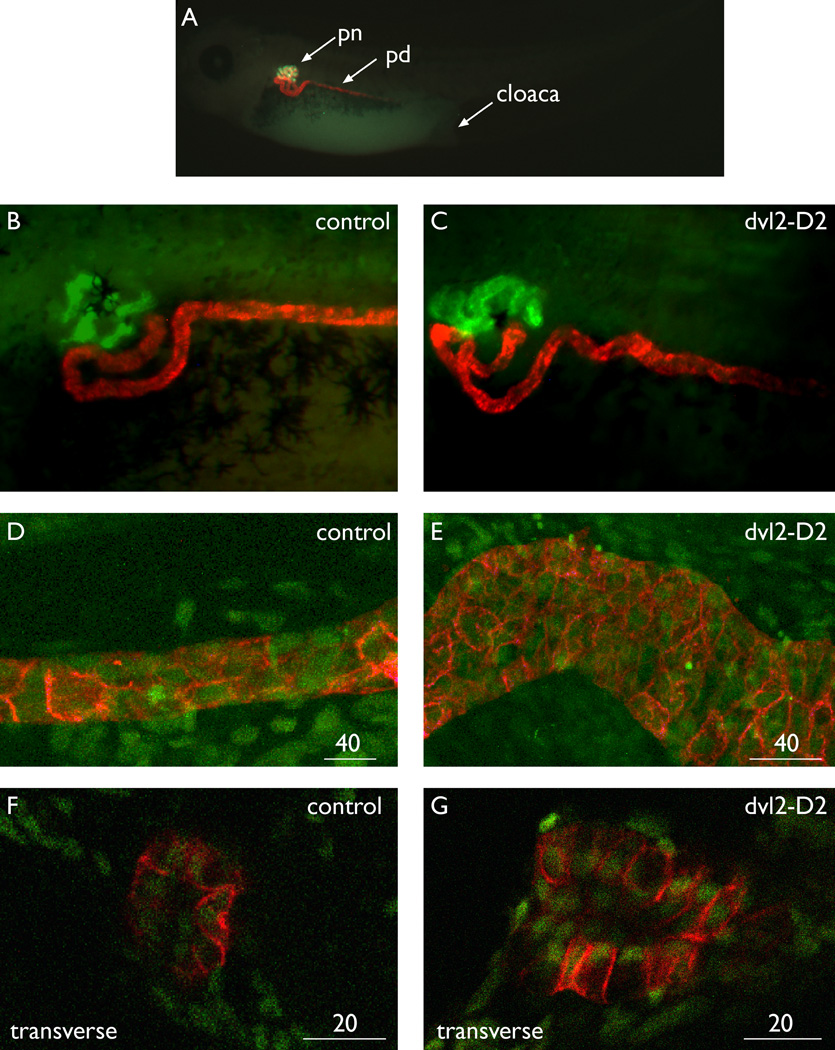
Roles for canonical wnt signaling in early kidney development are well established (e.g., Carroll and McMahon, 2003). To explore the role of non-canonical signaling in this same process, mutant forms of dishevelled homolog 2/dvl2 (Rothbacher et al., 2000) were tested for their effects on early kidney development using Xenopus embryo microinjection. Two different mutants were overexpressed by means of microinjection of synthetic mRNA into the ventro-vegetal blastomeres of 8- to 16-cell stage Xenopus embryos that are fated to form the pronephric kidney, blastomeres V2 and V2.2 respectively. Injected embryos were grown to stage 40/41, fixed, and stained with pronephric markers. Two different stains were used for the pronephric nephron, either the previously described monoclonal 3G8 (Vize et al., 1995) or fluorescein coupled Erythrina cristagalli lectin (ECL), while antibody 4A6 was used to stain distal tubules and pronephric (Wolffian) ducts. Only one side of an embryo was injected with mRNA plus tracer, and the contralateral side serves as an internal control. Blocking non-canonical signaling did not interfere with kidney specification or patterning as antibody staining indicated both proximal and distal pronephric epithelial tubules were present and in the correct place, very unlike the effect of blocking canonical signaling which effectively inhibits kidney development (Lyons et al., 2009). However, while control embryos possessed a long, narrow Wolffian duct running in a straight line toward the cloaca, embryos injected with mutant dvl2-D2 were found to have regions with broader than normal ducts and in some cases, concertina-shaped ducts that meander toward the cloaca (Fig. 1). More detailed examination of lateral and transverse views of dvl2-D2-expressing ducts using confocal microscopy confirmed that these were approximately 1.5 times as wide as control ducts, being on average 78 microns wide while controls were 50 microns wide (Table 1). The number of cells in transverse sections was found to be double the number observed in controls and ducts had a clear lumen (Table 1). This effect was observed in 41/207-injected embryos (19.8%) over many independent experiments. An mRNA encoding a different deletion of the PDZ domain of dvl2, dvl2-D4 (Sokol, 1996; Rothbacher et al., 2000), also generated wider Wolffian ducts but at a lower frequency (20/201, 9.9%). The difference in penetrance could be due to differences in translation efficacy or due to different impacts on canonical/non-canonical branches of the wnt pathway. However, as duplicated axis were never observed, as would be expected if either reagent were promoting canonical signaling, the most likely reason for a common phenotype is that inhibition of non-canonical signaling is responsible for the observed phenotype. Similar defects were never observed in control embryos or on the contralateral side of mRNA-injected embryos, or in any of the dozens of other mRNA injection experiments we have performed examining pronephric gene function in Xenopus. As dvl2-D2 failed to induce secondary axes at the mRNA doses used here (see the Experimental Procedures section), it is extremely unlikely that any canonical stimulation is responsible for the observed dvl2-D2 phenotype. Likewise, the dvl2-D2 reagent clearly blocked non-canonical signaling, as embryos injected into dorsal blastomeres failed to undergo normal convergence and extension movements and had short body axes (Supp. Fig. S1, which is available online).
Fig. 1.
Dominant-negative dvl2 generates Wolffian ducts that are abnormally shaped, wider, and contain more cells than control ducts. Staining details for each technique are available in the Experimental Procedures section. A: Control embryo stained for proximal tubules in green (3G8) and the Wolffian duct in red (4A6). B: Lateral view of control stained for proximal tubules with ECL (green) and 4A6 (red). C: Dvl2-D2 mRNA-injected pronephros. The Wolffian duct is an abnormal serpentine shape. D: Confocal maximal projection of a control duct, stained with 4A6 (red) to highlight the pronephros and counterstained with sytox (green) for nuclei. E: Confocal maximal projection of dvl2-D2 mRNA-injected pronephros, stained as described in D. The duct is wider and has more cells than controls. F,G: Control and dvl2-D2 mRNA-injected ducts stained with 4A6 (red) and sytox (green) then viewed in optical transverse sections. The dvl2-D2 duct contains more cells, but both have an open lumen and normal epithelialization. pn, pronephric nephron proximal domain; pd, pronephric distal tubule and duct; cl, cloaca. Panels A through E show lateral views, while F and G illustrate transverse views. Scale bar = 40 microns in D,E, 20 microns in F,G.
TABLE 1.
dvl2-D2 mRNA Injected Pronephroi Are Wider and Have More Cells Than Controls
| Sample | Diameter (microns) |
Diameter (microns) |
# Cells in transverse section |
# Cells in transverse section |
|---|---|---|---|---|
| Injected mRNA | Control | dvl2-D2 | Control | dvl2-D2 |
| 1 | 47 | 81 | 9 | 15 |
| 2 | 52 | 77 | 9 | 18 |
| 3 | 51 | 75 | 9 | 23 |
| 4 | 50 | 79 | ||
| 5 | 48 | 78 | ||
| 6 | 50 | |||
| 7 | 49 | |||
| 8 | 53 | |||
| mean | 50 | 78 | 9 | 18.7 |
The effect of dvl2-D2 on pronephric morphology could be caused by impacting pronephric specification, cell proliferation, apoptosis, morphogenesis, or a combination of these possibilities. As apoptotic rates are extremely low in developing pronephroi (Urban et al., 2006) inhibition of this process is very unlikely to generate the observed effects so was not investigated. As defects in morphogenesis could be caused by earlier effects on specification or cell division these later processes were explored first. To assay cell division rates in early kidney primordia without performing serial sectioning of a large number of samples, it was necessary to develop a novel implementation of fluorescent in situ hybridization. An effective protocol was developed by coinjecting dvl2-D2 mRNA with an mRNA encoding membrane bound green fluorescent protein (GFP; RhoA-GFP, Park et al., 2008). These embryos were raised to stage 21/22, when the pronephric primordium is first forming (Vize et al., 2003), then fixed. Following permeabilization, fluorescent in situ hybridization was performed with an lhx1 or a pax8 probe and a fluorescein tyramide, followed by anti-phospho-histone H3 antibody treatment detected by means of tyramide enhancement with Cy3-tyramide. Finally, GFP was detected by means of an anti-GFP antibody developed using Cy5.5 tyramide. Neither the anti-phospho-histone H3 or GFP antibodies generated sufficiently strong signals for confocal microscopy unless tyramide amplifications were performed. Following processing, embryos were cleared and analyzed by means of confocal microscopy (Fig. 2).
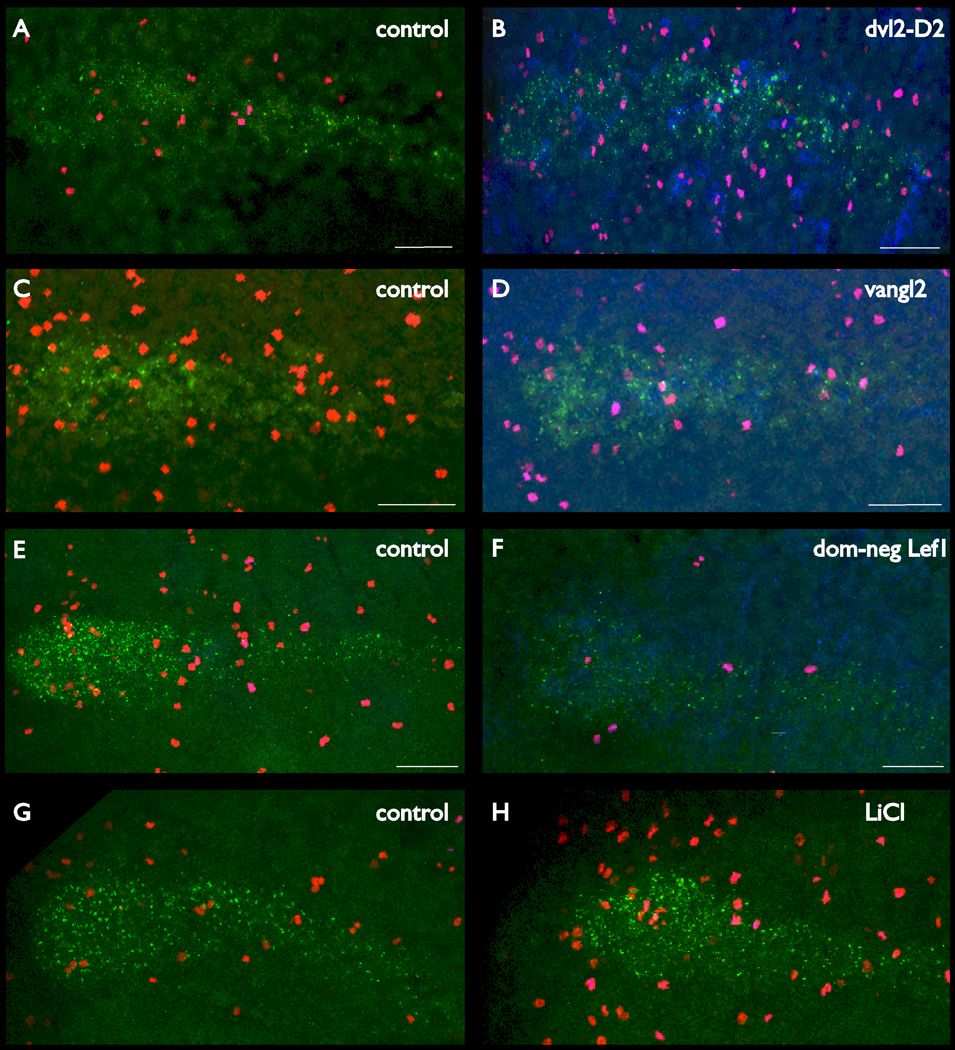
Fig. 2.
Microinjection of dvl2-D2 mRNA increases the number of phospho-H3 positive cells in the pronephros. Embryos were injected with dvl2-D2 plus membrane bound green fluorescent protein (GFP) mRNAs at the eight-cell stage, grown to stage 21/22, then fixed. Fluorescent in situ hybridization (FISH) was performed in the green channel to detect the pronephros, tyramide enhanced immunochemistry in the red channel to detect phospho-H3, and tyramide enhanced immunochemistry to detect GFP in the far red channel (shown in blue). A,C,E,G: The left column shows different channels (A,C,E) of a control sample with a merge of these presented in panel G. The right column shows a similar data set for a dvl2-D2 plus GFP mRNA-injected pronephros. F,H: Due to the sequence of development some of the phospho-H3 signal also shows up in panels E and F, but is double stained and appears as magenta in panels G and H allowing the clear distinction of phospho-H3 and GFP.
For confocal scanning, embryos were oriented in a lateral aspect relative to the microscope objective. All three wavelengths (fluorescein, Cy3 and Cy5.5) were then sampled in the Z plane in a stack that included the entire pronephros. The injected side of each sample was identified by means of GFP staining. Both injected and control stacks were then manually scored by scrolling through each Z stack and scoring with phospho-H3 positive nuclei laying within the lhx1 positive pronephric primordium (Supp. Movie S1). Images of typical results are shown in Figure 2 and tabulated phospho-H3 counts are shown in Table 2.
TABLE 2.
Pronephric Phospho-H3 Positive Cell Numbers in mRNA-Injected Embryos
| Injected mRNA | Treated mean (#) |
Control mean (#) |
P (t -test) |
|---|---|---|---|
| None | 11 (6) | 12.2 (6) | 0.3 |
| dvl2-D2 | 19.6 (14) | 12.4 (14) | 0.029 |
| vangl2 | 14.9 (11) | 22.3 (11) | 0.0001 |
| lithium | 29.8 (13) | 11.3 (6) | 0.0005 |
| dom-neg lef1 | 9.1 (9) | 31.4 (5) | 0.00001 |
A total of 14 injected primordia and 14 contralateral control primordia were counted in stage 21/22 stage embryos. Pronephroi from dvl2-D2 mRNA-injected embryos contained on average 19.6 phospho-h3 positive cells (SD = 10.44) while control pronephroi contain 12.4 positive cells (SD = 8.88). A student’s t-test indicates this difference is significant (P = 0.029; Table 2). Analysis by means of a Mann-Whitney U-test also indicated a significant difference in numbers between the two sets (P = 0.0003). The number of positive cells in two batches of unmanipulated control embryos of the same stage was also assayed and found to be similar to the controls in mRNA injection experiments, with an average of 11 and 12 dividing cells per stage 21/22 pronephric primordia.
More dividing cells could be caused by dvl2-D2 increasing the rate of cell division in the pronephroi, or by causing more cells to adopt a pronephric specification and the larger kidney undergoing normal rates division per cell. While no obvious deficiencies in other tissues or consistent enlargement of the pronephric primordium was observed in mRNA-injected embryos (some differences between the two pronephroi in an individual is normal- for example see http://www.xenbase.org/common/ViewImageActionNonAdmin.do?imageId=112), this does not completely exclude the later possibility. As canonical and non-canonical wnt signaling are antagonistic in other contexts, and as canonical signaling regulates cell proliferation in other organs, we hypothesized that the suppression of non-canonical signals by means of dvl2-D2 resulted in a loss of this antagonism and this is causing the increase in cell proliferation in pronephroi.
As suppression of non-canonical signaling enhanced rates of cell division (Table 2), the effect of enhancing non-canonical signaling was then tested. This was achieved by microinjection of vangl2 mRNA (Jenny et al., 2003). The protocol was identical to that of the prior experiment—embryos were injected with vangl2 plus GFP mRNAs, grown to stage 21/22, then fixed and stained for the pronephric primordium, phospho-H3 and the lineage tracer, then scanned using confocal microscopy and dividing cells within the pronephros counted manually. The results are presented in Table 2 and Figure 2. The effect of enhancing non-canonical signaling in the forming pronephros is the opposite of suppressing this branch of the wnt signaling pathway, and results in lower numbers of dividing cells (P = 0.0001).
Canonical wnt signaling was first suppressed by means of expression of En-R-LefN-GR744A mRNA (Deroo et al., 2004), hereafter referred to as dominant-negative lef1. This activity of this neomorphic form of lef1 is under the control of a fused glucocorticoid receptor ligand binding domain. Embryos were injected into one side (blastomere V2) with both dominant-negative lef1 and membrane bound GFP mRNAs, then grown to stage 21 in normal medium. They were then treated with dexamethasone to activate the neomorphic dominant-negative for 2 hr at 23°C followed by fixation and processing for lhx1, phospho-histone H3, and GFP as described above. As in other experiments, the injected side of the embryo was identified by means of the presence of the lineage tracer, and the number of dividing cells in the pronephros on the injected side of the embryo compared with the number on the uninjected side. Following confocal scoring, dominant-negative lef1-injected pronephroi were found to contain on average only 9.1 dividing cells, while contralateral control pronephroi had an average of 31.4. The number of dividing cells in uninjected pronephroi is higher in this experiment than that observed in other experiments, possibly because dexamethasone treatment enhances cell division rates, or it may simply be specific to the embryonic stage scored. The number of dividing cells was significantly different between dominant-negative lef1-injected and uninjected pronephroi (P = 0.00001; t-test).
Canonical wnt signaling was then activated by means of treatment of embryos with a pulse of lithium chloride. While nonspecific, this treatment is a powerful activator of this signaling pathway (Klein and Melton, 1996). Lithium treatment was performed at stage 21/22 for 4 min, then embryos kept for 1 hr at 23°C, fixed, and processed to detect diving cells. Pronephroi in control embryos had 11.3 dividing cells per pronephros (similar to other experiments), while lithium-treated pronephroi had 29.8 dividing cells. Once again, this difference was statistically significant (P = 0.0005, t-test).
In summary, suppression of non-canonical signaling enhances division rates in the pronephric primordium while enhancing this pathway decreases cell division rates. Suppression on the canonical branch has the opposite effect and decreases proliferation rates, while lithium treatment (which enhances canonical wnt signaling) increases rates of cell division within the forming pronephric primordium (Figs. 1–3).
Fig. 3.
Inhibiting non-canonical wnt signals or promoting canonical wnt signals leads to increases in cell division, while promoting non-canonical or suppressing canonical signals results in a decrease in division rates. Controls are shown on the left, and treated embryos on the right. The pronephros is stained green by means of fluorescent in situ hybridization (FISH), dividing cells stained red by means of anti-phospho-H3, and mRNA-injected samples identified by blue green fluorescent protein (GFP) staining in all panels. Three-dimensional scanning determined which phospho-H3 positive cells were within pronephroi in each case (Table 2). A–F: Images show contralateral sides of the same embryo. G/H: Representative images with average division rates were selected as both sides of each embryo received the same treatment. Scale bars = 100 microns.
DISCUSSION
Inhibiting non-canonical signaling results in the distal segment and Wolffian duct of the embryonic kidney becoming wider than usual and forming a serpentine duct rather than the usual very straight morphology (Fig. 1). This unusual phenotype has not been previously described in any detail. The effect is restricted to the posterior portion of the kidney, and no effects were observed in the proximal tubules. As different segments of the pronephros express different wnts and wnt receptors (e.g., Deardorff et al., 1998), different wnt signaling environments occur in a proximal to distal manner, with only the distal environment being capable of overproliferation in response to suppressing non-canonical signaling. Intriguingly, although not discussed, inhibition of wnt11b using a secreted dominant-negative also seems to have this effect (see Fig. 6B, panel C in Tetelin and Jones, 2009). This strongly suggests that, in addition to its role in pronephric patterning (Tetelin and Jones, 2009), the non-canonical wnt 11b also participates in balancing cell division rates in the forming kidney.
Two of the tested reagents led to increases in cell division rates in stage 21/22 pronephroi; dvl2-D2 mRNA and lithium treatment. Injected mRNAs have a half-life of approximately 2 days (Vize et al., 1991), so the mRNA injection will generate protein and inhibit non-canonical signaling up until the time at which embryos were fixed for analysis and the time at which the lithium pulse was administered. As the dvl2-D2 mRNA and protein are present throughout the time window in which pronephric specification occurs around stage 12 (Brennan et al., 1998), this treatment could affect either early specification or later cell division rates. However, the short time scale of the lithium treatment, 4 minutes plus a 60-minute recovery, and the lateness of the treatment, stage 22, long after specification is complete, indicates that this lithium regimen very likely increases cell division rates without impacting specification. Likewise, in the dominant-negative lef1 mRNA injections, the neomorphic protein was only activated with dexamethosone for a short time window long after specification is complete, and therefore tests cell division response separate from kidney patterning. As the effect on pronephric cell division rates is detectable by stage 22 in dvl2-D2, dom-neg lef1 and vangl2 microinjections non-canonical impacts on cell division must occur sometime before this. Likewise, as lithium treatments at stage 22 strongly promoted proliferation, the impact of wnts of cell division rates is still in place at this time. We cannot determine from our data how much longer the connection between wnt signaling and proliferation continues beyond stage 22.
Only one of the tested reagents, the canonical inhibiting dominant-negative lef1, suppressed cell division rates. Together these data strongly indicate that up-regulating canonical signaling in the developing pronephros is pro-proliferative while down-regulating this pathway is anti-proliferative. While dominant-negative proteins are always capable of additional unknown activities, the combination of reagents used, the clear suppression of PCP by dvl-D2 and the failure of this same reagent to induce secondary axes under these conditions, all point to an involvement of non-canonical signaling in the control of cell division rates and the generation of the pronephric phenotype.
Canonical wnt signaling has been found to promote proliferation by up-regulating cyclin d1/ccnd1 and cyclin d2/ccnd2 and by down-regulating cell cycle exit (Megason and McMahon, 2002) Concomitantly, G2/M progression is required for wnt receptor activity and canonical signaling (Davidson et al., 2009) thereby setting up a positive proliferation feedback loop. Non-canonical wnt signaling on the other hand has not previously been reported to have any effects on cell division rates. The non-canonical branches have been shown to have powerful effects on cell behavior, migration and the polarity of cell division planes (e.g., Wallingford et al., 2000; Tree et al., 2002; Karner et al., 2009) but have not been shown to directly regulate cell proliferation. The effect of suppressing non-canonical signaling we describe may well be acting indirectly on proliferation by suppressing signaling through the canonical branch. When cell division is promoted by suppressing non-canonical activity, a 1.6-fold increase is observed, while inhibiting cell division by means of lef1 results in a 3.5-fold decrease. While this may simply reflect a difference in the efficacy of the two very different blocking strategies and reagents, it may also be caused by the inherent inefficiency of inhibiting an inhibitor, versus a direct effect on an activator.
These data demonstrate that in the developing embryonic kidney dysregulation of the non-canonical branch of wnt signaling alters cell division rates. Suppression of this branch increases division rates, while enhancement increases them. It is also highlights another example of the canonical branch stimulating, and being required for, cell division. Cell proliferation can therefore be added to the list of antagonistic interactions between the canonical and non-canonical branches of the wnt signal transduction pathway.
EXPERIMENTAL PROCEDURES
Embryo Treatments
Xenopus laevis embryos were generated and raised using standard protocols using 0.1 × MMR as the medium (Sive et al., 2000). Lithium treatments were performed by treating stage 22 embryos with 0.15M LiCl at room temperature for 4 min, then culturing at room temperature for an additional 60 min before fixation and staining.
mRNA Injections
All mRNAs were generated in vitro using SP6 polymerase as previously described (Melton et al., 1984). Construct dvl2-D2 lacks amino acids 238–331 (Rothbacher et al., 2000) and dvl2-D4/Xdd1 lacks amino acids 296–381 (Sokol, 1996; Rothbacher et al., 2000) of NCBI protein NP_001084096. Dvl2-D2 and dvl2-D4 templates were kindly provided by J. Wallingford (University of Texas, Austin) and were cut with NotI and transcribed with SP6 RNA polymerase. En-R-LefN-GR744A mRNA was synthesized as previously described (Deroo et al., 2004). Vangl2 was cut with AspI and transcribed with SP6 RNA polymerase to generate mRNA encoding a vangl2/GFP fusion as previously described (Jenny et al., 2003). All mRNAs were purified by column chromatography with Sephadex G-50 medium (Sigma S6022). Injections of 5 to 10 nl of 200 pg mRNA were performed at the 8- to 16-cell stage into blastomere V2 or V2.2 blastomeres, respectively, (Moody, 1987) in 2% (w/v) Ficoll 400. Two to 4 hr after injection, embryos were transferred to 0.1 × MMR and cultured at 13°C until they reached the desired stage. For glucocorticoid receptor-regulated constructs, 4 µg/ml dexamethasone (Sigma D4902) was added to the medium at stage 21/22. Embryos were then cultured for 2 hr at room temperature in the presence of dexamethasone, followed by fixation in MEMFA (Sive et al., 2000) and storage in 100% (v/v) methanol.
Tyramides and Antibodies
Fluor-coupled tyramides were synthesized in-house as previously described (Vize et al., 2009). Fluorescent in situ hybridization was performed as described by Gerth et al. (2005) and Vize et al. (2009). For the lineage-traced fluorescent in situ hybridization (FISH)/phosphoH3 experiments, FISH was performed against either lhx1 (GenBank NM_001090659 cut with XhoI and transcribed with T7 RNA polymerase, Taira et al., 1994) for stage 20–30 samples, or atp1a1 (GenBank U49238 cut with SmaI and transcribed with T7 RNA polymerase, Zhou and Vize, 2004) for post-stage 30 samples then developed using a fluorescein-tyramide. Embryos were subsequently developed with a rabbit anti–phospho-histone H3 polyclonal (Millipore 06-570)/donkey anti–rabbit-horseradish perxidase (HRP) and a Cy3 tyramide, followed by development with anti-GFP-Alexa647 (Invitrogen A31852)/donkey anti–rabbit-HRP and a Cy5.5 tyramide. Fluorescein coupled Erythrina cristagalli lectin was from Vector Laboratories (FL-1141) and used at 50 µg/ml to stain proximal tubules.
Confocal Microscopy
Following FISH and immunofluorescence embryos were bleached, dehydrated, and cleared (Vize et al., 2009) using methyl salicylate (Sigma M6752). Confocal microscopy was performed using a Leica TCS-SL inverted confocal microscope and a HC PL APO long working distance 10× water immersion lens. A stack of 50 overlapping sections was collected for each pronephros, sampling in the fluorescein isothiocyanate (FITC), Cy3 and Cy5.5 spectra. Stacks were typically approximately 120 microns thick in toto. Each image stack was manually scanned and phospho-histone H3 positive nuclei that were within the kidney marker expression space scored.
Supplementary Material
ACKNOWLEDGMENTS
The En-R-LefN-GR744A construct was kindly provided by Jon Lyons and Pierre McCrea, vangl2, dvl2-D2, and dvl2-D4 constructs provided by John Wallingford, and 3G8 and 4A6 antibodies were from Elizabeth Jones. The image in panel 1A was provided by C. White. The work was initially supported by the AHFMR and through salary support to P.D.V. from the NICHD.
Grant sponsor: NICHD; Grant number: R01 HD45776; Grant number: P41 HD064556.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Brennan HC, Nijjar S, Jones EA. The specification of the pronephric tubules and duct in Xenopus laevis. Mech Dev. 1998;75:127–137. doi: 10.1016/s0925-4773(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, McMahon AP. Secreted molecules in metanephric induction. J Am Soc Nephrol. 2000;11 suppl 16:S116–S119. [PubMed] [Google Scholar]
- Carroll T, McMahon AP. The molecular basis of kidney development. In: Vize PD, Woolf A, Bard JB, editors. The Kidney; from normal development to congenital disease. Amsterdam: Academic Press; 2003. pp. 343–376. [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- Chi L, Zhang S, Lin Y, Prunskaite-Hyyrylainen R, Vuolteenaho R, Itaranta P, Vainio S. Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal gdnf and stromal Fgf7 signalling during kidney development. Development. 2004;131:3345–3356. doi: 10.1242/dev.01200. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene stabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Tan C, Conrad LJ, Klein PS. Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development. 1998;125:2687–2700. doi: 10.1242/dev.125.14.2687. [DOI] [PubMed] [Google Scholar]
- Davison G, Shen J, Huang YL, Su Y, Karaulanov E, Bartsherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of wnt receptor activation. Dev Cell. 2009;17:749–750. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Deroo T, Denayer T, Van Roy F, Vleminckx K. Global inhibition of Lef1/Tcf-dependent wnt signaling at its nuclear end point abrogates development in transgenic Xenopus embryos. J Biol Chem. 2004;279:50670–50675. doi: 10.1074/jbc.M408969200. [DOI] [PubMed] [Google Scholar]
- Gerth VE, Zhou X, Vize PD. Nephrin expression and three-dimensional morphogenesis of the Xenopus pronephric glomus. Dev Dyn. 2005;233:1131–1139. doi: 10.1002/dvdy.20415. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the structure and development of vertebrates. London: Macmillan and Co.; 1930. [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA. Xenopus: a prince among models for pronephric kidney development. J Am Soc Nephrol. 2005;16:313–321. doi: 10.1681/ASN.2004070617. [DOI] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–168. [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD. Wnt-4 activates the canonical beta-catenin-mediated wnt pathway and binds frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Lyons JP, Miller RK, Zhou X, Weidinger G, Deroo T, Denayer T, Park JI, Ji H, Hong JY, Li A, Moon RT, Jones EA, Vleminckx K, Vize PD, McCrea PD. Requirement of Wnt/beta-catenin signaling in pronephric kidney development. Mech Dev. 2009;126:142–159. doi: 10.1016/j.mod.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén L. Organogenesis of the kidney. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Sokol SY. Analysis of dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Taira M, Otani H, Jamrich M, Dawid IB. Expression of the LIM class homeobox gene xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development. 1994;120:1525–1536. doi: 10.1242/dev.120.6.1525. [DOI] [PubMed] [Google Scholar]
- Tetelin S, Jones EA. Xenopus Wnt11b is identified as a potential pronephric inducer. Dev Dyn. 2009;239:148–159. doi: 10.1002/dvdy.22012. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Urban AE, Zhou X, Ungos JM, Raible DW, Altmann CR, Vize PD. FGF is essential for both condensation and mesenchymal-epithelial transition stages of pronephric kidney tubule development. Dev Biol. 2006;297:103–117. doi: 10.1016/j.ydbio.2006.04.469. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Gottardi CJ. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev Dyn. 2010;239:34–44. doi: 10.1002/dvdy.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize PD, Hemmati-Brivanlou A, Harland R, Melton DA. Assays for gene function in developing Xenopus embryos. Methods Cell Biol. 1991;36:367–387. doi: 10.1016/s0091-679x(08)60288-5. [DOI] [PubMed] [Google Scholar]
- Vize PD, Jones EA, Pfister R. Development of the Xenopus pronephric system. Dev Biol. 1995;171:531–540. doi: 10.1006/dbio.1995.1302. [DOI] [PubMed] [Google Scholar]
- Vize PD, Seufert DW, Carroll TJ, Wallingford JB. Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev Biol. 1997;188:189–204. doi: 10.1006/dbio.1997.8629. [DOI] [PubMed] [Google Scholar]
- Vize PD, Carroll TJ, Wallingford JB. Induction, development and physiology of the pronephric tubules. In: Vize PD, Woolf AS, Bard JBL, editors. The kidney: from normal development to congenital disease. Amsterdam: Academic Press; 2003a. pp. 19–50. [Google Scholar]
- Vize PD, Woolf AS, Bard JBL. The kidney: from normal development to congenital disease. Amsterdam: Academic Press; 2003b. p. 520. [Google Scholar]
- Vize PD, McCoy KE, Zhou X. Multichannel wholemount fluorescent and fluorescent/chromogenic in situ hybridization in Xenopus embryos. Nat Protoc. 2009;4:975–983. doi: 10.1038/nprot.2009.69. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23:569–582. doi: 10.2165/00023210-200923070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Vize PD. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev Biol. 2004;271:322–338. doi: 10.1016/j.ydbio.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Zhou X, Vize PD. Pronephric regulation of acid-base balance; coexpression of carbonic anhydrase type 2 and sodium-bicarbonate cotransporter-1 in the late distal segment. Dev Dyn. 2005;233:142–144. doi: 10.1002/dvdy.20225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.