Proton-coupled electron transfer (PCET) is important to the generation and transport of amino acid radicals within class I E. coli ribonucleotide reductase (RNR).1–3 RNR catalyzes the conversion of nucleoside diphosphates to deoxynucleoside diphosphates and, as such, plays a crucial role in DNA replication and repair.4 The enzyme is composed of two subunits, α2, which contains binding sites for nucleotide substrates and effectors, and β2, which contains a diiron-tyrosyl radical (•Y122) cofactor. A complex between α2 and β2 is required for activity, and turnover is putatively initiated by the transfer of the oxidizing equivalent from •Y122 to a cysteine residue (C439) at the active site in α2, therein generating a transient thiyl radical. A docking model of the individual α2 and β2 subunits separate Y122 and C439 by 35 Å;5,6 recent PELDOR studies support this long distance.7
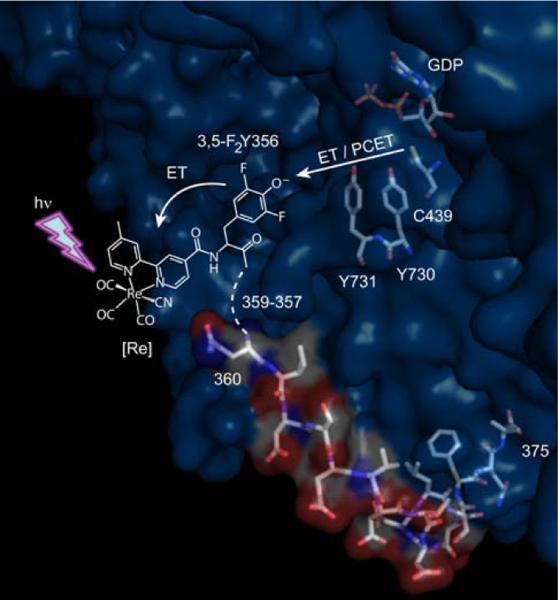
Several conserved aromatic amino acid residues between Y122 and C439 form β2(Y122↔W48↔Y356)↔(Y731↔Y730↔C439)α23 as a viable pathway for radical hopping in RNR though the precise mechanism defining the electron and proton coupling in radical transport remains to be defined.2 To study the PCET of radical transport in RNR, we have engineered photoRNRs. In these constructs, the β2 subunit is replaced by its C-terminal, 20-residue peptide (Y-R2C19), which contains both the binding determinant of β2 to α2 and the redox active Y356 residue. The RNR is transformed into a photoRNR by appending a photooxidant to Y356 on the peptide. Under these conditions, •Y356 formation and RNR turnover may be phototriggered. We have previously used Trp,8 benzophenone and anthraquinone9 to phototrigger •Y356. These studies show that radical transport in α2 most likely occurs via transfer of both a proton and an electron between the Y731↔Y730↔C439 residues shown in Figure 1; however, the •Y356 radical has not been directly detected. We now improve the photoRNR construct by the use of Re(bpy)(CO)3CN, [Re], as the photochemical Y• generator10 and the 3,5-difluorotyrosine (3,5-F2Y) unnatural amino acid11 at “position 356” in the Y-R2C19 peptide shown in Figure 1. With Y-R2C19 modified with [Re], we achieve the highest turnover yet recorded for a photoRNR and we observe, for the first time, a radical, 3,5-F2Y•, on the peptide bound to α2 that is competent for initiating deoxynucleotide formation.
Figure 1.
A photoRNR with [Re] as the photochemical radical initiator. Shown is the structure of the α2:Y-R2C19 complex from ref [6]. Only peptide residues 360–375 (β2 numbering) are visible in the structure. [Re] and 3,5-F2Y356 have been added for illustrative purposes. Excitation of [Re] with UV light initiates 3,5-F2Y• formation and radical transport in α2.
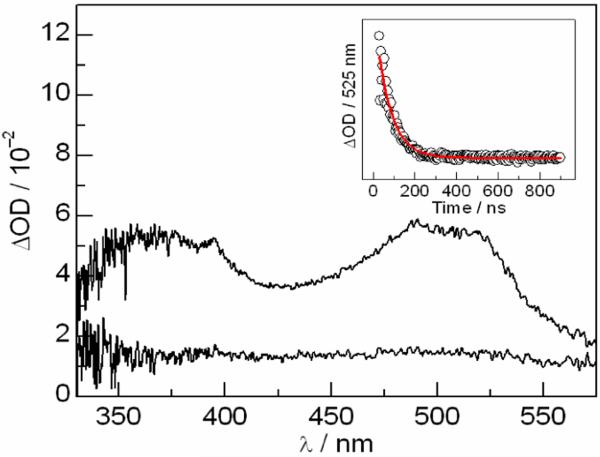
The [Re]-3,5-F2Y-R2C19 peptide was prepared using solid-phase peptide synthesis by coupling the N-terminal amino acid to COOH-functionalized bipyridine (bpy) ligand of the [Re] complex (see SI for synthetic details). Nanosecond transient absorption (TA) spectroscopy was employed to investigate photochemical tyrosyl radical generation on the modified peptide. Similar to what has previously described for the [Re]-3,5-F2Y model complex,12 excitation of the full length peptide in aqueous solutions at pH > pKa(3,5-F2Y) = 7.2 with 355 nm laser light produces the [Re0]-3,5-F2Y•-R2C19 charge separated state, which decays with a time constant of 70 ns (Figure 2). Of note, the peak at 395 nm resembles that previously observed for 3,5-F2Y• in dipeptides studies.11,12
Figure 2.
TA spectra of 100 μM [Re]-3,5-F2Y-R2C19 obtained 65 and 215 ns after a 355 ns flash. Conditions: 50 mM Tris buffer (pH 8.5), 20% glycerol, 1 mM CDP, 3 mM ATP, and 15 mM MgSO4. Insets: Single wavelength kinetics traces (∘) with single exponential fit ( ) recorded at the wavelength indicated. The peaks at 395 and 525 nm corresponds to 3,5-F Y• and bpy•- in the charge separated state.10,11
) recorded at the wavelength indicated. The peaks at 395 and 525 nm corresponds to 3,5-F Y• and bpy•- in the charge separated state.10,11
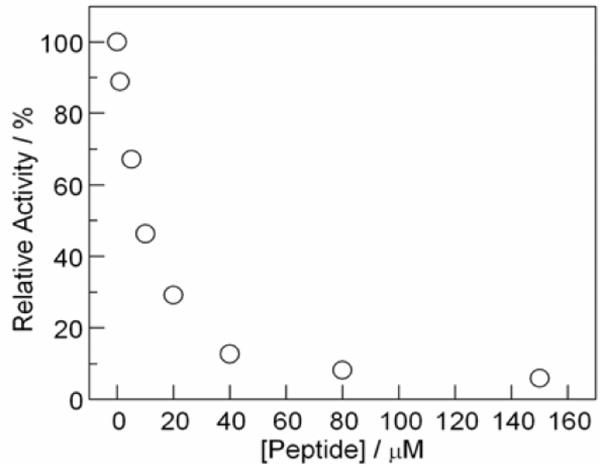
The binding of [Re]-Y-R2C19 to α2 was investigated using an established competitive inhibition assay of RNR in the presence of increasing concentrations of peptide.13 Figure 3 plots the relative activity of a solution α2 (0.1μM) and β2 (0.2 μM) towards dCDP production as a function of increasing concentrations of [Re]-Y-R2C19. As shown in the figure, this peptide effectively competes with β2 for binding to α2. The KD for the peptide:α2 interaction was estimated as 8 μM using the established competitive inhibition assay to approximate binding.9,13 This binding strength is similar to that obtained for other Y-R2C19 peptides, indicating that the large [Re] chromophore does not inhibit binding.
Figure 3.
Competitive inhibition binding assay of [Re]-Y-R2C19 to α2. The relative activity of a solution of α2 (0.1μM) and β2 (0.2 μM) was assayed spectrophotometrically as a function of increasing concentration of peptide as described in the Supporting Information.
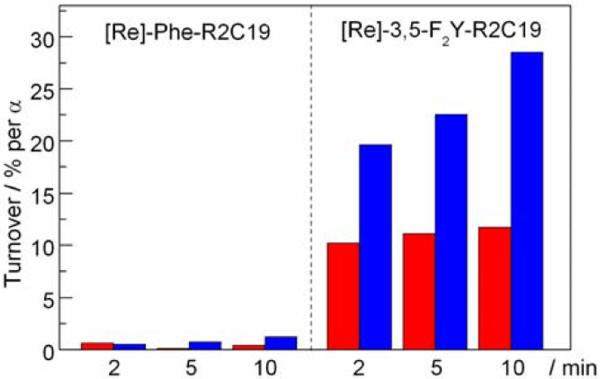
Figure 4 shows the results of the single turnover photochemical RNR assay with [Re]-3,5-F2Y-R2C19 and [Re]-Phe-R2C19 peptides. A 10:1 peptide/protein ratio was employed in order to bind the majority of α2 to peptide.14 Using the estimated KD of 8 μM and a 1:1 binding model of peptide to α, we calculate that 96% of α in solution is bound to peptide. Experiments were performed by steady state photolysis of a solutions of 200 μM peptide, 20 μM α2, 3 mM ATP effector and 0.75 mM [5-3H]-CDP substrate (with 15 mM MgSO4, 50 mM Tris buffer, and 20% glycerol) using light of λ > 348 nm. The details of the photochemical assay and data analysis are provided in the SI. The addition of glycerol in these experiments served to increase the stability of the protein and resulted in data that was more reproducible.
Figure 4.
Single turnover assays for α2 with [Re]-(Phe/3,5-F2Y)-R2C19 peptides in 20% glycerol. Data was collected after 2, 5, and 10 minutes of photolysis with light of λ > 348 nm. Red and blue bars are at pH 8.2 and 7.5, respectively.
Photolysis of the [Re]-3,5-F2Y-R2C19 peptide with α2 resulted in a modest time dependent turnover that approached, after 10 minutes, 12 and 29% (per α) at pH 8.2 and 7.5, respectively. RNR activity exhibits a pH rate profile with a maximum at pH ~8.1, although the nature of this phenomenon is not understood.15 Clearly, the photoRNR exhibits a different pH dependence than the wild-type. Mechanistic conclusions regarding the PCET of radical generation and transport cannot be drawn from pH dependent studies of a single photoRNR system, as protein conformations and electrostatics may vary with pH.
The time dependence of the turnover may be a result of the conformational flexibility of the C-terminus of the peptide bound to α2. As shown in Figure 1, this region is disordered and not located in the crystal structure of α2.6 The N-terminus of the peptide bound to α2 likely has a high degree of conformational flexibility, and sampling of the conformation that supports radical transport may occur many times during the steady state photolysis experiment. Similar time-dependent turnover numbers were measured on the minute timescale in the absence of glycerol (data not shown). We therefore do not attribute the time-dependence to an increased viscosity effect.
In contrast, the [Re]-Phe-R2C19 peptide essentially yields no turnover (< 1 %) throughout the photolysis experiment at both pHs. The lack of turnover with this peptide indicates that the 3[Re]* excited state cannot directly generate radicals in α2 that are competent for turnover, and that radical transport in the [Re]-3,5-F2Y-R2C19:α2 system must proceed through the transiently generated 3,5-F2Y• radical on the peptide.
The percent turnover obtained with the [Re]-3,5-F2Y-R2C19 peptide at pH 7.5 after 10 minutes (29 %) is the highest yet recorded for any photoRNR system. Previous photoRNRs were constructed using benzophenone and anthraquinone photooxidants.9 Upon excitation, these chromophores were shown to degrade the α2 protein, thus limiting the overall turnover yield. The photochemistry of the 3[Re]* excited state, however, is specific for oxidation of the proximal 3,5-F2Y residue on the peptide. Furthermore, the [Re]0–3,5-F2Y• charge separated state decays via charge recombination on the nanosecond timescale, thereby limiting spurious side reactions of 3,5-F2Y•.
Mounting evidence supports that the active α2:β2 complex is asymmetric. Studies of disulfide bond formation upon reduction of CDP reveal that the active site cysteines of a single α become oxidized to cystine in the pre-steady state.16 Pre-steady state and steady state analyses of dCDP formation reveal that up to 3.0 eq of dCDP may be formed per α2, rather than the 4 eq expected for a fully symmetric complex.17 3,4-Dihydroxyphenylalanine (DOPA) may be site-specifically incorporated into β2 at position 356 and therein serves as a thermodynamic trap during radical transport.18,19 The DOPA• radical is only observed upon mixing the α2 and β2 subunits in the presence of substrate and effector, and only ~50% of •Y122 radical becomes trapped as DOPA•. If the active complex of the photoRNR is also asymmetric, we may be limited to a maximum of 50% turnover per α, which is approached here using [Re] as the radical initiator.
Encouraged by the high turnover, we sought to observe the radical upon photolysis of the peptide:α2 complex using TA spectroscopy. Sufficient signal-to-noise ratios were obtained with a minimal amount of spectral averaging (~100 spectra) by exciting 100 μM solutions of the peptide with the third harmonic output of a nanosecond Nd:YAG laser (355 nm, 1–2 mJ per pulse). Sample sizes could be limited to 200 μL through the use of a 2 × 10 mm microcuvette. The white light probe and laser pump beams were focused down to 2 mm and strictly overlapped such that they could be passed through the cuvette along the 10 mm pathlength. This configuration allowed for optimum signal at the low concentrations of the peptide used.
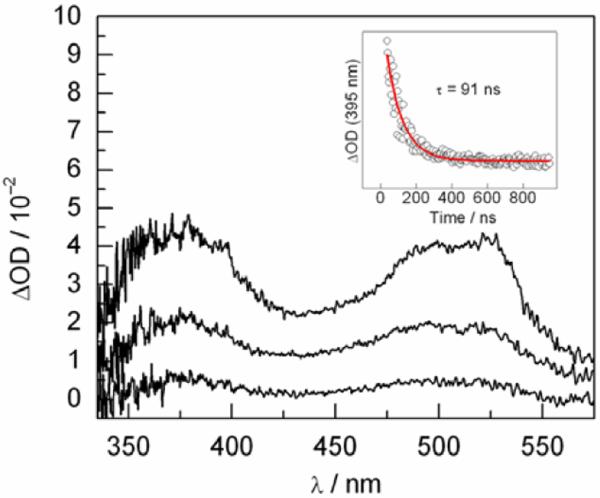
To observe the 3,5-F2Y• radical on the peptide, bound to α2, the Y731F-α2 variant was employed. This mutation inhibits photochemical RNR turnover8 by preventing radical transport from •Y356 on the peptide to Y730 in α2. 100 μM [Re]-3,5-F2Y-R2C19 peptide was mixed with 140 μM Y731F-α2 (with 1 mM CDP, 3 mM ATP, 15 mM MgSO4, 50 mM Tris, 20% glycerol) at pH 8.2 such that most of the peptide would be bound to protein (87% based on KD = 8 μM). Figure 5 shows the absorption spectra obtained following excitation of this solution with the 355 nm laser pulse. The spectrum at the earliest time point (65 ns) resembles an admixture of the 3[Re]* excited state and the [Re]0–3,5-F2Y• charge separated state,10,12 with a peak at 380 nm (3[Re]*) and a shoulder at 395 nm (3,5-F2Y•). Single wavelength kinetics recorded at 395 nm could be fit to a single exponential decay function with a lifetime of 91 ns.
Figure 5.
TA spectra of a 100 μM solution of [Re]-3,5-F2Y-R2C19 in the presence of 140 μM Y731F-α2, 1 mM CDP, and 3 mM ATP in 20% glycerol at pH 8.2. Spectra were recorded at 65, 115, and 215 ns. Inset: Single wavelength absorbance kinetics recorded at 395 nm.
The peak at 395 nm resembles that observed for 3,5-F2Y• in the TA spectrum of the full length [Re]-3,5-F2Y−-R2C19 peptide free in solution (Figure 1). Under the experimental conditions, 87% of the peptide should be bound. To illustrate that the peak at 395 nm in Figure 5 corresponds to 3,5-F2Y• bound to α2, we have calculated a spectrum consisting of 87% 3[Re]* excited state and 13% [Re]0-3,5-F2Y• (Figure S5), as would be the case if binding of the peptide inhibited radical formation. The peak at 395 nm is hardly visible in this theoretical spectrum, thus the radical observed in Figure 5 must be bound to α2.
The data of Figure 5 constitute a breakthrough in the investigation of the PCET pathway of radical transport in RNR. The [Re] chromophore, in combination with fluorotyrosines, provides a method for selectively generating tyrosyl radicals on the FnY-R2C19 peptide bound to α2 that are competent for turnover and that can be observed with TA spectroscopy. Future experiments will focus on the use of Y730F and C439S-α2 variants to trap the radical in α2 and monitor the kinetics for radical hopping from the peptide into the protein. These experiments will ultimately address whether conformational gating or PCET is rate limiting in •Y356→Y731 radical transport in this system.
Supplementary Material
Photo-ribonucleotide reductases (photoRNRs) are developed for the generation and transport of amino acid radicals by proton-coupled electron transfer (PCET) in this enzyme. The β2 subunit has been replaced with the [Re]-3,5-F2Y-R2C19 peptide, which substitutes 3,5-F2Y for Y at “position 356” and contains the Re(bpy)(CO)3CN ([Re]) photochemical radical generator. Excitation of this peptide with 355 nm light produces the [Re]0-3,5-F2Y• charge separated state within the nanosecond laser pulse, as characterized by transient absorption (TA) spectroscopy. Excitation of the bound peptide:α2 complex results in 29% turnover after 10 minutes of photolysis, while the corresponding [Re]-Phe-R2C19:α2 system is inactive. The 3,5-F2Y• radical on the peptide bound to the Y731F-α2 variant has been observed by TA spectroscopy. These data allow us to observe, for the first time, a peptide-derived, protein-bound radical that is competent for initiating RNR turnover.
Acknowledgments
We thank the National Institutes of Health for support of this work GM47274 (DGN) and GM29595 (JS).
Footnotes
Supporting Information Available: Synthetic details for peptide synthesis/purification and protein isolation; TA studies for the [Re]-(Phe/ 3,5-F2Y)-R2C19 peptides; binding assay for [Re]-Y-R2C19 to α2; details of the photochemical assay.
References
- 1.List of Abbreviations: α2 large subunit of RNR containing substrate and effector binding sites; ATP, adenosine-5'-triphosphate; β2, small subunit of RNR containing the diiron-tyrosyl radical cofactor; CDP, cytidine-5'-diphosphate; DNA, deoxyribonucleic acid; PCET, proton-coupled electron transfer; PELDOR, pulsed electron-electron double resonance; R2C19, 19-mer, C-terminal peptide tail of β2; [Re], Re(bpy)(CO)3CN; RNR, class I E. coli ribonucleotide reductase; TA, transient absorption; Tris, tris(hydroxymethyl)aminomethane
- 2.Reece SY, Hodgkiss JM, Stubbe J, Nocera DG. Phil. Trans. Roy. Soc. B. 2006;361:1351. doi: 10.1098/rstb.2006.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stubbe J, Nocera DG, Yee CS, Chang MCY. Chem. Rev. 2003;103:2167. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- 4.Jordan A, Reichard P. Annu. Rev. Biochem. 1998;67:71. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 5.Uhlin U, Eklund H. Nature. 1994;370:533. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson M, Uhlin U, Ramaswamy S, Ekberg M, Regnström K, Sjöberg BM, Eklund H. Structure. 1997;5:1077. doi: 10.1016/s0969-2126(97)00259-1. [DOI] [PubMed] [Google Scholar]
- 7.Bennati M, Robblee JH, Mugnaini V, Stubbe J, Freed JH, Borbat P. J. Am. Chem. Soc. 2005;127:15014. doi: 10.1021/ja054991y. [DOI] [PubMed] [Google Scholar]
- 8.Chang MCY, Yee CS, Stubbe J, Nocera DG. Proc. Nat. Acad. Sci. USA. 2004;101:6882. doi: 10.1073/pnas.0401718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reece SY, Seyedsayamdost MR, Stubbe J, Nocera DG. J. Am. Chem. Soc. 2007;129:8500. doi: 10.1021/ja0704434. [DOI] [PubMed] [Google Scholar]
- 10.Reece SY, Nocera DG. J. Am. Chem. Soc. 2005;127:9448. doi: 10.1021/ja0510360. [DOI] [PubMed] [Google Scholar]
- 11.Seyedsaymdost MR, Reece SY, Nocera DG, Stubbe J. J. Am. Chem. Soc. 2006;128:1569. doi: 10.1021/ja055926r. [DOI] [PubMed] [Google Scholar]
- 12.Reece SY, Seyedsayamdost MR, Stubbe J, Nocera DG. J. Am. Chem. Soc. 2006;128:13654. doi: 10.1021/ja0636688. [DOI] [PubMed] [Google Scholar]
- 13.Climent I, Sjöberg B-M, Huang CY. Biochemistry. 1991;30:5164. doi: 10.1021/bi00235a008. [DOI] [PubMed] [Google Scholar]
- 14.Chang MCY, Yee CS, Stubbe J, Nocera DG. Proc. Natl. Acad. Sci. USA. 2004;101:6882. doi: 10.1073/pnas.0401718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyedsaymdost MR, Yee CS, Reece SY, Nocera DG, Stubbe J. J. Am. Chem. Soc. 2006;128:1562. doi: 10.1021/ja055927j. [DOI] [PubMed] [Google Scholar]
- 16.Erikson HK. Biochemistry. 2001;40:9631. doi: 10.1021/bi010651n. [DOI] [PubMed] [Google Scholar]
- 17.Ge J, Yu G, Ator MA, Stubbe J. Biochemistry. 2003;42:10071. doi: 10.1021/bi034374r. [DOI] [PubMed] [Google Scholar]
- 18.Seyedsayamdost MR, Stubbe J. J. Am. Chem. Soc. 2006;128:2522. doi: 10.1021/ja057776q. [DOI] [PubMed] [Google Scholar]
- 19.Seyedsayamdost MR, Stubbe J. J. Am. Chem. Soc. 2007;129:2226. doi: 10.1021/ja0685607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.