Abstract
Study Objectives:
To assess whether changes in psychomotor vigilance during sleep deprivation can be estimated using heart rate variability (HRV).
Design:
HRV, ocular, and electroencephalogram (EEG) measures were compared for their ability to predict lapses on the Psychomotor Vigilance Task (PVT).
Setting:
Chronobiology and Sleep Laboratory, Duke-NUS Graduate Medical School Singapore.
Participants:
Twenty-four healthy Chinese men (mean age ± SD = 25.9 ± 2.8 years).
Interventions:
Subjects were kept awake continuously for 40 hours under constant environmental conditions. Every 2 hours, subjects completed a 10-minute PVT to assess their ability to sustain visual attention.
Measurements and Results:
During each PVT, we examined the electrocardiogram (ECG), EEG, and percentage of time that the eyes were closed (PERCLOS). Similar to EEG power density and PERCLOS measures, the time course of ECG RR-interval power density in the 0.02- 0.08-Hz range correlated with the 40-hour profile of PVT lapses. Based on receiver operating characteristic curves, RR-interval power density performed as well as EEG power density at identifying a sleepiness-related increase in PVT lapses above threshold. RR-interval power density (0.02-0.08 Hz) also classified subject performance with sensitivity and specificity similar to that of PERCLOS.
Conclusions:
The ECG carries information about a person's vigilance state. Hence, HRV measures could potentially be used to predict when an individual is at increased risk of attentional failure. Our results suggest that HRV monitoring, either alone or in combination with other physiologic measures, could be incorporated into safety devices to warn drowsy operators when their performance is impaired.
Citation:
Chua ECP; Tan WQ; Yeo SC; Lau P; Lee I; Mien IH; Puvanendran K; Gooley JJ. Heart rate variability can be used to estimate sleepiness-related decrements in psychomotor vigilance during total sleep deprivation. SLEEP 2012;35(3):325-334.
Keywords: Sleepiness, PVT, HRV, ECG, heart rate, EEG, PERCLOS
INTRODUCTION
Sleepiness is a major cause of vehicular and occupational accidents, resulting in a large societal and economic burden.1,2 Insufficient sleep results in a broad range of performance deficits, including decreased vigilance, slower response times, increased errors, and impaired decision making.3 These performance deficits and the inability to resist falling asleep contribute to automobile crashes and industrial accidents (e.g., the Exxon Valdez oil spill and nuclear accidents in Chernobyl and on Three Mile Island).4
Sleep loss can result from acute sleep deprivation, daily sleep restriction (e.g., ≤ 6 h of sleep per night), or when sleep is disrupted as part of a sleep disorder. The effects of sleepiness on performance have been compared with the effects of being intoxicated with alcohol. After 24 hours of sustained wakefulness, performance decrements on some tasks are similar to those observed at a blood alcohol concentration of 0.10%, beyond the legal limit for driving.5 A similar negative impact of sleep loss on performance has been demonstrated when individuals are tested after a week of sleep restriction with only 6 hours of time in bed per night.6 Of particular concern, self-rated sleepiness and performance can become dissociated during sleep deprivation. Hence, a person's perceived level of alertness often does not correspond with his or her actual decline in vigilance during sleep loss.
Given that sleepiness increases a person's risk for attentional failure, errors, and accidents, many technologies have been developed to assess vigilance and performance levels in real time.7 Such technologies aim to warn the user when a lapse in performance is likely to occur and, if coupled with an appropriate intervention, could potentially prevent an accident from taking place. Technologies that seek to monitor online an individual's physiologic state have primarily focused on features of the eyes, face, and head. Several ocular measures have been examined as indicators of fatigue, including eye blinks, pupil responses, saccadic eye movements, and percentage of eyelid closure over the pupil over time (PERCLOS). Of these measures, PERCLOS has been shown to correlate with lapses in visual attention and measures of simulated driving performance during sleep loss.8,9 Although PERCLOS data can be collected noninvasively from dashboard-mounted cameras, it can be difficult to obtain quality retinal reflections from some users' eyes, and PERCLOS does not always work well during the daytime when sunlight is being reflected inside the vehicle.10 Similar to ocular measures, electroencephalogram (EEG) monitoring can be used to track changes in drowsiness and fatigue.11,12 At present, however, such monitors are not very practical for ambulatory or in-vehicle use.Given that each fatigue-monitoring technology has its limitations, an integrated system that assesses multiple bio-signals simultaneously may perform better than any one measure taken alone. Here, we examined heart rate variability (HRV) as a potential adjunct to existing measures of sleepiness derived from the eyes, face, and head. As demonstrated by routine clinical use of 24-hour Holter monitoring, electrocardiogram (ECG) signals can be collected continuously in diverse operational settings. In addition, new technologies allow for noncontact recording of heart-beat measurements.13 Recently, it was shown that some measures of HRV can be used to predict daytime performance levels.14 In the present study, we extend these findings by examining whether HRV can be used to estimate changes in sustained visual attention during total sleep deprivation. We show that, similar to EEG and PERCLOS measures, RR-interval power density in the 0.02- to 0.08-Hz frequency range correlates strongly with lapses on the Psychomotor Vigilance Task (PVT) and can be used to estimate decrements in PVT performance caused by sleepiness.
METHODS
Subjects
Young healthy Chinese men (n = 24) (mean age ± SD = 25.9 ± 2.8 years) were enrolled in a 4-day study at the Chronobiology and Sleep Laboratory (CSL), Duke-NUS Graduate Medical School Singapore. Health was assessed by screening questionnaires and self-reported medical history. Participants had an average body-mass index of 22.3 ± 2.7 kg/m2 and reported no use of medications or nicotine products. Individuals with an extreme chronotype (< 31 or > 69 on the Horne-Östberg questionnaire) or Pittsburgh Sleep Quality Index score of greater than 7 were excluded (mean ± SD = 3.5 ± 1.6). Subjects were ineligible if they had a history of shift work or if they travelled across time zones within 3 weeks prior to the start of the study. For at least 1 week before being admitted to the CSL, participants were required to maintain a fixed sleep-wake cycle of their choice (8 hours sleep, 16 hours wake), which was verified by actigraphy monitoring (Actiwatch-L, MiniMitter, Inc., Bend, OR). During prestudy screening, subjects were asked to avoid caffeine, alcohol, and over-the-counter medications. Informed consent was obtained from all participants, and research procedures were approved by the SingHealth Centralized Institutional Review Board. Procedures were in compliance with the Health Insurance Portability and Accountability Act regulations and the Declaration of Helsinki.
Protocol
Subjects lived individually for 4 days at the CSL in a sound-attenuated and windowless environment that was shielded from external time cues. Participants arrived in the evening and went to bed at their regular prestudy sleep time. After an 8-hour sleep opportunity, subjects underwent a 40-hour constant routine (CR) procedure consisting of wakefulness enforced by research staff, semi-recumbent position in bed, and consumption of hourly equicaloric snacks.15 Ambient light was provided by ceiling-mounted light-emitting diode lamps that were dimmed to less than 5 lux (1.9 μW/cm2). Illuminance was measured at a height of 187 cm with an ILT1400 radiometer that was fitted with an SEL-033/Y/W detector (International Light Technologies, Inc., Peabody, MA) aimed directly at the ceiling lamps. Illuminance measured horizontally at the level of the subjects' eyes was approximately 0.6 lux (range = 0.59 to 0.63 lux; 1.1 to 2.4 μW/cm2). After completing the CR procedure, participants were given a 12-hour sleep opportunity before being discharged from the study. Researchers were present at all times during the 4-day study to carry out research procedures and to ensure protocol compliance.
Subjective Sleepiness and Performance Testing
Every 2 hours during the CR procedure, subjects completed the Karolinska Sleepiness Scale (KSS),16 which is a 9-point scale for assessing sleepiness with responses ranging from “very alert (1)” to “very sleepy, great effort to keep awake, fighting sleep (9).” Subjects also completed a visual analogue scale (VAS),17 which asked participants to rate how they feel on a line labeled with the word pair “sleepy” and “alert” at opposite ends. Sustained visual attention was assessed using a 10-minute PVT. During the PVT, participants were required to maintain their fastest possible reaction time (RT) to a simple visual stimulus with random interstimulus intervals between 2 and 10 seconds.18 PVT lapses were defined as RTs that exceeded 0.5 seconds. The first test battery was given 4.5 hours after wake time, resulting in a total of 18 PVT test sessions during the CR procedure.
Physiologic Measurements and Signal Processing
The ECG, EEG, and EOG were recorded continuously during the CR procedure. Signals were bandpass-filtered at 0.3 to 35 Hz and recorded at 200 Hz using a Comet Portable EEG system from Grass Technologies (Astro-Med, Inc., West Warwick, RI). Subjects were asked to press an event marker to mark the onset and offset of PVT testing in the EEG recording. Occasionally, participants forgot to press the event marker, in which case a research technician made an annotation manually in the EEG record using a separate event marker. Given that the timing of pressing the event marker did not always coincide exactly with the onset or offset of the PVT, performance and physiologic signals (see below) were assessed during the middle 8 minutes between marked events, to ensure that physiologic data were analyzed only during the PVT.
The ECG
The ECG was recorded with a single-channel modified V5 lead. QRS peaks were detected using a Hilbert transform-based method,19 and the RR-interval time series was determined. Spectral analysis was performed on each RR-interval series using the Lomb-Scargle periodogram method.20 We examined conventional HRV spectral analysis metrics defined by the Task Force for Heart Rate Variability Analysis.21 These metrics include RR-interval spectral power in very-low-frequency (VLF, ≤ 0.04 Hz), low-frequency (LF, 0.04 – 0.15 Hz) and high-frequency (HF, 0.15 – 0.40 Hz) bands, normalized LF and HF power, and the ratio of LF to HF power. In separate analyses, RR-interval power density was determined in 0.02-Hz bins from 0.0 to 0.4 Hz.
The EEG
The EEG was recorded from the z-line using the International 10-20 System for electrode placement, with frontal (Fz), central (Cz), parietal (Pz) and occipital (Oz) derivations and 2 mastoid references (A1 and A2). Each derivation was averaged online to obtain a single mastoid-referenced channel (e.g., Fz-AVG = [Fz-A1 + Fz-A2] / 2). Each EEG signal (i.e., Fz, Cz, Pz, Oz) was visually inspected for eye blinks, slow eye movements, and artifacts caused by body movements or cardiac activity. Two-second non-overlapping epochs containing eye blinks, slow eye movements, muscle activity, or cardiac activity were excluded. On average, 23.2% ± 2.9% of epochs were free of artifacts, corresponding to 55.7 ± 6.9 epochs per PVT session. There was a small but significant decline in artifact-free epochs within each PVT session, ranging from 24.8% ± 3.3% during the first minute, to 21.7% ± 2.8% during the last minute of each 8-minute EEG segment (1-way repeated-measures analysis of variance; F22,7 = 5.37, P < 0.001). Data from 1 subject could not be used due to technical problems in the EEG recording. Artifact-free epochs during each PVT were subjected to spectral analysis using the modified periodogram method (200-Hz sampling rate, Hamming window, 512-point fast Fourier transform, 0.39-Hz bin resolution). For each PVT session, EEG power spectral density (PSD) was determined by averaging PSD in artifact-free epochs in 0.5-Hz bins from 0.5 to 16 Hz. In separate analyses, EEG PSD was averaged across delta (1.0-4.5 Hz), theta (4.5-8.5 Hz), alpha (8.5-12.5 Hz), and beta (12.5-15.5 Hz) frequency bands.22 To generate group plots, the EEG PSD in each frequency band was log transformed and averaged between subjects.
The EOG
The EOG electrodes were placed lateral to the outer canthus of each eye, with leads located slightly above (right eye) and below (left eye) the bicanthal plane. Each EOG electrode was referenced to the contralateral mastoid electrode (A1 or A2). EOG data were manually scored for eye blinks by a single scorer. For each PVT session, the middle 8-minute EOG segment was divided into 240 two-second, non-overlapping epochs. The number of epochs containing at least 1 eye blink was determined for each PVT session.
Eye-tracking
To assess PERCLOS, eye-tracking data were collected during the PVT in a subset of subjects (n = 15). Pupil diameter of the left eye was recorded at 120 Hz using an ISCAN eye-tracker (ISCAN, Inc., Woburn, MA). PERCLOS was defined as the percentage of time that the pupil was at least 80% covered by the eyelid.8 Given that PERCLOS assesses slow eyelid closures, rather than blinks, we excluded eye-closure events shorter than 400 ms; this threshold for eye-blink duration falls within the range recommended for determining PERCLOS (300 to 500 ms).23
Core body temperature
Core body temperature was collected wirelessly using an ingestible VitalSense temperature transmitter (Minimitter, Inc., Bend, OR). Subjects swallowed the telemetric sensor just prior to bedtime on the first day of the study. Core temperature was transmitted to a VitalSense Integrated Physiologic Monitor placed near the subject. Five subjects who passed the sensor during the CR procedure were given a second telemetry transmitter, and data from 3 subjects were lost due to equipment failure. Temperature data were collected every minute and averaged during each PVT session.
Data Analysis and Statistics
Correlation between PVT lapses and physiologic measures
We performed Pearson correlation analysis to determine the strength of the linear relationship between PVT lapses and each physiologic measure. After applying a z-transformation to normalize PVT performance and physiologic data within subjects, we determined the Pearson correlation coefficient (r) in each participant and then averaged between subjects. We also performed group correlation analyses, in which data from all subjects were combined prior to determining the Pearson correlation coefficient. Since all analyses were performed using normalized data, the group correlation coefficient for any given pairwise comparison was identical to the average of individual r values. Within subjects, statistical comparisons between r values for different physiologic measures vs PVT lapses were performed using the method described by Steiger, in which a Z statistic is calculated.24 The critical significance level was corrected for multiple comparisons using the Bonferroni correction (0.05 / n).
Receiver operating characteristic analysis
Receiver operating characteristic (ROC) analysis was used to compare the relative performance of PERCLOS, RR-interval PSD (0.02-0.08 Hz), frontal EEG PSD (1.0-4.5 Hz), and subjective sleepiness (VAS) at predicting an increase in PVT lapses above threshold.25 The binary classification task was to identify a threshold increase (> 25%, > 50%, or > 75%) in the number of PVT lapses, measured relative to each subject's performance during baseline (see below). These arbitrary thresholds were chosen to span different degrees of performance impairment. In most individuals, PVT lapses exceeded the 25% threshold within 2 hours after habitual bedtime; at this threshold, participants began to show an unambiguous decline in PVT performance and an increase in subjective sleepiness. Thereafter, PVT lapses increased monotonically and, in most participants, exceeded the 50% threshold during the middle of the night. The 75% threshold occurred close to habitual wake time, when most subjects were struggling to keep awake. PVT performance usually improved within the next few hours, with PVT lapses dropping below the 50% and 25% thresholds in some but not all participants. Baseline performance in individuals was determined as the median number of PVT lapses per session during the first 16 hours of wakefulness. For each subject, the range of PVT lapses was then taken as the difference of the session with the maximum number of lapses during the CR procedure and the median number of lapses during baseline. Hence, the threshold for a 25% increase in PVT lapses was computed as 0.25 times the range plus baseline.
For each physiologic measure, data were z-transformed within subjects and then pooled to construct an ROC curve. The optimal classification threshold was one that maximized the function: Sensitivity – m(1–Specificity). Specifically, a slope of the tangent to the ROC curve m was defined as
where FP is false positive, FN is false negative, and p is the pretest probability.26 Cost ratios were selected using SigmaPlot 11 software (ROC Curves Module; Systat Software Inc., San Jose, CA) and were set at 1.0, 0.5, and 0.25 for 25%, 50%, and 75% PVT lapse thresholds. Pretest probability was estimated empirically from the data, giving p equal to 0.41 for a greater than 25% increase in PVT lapses, p equal to 0.25 for a greater than 50% increase in PVT lapses, and p equal to 0.13 for a greater than 75% increase in PVT lapses.
To assess the relative performance of different physiologic measures at classifying whether a subject had performance above threshold vs performance below threshold on the PVT, we compared the area under ROC curves (AUC) using a nonparametric approach, as previously described.25,27 The χ2 statistic and associated P value for pairwise AUC comparisons were determined using SigmaPlot 11 software.
RESULTS
Time Course of PVT Performance and Physiologic Measures
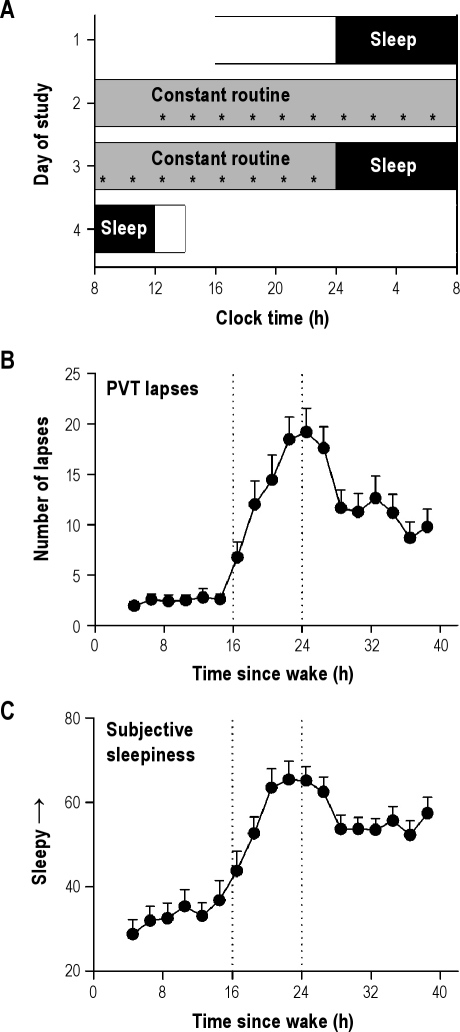
Subjects were kept awake continuously for 40 hours, during which they completed a 10-minute PVT every 2 hours to assess their ability to sustain visual attention (Figure 1A). During the usual hours of sleep, RT and the number of PVT lapses per session (RT > 0.5 s) increased sharply, reaching their highest levels at about 24 hours after wake (Figure 1B and Figure S1A). Thereafter, PVT performance improved but did not recover fully to baseline levels when subjects were rested (i.e., performance during the first 16 h of wake). This partial improvement in PVT performance after 24 hours of wakefulness was presumably due to increased circadian drive for alertness, which closely tracks the circadian rhythm of body temperature (Figure S1B).22 The profile of subjective sleepiness was similar to that observed for PVT lapses (r = 0.59 ± 0.04, P < 0.001), with highest levels of sleepiness reported between 20 and 26 hours after wake time (Figure 1C and Figure S1C).
Figure 1.
Protocol for measuring sleepiness-related changes in physiology and performance. (A) Subjects participated in a 4-day laboratory protocol that included a 40-h constant routine procedure in dim light (< 5 lux). The protocol shown is for a representative subject with habitual bedtime at midnight. Black bars show scheduled sleep episodes in darkness. Every 2 hours, subjects completed the Karolinska Sleepiness Scale, a visual analogue scale for sleepiness, and a 10-minute Psychomotor Vigilance Task (PVT; asterisks). (B) Lapses on the PVT increased during the usual hours of sleep and reached their highest levels at about 24 h after wake. Thereafter, performance showed partial recovery toward the baseline rested state. (C) Subjective sleepiness measured with the visual analogue scale (VAS) showed a similar profile to PVT lapses, with highest levels of sleepiness reported between 20 and 26 h after wake. Vertical reference lines in B and C indicate habitual bedtime and wake time. Error bars show SEM.
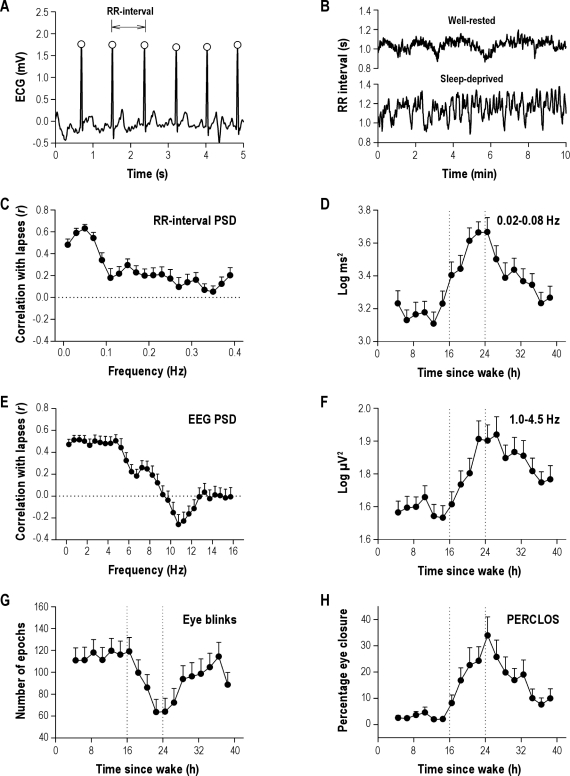
To determine whether HRV can be used to track changes in PVT performance during sleep deprivation, we examined the RR-interval time series during each PVT session (Figure 2A-B). Heart rate (i.e., 60 divided by the average of RR intervals) showed a strong circadian rhythm similar to the body-temperature profile, whereas the standard deviation of RR intervals (SDNN) showed a time course that more closely resembled the profile for PVT lapses (Figure S2). Next, we examined conventional HRV spectral analysis metrics, including spectral power in VLF, LF, and HF bands of the RR-interval time series. The profiles of VLF spectral power and PVT lapses were similar, suggesting that some measures of RR-interval PSD track a person's ability to sustain visual attention (Figure S2). To examine this possibility in greater detail, we assessed the time course of RR-interval PSD in 0.02-Hz bins from 0.0 to 0.4 Hz. PSD in the 0.02- to 0.08-Hz frequency range, which spans the upper part of the VLF band and the lower part of the LF band, correlated most strongly with PVT lapses (r = 0.68 ± 0.03, P < 0.001; Table 1 and Figure 2C). In this frequency range, the correlation coefficient (r) for RR-interval PSD versus PVT lapses was significantly greater than the r value for either VLF or LF PSD vs PVT lapses (Z = 4.66, P < 0.001; Z = 5.66, P < 0.001). Similar to the profile for PVT lapses, RR-interval PSD in the 0.02- to 0.08-Hz frequency range increased during the habitual hours of sleep and then showed partial recovery toward the baseline rested state after 24 hours of wakefulness (Figure 2D).
Figure 2.
Time course of heart rate variability, electroencephalographic (EEG) spectral power, and ocular measures during sleep deprivation. (A) The RR-interval time series during each Psychomotor Vigilance Task (PVT) was determined from the peak of consecutive QRS complexes (open circles) in the electrocardiogram (ECG). (B) As shown in a representative subject, variability in the RR-interval time series was low during the habitual hours of wakefulness, and increased during sleep deprivation. (C) RR-interval power spectral density (PSD) in the 0.02- to 0.08-Hz frequency band correlated most strongly with PVT lapses. (D) The time course of RR-interval PSD in this frequency range increased during the usual hours of sleep, and then decreased after 24 h of wake, similar to the profile of PVT lapses shown in Figure 1B. (E) For EEG PSD-derived measures, delta power (1.0-4.5 Hz) measured in the frontal derivation correlated most strongly with PVT performance. (F) The time course of delta power tracked the profile of PVT lapses and subjective sleepiness. (G) Eye blinks showed an inverted profile relative to PVT lapses, whereas (H) the time course of PERCLOS matched the profile of PVT performance during 40 h of sustained wakefulness. Vertical reference lines in D, F, G, and H indicate habitual bedtime and wake time. Error bars show SEM.
Table 1.
Correlation of different physiologic measures with PVT lapses during 40 hours of sustained wakefulness
| Measure | Signal type | Correlation coefficient (r ± SEM) | % of subjects with correlation P < 0.05 |
|---|---|---|---|
| PERCLOS | Eye-tracking | 0.77 ± 0.04* | 86.7 |
| RR-interval PSD (0.02 - 0.08 Hz) | ECG | 0.68 ± 0.03* | 90.0 |
| RR-interval SDNN | ECG | 0.67 ± 0.04* | 90.0 |
| Delta power (Fz) | EEG | 0.65 ± 0.04* | 87.0 |
| VAS (Sleepiness) | Self-report | 0.59 ± 0.04* | 66.7 |
| RR-interval VLF power | ECG | 0.57 ± 0.05* | 80.0 |
| RR-interval LF power | ECG | 0.56 ± 0.05* | 65.0 |
| KSS | Self-report | 0.56 ± 0.04* | 66.7 |
| Delta power (Cz) | EEG | 0.54 ± 0.06* | 60.9 |
| Eye blinks | EOG | −0.51 ± 0.07* | 60.9 |
| Theta power (Pz) | EEG | 0.49 ± 0.06* | 60.9 |
| Theta power (Cz) | EEG | 0.46 ± 0.07* | 52.2 |
| Time since wake | Time | 0.45 ± 0.04* | 50.0 |
| Delta power (Oz) | EEG | 0.45 ± 0.06* | 47.8 |
| Theta power (Oz) | EEG | 0.45 ± 0.09* | 65.2 |
| Delta power (Pz) | EEG | 0.44 ± 0.08* | 60.9 |
| Theta power (Fz) | EEG | 0.37 ± 0.07* | 47.8 |
| Core body temperature | Temperature | −0.30 ± 0.06* | 20.0 |
| Heart rate | ECG | −0.26 ± 0.07* | 25.0 |
| Alpha power (Oz) | EEG | −0.21 ± 0.11* | 56.5 |
| RR-interval HF power | ECG | 0.19 ± 0.06* | 20.0 |
| Alpha power (Pz) | EEG | −0.11 ± 0.10 | 39.1 |
| Alpha power (Fz) | EEG | −0.09 ± 0.10 | 39.1 |
| Alpha power (Cz) | EEG | −0.09 ± 0.10 | 43.5 |
| Beta power (Fz) | EEG | −0.05 ± 0.09 | 30.4 |
| Beta power (Oz) | EEG | −0.02 ± 0.08 | 30.4 |
| Beta power (Cz) | EEG | −0.02 ± 0.09 | 26.1 |
| Beta power (Pz) | EEG | 0.02 ± 0.10 | 26.1 |
The Pearson correlation coefficient (r) was determined for each physiologic measure vs Psychomotor Vigilance Task (PVT) lapses. Here, the mean r ± SEM is shown across subjects, with r values ranked in descending order of magnitude. Asterisks indicate significant group correlations (P < 0.001). The percentage of subjects with a significant correlation between each physiologic measure and PVT lapses is shown in the far right column.
ECG, electrocardiogram; EEG, electroencephalogram; EOG, electrooculogram; PERCLOS, percentage of eyelid closure over the pupil over time; PSD, power spectral density; SDNN: standard deviation of RR intervals; VLF, very low frequency; LF, low frequency; HF, high frequency; VAS, visual analogue scale; KSS, Karolinska sleepiness scale.
Next, we compared our findings for HRV with validated measures of sleepiness derived from EEG PSD and ocular measures (Table 1). We examined EEG PSD in 0.5-Hz bins from 0.5 to 16 Hz (Fz, Cz, Pz, and Oz; Figure 2E and Figure S3). The EEG frequency band that correlated most strongly with PVT lapses was delta (1.0-4.5 Hz) measured from the frontal derivation (r = 0.65 ± 0.04, P < 0.001). Although the correlation coefficient for RR-interval PSD (0.02-0.08 Hz) vs PVT lapses was higher than that observed for frontal EEG delta power (0.68 and 0.65, respectively), the difference in r values was not significant (Z = 1.05, P = 0.29). Similar to PVT lapses and RR-interval PSD, delta activity was relatively stable during the first 16 hours of wakefulness, followed by a sharp increase during the usual hours of sleep. After a peak in delta activity 22 to 26 hours after wake, delta power decreased but remained higher than levels measured 24 hours earlier at the same relative clock times (Figure 2F). By comparison, theta (4.5-8.5 Hz) activity increased during the night but remained elevated after 24 hours of wake, alpha power (8.5-12.5 Hz) was lowest during the usual hours of sleep, and power in the beta band increased monotonically shortly after habitual bedtime (Figure S4).
PERCLOS correlated positively with PVT performance (r = 0.77 ± 0.04, P < 0.001), whereas eye blinks showed an inverted profile relative to PVT lapses (r = −0.51 ± 0.07, P < 0.001; Figure 2G-H). Hence, as PVT performance became increasingly impaired from 16 to 24 hours after wake, subjects blinked less frequently, but the percentage of time that their eyes were closed increased.22 Of all physiologic measures examined, PERCLOS correlated most strongly with PVT lapses (Table 1), with an r value that was significantly greater than that observed for RR-interval PSD (0.02-0.08 Hz), frontal EEG delta power, and self-rated sleepiness (Z > 3.28 and P < 0.001 for all pairwise comparisons).
ROC Curves for Estimating PVT Lapses
To compare the relative performance of HRV at predicting an increase in PVT lapses vs other measures of sleepiness, we constructed ROC curves for RR-interval PSD (0.02-0.08 Hz), frontal EEG PSD (1.0-4.5 Hz), PERCLOS, and self-reported sleepiness (Table 2 and Figure 3A). For each PVT session, the binary classification task was to determine whether a subject would show a greater than 25% increase in lapses from baseline, measured relative to each individual's range of PVT performance during 40 hours of wakefulness.
Table 2.
Relative performance of different physiologic measures at identifying an increase in PVT lapses above threshold
| Measure | AUC ± SE | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | χ2 | P value |
|---|---|---|---|---|---|
| 25% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 78.0 (69.0 – 85.4) | 87.6 (81.5 – 92.2) | - | - |
| RR-interval PSD | 0.87 ± 0.02 | 75.8 (68.2 – 82.5) | 89.1 (84.1 – 93.0) | 0.56 | 0.45 |
| Sleepiness (VAS) | 0.83 ± 0.02 | 66.9 (59.4 – 73.7) | 83.5 (78.3 – 87.8) | 7.37 | 0.007 |
| Delta power (Fz) | 0.82 ± 0.02 | 76.3 (69.2 – 82.5) | 76.3 (70.5 – 81.5) | 8.74 | 0.003 |
| 50% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 76.9 (64.8 – 86.5) | 87.3 (82.0 – 91.6) | - | - |
| RR-interval PSD | 0.85 ± 0.02 | 73.4 (63.3 – 82.0) | 84.6 (79.7 – 88.7) | 6.71 | 0.01 |
| Delta power (Fz) | 0.82 ± 0.03 | 70.8 (61.1 – 79.2) | 83.8 (79.2 – 87.7) | 16.25 | < 0.0001 |
| Sleepiness (VAS) | 0.78 ± 0.02 | 60.6 (50.7 – 69.8) | 80.5 (75.8 – 84.7) | 16.38 | < 0.0001 |
| 75% PVT lapse threshold | |||||
| PERCLOS | 0.91 ± 0.02 | 81.3 (63.6 – 92.8) | 87.4 (82.5 – 91.3) | - | - |
| RR-interval PSD | 0.86 ± 0.02 | 87.5 (74.8 – 95.3) | 78.5 (73.6 – 83.0) | 2.58 | 0.11 |
| Delta power (Fz) | 0.82 ± 0.03 | 77.8 (64.4 – 88.0) | 76.9 (72.2 – 81.2) | 18.53 | < 0.0001 |
| Sleepiness (VAS) | 0.79 ± 0.03 | 60.0 (45.9 – 73.0) | 81.7 (77.4 – 85.5) | 18.75 | < 0.0001 |
Physiologic measures are ranked by area under the curve (AUC) of receiver operating characteristic (ROC) curves in descending order of magnitude at different Psychomotor Vigilance Task (PVT) lapse thresholds. Sensitivity and specificity values were determined at the optimal classification threshold, with 95% confidence intervals (CI) shown in parentheses. At each PVT lapse threshold tested, the AUC of ROC curves for RR-interval PSD (0.02-0.08 Hz), self-reported sleepiness (visual-analog scale [VAS]), and electroencephalographic (EEG) delta power were compared to the AUC for percentage of eyelid closure over the pupil over time (PERCLOS). For each pairwise comparison, the χ2 statistic is shown.
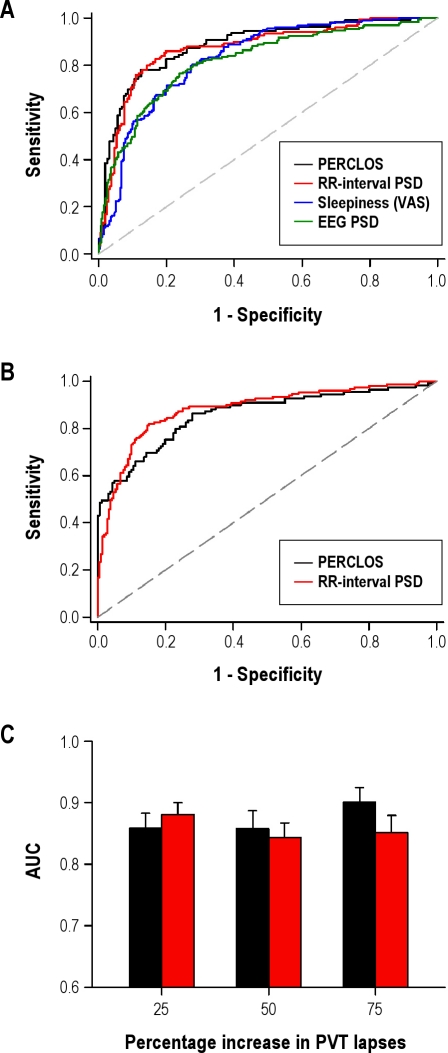
Figure 3.
RR-interval power spectral density (PSD) can be used to identify an increase in Psychomotor Vigilance Task (PVT) lapses above threshold. (A) Receiver operating characteristic (ROC) curves show the relative performance of different physiologic and subjective measures at identifying a > 25% increase in PVT lapses, measured relative to each individual's range of PVT performance. (B) Using leave-1-out cross-validation to normalize data for classification, ROC curves are shown for RR-interval PSD (0.02-0.08 Hz) versus percentage of eyelid closure over the pupil over time (PERCLOS) for classifying subject performance at the 25% PVT lapse threshold. (C) Based on area under the curve (AUC) for ROC curves at different PVT lapse thresholds, RR-interval PSD (red bars) and PERCLOS (black bars) were similar in their ability to predict a relative increase in PVT lapses. Error bars show the standard error of AUC values.
ROC curves for RR-interval PSD (0.02-0.08 Hz) and PERCLOS were similar, with mean AUC values of 0.87 ± 0.02 and 0.89 ± 0.02, respectively (χ2 = 0.56, P = 0.45; Table 2). At their respective optimal thresholds, RR-interval PSD classified subject performance with 76% sensitivity and 89% specificity, and PERCLOS classified subject performance with 78% sensitivity and 88% specificity. By comparison, self-reported sleepiness and frontal EEG delta power performed worse at determining a greater than 25% increase in PVT lapses; AUC values for these measures were 0.83 ± 0.02 and 0.82 ± 0.02, respectively (Table 2).
To address the possibility that the relative performance of RR-interval PSD at classifying subject performance would change at different thresholds for PVT lapses, we also compared AUC for greater than 50% and greater than 75% increases in PVT lapses (Table 2). Across different thresholds, RR-interval PSD provided the closest performance relative to PERCLOS at correctly identifying an increase in the number of PVT lapses. PERCLOS outperformed RR-interval PSD at the 50% threshold (χ2 = 6.71, P = 0.01), but not at the 75% threshold for PVT lapses (χ2 = 2.58, P = 0.11). By comparison, PERCLOS outperformed EEG PSD and self-rated sleepiness at all thresholds tested (χ2 > 7.3 and P < 0.01 for all pairwise comparisons).
Given that PERCLOS was measured in a subset of participants, we also compared AUC only in subjects in whom eye-tracking data were collected (n = 14 without any missing data). Consistent with our findings in the larger group, PERCLOS performed better than RR-interval PSD at the 50% threshold (χ2 = 6.18, P = 0.01) but not at the 25% or 75% thresholds for PVT lapses (χ2 = 1.70, P = 0.19; χ2 = 2.79, P = 0.10; Table S1). PERCLOS outperformed frontal EEG delta power at all thresholds tested (χ2 ≥ 6.03 and P ≤ 0.01), in addition to self-reported sleepiness at 50% and 75% thresholds for PVT lapses (χ2 ≥ 8.20 and P < 0.01). At the 25% threshold, however, the difference in AUC for PERCLOS vs self-rated sleepiness did not reach statistical significance (χ2 = 3.00 and P = 0.08).
In the analyses described above, physiologic measures that were collected during sleep deprivation were z-transformed within subjects prior to ROC analysis. In a real-world setting, a person's physiologic response to sleep deprivation would not be known in advance; hence, it would be necessary to estimate a person's normalization parameters using only his physiologic data at baseline. We therefore tested our HRV-based approach for estimating subject performance using leave-1-subject-out cross-validation to z-transform each individual's RR-interval PSD data. In the training group, the normalization parameters μ and σ were regressed linearly on mean and standard deviation of RR-interval PSD (0.02-0.08 Hz) measured during the first 16 hours of wakefulness. The resulting curves were then used to estimate μ and σ in each test subject, based on his RR-interval PSD measured during baseline. Transformed data were then pooled across subjects to generate ROC curves (Figure 3B). At the optimal classification threshold, RR-interval PSD in the 0.02- to 0.08-Hz range classified a greater than 25% increase in PVT lapses with 81% sensitivity and 85% specificity. By comparing AUC of ROC curves, we found that RR-interval PSD and PERCLOS performed equally well at identifying a greater than 25% increase in PVT lapses relative to baseline (χ2 = 1.07, P = 0.30; Figure 3C). AUC was also similar for RR-interval PSD vs PERCLOS at 50% and 75% PVT performance thresholds (χ2 = 1.07, P = 0.30; χ2 = 1.07, P = 0.30; Figure 3C). These results indicate that it is possible to estimate whether a subject will show an increase in PVT lapses above threshold by comparing his RR-interval PSD (0.02-0.08 Hz) at any point in time with his RR-interval PSD when he is well rested.
DISCUSSION
Our results demonstrate that HRV can be used to estimate sleepiness-related lapses in psychomotor vigilance. During 40 hours of sustained wakefulness, RR-interval PSD in the 0.02- to 0.08-Hz range performed nearly as well as PERCLOS at classifying subject performance on the PVT. These laboratory findings suggest that ECG-derived measures could potentially be used to assess an individual's sleepiness level, similar to existing technologies that derive fatigue or sleepiness estimates from ocular measures or the EEG.
Estimating Performance Using ECG-Derived Measures
In humans, sleepiness is regulated by the interaction of homeostatic and circadian processes.28 Hence, physiologic signals that are used to estimate sleepiness-related decrements in performance should be sensitive to homeostatic and circadian modulation. Similar to the profile of PVT lapses, we found that RR-interval PSD (0.02-0.08 Hz) increased monotonically during the usual hours of sleep, as subjects became increasingly sleep deprived. After about 24 hours of sustained wakefulness, however, RR-interval PSD and PVT lapses decreased, as the circadian rhythm of alertness opposed the build-up of homeostatic sleep pressure. These results indicate that RR-interval PSD tracks changes in PVT performance induced by interaction of the homeostatic drive to sleep and the circadian sleep-wake cycle. Our findings also extend those by Chua et al., in which it was shown that some measures of HRV correlate with PVT performance during the daytime, in the absence of sleep deprivation.14
We found that RR-interval PSD in the 0.02- to 0.08-Hz range correlated more strongly with PVT lapses compared to PSD in the VLF and LF bands (≤ 0.04 Hz and 0.04 to 0.15 Hz, respectively).21 Our findings for heart rate, its overall variability, and PSD in the VLF, LF, and HF bands are consistent, however, with previous reports using similar methodology.29,30–31 For example, the time course for PSD in the HF band, which is thought to reflect vagal activity, showed strong circadian variation during the first 24 hours of sustained wakefulness.31–33 By examining HRV over a longer CR procedure than in previous studies, however, we show that HF power was much lower during the second day of sleep deprivation compared with HF power measured 24 hours earlier at the same relative clock times. Additionally, normalized LF power and the LF/HF power ratio remained elevated beyond 24 hours of continuous wakefulness, with little or no recovery toward the baseline rested state.34 These findings are consistent with previous reports that sleep deprivation increases sympathetic tone.34–36
Taken together, our results demonstrate that various measures of HRV are differentially sensitive to the effects of total sleep deprivation. By examining PSD of the RR-interval time series across different frequency bands, we found that power in the 0.02- to 0.08-Hz range tracked PVT performance closely during sleep deprivation, suggesting that this measure of HRV could potentially be used to predict sleepiness-related decrements in psychomotor vigilance.
Technical Considerations
The primary behavioral outcome in our study was PVT lapses, which is a measure of sustained visual attention. The PVT has been used extensively to assess the effects of sleep deprivation,22 sleep restriction,6 and sleep-disordered breathing37 on vigilance levels. We did not, however, test whether sleepiness-related decrements in other cognitive functions can be estimated by RR-interval PSD, PERCLOS, or EEG PSD.
Similar to other studies, we observed large interindividual differences in PVT performance during sleep deprivation.38 Based on cumulative distribution plots for PVT lapses per session (Figure S5), 5 lapses represented roughly the 10th percentile of performance in some participants, corresponding to low levels of sleepiness during the first 16 hours of wakefulness. In other subjects, the same number of lapses corresponded to the 80th percentile of performance, when subjects were sleep deprived and fighting the urge to fall asleep. To account for these interindividual differences, we used a relative threshold for defining performance that was based on each person's range of PVT lapses during sustained wakefulness. A drawback of this approach is that our model does not predict absolute performance levels. Rather, our method addresses the question: How much worse is this person's performance right now, relative to his normal ‘rested’ performance level? As such, our approach requires individualized calibration with a baseline measure of RR-interval PSD when the subject is not deprived of sleep. This could prove difficult in a real-world setting, as many individuals are chronically sleep deprived. Additionally, for our approach to work, the user's RR-interval PSD would need to be z-transformed using normalization parameters derived from population data; such data would need to be collected prospectively in field studies.
For our laboratory findings to translate to a real-world setting, it will be important to demonstrate that RR-interval PSD can be used to predict lapses when other factors that can modulate HRV are present. For example, changes in HRV occur in cardiovascular disease21 and in response to some medications21 and may vary by sex and age.39,40 Also, it is possible that changes in posture or activity may affect RR-interval PSD in the 0.02- to 0.08-Hz frequency range. Given that we studied only young healthy men in a highly controlled laboratory environment, more studies are required to determine the influence of sex, age, health status, and activity on the relationship between HRV and sleepiness. To reduce variability in HRV in a field setting, it may be necessary to collect a person's HRV data under a standardized set of conditions or during a task in which changes in posture and activity are minimized.
Potential Applications of HRV in Fatigue Monitoring and Detection
Our laboratory results suggest that HRV deserves further evaluation as a potential indicator of sleepiness. A significant advantage of HRV over most other bio-signals is that portable and nonintrusive technologies already exist for long-term physiologic monitoring of the ECG. Telemetric cardiac monitoring has been used clinically for 50 years, and methods for collecting RR-interval data have matured substantially, with the first generation of noncontact heart rate monitors now available.13
Current approaches for assessing a person's level of sleepiness include ocular, EEG, and video-based monitoring. Consistent with a previous study that compared different fatigue-monitoring technologies,8 we found that PERCLOS outperformed EEG and eye-blink measures at estimating PVT performance. By comparison, RR-interval PSD and PERCLOS classified PVT performance with similar sensitivity and specificity. Given that PERCLOS associates reliably with lapses in attention and driving performance,8 in future studies it will be important to test whether RR-interval PSD can also be used to predict drowsiness-related lapses in simulated driving performance. Also, because we examined HRV only during total sleep deprivation, in future studies, it will be important to test whether RR-interval PSD can be used to estimate sleepiness-related decrements in performance during chronic sleep restriction or acute partial sleep loss.
In the present study, we did not examine whether HRV measures can be used to assess sleepiness on a moment-to-moment basis. It is possible that a person could temporarily increase his alertness level or RR-interval PSD in the 0.02- to 0.08-Hz frequency range by performing a stimulating activity (e.g., by singing aloud to music, exercising, or pinching himself). Such strategies are sometimes employed by drowsy drivers in an attempt to override the urge to fall asleep at the wheel. Although ocular measures, EEG, or video-based monitoring systems may outperform HRV metrics in detecting when an operator has fallen sleep, our results nonetheless suggest that HRV measures could potentially be used to track an individual's overall sleepiness level during tasks that require sustained visual attention. Such information could be used to appropriately time breaks, naps, or pharmacologic interventions to reduce sleepiness levels.
In conclusion, our findings demonstrate that HRV can be used to estimate decrements in PVT performance resulting from total sleep deprivation. In a laboratory setting, RR-interval PSD in the 0.02- to 0.08-Hz frequency range performed nearly as well as PERCLOS at identifying a relative increase in PVT lapses caused by sleepiness. In future studies, it will be important to test whether our findings can be translated to a real-world environment. Given that ECG acquisition is minimally intrusive and potentially does not require contact with the body, our results suggest that HRV measures could be incorporated into fatigue-monitoring systems in diverse operational settings.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Jonathan Bostick, Victor Pachas, Isabelle Jang, Hui-Ning Lim, Luuan-Chin Tan, and Eric Fang for their assistance in carrying out these studies, and the staff at the SingHealth Investigational Medicine Unit for medical supervision. Work for this study was performed at Duke-NUS Graduate Medical School Singapore. This work was supported by the Duke-NUS Signature Research Program funded by the Agency for Science, Technology and Research, Singapore, and the Ministry of Health, Singapore; National Medical Research Council, Singapore under NIG09may007 (to Dr. Gooley); SingHealth Foundation, Singapore, under SHF/FG410P/2009 (to Dr. Puvanendran).
ABBREVIATIONS
- ECG
electrocardiogram
- EEG
electroencephalogram
- EOG
electrooculogram
- HF
high frequency
- HRV
heart rate variability
- KSS
Karolinska Sleepiness Scale
- LF
low frequency
- PSD
power spectral density
- PERCLOS
percentage of eyelid closure over the pupil over time
- PVT
psychomotor vigilance task
- RT
reaction time
- ROC
receiver operating characteristic
- VAS
visual analogue scale
- VLF
very low frequency
Footnotes
A commentary on this article appears in this issue on page 307.
Time course of performance and subjective sleepiness during 40 h of sustained wakefulness. (A) Mean log reaction time (RT) on the Psychomotor Vigilance Task (PVT) increased during the usual hours of sleep. RTs were slowest at about 24 h after wake and then improved over the next several hours. (B) This improvement in performance coincided with the rising phase of core body temperature (CBT) rhythm. (C) Subjective ratings of sleepiness on the Karolinska Sleepiness Scale (KSS) showed a time course that was similar to the profile for PVT performance. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Time course of heart rate variability measures during 40 h of sustained wakefulness. Heart rate variability was measured during a 10-min PVT given every two hours. From top to bottom: HR, heart rate; SDNN, standard deviation of RR intervals; VLF, spectral power in the very low frequency band of the RR-interval time series (≤ 0.04 Hz); LF(n), normalized power in the low frequency band (0.04-0.15 Hz); LF, spectral power in the low frequency band; HF(n), normalized power in the high frequency band (0.15-0.40 Hz); HF, spectral power in the high frequency band; LF/HF; ratio of LF to HF power. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Correlation of electroencephalographic (EEG) spectral power with PVT lapses during 40 h of sustained wakefulness. EEG power was determined in 0.5 Hz bins during each Psychomotor Vigilance Task (PVT) session. Pearson correlation coefficients (r) are shown for power measured in each frequency bin vs PVT lapses. The r values were averaged across subjects, and error bars show SEM. Correlations are shown for EEG power spectral density (PSD) versus PVT lapses in (A) central, (B) parietal, and (C) occipital derivations.
Time course of electroencephalographic (EEG) power spectral density (PSD) during 40 h of sustained wakefulness. From left to right: EEG PSD measured in frontal (Fz), central (Cz), parietal (Pz) and occipital (Oz) derivations along the z-line. From top to bottom: delta (1.0-4.5 Hz), theta (4.5-8.5 Hz), alpha (8.5-12.5 Hz), and beta (12.5-15.5 Hz) frequency bands. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Interindividual differences in Psychomotor Vigilance Task (PVT) performance. (A) The time course of PVT lapses is shown in all subjects (n = 24), demonstrating large intersubject variability in performance. (B) Cumulative distribution plots are shown for PVT lapses (18 sessions) measured during 40 h of sustained wakefulness. Each trace shows PVT performance in a different subject.
Table S1.
Relative performance of different physiologic measures at identifying an increase in PVT lapses above threshold (Sub-group analysis for subjects with PERCLOS measurements and no missing data for other physiological measurements; n = 14).
| Measure | AUC ± SE | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | χ2 | P value |
|---|---|---|---|---|---|
| 25% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 79.2 (70.0 – 86.6) | 87.4 (81.5 – 92.3) | - | - |
| RR-interval PSD | 0.86 ± 0.02 | 81.2 (72.2 – 88.3) | 82.8 (75.8 – 88.4) | 1.70 | 0.19 |
| Sleepiness (VAS) | 0.84 ± 0.03 | 75.3 (65.7 – 83.3) | 83.4 (76.5 – 89.0) | 3.00 | 0.08 |
| Delta power (Fz) | 0.79 ± 0.03 | 65.2 (55.2 – 74.5) | 86.6 (80.3 – 91.7) | 6.59 | 0.01 |
| 50% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 79.0 (66.8 – 88.3) | 86.3 (80.6 – 90.9) | - | - |
| RR-interval PSD | 0.82 ± 0.03 | 72.6 (59.8 – 83.2) | 84.7 (78.8 – 89.5) | 6.18 | 0.01 |
| Sleepiness (VAS) | 0.79 ± 0.03 | 59.7 (46.5 – 72.0) | 84.7 (78.8 – 89.5) | 8.20 | 0.004 |
| Delta power (Fz) | 0.79 ± 0.03 | 81.4 (68.6 – 89.6) | 82.3 (75.9 – 87.3) | 6.03 | 0.01 |
| 75% PVT lapse threshold | |||||
| PERCLOS | 0.90 ± 0.02 | 80.7 (62.5 – 92.6) | 86.4 (81.2 – 90.7) | - | - |
| RR-interval PSD | 0.86 ± 0.03 | 87.1 (70.2 – 96.4) | 79.2 (73.2 – 84.3) | 2.79 | 0.10 |
| Sleepiness (VAS) | 0.77 ± 0.04 | 61.3 (42.2 – 78.2) | 80.1 (74.2 – 85.2) | 11.94 | < 0.0001 |
| Delta power (Fz) | 0.72 ± 0.05 | 60.0 (42.2 – 78.2) | 85.8 (80.1 – 90.3) | 11.68 | < 0.0001 |
Physiologic measures are ranked by area under the curve (AUC) of receiver operating characteristic (ROC) curves in descending order of magnitude at different PVT lapse thresholds. Sensitivity and specificity values were determined at the optimal classification threshold, with 95% confidence intervals shown in parentheses. At each PVT lapse threshold tested, the AUC of ROC curves for RR-interval PSD (0.02-0.08 Hz), self-reported sleepiness (visual-analog scale [VAS]), and electroencephalographic (EEG) delta power were compared to the AUC for percentage of eyelid closure over the pupil over time (PERCLOS). For each pairwise comparison, the χ2 statistic is shown in the far right column.
REFERENCES
- 1.Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1908–13. doi: 10.1001/jama.279.23.1908. [DOI] [PubMed] [Google Scholar]
- 2.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17:84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 4.Williamson A, Lombardi DA, Folkard S, Stutts J, Courtney TK, Connor JL. The link between fatigue and safety. Accid Anal Prev. 2011;43:498–515. doi: 10.1016/j.aap.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 7.Balkin TJ, Horrey WJ, Graeber RC, Czeisler CA, Dinges DF. The challenges and opportunities of technological approaches to fatigue management. Accid Anal Prev. 2011;43:565–72. doi: 10.1016/j.aap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Dinges DF, Mallis M., Maislin G., Powell JW. Washington, DC: National Highway Traffic Safety Administration; 1998. Final report: Evaluation of techniques for ocular measurement as an index of fatigue and the basis for alertness management. Report No.: DOT HS 808 762. [Google Scholar]
- 9.Wierwille WW, Lewin MG, Fairbanks RJ., III . Washington, DC: National Highway Traffic Safety Administration; 1996. Final report: Research on vehicle-based driver status/performance monitoring, Part III. Report No.: DOT HS 808 640. [Google Scholar]
- 10.Hartley L, Horberry T, Mabbott N, Krueger GP. Melbourne, Australia: National Road Transport Commission; 2000. Review of fatigue detection and prediction technologies. [Google Scholar]
- 11.Lal SK, Craig A. Driver fatigue: electroencephalography and psychological assessment. Psychophysiology. 2002;39:313–21. doi: 10.1017/s0048577201393095. [DOI] [PubMed] [Google Scholar]
- 12.Lal SK, Craig A, Boord P, Kirkup L, Nguyen H. Development of an algorithm for an EEG-based driver fatigue countermeasure. J Safety Res. 2003;34:321–8. doi: 10.1016/s0022-4375(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 13.Droitcour AD, Boric-Lubecke O, Lubecke VM, Lin J, Kovacs GTA. Range correlation and I/Q performance benefits in single-chip silicon doppler radars for noncontact cardiopulmonary monitoring. IEEE T Microw Theory. 2004;52:838–48. [Google Scholar]
- 14.Chua CP, McDarby G, Heneghan C. Combined electrocardiogram and photoplethysmogram measurements as an indicator of objective sleepiness. Physiol Meas. 2008;29:857–68. doi: 10.1088/0967-3334/29/8/001. [DOI] [PubMed] [Google Scholar]
- 15.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 16.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 17.Babkoff H, Caspy T, Mikulincer M. Subjective sleepiness ratings: the effects of sleep deprivation, circadian rhythmicity and cognitive performance. Sleep. 1991;14:534–9. doi: 10.1093/sleep/14.6.534. [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 19.Benitez D, Gaydecki PA, Zaidi A, Fitzpatrick AP. The use of the Hilbert transform in ECG signal analysis. Comput Biol Med. 2001;31:399–406. doi: 10.1016/s0010-4825(01)00009-9. [DOI] [PubMed] [Google Scholar]
- 20.Moody G. B. Spectral analysis of heart rate without resampling. Comput Cardiol. 1993;20:715–8. [Google Scholar]
- 21.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 22.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 23.Hammoud RI, Zhang H. Alertometer: Detecting and mitigating driver drowsiness and fatigue using an integrated human factors and computer vision approach. In: Hammoud RI, editor. Passive Eye Monitoring: Algorithms, Applications and Experiments. 1st ed. Berlin, Germany: Springer; 2008. pp. 301–22. [Google Scholar]
- 24.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–51. [Google Scholar]
- 25.Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L. The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform. 2005;38:404–15. doi: 10.1016/j.jbi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 28.Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- 29.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 30.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295:H2156–H2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandewalle G, Middleton B, Rajaratnam SM, et al. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res. 2007;16:148–55. doi: 10.1111/j.1365-2869.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 32.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 33.van Eekelen AP, Houtveen JH, Kerkhof GA. Circadian variation in base rate measures of cardiac autonomic activity. Eur J Appl Physiol. 2004;93:39–46. doi: 10.1007/s00421-004-1158-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ruger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Rev Endocr Metab Disord. 2009;10:245–60. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell NB, Riley RW, Schechtman KB, Blumen MB, Dinges DF, Guilleminault C. A comparative model: reaction time performance in sleep-disordered breathing versus alcohol-impaired controls. Laryngoscope. 1999;109:1648–54. doi: 10.1097/00005537-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 39.Stein PK, Kleiger RE, Rottman JN. Differing effects of age on heart rate variability in men and women. Am J Cardiol. 1997;80:302–5. doi: 10.1016/s0002-9149(97)00350-0. [DOI] [PubMed] [Google Scholar]
- 40.Sinnreich R, Kark JD, Friedlander Y, Sapoznikov D, Luria MH. Five minute recordings of heart rate variability for population studies: repeatability and age-sex characteristics. Heart. 1998;80:156–62. doi: 10.1136/hrt.80.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course of performance and subjective sleepiness during 40 h of sustained wakefulness. (A) Mean log reaction time (RT) on the Psychomotor Vigilance Task (PVT) increased during the usual hours of sleep. RTs were slowest at about 24 h after wake and then improved over the next several hours. (B) This improvement in performance coincided with the rising phase of core body temperature (CBT) rhythm. (C) Subjective ratings of sleepiness on the Karolinska Sleepiness Scale (KSS) showed a time course that was similar to the profile for PVT performance. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Time course of heart rate variability measures during 40 h of sustained wakefulness. Heart rate variability was measured during a 10-min PVT given every two hours. From top to bottom: HR, heart rate; SDNN, standard deviation of RR intervals; VLF, spectral power in the very low frequency band of the RR-interval time series (≤ 0.04 Hz); LF(n), normalized power in the low frequency band (0.04-0.15 Hz); LF, spectral power in the low frequency band; HF(n), normalized power in the high frequency band (0.15-0.40 Hz); HF, spectral power in the high frequency band; LF/HF; ratio of LF to HF power. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Correlation of electroencephalographic (EEG) spectral power with PVT lapses during 40 h of sustained wakefulness. EEG power was determined in 0.5 Hz bins during each Psychomotor Vigilance Task (PVT) session. Pearson correlation coefficients (r) are shown for power measured in each frequency bin vs PVT lapses. The r values were averaged across subjects, and error bars show SEM. Correlations are shown for EEG power spectral density (PSD) versus PVT lapses in (A) central, (B) parietal, and (C) occipital derivations.
Time course of electroencephalographic (EEG) power spectral density (PSD) during 40 h of sustained wakefulness. From left to right: EEG PSD measured in frontal (Fz), central (Cz), parietal (Pz) and occipital (Oz) derivations along the z-line. From top to bottom: delta (1.0-4.5 Hz), theta (4.5-8.5 Hz), alpha (8.5-12.5 Hz), and beta (12.5-15.5 Hz) frequency bands. Vertical reference lines indicate habitual bedtime and wake time. Error bars show SEM.
Interindividual differences in Psychomotor Vigilance Task (PVT) performance. (A) The time course of PVT lapses is shown in all subjects (n = 24), demonstrating large intersubject variability in performance. (B) Cumulative distribution plots are shown for PVT lapses (18 sessions) measured during 40 h of sustained wakefulness. Each trace shows PVT performance in a different subject.
Table S1.
Relative performance of different physiologic measures at identifying an increase in PVT lapses above threshold (Sub-group analysis for subjects with PERCLOS measurements and no missing data for other physiological measurements; n = 14).
| Measure | AUC ± SE | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | χ2 | P value |
|---|---|---|---|---|---|
| 25% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 79.2 (70.0 – 86.6) | 87.4 (81.5 – 92.3) | - | - |
| RR-interval PSD | 0.86 ± 0.02 | 81.2 (72.2 – 88.3) | 82.8 (75.8 – 88.4) | 1.70 | 0.19 |
| Sleepiness (VAS) | 0.84 ± 0.03 | 75.3 (65.7 – 83.3) | 83.4 (76.5 – 89.0) | 3.00 | 0.08 |
| Delta power (Fz) | 0.79 ± 0.03 | 65.2 (55.2 – 74.5) | 86.6 (80.3 – 91.7) | 6.59 | 0.01 |
| 50% PVT lapse threshold | |||||
| PERCLOS | 0.89 ± 0.02 | 79.0 (66.8 – 88.3) | 86.3 (80.6 – 90.9) | - | - |
| RR-interval PSD | 0.82 ± 0.03 | 72.6 (59.8 – 83.2) | 84.7 (78.8 – 89.5) | 6.18 | 0.01 |
| Sleepiness (VAS) | 0.79 ± 0.03 | 59.7 (46.5 – 72.0) | 84.7 (78.8 – 89.5) | 8.20 | 0.004 |
| Delta power (Fz) | 0.79 ± 0.03 | 81.4 (68.6 – 89.6) | 82.3 (75.9 – 87.3) | 6.03 | 0.01 |
| 75% PVT lapse threshold | |||||
| PERCLOS | 0.90 ± 0.02 | 80.7 (62.5 – 92.6) | 86.4 (81.2 – 90.7) | - | - |
| RR-interval PSD | 0.86 ± 0.03 | 87.1 (70.2 – 96.4) | 79.2 (73.2 – 84.3) | 2.79 | 0.10 |
| Sleepiness (VAS) | 0.77 ± 0.04 | 61.3 (42.2 – 78.2) | 80.1 (74.2 – 85.2) | 11.94 | < 0.0001 |
| Delta power (Fz) | 0.72 ± 0.05 | 60.0 (42.2 – 78.2) | 85.8 (80.1 – 90.3) | 11.68 | < 0.0001 |
Physiologic measures are ranked by area under the curve (AUC) of receiver operating characteristic (ROC) curves in descending order of magnitude at different PVT lapse thresholds. Sensitivity and specificity values were determined at the optimal classification threshold, with 95% confidence intervals shown in parentheses. At each PVT lapse threshold tested, the AUC of ROC curves for RR-interval PSD (0.02-0.08 Hz), self-reported sleepiness (visual-analog scale [VAS]), and electroencephalographic (EEG) delta power were compared to the AUC for percentage of eyelid closure over the pupil over time (PERCLOS). For each pairwise comparison, the χ2 statistic is shown in the far right column.